Abstract
Over the last half century, climate change, coral disease, and other anthropogenic disturbances have restructured coral‐reef ecosystems on a global scale. The disproportionate loss of once‐dominant, reef‐building taxa has facilitated relative increases in the abundance of “weedy” or stress‐tolerant coral species. Although the recent transformation of coral‐reef assemblages is unprecedented on ecological timescales, determining whether modern coral reefs have truly reached a novel ecosystem state requires evaluating the dynamics of reef composition over much longer periods of time. Here, we provide a geologic perspective on the shifting composition of Florida's reefs by reconstructing the millennial‐scale spatial and temporal variability in reef assemblages using 59 Holocene reef cores collected throughout the Florida Keys Reef Tract (FKRT). We then compare the relative abundances of reef‐building species in the Holocene reef framework to data from contemporary reef surveys to determine how much Florida's modern reef assemblages have diverged from long‐term baselines. We show that the composition of Florida's reefs was, until recently, remarkably stable over the last 8000 yr. The same corals that have dominated shallow‐water reefs throughout the western Atlantic for hundreds of thousands of years, Acropora palmata, Orbicella spp., and other massive coral taxa, accounted for nearly 90% of Florida's Holocene reef framework. In contrast, the species that now have the highest relative abundances on the FKRT, primarily Porites astreoides and Siderastrea siderea, were rare in the reef framework, suggesting that recent shifts in species assemblages are unprecedented over millennial timescales. Although it may not be possible to return coral reefs to pre‐Anthropocene states, our results suggest that coral‐reef management focused on the conservation and restoration of the reef‐building species of the past, will optimize efforts to preserve coral reefs, and the valuable ecosystem services they provide into the future.
Keywords: coral reefs, coral restoration, ecosystem stability, Florida, Holocene, species composition, western Atlantic
Introduction
The catastrophic decline of coral populations over the last half century has resulted in significant shifts in the composition of the world's coral reefs (Graham et al. 2013, Kuffner and Toth 2016). The species assemblages of many reefs have diverged so dramatically from their pre‐Anthropocene (Waters et al. 2016) states that they may no longer have historical or geological analogues (Aronson and Precht 2001a, Precht and Miller 2007, Hobbs et al. 2009, Graham et al. 2013; cf. Williams and Jackson 2007). The emergence of these so‐called “novel ecosystems” (sensu Hobbs et al. 2006), presents a unique set of challenges for coral‐reef management, particularly as climate change and other anthropogenic disturbances continue to push reefs further away from natural baselines of reef composition and function (Hobbs et al. 2006, Graham et al. 2013).
In the western Atlantic, three coral taxa, Acropora palmata, A. cervicornis, and Orbicella spp., have dominated shallow‐water coral‐reef habitats throughout the region since at least the late Pleistocene (~600 thousand years ago; Jackson 1992, Budd and Johnson 1999, Pandolfi and Jackson 2006, Precht and Miller 2007). Beginning in the late 1970s, however, coral disease, coral bleaching, and a suite of local‐scale stressors have driven a precipitous decline in the populations of these foundation species (Aronson and Precht 2001a , b, Precht and Miller 2007, Schutte et al. 2010; Kuffner and Toth 2016, Hughes et al. 2017, Bruno et al. 2019). As a result, the Acropora and Orbicella spp. that had characterized western Atlantic reefs for hundreds of thousands of years are now listed as threatened under the U.S. Endangered Species Act (71 FR 26852, 79 FR 53852, and 79 FR 53852).
On many western Atlantic reefs, declines in Acropora and Orbicella spp. have coincided with absolute or relative increases in the populations of “weedy” or “stress‐tolerant” taxa (sensu Darling et al. 2012) such as Porites astreoides (Green et al. 2008), Siderastrea spp. (Burman et al. 2012, Toth et al. 2014), Agaricia spp. (Aronson and Precht 1997, Aronson et al. 2004, 2006), Millepora spp. (Toth et al. 2014), or even non‐scleractinian biota (e.g., Norström et al. 2009, Aronson et al. 2012, Ruzicka et al. 2013, Toth et al. 2014). Although the high reproductive rates of these taxa make them well‐poised to continue to take advantage of open space in the short term (see Darling et al. 2012), they cannot fill the same functional role as the reef‐building corals they have supplanted (Kuffner and Toth 2016, González‐Barrios and Álvarez‐Filip 2018). Most remaining corals are small in size, have slow growth rates and short lifespans, and, therefore, have limited capacity for carbonate production and the construction of reef framework (Alvarez‐Filip et al. 2013, Perry et al. 2015, Kuffner and Toth 2016). Continued growth and maintenance of three‐dimensional reef structure is essential to ensuring the persistence of the valuable ecosystem services coral reefs provide (Kuffner and Toth 2016, Storlazzi et al. 2019); however, shifts toward the dominance of weedy coral taxa may be accelerating the transition of reefs to a novel ecosystem state in which the biological, chemical, and physical processes of reef erosion will degrade reef structure faster than it can be built (Williams et al. 1999, Alvarez‐Filip et al. 2013, Perry et al. 2015, Yates et al. 2017, Perry and Alvarez‐Filip 2018, Kuffner et al. 2019).
Designing management solutions that mitigate the erosion of reef structure and promote reef accretion will be key to maintaining the functional integrity of coral‐reef ecosystems (Kuffner and Toth 2016). The increasing focus on coral restoration (e.g., Lirman and Schopmeyer 2016) may be one important step toward this goal; however, whether and to what degree reef‐building coral assemblages can be restored will depend on how far today's ecosystems have shifted from pre‐Anthropocene baselines (Hobbs et al. 2009, Graham et al. 2013). There are challenges associated with accounting for biases in geological records of reef development (Greenstein 2007, Hubbard 2011), including those from the most recent period of reef growth, known as the Holocene epoch (11,700 yr ago to present). Nonetheless, the Holocene foundations of modern reefs can provide valuable insights into long‐term trends in reef‐framework composition that can be used to evaluate the recent shift to novel ecosystem states and to guide future coral‐reef management (Jackson 1992, Greenstein et al. 1998, Aronson et al. 2004, Precht and Miller 2007, Hubbard 2011, Precht and Aronson 2016).
In this study, we analyzed the species composition of an extensive archive of Holocene reef cores from throughout the Florida Keys reef tract (FKRT) and compared the millennial‐scale variability in reef framework composition to contemporary reef assemblages. Florida's reefs are no exception to the recent trends of declining coral cover and changes in species dominance on reefs around the world (Bruno and Selig 2007, Precht and Miller 2007, Schutte et al. 2010, Burman et al. 2012, Ruzicka et al. 2013, Toth et al. 2014, Guest et al. 2018). Unlike most reefs in the western Atlantic, however, the FKRT has grown little for the last 3,000 yr, most likely because of long‐term, regional climatic cooling (Toth et al. 2018a). This suggests that, in Florida, geologic declines predated modern, ecological coral‐reef degradation (Toth et al. 2018a). Our study, therefore provides a unique opportunity both to establish a pre‐anthropogenic baseline for the composition of Florida's reefs, and, by evaluating changes in reef assemblages surrounding the geologic shutdown of the FKRT, to determine whether there is precedent for the modern shifts in species composition. Our specific objectives were to (1) identify the primary coral taxa responsible for Holocene reef‐building in the region, (2) evaluate the spatial and temporal variability in reef assemblages during the Holocene, (3) determine if there were changes in species composition associated with the shutdown of reef accretion, and (4) compare the relative composition of Florida's Holocene reef framework to contemporary reef assemblages.
Methods
Regional setting
The FKRT is located along the edge of the Florida shelf, 5–7 km offshore of the Florida Keys, USA and extends more than 350 km from Biscayne National Park (N.P.) in the northeast, through the Florida Keys National Marine Sanctuary, to Dry Tortugas N.P. in the southwest (Fig. 1; Lidz et al. 2003, Shinn and Lidz 2018). It is a subset of the broader Florida coral‐reef ecosystem, which extends north to Palm Beach County, Florida and includes numerous patch reefs and hardground environments inshore of the shelf‐edge reefs (Lidz et al. 2003, Stathakopoulos and Riegl 2015). The FKRT can be divided into six biogeographic subregions based on hydrographic variability: Dry Tortugas N.P., the Marquesas, the Lower Keys, the Middle Keys, the Upper Keys, and Biscayne N.P. Whereas Dry Tortugas N.P. and the Marquesas are relatively open‐ocean environments, the reefs of the Keys subregions and Biscayne N.P. are influenced by groundwater, terrestrial runoff, and variability associated with the influx of water masses from Florida and Biscayne Bays (Marszalek et al. 1977, Ginsburg and Shinn 1994, Precht and Miller 2007, Toth et al. 2017, 2018a).
Figure 1.
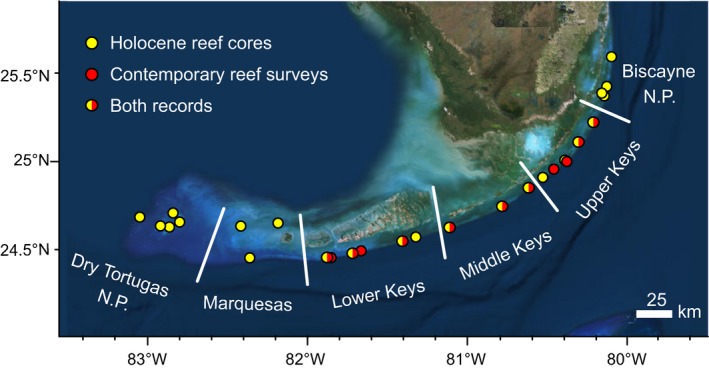
Map of the six subregions of the Florida Keys Reef Tract (FKRT). Points indicate locations where Holocene reef cores were collected (yellow), contemporary (CREMP) reef surveys were conducted (red), and where both data sets were available (yellow and red). Imagery from Esri, DigitalGlobe, GeoEye, Earthstar Geographics, CNES/Airbus Ds, USDA, USGS, AeroGRID, IGN, and the GIS User Community. N.P., National Park.
Description of Holocene reef cores
We evaluated 59 Holocene reef cores from the six subregions of the FKRT (Fig. 1): 17 from Dry Tortugas N.P., 3 from the Marquesas, 10 from the Lower Keys, 10 from the Middle Keys, nine from the Upper Keys, and 10 from Biscayne N.P. The cores, which are housed in the U.S. Geological Survey Core Archive, were collected between 1976 and 2017 using underwater hydraulic drilling systems based on the design pioneered by I. G. Macintyre (Macintyre 1975; core archive data available online).1 The coring systems allow successive sections, or “intervals” (typically ~1.5 m long), of reef framework to be recovered from a single borehole (see Hubbard 2011, Toth et al. 2018a). Although a total of 232 intervals of Holocene coral‐reef framework were recovered in the 59 cores, we only analyzed the 216 intervals from shallow (i.e., paleodepths < 10 m below modern mean sea level [bMSL]) fore‐reef environments and those for which we could generate reliable age models (described in Age and paleodepth calculations and in Toth et al. 2019). Core metadata, radiometric ages, and descriptive core logs for each of the cores are provided in Toth et al. (2018b).
Age and paleodepth calculations
We estimated the ages of the boundaries of each interval in the 59 cores by linear interpolation of 202 calibrated radiometric ages measured in a previous study (Toth et al. 2018a , b). We then averaged the estimated ages of the beginning and end of each the interval to determine its mean age. We did not include any cores in our reconstruction that did not have at least two ages. The mean age of the intervals ranged from 10,222–121 yr before present (yr BP, where “present” is 1950; Appendix S1: Fig. S1); however, most ages, ~75%, were from the middle Holocene (8,200–4,200 yr BP). We note that whereas Toth et al. (2018a) excluded ages from their reef accretion calculations when adjacent ages in the cores were not statistically different from one another, we retained these ages when creating age models for this study.
Next, we determined the average paleodepth for each interval using the reconstruction of Holocene sea‐level variability in south Florida developed by Khan et al. (2017). First, we used the model to estimate the position of sea level relative to modern MSL at the beginning and end of each depth interval in the cores based on the estimated ages of those points. Depths of intervals were converted to MSL by adding the water depth at the coring location (see Toth et al. 2018b). We then calculated mean paleodepths for each interval by averaging its starting and ending paleodepths. Most, ~72%, of the estimated paleodepths fell between 0 and 6 m bMSL, which would represent reef crest to shallow reef slope environments (Precht and Miller 2007); however, the overall range of interval paleodepths was 23.7 m bMSL to −2.7 m bMSL (Appendix S1: Fig. S2). To ensure that our analyses compared reefs from similar fore‐reef environments, we excluded data from 13 intervals in Dry Tortugas N.P. with paleodepths < 10 m (Toth et al. 2019).
We emphasize that both the average ages and paleodepths we calculated are estimates. The average uncertainties (2σ range) associated with the radiometric ages is ~250 yr. Significant uncertainty in our paleodepth estimates stems from both uncertainty in Khan et al.'s (2017) sea‐level model (1σ = ~0.9 m) and uncertainties in assigning depths to samples within the core intervals. Because of these uncertainties, we only use the paleodepths as a basis for excluding data from anomalously deep paleoenvironments (i.e., >10 m bMSL) and we only evaluate broad‐scale temporal variability in fore‐reef composition.
Quantifying Holocene reef composition
We analyzed the composition of the 216 core intervals through digital analysis of high‐resolution, photographic, core logs (provided in Toth et al. 2018b) using the methodology described by Toth et al. (2015). First, all visible material in the core photographs including corals, unconsolidated sediments, and carbonate reef rock, were identified using the physical core records for reference and were digitally traced using the Area Analysis Tool in the program Coral Point Count (CPCe; Kohler and Gill 2006). Coral taxa were generally identified to the species level; however, Agaricia, Madracis, Oculina, and Millepora spp., which were all rare in our records (Appendix S1: Tables S1, S2), were only identified to the genus level. Additionally, Porites spp. with branching morphologies, P. porites, P. divaricata, and P. furcata, were grouped as “branching Porites spp.” and Orbicella annularis, O. faveolata, and O. franksi were classified as “Orbicella spp.” Corals that were too taphonomically degraded or too small to be confidently identified to even the genus level were categorized as “unidentified corals”.
To calculate percent recovery of reef framework and the absolute and relative percent composition of each coral taxon in each interval, we first calculated the intervals’ theoretical surface areas by multiplying the penetration length of the intervals (e.g., 1.5 m) by the average diameter of the core as measured in CPCe (~3.5–5.0 cm depending on coring equipment used). Percent recovery and absolute percent composition of core constituents were then calculated by dividing the total measured area of each interval or each type of substrate, respectively, by the theoretical surface area of that interval and multiplying those values by 100%. Relative percent composition of coral taxa was calculated by dividing the surface area measured for each taxon in an interval by the total surface area of all coral taxa in that interval. Although we provide data on the absolute percent composition of the Holocene reef cores in Toth et al. (2019), the analyses presented here are all based on the relative percent composition of coral taxa.
Evaluating trends in Holocene reef composition
To evaluate temporal variability in reef composition during the Holocene, we used data from the 208 core intervals from Dry Tortugas N.P., the Lower, Middle, and Upper Keys, and Biscayne N.P. subregions. Because of the low sample size from the Marquesas (Appendix S1: Table S1), we excluded the eight intervals from that subregion from all statistical analyses. For the analysis of spatial variability as well as the comparisons with the modern reef composition (described in the following section), we only used the 158 intervals representing the middle Holocene (8,200–4,200 yr BP), because of the paucity of data from most sub‐subregions during the early and late Holocene (i.e., before 8,200 yr BP and after 4,200 yr BP, respectively; Appendix S1: Table S2).
We examined the spatial and temporal variability in Holocene reef composition by constructing two‐dimensional ordinations of Bray‐Curtis dissimilarity matrices after Wisconsin double standardization using nonmetric multidimensional scaling (NMDS) with the metaMDS function in the R package vegan (Oksanen et al. 2017). The spatial variability in middle Holocene reef composition was also evaluated by conducting an analysis of similarities (ANOSIM) of square‐root transformed Bray‐Curtis dissimilarities with site nested within subregion using Primer 6 software (Quest Research Ltd., Auckland, New Zealand). We further investigated the changes in composition responsible for any significant spatial variability in Holocene reef composition using SIMPER analysis (vegan's “simper” function).
We used linear mixed‐effects models to more closely evaluate the spatial variability of the relative percent composition of the two dominant taxa in the middle Holocene reef framework: A. palmata and Orbicella spp. Those data did not conform to the assumptions of homoscedasticity of variance or normality and were, therefore, rank transformed prior to analysis. Using the R package nlme, we used the lme function to construct linear mixed‐effects models that treated subregion as a fixed effect and site as a random effect and used the anova.lme function to test the significance of the fixed effect based on Wald's test (Pinheiro et al. 2017). Pairwise, post‐hoc analyses were performed using the R package lsmeans (Lenth 2016). We also evaluated the impact of reef composition on Holocene reef accretion rates, by characterizing 77 intervals in the cores for which accretion rates had previously been calculated (Toth et al. 2018a , b) as being dominated (>75% composition) by A. palmata, massive taxa, or as “mixed.” We compared accretion rates among groups using an ANOVA after rank transformation because the model residuals failed to meet the assumption of normality.
Bias in the Holocene record
Uplifted coral reefs generally provide the most complete records of past coral‐reef assemblages (Jackson 1992, Pandolfi and Jackson 2006, Lescinsky et al. 2012); however, uplifted western Atlantic reefs are isolated to a few, tectonically active locations. Sub‐aerially exposed reefs that formed during the sea‐level highstand ~125 thousand years ago are more ubiquitous, but in south Florida, these reefs grew under different environmental conditions than contemporary ecosystems (Precht and Miller 2007). Although reconstructing the composition of Holocene reef assemblages from submerged, drill core records is not without its challenges, core‐based records of reef framework composition likely provide the best available pre‐anthropogenic baseline for south Florida.
The first, and most obvious challenge, is how to make reef‐scale interpretations from two‐dimensional core records (Hubbard 2011). Cores cannot provide a complete picture of the composition of reefscapes in the past; however, the unprecedented size of our data set, suggests that we should have the ability to reconstruct general trends in reef‐framework composition through space and time (see Hubbard 2011). A second complication relates to preservation biases associated with drill coring in particular. Like many reefs in the western Atlantic (Hubbard et al. 1990), a large proportion (>50% based on average recovery in our cores; Appendix S1: Fig. S3) of the Holocene reef structure in the Florida Keys is composed of void spaces or unconsolidated sediments, which are not generally recovered with our drilling system (Shinn et al. 1981, Hubbard 2011). Similarly, sections of reef built by unconsolidated coral rubble (e.g., A. cervicornis, branching Porites spp., or Agaricia spp.) are typically only recovered in rotary cores when they are bounded by larger coral skeletons in the core barrel (L. T. Toth, A. Stathakopoulos, and E. A. Shinn, personal observation). Rotary coring can also fragment the skeletons of corals with fragile morphologies, which further biases the record toward corals with robust growth forms (Hubbard 2011). Thus, our reconstruction represents a record of the composition of consolidated reef framework built during the Holocene, rather than a complete record of Holocene reef composition.
Our records are also subject to the same issues of preservation bias and time averaging as studies of Pleistocene outcrops (Greenstein et al. 1998, Greenstein 2007). Branching corals such as A. cervicornis tend to be overrepresented in coral‐reef death assemblages relative to those with massive morphologies, primarily as a result of frequent storm deposition (Shinn et al. 2003, Greenstein 2007, Blanchon et al. 2017); however, preservation in the fossil record is biased toward corals with larger, more robust morphologies (see Enos and Perkins 1977 for a list of the relative preservation potential of corals in the Florida Keys). Small corals or those with finely branching, encrusting, or plating morphologies (see Appendix S1: Table S3) are easily eroded or fragmented during storms and their rubble may be exported off the reef to back‐reef environments or sediment‐filled grooves instead of being incorporated into the reef framework (Shinn et al. 1981, 2003, Hubbard et al. 1990, Hubbard 2011). Although storm‐related deposition is common on many reefs in the western Atlantic (e.g., Blanchon et al. 2017), the lack of significant age reversals in our cores (Toth et al. 2018b), suggests that the fore‐reef framework described in this study mostly grew in situ. Because of the various biases associated with preservation and incorporation into the fossil record as well as the issue of time averaging, the geological record generally cannot be used to reconstruct past coral cover or coral abundance (but see Lescinsky et al. 2012). Instead, we suggest that our reconstruction be interpreted as a record of millennial‐scale variability in reef framework composition in the fore‐reef environments of the FKRT rather than a complete representation of the corals that grew there during the Holocene.
Comparison of modern and middle Holocene reef composition
To determine how modern reef assemblages of the FKRT compare with the assemblages of the middle Holocene, we obtained data on the contemporary reef assemblages of the FKRT from Florida Fish and Wildlife Conservation Commission's Coral Reef Evaluation and Monitoring Program (CREMP; Ruzicka et al. 2010, Colella et al. 2012, Ruzicka et al. 2013). The CREMP monitoring program has surveyed 40 sites throughout the Florida Keys annually since 1996. For each site, percent cover of hard corals and other benthos was estimated using a random‐point‐count method on images taken at two to four permanent, 0.4 × 22 m transects. We used data from the 12 shallow (~2–7 m bMSL), offshore CREMP sites (Ruzicka et al. 2013) in the Keys subregions, which are in the most similar fore‐reef depth environment as the middle Holocene data (>10 m bMSL, but generally 0–6 m bMSL). Eight of the CREMP sites included in this study are actually from the same reefs where Holocene reef cores were collected (Fig. 1).
Because of the potential for poor preservation of finely branching, plating, or encrusting corals and small mounding corals (maximum colony diameter < 0.5 m) in the fossil record in general, and core records, in particular (see previous section; Enos and Perkins 1977, Greenstein et al. 1998, Hubbard et al. 2005, Greenstein 2007, Hubbard 2011), we took a conservative approach and excluded these taxa from the comparative analysis of modern and middle Holocene reef composition (see Appendix S1: Table S3 for justifications for why particular taxa were excluded). We also excluded Solenastrea bournoni because it was absent from the modern data set and Dendrogyra cylindrus and Meandrina meandrites because they were not observed in the middle Holocene data set. Although a total of 25 coral species were identified in the shallow shelf‐edge reef habitats during the CREMP surveys in 1996 and 2015 (Appendix S1: Table S4), and 18 coral species were identified in the middle Holocene intervals of the reef cores (Appendix S1: Table S2), our comparative analysis only includes the 10 taxa in the CREMP data set that were also likely to be preserved in the reef‐core records (after Enos and Perkins 1977): A. palmata, Orbicella spp., Pseudodiploria strigosa, Pseudodiploria clivosa, Diploria labrynthiformis, Colpophyllia natans, Porites astreoides, Montastraea cavernosa, Siderastrea siderea, and Stephanocoenia intersepta. In general, these were the most common taxa in both the modern surveys and middle Holocene reef framework (Appendix S1: Table S2, S4; Ruzicka et al. 2013); however, S. intercepta was uncommon in both records and Millepora spp. was relatively abundant in the modern surveys (Appendix S1: Table S4). It is possible that the abundance of some of the excluded taxa have changed relative to the middle Holocene, but because of the potential for sampling biases associated with most of these taxa, we were not able to assess changes in their relative composition in this study. In both data sets, we recalculated percent composition of the taxa we did include relative to the total percent composition of those ten taxa (i.e., their sum equaled 100%). We examined overall changes in the composition in the Keys subregions between the middle Holocene, 1996, the first year of the CREMP surveys, and the more recent surveys in 2015, with NMDS and ANOSIM using the methods described previously. The overall results were similar whether we analyzed all 12 of the CREMP sites or just the eight sites where there were data from both the middle Holocene and CREMP (see Appendix S1: Tables S6–S10), so we present the results from the analysis using all 12 CREMP sites.
Results
Holocene reef composition
Percent recovery in the cores was highly variable (25.6–55.0% on average among subregions; Appendix S1: Fig. S3; Toth et al. 2019), but averaged ~42.3% ± 1.7% (mean ± SE) across the 216 intervals included in our analysis. This degree of recovery is typical of western Atlantic reef frameworks, which are often dominated by unconsolidated sediments and void spaces (Hubbard et al. 1990, 2005, Gischler and Hudson 2004). Carbonate reef rock (generally grainstones and/or packstones) made up ~10.0% ± 1.3% of the recovered material on average (range 5.5–17.8%; Appendix S1: Fig. S4; Toth et al. 2019); however, sub‐fossil corals accounted for the majority of the reef framework.
We identified 21 unique coral taxa in the Holocene cores from the FKRT (Appendix S1: Table S1; Toth et al. 2019). Two coral taxa, Orbicella spp. and A. palmata, dominated the Holocene reef framework, accounting for ~64.8% of the relative coral composition in our cores (39.7% ± 2.7% and 25.2% ± 2.7%, respectively). The brain corals, Ps. strigosa, D. labyrinthiformis, and C. natans, were subdominant, averaging 10.0% ± 1.4%, 5.4% ± 1.2%, and 5.1% ± 1.1% of the reef framework, respectively. Montastraea cavernosa, which had an average relative abundance of 3.2% ± 1.0% and A. cervicornis, which had an average relative abundance of 2.2% ± 0.7% were the only other corals that made up >2% of the Holocene record. Together, these seven taxa comprise >90% of all corals found in the Holocene reef framework. The remaining 14 taxa were rare and, except for S. siderea in the Marquesas and P. astreoides in the Upper Keys, they always made up <5% of the Holocene reef framework in any given subregion. Fewer than 1% (± <0.1%) of corals could not be identified to the genus level.
Spatial variability
The species composition of middle Holocene reef framework varied significantly among subregions (Fig. 2; Appendix S1: Tables S1, S6; ANOSIM, R = 0.396, P < 0.001) and to a lesser degree among sites (ANOSIM, R = 0.126, P < 0.002). Although the two dominant reef‐building taxa, A. palmata and Orbicella spp., were consistently top ranked in the contrasts between subregions (SIMPER analysis; Appendix S1: Table S6), there were spatial differences in their relative abundances. Dry Tortugas N.P. was unique compared with all other subregions (R = 0.317–1.000; P < 0.05) because of the relatively low abundance of A. palmata in the middle Holocene reef framework of this subregion: just 3.2% on average (± 2.0%; Fig. 2c; Appendix S1: Tables S1, S6). Because A. palmata was generally absent Dry Tortugas N.P., the relative abundances of Orbicella spp. and Ps. strigosa were higher there than elsewhere on the FKRT (Fig. 2; Appendix S1: Table S1, S6). In contrast, A. palmata had the highest abundance in the Middle Keys and Orbicella spp. was relatively uncommon there (Fig. 2; Appendix S1: Tables S1, S6). Indeed, the relative abundance of A. palmata was significantly higher and the relative abundance of Orbicella spp. was significantly lower in the Middle Keys compared with the Dry Tortugas N.P. (LME, F 4,15 = 5.62, P < 0.01; Tukey‐like test, P < 0.01 and LME, F 4,15 = 3.55, P = 0.03; Tukey‐like test, P = 0.03 for A. palmata and Orbicella spp., respectively). The differences between the Lower Keys, Upper Keys, and Biscayne N.P. were more subtle; however, the distinctions between these subregions were also driven by difference in the relative abundance of Orbicella spp., A. palmata, and, to a lesser extent D. labyrinthiformis (Fig. 2; Appendix S1: Table S6).
Figure 2.
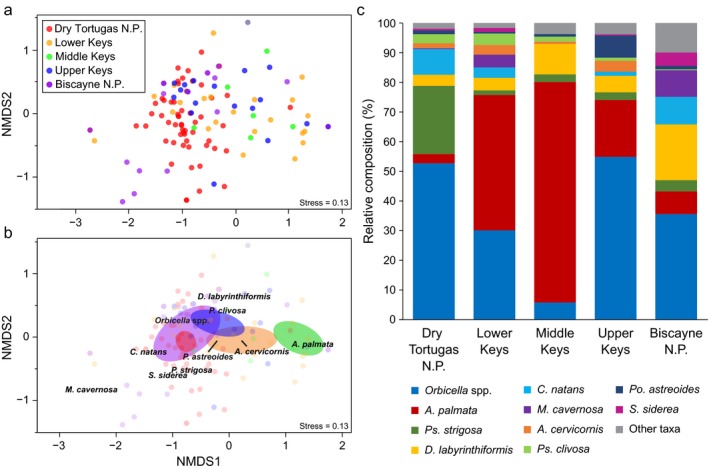
Nonmetric multidimensional scaling (NMDS) ordination of the relative composition of the 18 coral taxa found in the middle Holocene reef framework (a, b). (a) Site scores of intervals in the cores, with colors representing subregion of the FKRT. In (b), the colors of site scores are faded so that trends in the data can be more clearly displayed. Colored ellipses representing 95% CIs around the weighted average of site scores for each subregion. The names of the 10 most abundant taxa are plotted at the weighted average of their species scores. To simplify the plot, an extreme outlier from the Lower Keys (NMDS1 = −3.86) was not displayed. (c) Average relative composition (%) of the 10 most abundant coral taxa in the five main subregions of the FKRT during the middle Holocene. Taxa with relative abundances < 1% throughout the FKRT were combined into the “other taxa” category. Genera are Orbicella, Acropora, Pseudodiploria, Diploria, Colpophyllia, Montastraea, Porites, Siderastrea.
Temporal variability
Because most of our data were from the middle Holocene (Appendix S1: Fig. S2), the composition of the subset of cores from this period were very similar to the averages across the entire data set (Appendix S1: Table S2). The sample sizes from the early and late Holocene are much smaller and the data were not distributed equally across the subregions of the FKRT (Appendix S1: Fig. S2). Because of this, we only present exploratory statistical analyses of temporal variability during the Holocene (Appendix S1: Fig. S5).
The majority of the records from the early Holocene (before 8200 yr BP) are from Fowey Rocks outlier reef in Biscayne N.P. (Lidz et al. 2003; N = 17 intervals), as most reefs elsewhere on the FKRT had not initiated until after ~8,000 yr BP (Toth et al. 2018a). There was one interval from the Dry Tortugas N.P. from this period; however, because there was no replication in this subregion, we rely on the data from Fowey Rocks to evaluate average composition during this period. The reef at Fowey Rocks was dominated by A. palmata (Appendix S1: Table S2), which accounted for an average of 76.9% ± 7.1% of corals in those cores during the early Holocene. Orbicella spp. was the next most abundant taxon, averaging 7.5% (± 5.9% of the early Holocene reef framework. Interestingly, Millepora spp. has a similar abundance to Orbicella spp., averaging 7.5% ± 2.7% composition. Although milleporids generally do not preserve well in drill‐core records and were rare in our records overall, both cores from this site contained multiple sections where blades or branches of Millepora spp. corals had been secondarily cemented into the reef framework. The remaining ~10% of the cores were primarily composed of S. siderea (2.4% ± 2.4%), A. cervicornis (2.2% ± 1.8%), and branching Porites spp. (1.4% ± 1.4%). All of the other taxa we observed accounted for <1% of the early Holocene record (Appendix S1: Table S2). Data from the Middle Keys, Lower Keys, and Marquesas were especially limited during the late Holocene (Appendix S1: Fig. S2); however, the composition of FKRT reefs during the late Holocene (after 4,200 yr BP) was remarkably similar to both the middle Holocene and the averages across the entire record (Appendix S1: Table S2; Fig. S5).
Comparison between Holocene and contemporary assemblages
The relative species composition of contemporary reef assemblages of the Lower, Middle, and Upper Keys was significantly different from that of the middle Holocene reef framework in those subregions (Fig. 2; Appendix S1: Table S5, S7; ANOSIM, R = 0.327, P = 0.001). The results further suggest that the relative composition of reefs has continued to diverge from the middle Holocene baseline in recent decades, as the reefs of the Keys were more similar in composition to the middle Holocene reef framework in 1996 (ANOSIM, R = 0.275, P = 0.001) than in 2015 (ANOSIM, R = 0.478, P = 0.001). The changes in reef composition between the 1996 and 2015 surveys were minor compared with the changes that have occurred since the middle Holocene (ANOSIM, R = 0.140, P = 0.001).
The most striking differences between the contemporary and middle Holocene reef assemblages of the Keys subregions are the relative decreases in A. palmata and Orbicella spp., and the relative increases in P. astreoides and S. siderea (Appendix S1: Tables S5, S7; Fig. 3c). Acropora palmata and Orbicella dominated reef frameworks throughout the FKRT during the Holocene (Appendix S1: Table S2) and, together, accounted for an average of 81.4% ± 3.5% of the middle Holocene reef framework in the Keys subregions (Appendix S1: Table S2); however, the relative composition of these taxa averaged just 44.1% ± 5.8% in 1996 and by 2015 their average relative composition had dropped to 25.6% ± 4.9%. In contrast, P. astreoides and S. siderea, which only made up an average of 3.2% ± 1.2% and 0.7% ± 0.5% of the middle Holocene reef framework, respectively, had high relative abundances on the reefs of the Keys subregions by 1996 (averaging 21.5% ± 4.7% and 11.6% ± 3.6%, respectively) and were the dominant coral taxa by 2015 (Appendix S1: Tables S5, S7; averaging 32.4% ± 5.5% and 29.7% ± 5.2%, respectively).
Figure 3.
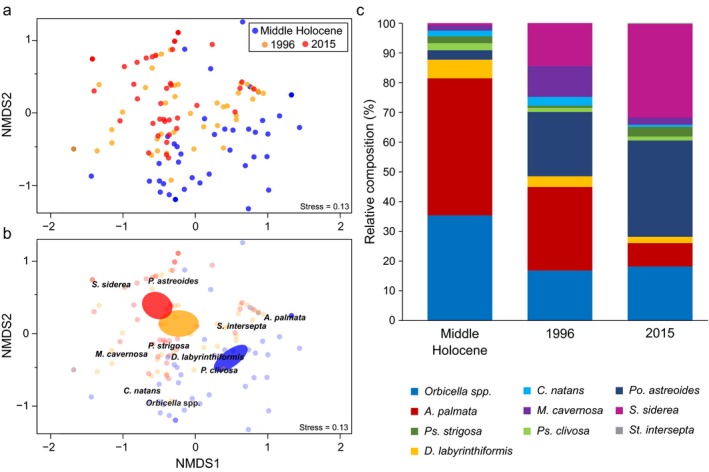
NMDS ordination of the relative composition of the 10 coral taxa compared between the middle Holocene reef framework (cores) and contemporary (CREMP) assemblages of the Lower, Middle, and Upper Keys (a, b). (a) Site scores, with colors representing time period. In (b), the colors of site scores are faded so that trends in the data can be more clearly displayed. Colored ellipses representing 95% CIs around the weighted average of site scores for each time period. The names of the coral taxa are plotted at the weighted average of their species scores. (c) Average relative composition (%) of corals during the middle Holocene compared with 1996 and 2015. Includes the 10 coral taxa likely to be preserved in the Holocene record and present in both Holocene reef cores and modern surveys and a category for “other taxa.” Genera are as in Fig. 2, with the addition of Stephanocoenia.
The spatial variability in reef composition across the FKRT was still present in the combined middle Holocene and contemporary data set (ANOSIM, R = 0.169, P = 0.001; Appendix S1: Fig. S6); however, the spatial differences in reef composition were small relative to the temporal difference between the middle Holocene and contemporary assemblages. These differences were driven by the four taxa that had the highest relative abundance in either the middle Holocene or contemporary data sets: A. palmata, Orbicella spp., P. astreoides, and S. siderea (Appendix S1: Table S8).
Discussion
The geological foundations of Florida's coral reefs
In south Florida, just two taxa, A. palmata and Orbicella spp., accounted for the majority of reef‐building during the Holocene. The remaining reef framework was primarily composed of the massive corals, Ps. strigosa, Ps. clivosa, D. labyrinthiformis, C. natans, and M. cavernosa (cumulative percentage: 96%). Similar reef‐coring studies in Buck Island in St. Croix, U.S. Virgin Islands (Hubbard et al. 2005) and the Belize Barrier Reef (Gischler and Hudson 2004) suggested that Holocene reef frameworks in those locations were also dominated by A. palmata and Orbicella spp. The resemblance between the composition of all three Holocene reefs to Pleistocene and to pre‐Anthropocene reefs throughout the western Atlantic, supports the conclusion that reefs in this region have, until recently, supported a similar assemblage of reef‐building taxa for hundreds of thousands of years (Jackson 1992, Budd and Johnson 1999, Aronson and Precht 2001a; Pandolfi and Jackson 2006, Precht and Miller 2007).
Although contemporary reef assemblages in the western Atlantic have been broadly similar over large spatial scales (Precht and Aronson 2016), the composition of individual reefs can vary significantly as a result of local‐ to mesoscale variability in geology, hydrography, or ecology (Murdoch and Aronson 1999, Toth et al. 2017). This trend was reflected in the middle Holocene (8,200–4,200 yr BP) reef assemblages as well: there was significant spatial variability in reef framework composition both within and among subregions of the FKRT. The largest differences were at the subregional scale (c.f., Murdoch and Aronson 1999) because of the anomalously low abundance of A. palmata in the Dry Tortugas and its prevalence in the middle Holocene reef framework of the Middle Keys (Fig. 2; Appendix S1: Table S1). The Dry Tortugas was the most extensively sampled subregion in this study (i.e., 80 intervals analyzed from 17 cores collected at six unique sites), so it is unlikely that the paucity of A. palmata there is sampling artifact. Furthermore, although the relatively shallow paleodepths represented in the Middle Keys may help explain the comparably high abundance of A. palmata in the middle Holocene reef framework of that subregion, it is unlikely that the rarity of A. palmata in Dry Tortugas was entirely a function of depth (see Supplemental Analyses in Appendix S1).
Shinn et al. (1977) was the first to note the conspicuous absence of A. palmata on modern reefs and in Holocene reef cores from throughout the Dry Tortugas. In our compilation, which includes Shinn et al.'s (1977) records, we found that although ~63% of the paleodepths of sections of reef framework from Dry Tortugas N.P. fall within A. palmata's preferred depth range (i.e., <5 m bMSL), this taxon was primarily found in a narrow, superficial layer in cores from the northeast area of the Park near Pulaski Light (Toth et al. 2018b). For comparison, A. palmata accounted for ~80% of the reef framework deposited at paleodepths shallower than 5 m bMSL in the Middle Keys, but, in the Dry Tortugas it only represented ~5% of the reef framework from that same depth zone. Whereas historical accounts suggest that there may have been larger populations of A. palmata in the Dry Tortugas in the late 1800s (Davis 1982), at present, A. palmata is restricted to a small, relic population near Garden Key (Ruzicka et al. 2010). The paucity of A. palmata in the Holocene reef framework suggests that this species may have never been an important reef builder in the Dry Tortugas. Studies aimed at better constraining the past spatial and temporal extent of A. palmata populations in Dry Tortugas N.P. are needed to address the question of why this location only supported marginal populations of A. palmata during the Holocene.
Stability of Holocene reef assemblages
Previous paleoecological studies from Pleistocene reef outcrops have demonstrated that, during periods of relatively stable sea level (i.e., highstands), the spatial variability in species assemblages among reef environments is generally greater than the temporal variability within individual locations (Aronson and Precht 2001a, Precht and Aronson 2016) and our Holocene reconstruction generally supports this conclusion. We did find that A. palmata was somewhat more abundant in our limited records from the early Holocene (i.e., before 8,200 yr BP) when the rates of sea‐level rise were most rapid (Khan et al. 2017). This taxon has especially high growth rates, which has allowed shallow‐water A. palmata reefs to keep pace with even the most extreme rates of sea‐level change during the Holocene (Precht and Aronson 2016) and explains why A. palmata is often dominant during the initiation phase of Holocene reef‐building in the Caribbean (e.g., Hubbard et al. 2005). Records from the outer reef tract of southeast Florida, which began growing during the early Holocene, could help to further elucidate the paleoecology of Florida's reefs during the early phases of reef development (Stathakopoulos and Riegl 2015). By the middle Holocene, however, the rate of sea‐level rise had slowed (Khan et al. 2017), and the relative abundances of reef‐building taxa were broadly similar over the last ~8,000 yr (Appendix S1: Table S2; Figs. S5, S7).
One important question for contextualizing declines in the populations of reef‐building corals observed over the last several decades is whether there have been hiatuses in the records of any dominant coral taxa in the past (c.f. Greenstein et al. 1998, Aronson et al. 2004, 2006). For example, based on the paucity of dated A. palmata samples from St. Croix and elsewhere in the Caribbean from ~5,900–5,400 and 3,300–2,900 yr BP, Hubbard et al. (2005) and Hubbard (2014) suggested that there may be a historic precedent for the modern decline of Caribbean acroporids (Aronson and Precht 2001b). In contrast, we found no evidence of significant hiatuses in A. palmata growth in the Florida Keys: 10 of the calibrated radiocarbon ages of A. palmata from our cores overlap with the period 5,900–5,400 yr BP and one sample overlaps the interval from 3,300–2,900 yr BP (Toth et al. 2018b). Furthermore, a recent study of Holocene coral‐reef development in southeast Florida found four additional A. palmata samples with ages overlapping the earlier gap in Hubbard's record (Stathakopoulos and Riegl 2015). Together, the Holocene records from throughout south Florida include 14 A. palmata ages between 5,900 and 5,400 yr BP, when A. palmata was putatively rare or absent from western Atlantic reef frameworks, negating the possibility of a Caribbean‐wide hiatus in acroporid reef growth at that time.
The fact that only one A. palmata sample from south Florida dates during the younger proposed gap, 3,300–2,900 yr BP, is not surprising considering that reef development throughout south Florida had largely ceased by that time (Stathakopoulos and Riegl 2015, Toth et al. 2018a). Indeed, only five corals of any species in our cores from the FKRT had ages during this period (Toth et al. 2018b). Shinn et al. (2003) documented a similar hiatus in the ages of storm‐deposited A. cervicornis throughout the Florida Keys (~3,150–2,600 yr BP; recalibrated from Shinn et al. 2003), which corresponds recent proposed acroporid gap (Hubbard et al. 2005, Hubbard 2014); however, Aronson et al. (2004) demonstrated that A. cervicornis‐dominated reefs in Belize and Panamá grew continuously through that interval. Although there may have been localized extirpations of acroporids in some locations in the past, we conclude that there is little evidence for Caribbean‐wide declines in Acropora spp. during the middle or late Holocene. Instead, our record supports the conclusion that the widespread loss of Caribbean acroporids beginning in the 1970s due to white‐band disease was unprecedented over millennial time scales (Aronson and Precht 2001b, Aronson et al. 2004, 2006, Wapnick et al. 2004).
The stability of reef framework composition in south Florida from ~8,000 yr BP until the last several decades is especially remarkable in light of the major changes in reef building that occurred on the FKRT during this period. Using the same core records as the present study, Toth et al. (2018a), demonstrated that although reefs on the FKRT accreted rapidly during the Holocene thermal maximum (HTM) ~7,000 yr BP, as climate cooled during the late Holocene, reef accretion declined dramatically. By 3,000 yr BP, reefs throughout the FKRT were no longer growing on pace with the rate of sea‐level rise, making them geologically senescent. Holocene reef accretion in south Florida was modulated by the negative influence of variable water masses from Florida Bay (Marszalek et al. 1977, Ginsburg and Shinn 1994, Precht and Miller 2007) and by the declining rate relative sea‐level rise after ~6,000 yr BP (Khan et al. 2017), but Toth et al. (2018a) concluded that climate was most likely the ultimate cause of the region‐wide shutdown of reef‐building by 3,000 yr BP (Toth et al. 2018a). Whereas Stathakopoulos and Riegl (2015) found that some reefs in southeast Florida shifted from assemblages dominated by massive corals to A. palmata as those reefs approached senescence, we found no evidence for a consistent shift in species composition associated with declining reef accretion (Appendix S1: Fig. S7; Toth et al. 2018b). Our results also support other studies that concluded that there is no significant difference in the accretion rate of western Atlantic reefs dominated by A. palmata, massive corals, or mixed assemblages (Appendix S1: Fig. S8; ANOVA, F 3,73 = 1.27, P = 0.29; e.g., Hubbard 2009, Toth et al. 2018a).
Acroporids would have been the most likely species to be negatively affected by the cooling that occurred after the HTM, as they are highly sensitive to both low and high temperature stress (Precht and Miller 2007); however, although the latitudinal range of Acropora populations in south Florida contracted during the middle Holocene as the climate cooled, resulting in its disappearance from the reefs in southeast Florida located north Biscayne N.P. (Fig. 1; Precht and Aronson 2004), A. palmata was present in all subregions of the FKRT except for the Dry Tortugas N.P. throughout the middle and late Holocene (Appendix S1: Fig. S7; Toth et al. 2018b, 2019). The persistence of A. palmata and the lack of any detectable change in the composition of Florida's reefs during the period surrounding the shutdown of reef accretion underscores the stability of western Atlantic reef assemblages over millennial timescales (Jackson 1992, Budd and Johnson 1999, Pandolfi and Jackson 2006, Precht and Miller 2007). The regional environmental changes during the last ~6,000 yr were sufficient to stall reef growth, but they were apparently not severe enough to cause significant changes to reef assemblages. This result supports the conclusions of Toth et al. (2018a) that reef accretion may be one of the most sensitive processes to environmental disturbance and that, although Florida's reefs were geologically senescent by ~3,000 yr BP, they maintained most ecological functions until recently. The fact that Florida's reef assemblages remained relatively stable until the last several decades indicates that conditions associated with recent disturbances must have been fundamentally different than what coral reefs have experienced over the last ~8,000 yr.
The emergence of novel assemblages
Climate change and other anthropogenic disturbances are reshaping the world's marine and terrestrial ecosystems through the disproportionate loss of habitat‐forming, ecosystem engineers, such as trees, kelps, and reef‐building corals (Hobbs et al. 2006, 2009, Williams and Jackson 2007, Anderegg et al. 2013, Perry et al. 2015, Krumhansl et al. 2016, Kuffner and Toth 2016, Bruno et al. 2019). The loss of these foundation species and the resulting shifts in species dominance can have cascading and often destabilizing effects on ecological structure and function, and the capacity of these habitats to continue providing ecosystem services to society (Hobbs et al. 2009, Anderegg et al. 2013, Krumhansl et al. 2016). Unfortunately, the emergence of novel ecosystems is predicted to become an increasingly common global phenomenon under the continuing influence of anthropogenic climate change (Williams and Jackson 2007).
Since the 1970s, coral populations around the world have declined by more than 50%, primarily as a result of climate‐driven coral bleaching and disease, and average coral cover throughout the region is now just over 10% (Aronson and Precht 2001a , b, Precht and Miller 2007; Schutte et al. 2010; Kuffner and Toth 2016, Hughes et al. 2017, Bruno et al. 2019). On the FKRT, coral cover at our study sites decreased from ~12% ± 1.3% on average in 1996 to just 4.9% ± 0.7% in 2015. The disproportionate loss of reef‐building corals on these reefs (see Ruzicka et al. 2013, Toth et al. 2014) has led to a shift in coral species composition that is without precedent in at least the last ~8,000 yr. The absolute percent coverage of S. siderea and P. astreoides on the shallow, offshore reefs of the FKRT is low (~0.5% and ~1%, respectively, in 2015) and has changed little since 1996 (Ruzicka et al. 2013); however, S. siderea now has the highest relative abundance across a variety of reef habitats on the FKRT (Burman et al. 2012, Toth et al. 2014) and the relative abundance of P. astreoides has increased in the shallowest reef zones vacated by A. palmata (Fig. 3c; Appendix S1: Table S5). These weedy coral taxa were rare in the Holocene reef framework of the FKRT (Fig. 2; Appendix S1: Table S5), as well as in the U.S. Virgin Islands (Hubbard et al. 2005), and on Pleistocene reefs in Barbados (Jackson 1992, Pandolfi and Jackson 2006). Our study, therefore, adds to a growing body of research suggesting that modern coral‐reef degradation has caused a restructuring of coral‐reef assemblages that has no historic analogue (Aronson and Precht 2001a, Aronson et al. 2004, 2006, Pandolfi and Jackson 2006; c.f. Williams and Jackson 2007) and indicates that Florida's reefs have likely transitioned into a novel ecosystem state (Hobbs et al. 2006, 2009, Graham et al. 2013).
The loss of foundation species often results in significant changes in habitat structure and quality (e.g., in forest [Anderegg et al. 2013] and kelp [Krumhansl et al. 2016] ecosystems) and this has certainly been the case for coral reefs in the western Atlantic (Kuffner and Toth 2016). In Florida, reefs have become more spatially homogenous than in the past (Burman et al. 2012) and the characteristic zonation of western Atlantic reefs has largely been lost (Jackson 1992, Aronson and Precht 2001a, Aronson et al. 2006, Precht and Miller 2007, Burman et al. 2012, Kuffner and Toth 2016, Precht and Aronson 2016). On the FKRT, the composition of the middle Holocene reef framework was more similar to the modern assemblages in 1996 than in 2015 (Fig. 3; Appendix S1: Table S7), likely reflecting the continuing decline of reef‐building A. palmata and Orbicella spp. over this period (Ruzicka et al. 2013, Toth et al. 2014). Hubbard et al. (2005) made a similar observation in Buck Island, U.S. Virgin Islands. There, the composition of Holocene reef framework more closely resembled reefs growing in the 1970s than in the 1980s and 1990s. The ongoing shifts in species dominance and the resulting homogenization of reefscapes has important consequences for the ecological and geological functions that reefs provide (Perry and Alvarez‐Filip 2018).
Although sustaining a minimum level of living coral is crucial to maintaining the balance between reef accretion and erosion, the composition of the remnant coral assemblages also has a significant impact on structural complexity and carbonate production (Williams et al. 1999, Alvarez‐Filip et al. 2013, Perry et al. 2015, González‐Barrios and Álvarez‐Filip 2018, Perry and Alvarez‐Filip 2018). Compared with the reef‐building corals they replaced, the relatively small, flat (Green et al. 2008), weedy taxa that now dominate reef assemblages throughout the western Atlantic have limited capacity for carbonate production and reef framework construction (Perry et al. 2015, Kuffner and Toth 2016). The transition to a novel reef state in which weedy corals and other, non‐calcifying organisms (Ruzicka et al. 2013, Toth et al. 2014) are the most abundant reef biota may, therefore, be accelerating the erosion and flattening of reef framework and facilitating the loss of critical ecosystem services (Perry et al. 2015, Perry and Alvarez‐Filip 2018).
Management implications
As coral‐reef degradation continues to drive the transition of reefs into states in which reef erosion, rather than accretion, is the dominant process (Williams et al. 1999, Perry et al. 2015, Perry and Alvarez‐Filip 2018), preserving the physical structure of coral reefs will become an increasingly important goal of coral‐reef management (Kuffner and Toth 2016). Like many reefs in the western Atlantic, the reef framework built over millennia in south Florida is now rapidly being eroded away (Yates et al. 2017, Kuffner et al. 2019). Because accretion has been negligible on the FKRT for at least 3000 yr (Toth et al. 2018a), it may be unrealistic to expect that the balance can be easily be tipped back to a state of net accretion; however, preserving the remaining geological structure of Florida's reefs, and the valuable ecosystem services it provides, is a reasonable minimum benchmark for successful coral‐reef management. One increasingly popular tool for coral‐reef management that has the potential to help to mitigate the problem reef erosion is coral restoration (Rinkevich 2008, Lirman and Schopmeyer 2016).
Most coral restoration efforts in the western Atlantic to date have focused on A. cervicornis, because of its high growth rates and potential for reproduction through fragmentation (Lirman and Schopmeyer 2016, Kuffner et al. 2017). Although this coral can provide valuable habitat in the short‐term (Lirman and Schopmeyer 2016), it is highly susceptible to mortality from disease and coral bleaching (Aronson and Precht 2001b, Muller et al. 2018). It also contributes only minimally to reef building in the long‐term in most locations (but see Aronson et al. 2004, Wapnick et al. 2004), because its fragile morphology makes it susceptible to post‐depositional loss from reef framework (Enos and Perkins 1977, Shinn et al. 1981, 2003). In our cores, A. cervicornis only comprised ~2% of the Holocene reef framework (Appendix S1: Tables S1, S2) and it was absent from Holocene reef cores from Buck Island, St. Croix (Hubbard et al. 2005). Continuing to invest time and resources into restoration of such an ephemeral space occupier may, therefore, not be the best means for achieving long‐term management goals, particularly in places where shoreline protection from storms is a desirable ecosystem service.
Instead, we suggest shifting the focus of restoration programs to more robust, reef‐building species, such as A. palmata and Orbicella spp., which produce reef structure that persists for centuries to millennia after the corals die. Fortunately, several countries in the western Atlantic have recently initiated successful A. palmata restoration programs (Lirman and Schopmeyer 2016). At the same time, novel methods for jump‐starting the restoration of Orbicella spp. and other massive taxa are gaining traction (i.e., microfragmenting; Forsman et al. 2015, Page et al. 2018). In particular, the concept of “reskinning” the surface of large, dead, coral colonies through the fusion of coral microfragments (Forsman et al. 2015), provides a potential mechanism for rapidly restoring a veneer of living coral tissue that could protect the reef framework from erosion (Kuffner and Toth 2016). There remain a number of important challenges when it comes to coral restoration, including questions of how, where, and when to outplant corals to maximize growth and survival (Lirman and Schopmeyer 2016, Kuffner et al. 2017, Guest et al. 2018, Page et al. 2018) and how to scale restoration to a level at which it will have a significant impact on reef ecology and function (Rinkevich 2008, Ladd et al. 2019); however, using the geological foundations of western Atlantic reefs to guide what species should be restored is a critical first step in optimizing the outcomes of coral‐reef management.
The unprecedented scale and magnitude of anthropogenic disturbances over the last half a century has led to the emergence of novel species configurations on coral reefs and other natural ecosystems around the world (Hobbs et al. 2006, 2009, Graham et al. 2013). Whereas human intervention has the potential to manage or even reverse transitions to novel ecosystem states in cases where biotic transitions arose from local‐scale human perturbations, such as land‐use change or localized introductions of invasive species (Hobbs et al. 2006, 2009), global climate change is increasingly becoming the primary driver of change in natural systems (Williams and Jackson 2007, Hobbs et al. 2009, Anderegg et al. 2013, Graham et al. 2013, Bruno et al. 2019). Active restoration of reef‐building corals may help to preserve some ecological and geological reef functions in the short short‐term; however, it is unlikely that local‐scale management alone can return reefs to natural historical and geological baselines (Hobbs et al. 2006, Graham et al. 2013, Hughes et al. 2017). Ensuring the long‐term persistence and recovery of coral reefs, and the valuable ecosystem services they provide, will ultimately require aggressive global‐scale action to combat the human fingerprint on the world's climate (Hughes et al. 2017, Bruno et al. 2019).
Supporting information
Correction statement: The corrected finalized version of the supporting information file was posted to accompany this article on September 16, 2019. We apologize to the authors and readers for this error.
Acknowledgments
We thank Richard Aronson and William Precht whose ideas and insights provided the foundations for many of the concepts we presented. E. Ashe provided the outputs of the sea‐level models of Khan et al. (2017); J. McGeehin and the USGS Radiocarbon Laboratory facilitated the radiocarbon analysis; and H. Cheng and R. L. Edwards provided the U‐series ages. We thank John Bruno, Chris Perry, and Lorenzo Alvarez‐Filip for their constructive reviews of the manuscript. Fieldwork was conducted under permits from the National Park Service and the Florida Keys National Marine Sanctuary including DRTO‐2017‐SCI‐0006 and FKNMS‐2015‐058, respectively, for the recent reef‐drilling studies. Funding for the study was provided by the Coastal/Marine Hazards and Resources Program of the U.S. Geological Survey. Toth's research is also partially supported by the U.S. National Science Foundation (OCE‐1535007). Funding for the CREMP program was provided by the US EPA Water Quality Protection Program award X7‐00D39315‐5. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Toth, L. T. , Stathakopoulos A., Kuffner I. B., Ruzicka R. R., Colella M. A., and Shinn E. A.. 2019. The unprecedented loss of Florida's reef‐building corals and the emergence of a novel coral‐reef assemblage. Ecology 100(9):e02781 10.1002/ecy.2781
Corresponding Editor: John Bruno.
Correction statement: Copyright information was updated to version of record.
Note
https://doi.org/10.5066/f7319tr3
Data Availability
All paleoecological data used in this study are archived in USGS Data Releases available at: https://doi.org/10.5066/f7p8492q, https://doi.org/10.5066/f7nv9hjx, and https://doi.org/10.5066/p93xxxa0. The CREMP data can be accessed at: http://ocean.floridamarine.org/FKNMS_WQPP/coral.htm.
Literature Cited
- Alvarez‐Filip, L. , Carricart‐Ganivet J. P., Horta‐Puga G., and Iglesias‐Prieto R.. 2013. Shifts in coral‐assemblage composition do not ensure persistence of reef functionality. Scientific Reports 3:srep03486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg, W. R. L. , Kane J. M., and Anderegg L. D. L.. 2013. Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change 3:30–36. [Google Scholar]
- Aronson, R. B. , and Precht W. F.. 1997. Stasis, biological disturbance, and community structure of a Holocene coral reef. Paleobiology 23:326–346. [Google Scholar]
- Aronson, R. B. , and Precht W. F.. 2001a. Evolutionary paleoecology of Caribbean coral reefs Pages 171–201 in Allmon W. D., and Bottjer D. J., editors. Evolutionary paleoecology. Columbia University Press, New York, New York, USA. [Google Scholar]
- Aronson, R. B. , and Precht W. F.. 2001b. White band disease and the changing face of Caribbean coral reef. Hydrobiologia 406:25–38. [Google Scholar]
- Aronson, R. B. , Macintyre I. G., Wapnick C. M., and O'Neill M. W.. 2004. Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology 85:1876–1891. [Google Scholar]
- Aronson, R. B. , Precht W. F., and Macintyre I. G.. 2006. Global change and biotic homogenization of coral reefs. Geological Society American Abstracts 38:535. [Google Scholar]
- Aronson, R. B. , Precht W. F., Macintyre I. G., and Toth L. T.. 2012. Catastrophe and the life span of coral reefs. Ecology 93:303–313. [DOI] [PubMed] [Google Scholar]
- Blanchon, P. , Richards S., Bernal J. P., Cerdeira‐Estrada S., Ibarra M. S., Corona‐Martínez L., and Martell‐Dubois R.. 2017. Retrograde accretion of a Caribbean fringing reef controlled by hurricanes and sea‐level rise. Frontiers in Earth Science 10.3389/feart.2017.00078 [DOI] [Google Scholar]
- Bruno, J. F. , and Selig E. R.. 2007. Regional decline of coral cover in the Indo‐Pacific: timing, extent, and subregional comparisons. PLoS ONE 2:e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, J. B. , Côté I. M., and Toth L. T.. 2019. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don't MPAs improve resilience? Annual Review of Marine Science 11:307–334. [DOI] [PubMed] [Google Scholar]
- Budd, A. F. , and Johnson K. G.. 1999. Origination preceding extinction during late Cenozoic turnover of Caribbean reefs. Paleobiology 25:188–200. [Google Scholar]
- Burman, S. G. , Aronson R. B., and Van Woesik R.. 2012. Biotic homogenization of coral assemblages along the Florida reef tract. Marine Ecology Progress Series 467:89–96. [Google Scholar]
- Colella, M. , Ruzicka R. R., Kidney J. A., Morrison J. M., and Brinkhuis V. B.. 2012. Cold‐water event of January 2010 results in catastrophic benthic mortality on patch reefs in the Florida Keys. Coral Reefs 31:621–632. [Google Scholar]
- Darling, E. S. , Alvarez‐Filip L., Oliver T. A., McClanahan T. R., and Côté I. M.. 2012. Evaluating life‐history strategies of reef corals from species traits. Ecology Letters 15:1378–1386. [DOI] [PubMed] [Google Scholar]
- Davis, G. E. 1982. A century of natural change in coral distribution at the Dry Tortugas: a comparison of reef maps from 1881 and 1976. Bulletin of Marine Science 32:608–623. [Google Scholar]
- Enos, P. , and Perkins R. D.. 1977. Quaternary sedimentation in south Florida. Geological Society of America. Memoir 147:198. [Google Scholar]
- Forsman, Z. H. , Page C. A., Toonen R. J., and Vaughn D.. 2015. Growing coral larger and faster: micro‐colony‐fusion as a strategy for accelerating coral cover. PeerJ 3:e1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg, R. N. , and Shinn E. A.. 1994. Preferential distribution of reefs in the Florida Reef Tract: the past is the key to the present Pages 21–26 in Ginsburg R. N., editor. Colloquium on global aspects of coral reefs: health, hazards, and history. Rosenstiel School of Marine and Atmospheric Science, University of Miami, Coral Gables, Florida, USA. [Google Scholar]
- Gischler, E. , and Hudson J. H.. 2004. Holocene development of the Belize Barrier Reef. Sedimentary Geology 164:223–236. [Google Scholar]
- González‐Barrios, F. J. , and Álvarez‐Filip L.. 2018. A framework for measuring coral species‐specific contribution to reef functioning in the Caribbean. Ecological Indicators 95:877–886. [Google Scholar]
- Graham, N. A. , Cinner J. E., Norström A. V., and Nyström M.. 2013. Coral reefs as novel ecosystems: embracing new futures. Current Opinion in Environmental Sustainability 7:9–14. [Google Scholar]
- Green, D. H. , Edmunds P. J., and Carpenter R. C.. 2008. Increasing abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Marine Ecology Progress Series 359:1–10. [Google Scholar]
- Greenstein, B. J. 2007. Taphonomy: detecting critical events in fossil reef‐coral assemblages Pages 31–60 in Aronson R. B., editor. Geological approaches to coral reef ecology. Springer, New York, New York, USA. [Google Scholar]
- Greenstein, B. J. , Curran H. A., and Pandolfi J. M.. 1998. Shifting ecological baselines and the demise of Acropora cervicornis in the western North Atlantic and Caribbean Province: a Pleistocene perspective. Coral Reefs 17:249–261. [Google Scholar]
- Guest, J. R. , et al. 2018. Identifying and characterising coral reef “oases” against a backdrop of degradation. Journal of Applied Ecology 55:2865–2875. [Google Scholar]
- Hobbs, R. J. , et al. 2006. Theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography 15:1–7. [Google Scholar]
- Hobbs, R. J. , Higgs E., and Harris J. A.. 2009. Novel ecosystems: implications for conservation and restoration. Trends in Ecology and Evolution 24:599–605. [DOI] [PubMed] [Google Scholar]
- Hubbard, D. K. 2009. Depth‐related and species‐related patterns of Holocene reef accretion in the Caribbean and western Atlantic: a critical assessment of existing models. International Association of Sedimentologists Special Publication 41:1–18. [Google Scholar]
- Hubbard, D. K. 2011. Reef drilling Pages 856–869 in Hopley D., editor. The encyclopedia of modern coral reefs. Springer, Dordrecht, Netherlands. [Google Scholar]
- Hubbard, D. K. 2014. Holocene accretion rates and styles for Caribbean coral reefs: lessons for the past and future. SEPM Special Publication 105:264–281. [Google Scholar]
- Hubbard, D. K. , Miller A. I., and Scaturo D.. 1990. Production and cycling of calcium carbonate in a shelf‐edge reef system (St. Croix, U.S. Virgin Islands): applications to the nature of reef systems in the fossil record. Journal of Sedimentary Petrology 60:335–360. [Google Scholar]
- Hubbard, D. K. , Zankl H., Van Heerden I., and Gill I. P.. 2005. Holocene reef development along the northeastern St. Croix shelf, Buck Island, U.S. Virgin Islands. Journal of Sedimentary Research 75:97–113. [Google Scholar]
- Hughes, T. P. , et al. 2017. Coral reefs in the Anthropocene. Nature 546:82. [DOI] [PubMed] [Google Scholar]
- Jackson, J. B. 1992. Pleistocene perspectives on coral reef community structure. American Zoologist 32:719–731. [Google Scholar]
- Khan, N. S. , et al. 2017. Drivers of Holocene sea‐level change in the Caribbean. Quaternary Science Reviews 155:13–36. [Google Scholar]
- Kohler, K. E. , and Gill S. M.. 2006. Coral Point Count with Excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Computers and Geosciences 32:1259–1269. [Google Scholar]
- Krumhansl, K. A. , et al. 2016. Global patterns of kelp forest change over the past half‐century. Proceedings of the National Academy of Sciences USA 113:13785–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner, I. B. , and Toth L. T.. 2016. A geological perspective on the degradation and conservation of western Atlantic coral reefs. Conservation Biology 30:706–715. [DOI] [PubMed] [Google Scholar]
- Kuffner, I. B. , Bartels E., Stathakopoulos A., Enochs I. C., Kolodziej G., Toth L. T., and Manzello D. P.. 2017. Plasticity in skeletal characteristics of nursery‐raised staghorn coral, Acropora cervicornis . Coral Reefs 36:679–684. [Google Scholar]
- Kuffner, I. B. , Toth L. T., Hudson J. H., Goodwin W. B., Stathakopoulos A., Bartlett L. A., and Whitcher E. A.. 2019. Improving estimates of coral reef construction and erosion with in‐situ measurements. Limnology and Oceanography 10.1002/lno.11184 [DOI] [Google Scholar]
- Ladd, M. C. , Burkepile D. E., and Shantz A. A.. 2019. Near‐term impacts of coral restoration on target species, coral reef community structure, and ecological processes. Restoration Ecology 10.1111/rec.12939 [DOI] [Google Scholar]
- Lenth, R. V. 2016. Least‐squares means: the R package lsmeans. Journal of Statistical Software 69:1–33. [Google Scholar]
- Lescinsky, H. , Titus B., and Hubbard D.. 2012. Live coral cover in the fossil record: an example from Holocene reefs of the Dominican Republic. Coral Reefs 31:335–346. [Google Scholar]
- Lidz, B. H. , Reich C. D., and Shinn E. A.. 2003. Regional Quaternary submarine geomorphology in the Florida Keys. Geological Society of America Bulletin 115:845–866. [Google Scholar]
- Lirman, D. , and Schopmeyer S.. 2016. Ecological solutions to reef degradation: optimizing coral reef restoration in the Caribbean and Western Atlantic. PeerJ 4:e2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre, I. 1975. A diver‐operated hydraulic drill for coring submerged substrates. Atoll Research Bulletin 185:21–26. [Google Scholar]
- Marszalek, D. S. , Babashoff G., Noel M. R., and Worley D. R.. 1977. Reef distribution in south Florida. Pages 223–229 in Proceedings of the 3rd International Coral Reef Symposium. Miami, Florida, USA. [Google Scholar]
- Muller, E. M. , Bartels E., and Baums I. B.. 2018. Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis . eLife 7:e35066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch, T. J. T. , and Aronson R. B.. 1999. Scale‐dependent spatial variability of coral reef assemblages. Coral Reefs 18:341–351. [Google Scholar]
- Norström, A. V. , Nyström M., Lokrantz J., and Folke C.. 2009. Alternative stable states on coral reefs: beyond coral‐macroalgal phase shifts. Marine Ecology Progress Series 376:295–306. [Google Scholar]
- Oksanen, J. , et al. 2017. vegan: community ecology package. R package version 2.4‐3. https://CRAN.R-project.org/package=vegan
- Page, C. A. , Muller E. M., and Vaughn D. E.. 2018. Microfragmenting for the successful restoration of slow growing massive coral. Ecological Engineering 123:86–94. [Google Scholar]
- Pandolfi, J. , and Jackson J. B. C.. 2006. Ecological persistence interrupted in Caribbean coral reefs. Ecology Letters 9:818–826. [DOI] [PubMed] [Google Scholar]
- Perry, C. T. , and Alvarez‐Filip L.. 2018. Changing geo‐ecological functions of coral reefs in the Anthropocene. Functional Ecology 10.1111/1365-2435.13247 [DOI] [Google Scholar]
- Perry, C. T. , Steneck R. S., Murphy G. N., Kench P. S., Edinger E. N., Smithers S. G., and Mumby P. J.. 2015. Regional‐scale dominance of non‐framework building coral on Caribbean reefs affects carbonate production and future reef growth. Global Change Biology 21:1153–1164. [DOI] [PubMed] [Google Scholar]
- Pinheiro, J. , Bates D., DebRoy S., Sarkar D., and R Core Team . 2017. nlme: linear and nonlinear mixed effects models. R package version 3.1‐131. https://CRAN.Rproject.org/package=nlme
- Precht, W. F. , and Aronson R. B.. 2004. Climate flickers and range shifts of reef corals. Frontiers in Ecology and the Environment 2:307–314. [Google Scholar]
- Precht, W. F. , and Aronson R. B.. 2016. Stability of reef‐coral assemblages in the Quaternary Pages 155–173 in Hubbard D. K., editor. Coral reefs at the crossroads. Springer, New York, New York, USA. [Google Scholar]
- Precht, W. F. , and Miller S. L.. 2007. Ecological shifts along the Florida reef tract: the past as a key to the future Pages 237–312 in Aronson R. B., editor. Geological approaches to coral reef ecology. Springer, New York, New York, USA. [Google Scholar]
- Rinkevich, B. 2008. Management of coral reefs: We have gone wrong when neglecting active reef restoration. Marine Pollution Bulletin 56:1821–1824. [DOI] [PubMed] [Google Scholar]
- Ruzicka, R. , Colella M., Semon K., Brinkhuis V., Morrison J., Kidney J., Porter J., Meyers M., Christman M., and Colee J.. 2010. CREMP 2009 Final Report. Fish & Wildlife Research Institute/Florida Fish & Wildlife Conservation Commission. Saint Petersburg, Florida, USA. [Google Scholar]
- Ruzicka, R. R. , et al. 2013. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Marine Ecology Progress Series 489:125–141. [Google Scholar]
- Schutte, V. G. W. , Selig E. R., and Bruno J. F.. 2010. Regional spatio‐temporal trends in Caribbean coral reef benthic communities. Marine Ecology Progress Series 402:115–122. [Google Scholar]
- Shinn, E. A. , and Lidz B. H.. 2018. Page 154 Geology of the Florida keys. University Press of Florida, Gainesville, Florida, USA. [Google Scholar]
- Shinn, E. A. , Hudson J. H., Halley R. B., and Lidz B. H.. 1977. Topographic control and accumulation rate of some Holocene coral reefs: South Florida and Dry Tortugas. Proceedings of the 3rd International Coral Reef Symposium 1:1–7.
- Shinn, E. A. , Hudson J. H., Robbin D. M., and Lidz B.. 1981. Spurs and grooves revisited: construction versus erosion Looe Key reef, Florida. Proceedings of the 4th International Coral Reef Symposium, Manila, Philippines 1:475–483.
- Shinn, E. A. , Reich C. D., Hickey T. D., and Lidz B. H.. 2003. Staghorn tempestites in the Florida Keys. Coral Reefs 22:91–97. [Google Scholar]
- Stathakopoulos, A. , and Riegl B. M.. 2015. Accretion history of mid‐Holocene reefs from the southeast Florida continental reef tract, USA. Coral Reefs 34:173–187. [Google Scholar]
- Storlazzi, C. D. , Reguero B. G., Cole A. D., Lowe E., Shope J. B., Gibbs A. E., Nickel B. A., McCall R. T., van Dongeren A. R., and Beck M. W.. 2019. Rigorously valuing the role of U.S. coral reefs in coastal hazard risk reduction. USGS Open‐File Report 2019‐1027. U. S. Geological Survey, Reston, Virginia, USA
- Toth, L. T. , van Woesik R., Murdoch T. J. T., Smith S. R., Ogden J. C., Precht W. F., and Aronson R. B.. 2014. Do no‐take reserves benefit Florida's corals? 14 years of change and stasis in the Florida Keys National Marine Sanctuary. Coral Reefs 33:565–577. [Google Scholar]
- Toth, L. T. , Kuffner I. B., Cheng H., and Edwards R. L.. 2015. A new record of the late Pleistocene coral Pocillopora palmata from the Dry Tortugas, Florida reef tract, USA. Palaios 30:1–9. [Google Scholar]
- Toth, L. T. , Cheng H., Edwards R. L., Ashe E., and Richey J. N.. 2017. Millennial‐scale variability in the local radiocarbon reservoir age of south Florida during the Holocene. Quaternary Geochronology 42:130–143. [Google Scholar]
- Toth, L. T. , Kuffner I. B., Stathakopoulos A., and Shinn E. A.. 2018a. A 3000‐year lag between the geological and ecological collapse of Florida's coral reefs. Global Change Biology 24:5471–5483. [DOI] [PubMed] [Google Scholar]
- Toth, L. T. , Stathakopoulos A., and Kuffner I. B.. 2018b. Descriptive core logs, core photographs, radiocarbon ages, and data on reef development for cores of Holocene reef framework from the Florida Keys reef tract. USGS Data Release. U.S. Geological Survey, Reston, Virginia, USA.
- Toth, L. T. , Stathakopoulos A., Ruzicka R. A., and Collela M. A.. 2019. The absolute and relative composition of Holocene reef framework and modern reefs on the Florida Keys reef tract. USGS Data Release. U.S. Geological Survey, Reston, Virginia, USA.
- Wapnick, C. M. , Precht W. F., and Aronson R. B.. 2004. Millennial‐scale dynamics of staghorn coral in Discovery Bay, Jamaica. Ecology Letters 7:354–361. [Google Scholar]
- Waters, C. N. , et al. 2016. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351:aad2622. [DOI] [PubMed] [Google Scholar]
- Williams, J. W. , and Jackson S. T.. 2007. Novel climates, no‐analog communities, and ecological surprises. Frontiers in Ecology and the Environment 5:475–482. [Google Scholar]
- Williams, E. H. , Bartels P. J., and Bunkley‐Williams L.. 1999. Predicted disappearance of coral‐reef ramparts: a direct result of major ecological disturbances. Global Change Biology 5:839–845. [Google Scholar]
- Yates, K. K. , Zawada D. G., Smiley N. A., and Tiling‐Range G.. 2017. Divergence of seafloor elevation and sea level rise in coral reef ecosystems. Biogeosciences 14:1739–1772. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correction statement: The corrected finalized version of the supporting information file was posted to accompany this article on September 16, 2019. We apologize to the authors and readers for this error.
Data Availability Statement
All paleoecological data used in this study are archived in USGS Data Releases available at: https://doi.org/10.5066/f7p8492q, https://doi.org/10.5066/f7nv9hjx, and https://doi.org/10.5066/p93xxxa0. The CREMP data can be accessed at: http://ocean.floridamarine.org/FKNMS_WQPP/coral.htm.


