Abstract
Aim
The present study used a deep learning model (recurrent neural network) for testing: (i) whether social determinants are major determinants of the association among cerebrovascular disease, hearing loss and cognitive impairment in a middle‐aged or older population (hypothesis 1); and (ii) whether the association among the three diseases is very strong in the middle‐aged or older population (hypothesis 2).
Methods
Data came from the Korean Longitudinal Study of Aging (2014–2016), with 6060 participants aged ≥53 years. The association among the three diseases was divided into eight categories: one category for having no disease, three categories for having one disease, three categories for having two diseases and one category for having three diseases. Variable importance, the effect of a variable on model performance, was used for evaluating the two hypotheses. Hypothesis 1 was based on whether family support, socioeconomic status and social activity in the year 2014 were the top 10 determinants of the association in the year 2016. Hypothesis 2 was based on whether cerebrovascular disease, hearing loss and cognitive impairment in the year 2014 were the top five determinants of the association in the year 2016.
Results
Based on variable importance from the recurrent neural network, cerebrovascular disease (0.0386), cognitive impairment (0.0151) and hearing loss (0.0092) in 2014 were the top three determinants of the association in 2016. Children alive (0.0072), education (0.0049), income (0.0075), friendship activity (0.0042) and marriage (0.0036) in 2014 were the top 10 determinants of the association in 2016.
Conclusions
The findings of the present study support the two hypotheses, highlighting the importance of preventive measures, family support, socioeconomic status and friendship activity for managing the three diseases. Geriatr Gerontol Int 2019; 19: 711–716.
Keywords: association, cerebrovascular disease, cognitive impairment, hearing loss, social determinant
Introduction
Cerebrovascular disease, hearing loss and cognitive impairment are the leading causes of disease burden in the world and Korea.1, 2, 3, 4, 5, 6 Stroke accounted for the second greatest part of global mortality in 2013; that is, 12% (7 million) of 54 million deaths in the world.1 The estimated number of those with hearing loss in the world registered a rapid growth of 757% from 42 million in 1985 to 360 million in 2011.2 The global prevalence of dementia is expected to nearly double every 20 years; that is, from 36 million in 2010 to 66 million (or 115 million) in 2030 (or 2050).3 This global pattern is consistent with its local counterpart in Korea. Cerebrovascular disease was the third cause of death in Korea for 2016 (45.8/100 000),4 and otitis media was the seventh cause of disease burden in the nation for 2010 (294 disability‐adjusted life years per 100 000).5 The disability‐adjusted life years of dementia for Korea in 2010 (274 849) is projected to almost triple by 2050 (814 629).6
Then, is there strong comorbidity (or association) among the three aforementioned diseases above; that is, cerebrovascular disease, hearing loss and cognitive impairment? What determines the association? Is it true that social determinants (i.e. family support, socioeconomic status, social activity) are major determinants of the association? In fact, existing literature centers on a pair of chronic diseases only. For instance, several studies report a positive linkage between a pair of cerebrovascular disease, hearing loss and cognitive impairment, which is being affected by age, education, stroke history and vascular disorder (e.g. hypertension, diabetes).7, 8, 9, 10, 11, 12 This line of research also suffers from paying attention to only a small set of factors for the association (excluding social determinants, such as family support and social activity) and/or having a cross‐sectional research design (which cannot analyze a causal relationship between the association and its determinants). Indeed, new methods might be required for the prediction of the association as a set of multiple dependent variables, given that making a prediction over a set of multiple dependent variables is much less accurate and effective than doing so for a single dependent variable. It might be a significant contribution to develop a framework: (i) for identifying major determinants of the association among multiple chronic diseases (e.g. all 8 combinations of cerebrovascular disease, hearing loss and cognitive impairment); and (ii) for testing whether the association is very strong. It might be desirable for this framework to satisfy the following conditions as well: (iii) including a large set of demographic, socioeconomic and health‐related determinants for the association; (iv) using nationally representative longitudinal data; and (v) introducing new approaches that are much more accurate and effective for making a prediction over a set of multiple dependent variables.
In this context, the present study developed a framework based on a recurrent neural network (RNN): (i) to identify major determinants of the association among all eight combinations of cerebrovascular disease, hearing loss and cognitive impairment (as examples of chronic diseases in a middle‐aged or older population); and (ii) to test whether the association is very strong. The RNN, the central model of the present study, has been known for its performance comparable or superior to those of traditional methods, such as (multinomial) logistic regression and the random forest, regarding the prediction of chronic diseases.13, 14, 15, 16, 17, 18 Indeed, this study is characterized by: (iii) nationally representative longitudinal data for Koreans aged ≥53 years; (iv) 30 demographic, socioeconomic and health‐related determinants for the association; and (v) a new approach called “power set methods,” which changes a “multi‐label” classification design (with three dependent variables) to its “multi‐class” counterpart (with 8 categories of one dependent variable). Specifically, the present study tests the following hypotheses from the literature and discussion above:
Hypothesis 1: Social determinants are major determinants of the association among cerebrovascular disease, hearing loss and cognitive impairment in a middle‐aged or older population.
Hypothesis 2: The association among cerebrovascular disease, hearing loss and cognitive impairment is very strong in a middle‐aged or older population.
Methods
Participants
Data came from the Korean Longitudinal Study of Aging (KLoSA) in 2014 and 2016. Data were publicly available and de‐identified. The KLoSA is designed to create nationally representative longitudinal data on Koreans aged ≥45 years, which help to trace their characteristics over time and develop socioeconomic policies for these rapidly growing populations. This biennial survey involves a multi‐stage stratified sampling based on geographical areas and housing types across the nation. It uses computer‐assisted personal interviewing, and covers a wide range of demographic, socioeconomic and health‐related topics. The panels in the first, fifth and sixth waves for 2006, 2014 and 2016 consisted of 10 254, 8387 and 7893 participants aged ≥45 years, respectively. Among these 7893 participants, 1773 were excluded from this study, given that they lacked demographic, socioeconomic or health‐related information. The final sample of this study consisted of 6060 participants aged ≥53 years (75% of whom were aged >60 years, as in 2014).
Measures
Cerebrovascular disease, hearing loss and cognitive impairment in 2014 and 2016: the KLoSA question on cerebrovascular disease in 2014 and 2016 was “Since the last survey, have you ever been diagnosed by a doctor as cerebrovascular disease? 1. Yes. 5. No.” [C038]. The inquiry on hearing loss in 2014 and 2016 was “Do you experience difficulty in daily activity because of hearing loss? 1. Yes. 5. No.” [C092]. The Mini‐Mental State Examination score [C401‐C419 in the KLoSA inquiries] was recoded as no cognitive impairment (at least as high as a cut‐off) versus yes (lower than the cut‐off) with the different cut‐offs based on age, education and sex (Table S1).19
Disease–disease association in 2016: the association among cerebrovascular disease, hearing loss and cognitive impairment in 2016 was divided into eight categories: “0” for having no disease; “1,” “2” and “3” for having cerebrovascular disease only, hearing loss only and cognitive impairment only, respectively; “4,” “5” and “6” for having cerebrovascular disease and hearing loss, cerebrovascular disease and cognitive impairment, and hearing loss and cognitive impairment, respectively; and “7” for having all the three diseases. This approach, called “power set methods,” changes a “multi‐label” classification design (with 3 dependent variables) to its “multi‐class” counterpart (with 8 categories of 1 dependent variable). Here, “multi‐label” means “many (dependent) variables,” whereas “multi‐class” means “many categories” of one dependent variable.
Demographic, socioeconomic and health‐related factors in 2014: the following independent variables were also included in this study: (i) demographic factors, such as sex, age, marital status (married, separated, divorced, widowed, unmarried), the number of children alive, the number of brothers and sisters cohabiting, and parents alive (father and mother, father, mother, none); (ii) socioeconomic status including educational level (elementary school or below, junior high school, senior high school, college or above), personal income (normalized between 0 and 1), health insurance (Medicare, Medicaid) and economic activity (employed, unemployed); (iii) social activity, such as monthly frequencies of meeting with friends, religious activity, friendship activity, leisure activity, family activity, voluntary activity and political activity; (iv) health‐related factors, such as subjective health (very good, good, middle [neither good nor poor], poor, very poor), body mass index, smoker (non, former, current), drinker (non, former, current) and drug/medication intake (yes, no); (v) and other determinants, such as religion (no religion, Protestant, Catholic, Buddhist, Won Buddhist, other), residential type (apartment, other), region (big urban, small urban, rural), life satisfaction for economic status (0 to 100) and life satisfaction for overall life (0 to 100).
Statistical analysis
Six machine learning methods were compared for the prediction of the association to check the reliability of the RNN: RNN, logistic regression, decision tree, naïve Bayes, random forest and support vector machine. A decision tree consists of: (i) internal nodes (each meaning a test on an attribute [or independent variable]); (ii) branches (each denoting an outcome of the test); and (iii) terminal nodes (each representing a class label [or dependent variable]). A naïve Bayesian classifier is a predictor based on Bayes’ theorem. A random forest creates many training sets, trains many decision trees and makes a prediction with a majority vote (“bootstrap aggregation”). A support vector machine makes a prediction by maximizing a margin among hyperplanes separating data.20 Neurons in the input or previous hidden layer combine with weights in the next hidden or output layer of the RNN (feedforward algorithm). Then, the weights in the output layer and its previous hidden layers are adjusted based on how much they contributed to the loss of the RNN; that is, a gap between the actual and predicted class labels (backpropagation algorithm). Initially the weights are set as small random numbers around 0, and the feedforward and backpropagation algorithms iterate until certain criteria meet for the accurate prediction of a class label. Indeed, a long short‐term memory, a popular RNN, includes three gates (input, forget, output), the block input, the cell (or internal memory) and the block output. Given the current input and the previous hidden state, this RNN estimates the next hidden state.13, 14, 15, 16, 17, 18
The association among the three diseases with the eight categories in 2016 served as the dependent variable of the models. Cerebrovascular disease, hearing loss and cognitive impairment in 2014, and the demographic, socioeconomic and health‐related factors in 2014 served as the independent variables of the models. Data on 6060 participants were divided into training and validation sets with a 50:50 ratio (Tables S2,S3). The models were built (or trained) based on the training set with 3030 observations, then the models trained were validated based on the validation set with 3030 observations. Accuracy, a ratio of correct predictions among 3030 observations, was introduced as a criterion for validating the models trained. Variable importance from the RNN, an accuracy gap between a complete model and a model excluding a certain variable, was used for testing the two hypotheses of this study: (i) hypothesis 1 was evaluated based on whether family support (e.g. parents/children alive), socioeconomic status (e.g. education/income) and social activity (e.g. friendship/leisure activity) in 2014 were the top 10 determinants of the association in 2016; and (ii) hypothesis 2 was evaluated based on whether cerebrovascular disease, hearing loss and cognitive impairment in 2014 were the top five determinants of the association in 2016 (this can be considered to be one way of testing the correlation among the three diseases over time). Here, the greater “accuracy decrease” leads to the greater variable importance. Python 3.52 (Centrum voor Wiskunde en Informatica, Amsterdam, Netherlands) was used for the analysis in December 2018.
Results
Table 1 shows frequency tables for participants’ disease‐disease association and categorical attributes. Among the 6060 participants in Y2016, 1399 (23%) was diagnosed with at least one of the three diseases (cerebrovascular disease, hearing loss, cognitive impairment), and 181 (3%) was characterized by the diagnosis of the two or three symptoms. Among the participants in 2014, indeed, 321 (5%), 101 (2%) and 1057 (17%) were diagnosed as cerebrovascular disease, hearing loss and cognitive impairment, respectively. Table 2 shows the descriptive statistics for participants’ continuous attributes. All (or 75%) of the participants in 2014 were aged >53 years (or 60 years). On average, the age of the participants was 68 years, the number of children alive was three, the monthly income was $1266 and the monthly frequency of friendship activity was four. Based on Table 3, the accuracy of the RNN (0.8125) was higher than those of logistic regression and the random forest (0.7828 and 0.7881, respectively). In addition, variable importance from the RNN was derived by subtracting, from the accuracy of the model with all variables (the RNN full; 0.8125), the measure of the model excluding a certain variable (e.g., 0.7739 and 0.7974 for the RNN excluding cerebrovascular disease and cognitive impairment, respectively).
Table 1.
Frequency tables for participants’ disease–disease association and categorical attributes
| Count | Percentage (%) | |
|---|---|---|
| Association (in 2016) | ||
| N‐N‐N† | 4661 | 77.0 |
| Y‐N‐N‡ | 256 | 4.2 |
| N‐Y‐N | 73 | 1.2 |
| N‐N‐Y | 889 | 14.7 |
| Y‐Y‐N | 8 | 0.1 |
| Y‐N‐Y | 111 | 1.8 |
| N‐Y‐Y | 55 | 0.9 |
| Y‐Y‐Y | 7 | 0.1 |
| Education (in 2014 hereafter) | ||
| Elementary or below | 2697 | 44.5 |
| Junior high | 1053 | 17.4 |
| Senior high | 1697 | 28.0 |
| College or above | 613 | 10.1 |
| Sex | ||
| Male | 2556 | 42.2 |
| Female | 3504 | 57.8 |
| Marriage | ||
| Married | 4675 | 77.2 |
| Separated | 31 | 0.5 |
| Divorced | 126 | 2.1 |
| Widowed | 1189 | 19.6 |
| Unmarried | 39 | 0.6 |
| Religion | ||
| No religion | 3393 | 56.0 |
| Protestant | 1097 | 18.1 |
| Catholic | 381 | 6.3 |
| Buddhist | 1150 | 19.0 |
| Won Buddhist | 13 | 0.2 |
| Other | 26 | 0.4 |
| Residential type | ||
| Apartment | 4043 | 66.7 |
| Other | 2017 | 33.3 |
| Region | ||
| Urban (big) | 2492 | 41.1 |
| Urban (small) | 1955 | 32.3 |
| Rural | 1613 | 26.6 |
| Parents alive | ||
| Father and mother | 267 | 4.4 |
| Father | 93 | 1.5 |
| Mother | 1024 | 16.9 |
| None | 4676 | 77.2 |
| Health insurance | ||
| Medicare | 5737 | 94.7 |
| Medicaid | 323 | 5.3 |
| Economic activity | ||
| Employed | 2374 | 39.2 |
| Unemployed | 3686 | 60.8 |
| Subjective health | ||
| Very good | 68 | 1.1 |
| Good | 1609 | 26.6 |
| Middle (neither good nor poor) | 2729 | 45.0 |
| Poor | 1363 | 22.5 |
| Very poor | 291 | 4.8 |
| Smoker | ||
| Non | 4206 | 69.4 |
| Former | 1069 | 17.6 |
| Current | 785 | 13.0 |
| Drinker | ||
| Non | 3131 | 51.7 |
| Former | 905 | 14.9 |
| Current | 2024 | 33.4 |
| Drug/medicine intake | ||
| Yes | 3809 | 62.9 |
| No | 2251 | 37.1 |
| Cerebrovascular disease | ||
| Yes | 321 | 5.3 |
| No | 5739 | 94.7 |
| Hearing loss | ||
| Yes | 101 | 1.7 |
| No | 5959 | 98.3 |
| Cognitive impairment | ||
| Yes | 1057 | 17.4 |
| No | 5003 | 82.6 |
N‐N‐N for, cerebrovascular disease no, hearing loss no, cognitive impairment no.
Y‐N‐N for, cerebrovascular disease yes, hearing loss no, cognitive impairment no.
Table 2.
Descriptive statistics for participants’ continuous attributes
| Mean | SD | Minimum | 25% | 50% | 75% | Maximum | |
|---|---|---|---|---|---|---|---|
| Age (years) | 67.8 | 9.6 | 53 | 60 | 67 | 75 | 105 |
| Meeting with friends | 3.6 | 2.5 | 1 | 2 | 3 | 5 | 10 |
| Activity – religious | 2.1 | 0.7 | 1 | 2 | 2 | 2 | 10 |
| Activity – friendship | 4.0 | 1.5 | 1 | 4 | 4 | 4 | 10 |
| Activity – leisure | 3.0 | 0.4 | 1 | 3 | 3 | 3 | 10 |
| Activity – family | 5.0 | 0.6 | 1 | 5 | 5 | 5 | 10 |
| Activity – voluntary | 4.0 | 0.2 | 1 | 4 | 4 | 4 | 10 |
| Activity – political | 4.0 | 0.1 | 4 | 4 | 4 | 4 | 8 |
| No. children alive | 2.9 | 1.4 | 0 | 2 | 3 | 4 | 9 |
| No. brothers/sisters cohabiting | 3.5 | 1.7 | 1 | 2 | 3 | 5 | 11 |
| Monthly income ($) | 1265.8 | 1647.5 | 0 | 300 | 684 | 1666 | 36 000 |
| BMI | 23.3 | 2.9 | 12 | 22 | 23 | 25 | 82 |
| Life satisfaction – economic | 53.8 | 19.8 | 0 | 40 | 60 | 70 | 100 |
| Life satisfaction – overall | 60.5 | 16.7 | 0 | 50 | 60 | 70 | 100 |
BMI, body mass index.
Table 3.
Model performance and variable importance from the recurrent neural network
| Model | Accuracy | Variable importance |
|---|---|---|
| Multinomial logistic regression | 0.7828 | |
| Decision tree | 0.6640 | |
| Naive Bayes | 0.1680 | |
| Random forest – 1000 trees | 0.7891 | |
| Support vector machine | 0.7690 | |
| RNN full | 0.8125 | |
| RNN excluding cerebrovascular disease | 0.7739 | 0.0386 |
| RNN excluding cognitive impairment | 0.7974 | 0.0151 |
| RNN excluding hearing loss | 0.8033 | 0.0092 |
| RNN excluding age | 0.8036 | 0.0089 |
| RNN excluding No. children alive | 0.8053 | 0.0072 |
| RNN excluding education | 0.8076 | 0.0049 |
| RNN excluding income | 0.8079 | 0.0046 |
| RNN excluding activity – friend | 0.8083 | 0.0042 |
| RNN excluding sex | 0.8086 | 0.0039 |
| RNN excluding marriage | 0.8089 | 0.0036 |
| RNN excluding life satisfaction – overall | 0.8089 | 0.0036 |
| RNN excluding religion | 0.8096 | 0.0029 |
| RNN excluding meeting – friend | 0.8099 | 0.0026 |
| RNN excluding BMI | 0.8099 | 0.0026 |
| RNN excluding drug intake | 0.8099 | 0.0026 |
| RNN excluding health insurance | 0.8102 | 0.0023 |
| RNN excluding life satisfaction – economic | 0.8102 | 0.0023 |
| RNN excluding activity – religious | 0.8106 | 0.0019 |
| RNN excluding No. brothers/sisters cohabiting | 0.8106 | 0.0019 |
| RNN excluding parents alive | 0.8109 | 0.0016 |
| RNN excluding residential type | 0.8116 | 0.0009 |
| RNN excluding subjective health | 0.8116 | 0.0009 |
| RNN excluding activity – political | 0.8119 | 0.0006 |
| RNN excluding drinking | 0.8119 | 0.0006 |
| RNN excluding economic activity | 0.8129 | 0.0001 |
| RNN excluding smoking | 0.8129 | 0.0001 |
| RNN excluding activity – family | 0.8135 | 0.0001 |
| RNN excluding region | 0.8139 | 0.0001 |
| RNN excluding activity – voluntary | 0.8145 | 0.0001 |
| RNN excluding activity – culture leisure sports | 0.8149 | 0.0001 |
BMI, body mass index; RNN, recurrent neural network.
According to variable importance from the RNN (Fig. 1), cerebrovascular disease (0.0386), cognitive impairment (0.0151) and hearing loss (0.0092) in 2014 were the top three determinants of the association in 2016 (this supports hypothesis 2). Indeed, children alive (0.0072), education (0.0049), income (0.0075), friendship activity (0.0042) and marriage (0.0036) in 2014 were the top 10 determinants of the association in 2016 (this supports hypothesis 1). The logistic regression results (Table S4) provide useful information about the sign and magnitude for the effect of the major determinant on the association. For example, the odds of cerebrovascular disease in 2016 were 100‐fold as high for those with the disease in 2014 as for those without the disease in 2014. In addition, based on variable importance from the random forest (Fig. 2), the top 10 determinants of the association in 2016 were age (0.1062), income (0.0974) and drug intake (0.0848), as well as cerebrovascular disease (0.0581), hearing loss (0.0573), friendship meeting (0.0568), children alive (0.0538) and brothers/sisters cohabiting (0.0486) in 2014. The results of the RNN and the random forest both highlight the significance of promoting family support, socioeconomic status and social activity in comorbidity control.
Figure 1.
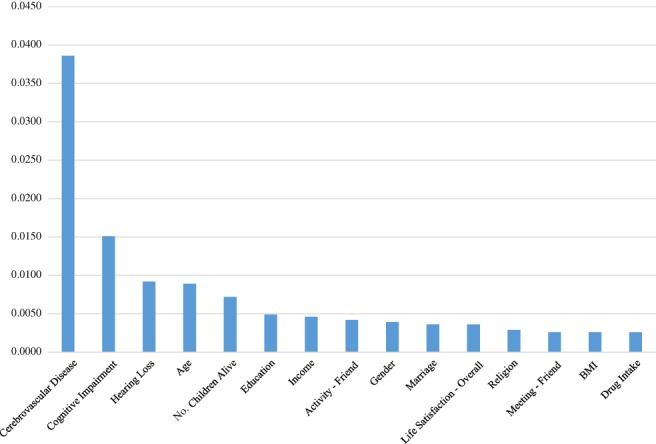
Variable importance from the artificial neural network.
Figure 2.
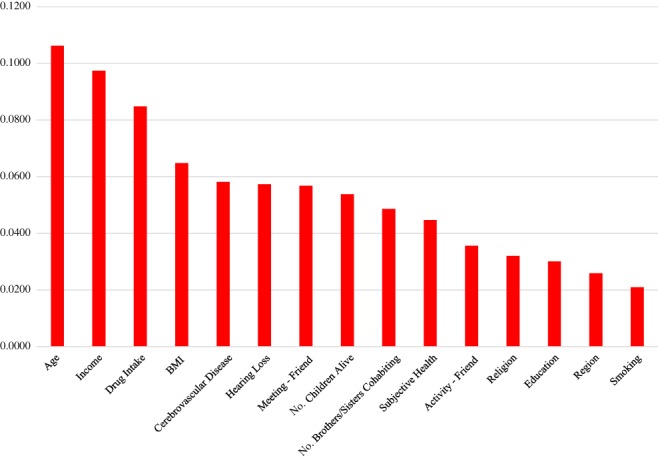
Variable importance from the random forest.
Discussion
According to the results of the present study, the RNN put more focus on cerebrovascular disease, hearing loss and cognitive impairment than the random forest (this highlights the importance of preventing the three diseases). The feedforward and backpropagation algorithms with constant learning (i.e. continued updates of weights) iterate in the RNN until certain criteria meet for the accurate prediction of a class label. This unique process of the RNN might lead to its distinctive outcomes from other machine learning methods, including the random forest. These findings also suggest that preventive measures for the three diseases should become central for health policies in Korea. Much more effort should be made for developing the effective prevention programs based on rigorous clinical trials and promoting the programs among all risk groups in the nation.
The present study also draws the following policy implications, given that family support (e.g. children alive, marriage), socioeconomic status (e.g. education/income) and social activity (e.g. friendship activity) are major determinants of the association among cerebrovascular disease, hearing loss and cognitive impairment in middle‐aged or older Koreans. First, the promotion of family support and friendship activity among those aged ≥53 years might be required to manage their cerebrovascular disease, hearing loss and cognitive impairment in Korea. As a matter of fact, family support and social activity among Koreans aged ≥60 was found to be still low, and economic burden was reported to be a major reason for the result.21, 22, 23 In this context, the following strategies and actions might be imperative for encouraging family support and social activity to improve the health conditions of older adults in the nation: (i) strengthening family services for older adults, especially with chronic disease, but no family either alive or nearby; (ii) expanding the system of vouchers and discount rates for social activity among older populations; (iii) creating more social institutions for older adults, especially in rural areas; (iv) and bringing more variety into family support and social activity in these institutions.23, 24, 25 Second, Korea's social policy needs to be updated to improve the socioeconomic status and health conditions of older adults in the nation. Korea has recently experienced an abrupt rise of the one‐person family and a sudden advent of an aged society.26, 27 Amidst these dramatic social transformations, however, social protection for “unprepared” older adults still remains much lower in Korea than in other advanced nations.28, 29 A continued expansion of social expenditure for older adults might be a priority for Korea's government policy to improve their socioeconomic status and health conditions.
The present study had some limitations. First, this study used a weak form of the longitudinal design because of constraints on memory capacity. The association among cerebrovascular disease, hearing loss and cognitive impairment with the eight categories in 2016 (wave 6) served as the dependent variable of the models, whereas the three diseases in 2014 (wave 5) and the demographic, socioeconomic and health‐related factors in 2014 (wave 5) served as the independent variables of the models. Using data from all six waves with a strong form of the longitudinal design is expected to significantly improve the accuracy of the RNN. Second, expanding this study to other chronic diseases and other determinants of association, such as health utility use, might add a great contribution to this line of research. Third, the present study did not consider possible mediating effects among variables. Fourth, this study did not consider the distributions of variables and the effects of their outliers. This study did not exclude any observation with complete information in order to make the sample size big enough. However, different management strategies for outliers in some variables (e.g. age, income) might lead to different results of variable importance from the RNN versus the random forest, and this might be a good topic for further research. Finally, subgroup analysis; e.g. 53–64 years, 65–74 years and ≥75 years in age, might have offered more insight into the major determinants of the association among the three diseases.
In conclusion, the association among cerebrovascular disease, hearing loss and cognitive impairment would be very strong in a middle‐aged or older population. For managing the association among the three diseases, the promotion of their preventive measures, family support, socioeconomic status and friendship activity would be required.
Disclosure statement
The authors declare no conflict of interest.
Supporting information
Table S1 Mini‐Mental State Examination score cut‐off for cognitive impairment.
Table S2 Data.
Table S3 Data codebook.
Table S4 Multinomial logistic regression results: odds ratio for variable/association.
Lee K‐S, Park KW. Social determinants of the association among cerebrovascular disease, hearing loss and cognitive impairment in a middle‐aged or older population: Recurrent neural network analysis of the Korean Longitudinal Study of Aging (2014–2016). Geriatr. Gerontol. Int. 2019;19:711–716. 10.1111/ggi.13716
References
- 1. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017; 120: 439–448. [DOI] [PubMed] [Google Scholar]
- 2. Olusanya BO, Neumann KJ, Saunders JE. The global burden of disabling hearing impairment: a call to action. Bull World Health Organ 2014; 92: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013; 9: 63–75.e2. [DOI] [PubMed] [Google Scholar]
- 4. Statistics Korea . Year 2016 Statistics on Causes of Death in Korea. Sejong: Statistics Korea, 2017. [Google Scholar]
- 5. Lee KS, Park JH. Burden of disease in Korea during 2000‐10. J Public Health (Oxf) 2014; 36: 225–234. [DOI] [PubMed] [Google Scholar]
- 6. Park JH, Eum JH, Bold B, Cheong HK. Burden of disease due to dementia in the elderly population of Korea: present and future. BMC Public Health 2013; 13: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuo CL, Shiao AS, Wang SJ, Chang WP, Lin YY. Risk of sudden sensorineural hearing loss in stroke patients: a 5‐year nationwide investigation of 44,460 patients. Medicine (Baltimore) 2016; 95: e4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorrel JE, Bishop CE, Spankovich C et al Relationship of stroke risk and hearing loss in African Americans: the Jackson Heart Study. Laryngoscope 2018; 128: 1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohd Zulkifly MF, Ghazali SE, Che Din N, Singh DK, Subramaniam P. A review of risk factors for cognitive impairment in stroke survivors. ScientificWorldJournal 2016; 2016: 3456943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag 2016; 12: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heywood R, Gao Q, Nyunt MSZ et al Hearing loss and risk of mild cognitive impairment and dementia: findings from the Singapore Longitudinal Ageing Study. Dement Geriatr Cogn Disord 2017; 43: 259–268. [DOI] [PubMed] [Google Scholar]
- 12. Fortunato S, Forli F, Guglielmi V et al A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol Ital 2016; 36: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi E, Schuetz A, Stewart WF, Sun J. Using recurrent neural network models for early detection of heart failure onset. J Am Med Inform Assoc 2017; 24: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pham T, Tran T, Phung D, Venkatesh S. Predicting healthcare trajectories from medical records: a deep learning approach. J Biomed Inform 2017; 69: 218–229. [DOI] [PubMed] [Google Scholar]
- 15. Suo Q, Ma F, Canino G et al A multi‐task framework for monitoring health conditions via attention‐based recurrent neural networks. AMIA Annu Symp Proc 2018; 2017: 1665–1674. eCollection 2017. [PMC free article] [PubMed] [Google Scholar]
- 16. Wang T, Qiu RG, Yu M. Predictive modeling of the progression of Alzheimer's disease with recurrent neural networks. Sci Rep 2018; 8: 9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sousa RT, Pereira LA, Soares AS. Predicting diabetes disease evolution using financial records and recurrent neural networks. arXiv 2018; 1811.09350(: cs.LG).
- 18. Liu J, Zhang Z, Razavian N. Deep HER. Chronic disease prediction using medical notes. arXiv 2018; 1808.04928(: cs.LG).
- 19. Kim KW, Kim MH, Kim JR et al The Standardization of the Dementia Diagnosis Instrument. Boondang: Seoul National University Boondang Hospital, 2009. [Google Scholar]
- 20. Han J, Micheline K. Data Mining: Concepts and Techniques, 2nd edn. San Francisco, CA: Elsevier, 2006. [Google Scholar]
- 21. Statistics Korea . The results of social survey for year. Seoul: Statistics Korea, 2009. [Google Scholar]
- 22. Lee YK, Jung KH, Oh YH, Yeom JH, Kim HA. Plans for Improving Leisure and Welfare Service among the Old in Korea. Seoul: Korea Institute for Health and Social Affairs, 2012. [Google Scholar]
- 23. Lee JS. Ways of Improving the Efficiency of Family Services for Diverse Families. Health & Social Welfare Forum, 2017. October; 78–91. [Google Scholar]
- 24. Ministry of Health and Welfare in Korea . Plan for Ageing Society and Population. Seoul: MHWK, 2011. [Google Scholar]
- 25. Choi Y, Park EC, Kim JH, Yoo KB, Choi JW, Lee KS. A change in social activity and depression among Koreans aged 45 years and more: analysis of the Korean Longitudinal Study of Aging (2006‐2010). Int Psychogeriatr 2015; 27: 629–637. [DOI] [PubMed] [Google Scholar]
- 26. Statistics Korea . Korean Statistical Information Service. Sejong: Statistics Korea 2018. [Cited 1 Dec 2018.] Available from URL: http://kosis.kr/index/index.do
- 27. Stephen EH. Bracing for low fertility and a large elderly population in South Korea. Korea Economic Institute Academic Paper Series 51. [Cited 1 Dec 2018.] Available from URL: http://www.keia.org/publications?pcat=421&%3Bauthors=All&%3Btitle=&%3Bitems_per_page=60
- 28. Organisation for Economic Cooperation and Development . A Framework for Growth and Social Cohesion in Korea. Paris: OECD, 2011. [Google Scholar]
- 29. Organisation for Economic Cooperation and Development . Social Expenditure Database. Paris: OECD, 2018. [Cited 1 Dec 2018.] Available from URL: http://www.oecd.org/social/expenditure.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Mini‐Mental State Examination score cut‐off for cognitive impairment.
Table S2 Data.
Table S3 Data codebook.
Table S4 Multinomial logistic regression results: odds ratio for variable/association.


