Abstract
Aim
In the EMPA‐REG OUTCOME trial, empagliflozin therapy reduced cardiovascular death by 38% compared with placebo when added to standard of care. Using the trial results, we created a discrete‐event simulation model to assess lifetime health economic outcomes in people with Type 2 diabetes and established cardiovascular disease.
Methods
Time‐dependent survival regression analysis was performed on data from EMPA‐REG OUTCOME for 10 cardiovascular and renal events (e.g. stroke, heart failure hospitalization, macroalbuminuria, cardiovascular mortality) to capture event rates over time, and interaction between events. Model performance was assessed by comparing predicted and observed outcomes at 3 years. Costs in the United Kingdom (UK) and health utilities were obtained from published literature. Outcomes included cumulative event rates, life‐years, costs and quality‐adjusted life‐years (QALYs).
Results
The model predicted an 18% relative increase (by 2.1 life‐years) in survival for empagliflozin (14.0 life‐years) vs. standard of care (11.9 life‐years), attributable to direct treatment effect on cardiovascular mortality, and to indirect effect via reductions in other events. Participants treated with empagliflozin may experience improved quality of life (1.0 QALY) and higher costs (£3737/participant), yielding an incremental cost‐effectiveness ratio (ICER) of £4083/QALY. Sensitivity analyses confirmed the robustness of these results to changes in input parameters.
Conclusions
Based on extrapolation of EMPA‐REG OUTCOME trial data using a participant‐level simulation model, empagliflozin in addition to standard of care is projected to be highly cost‐effective using UK healthcare costs. The impact in other countries will vary due to differences in drug pricing and accrual of other costs. (Clinical Trial Registry No: NCT01131676)
What's new?
The sodium–glucose co‐transporter 2 inhibitor empagliflozin has been shown to mitigate cardiovascular risk and cardiovascular death in people with Type 2 diabetes with established cardiovascular disease.
An economic model was developed to extrapolate the outcomes of empagliflozin plus standard of care compared with standard of care alone over peoples’ remaining lifetime in the United Kingdom.
Patient‐level data from EMPA‐REG OUTCOME were analysed to generate time‐to‐event distributions for 10 cardiovascular and renal outcomes, including myocardial infarction, stroke, heart failure hospitalization, development of chronic kidney disease and cardiovascular mortality.
Empagliflozin may have a positive benefit for people at costs acceptable to payers.
What's new?
The sodium–glucose co‐transporter 2 inhibitor empagliflozin has been shown to mitigate cardiovascular risk and cardiovascular death in people with Type 2 diabetes with established cardiovascular disease.
An economic model was developed to extrapolate the outcomes of empagliflozin plus standard of care compared with standard of care alone over peoples’ remaining lifetime in the United Kingdom.
Patient‐level data from EMPA‐REG OUTCOME were analysed to generate time‐to‐event distributions for 10 cardiovascular and renal outcomes, including myocardial infarction, stroke, heart failure hospitalization, development of chronic kidney disease and cardiovascular mortality.
Empagliflozin may have a positive benefit for people at costs acceptable to payers.
Introduction
Most of the Type 2 diabetes economic burden pertains to the diagnosis and management of related complications, mainly macrovascular, e.g. myocardial infarction (MI) and stroke, but also includes indirect costs of disease related to productivity loss and reduced life expectancy. The ultimate goal of anti‐glycaemic therapy in people with Type 2 diabetes is to prevent microvascular and macrovascular complications to increase life expectancy and quality of life (QoL) and to reduce costs 1, 2, 3, 4. However, demonstrating improvements in cardiovascular outcomes with anti‐hyperglycaemic therapies has been challenging. Major studies, including UKPDS, ACCORD, ADVANCE and VADT 5, performed to assess the impact of glycaemic control, have been unable to demonstrate unequivocal improvements in the occurrence of complications or mortality 5.
The EMPA‐REG OUTCOME study (Clinical Trial Registry No: NCT01131676) evaluated the effect of adding empagliflozin (10 or 25 mg once‐daily) to the standard of care on cardiovascular events and mortality in people with diabetes with established cardiovascular disease. Trial participants were taking a mix of background medications (metformin, dipeptidyl peptidase‐4 inhibitors, sulfonylureas, thiazolidinedione, glucagon‐like peptide‐1 agonists and insulin) alone or in combination for glycaemic control, and were receiving therapies to manage cardiovascular risk (lipid‐lowering therapy, anti‐hypertensive therapy and anti‐coagulants). EMPA‐REG‐OUTCOME was the first placebo‐controlled, randomized controlled trial of a glucose‐lowering therapy in Type 2 diabetes to demonstrate a significant reduction in cardiovascular outcomes, with a 38% reduction in cardiovascular mortality [hazard ratio (HR) 0.62; 95% confidence interval (CI) 0.49–0.77] 6. These results led to European Union and United States approval of empagliflozin to prevent cardiovascular death in people with Type 2 diabetes and established cardiovascular disease – the first glucose‐lowering drug to be granted a mortality indication.
We developed an economic model to extrapolate the clinical and cost outcomes of people with Type 2 diabetes and established cardiovascular disease who were recruited to the EMPA‐REG OUTCOME trial. The availability of quantitative outcome data allows this model to rely exclusively on observed event rates, rather than estimating event rates from extrapolated changes in biomarker values (e.g. HbA1c).
Participants and methods
Model approach and description
The discrete‐event simulation approach was used to simulate the rates of relevant clinical events in people with Type 2 diabetes with established cardiovascular disease from EMPA‐REG OUTCOME and to assign associated lifetime costs and QoL consequences. The number of complications and deaths that occur during the lifetime of a group of individuals with different characteristics are estimated based on event‐free survival curves from analyses of the trial data. As events accumulate, they can alter the risk of future events and management costs, and QoL of the simulated participant. This approach was selected based on a systematic literature review of approaches for modelling event‐driven clinical trial data 7. It permits modelling of multiple events for each participant, with the probability of events contingent on the type of events the participant has already experienced and their clinical characteristics (e.g. age, HbA1c).
At model initiation, a cohort of people with Type 2 diabetes and established cardiovascular disease is generated based on EMPA‐REG OUTCOME data (Fig. 1). Identical participants are assigned to treatment with standard of care with empagliflozin (empagliflozin regimen) or standard of care with no additional active treatment (placebo regimen). Predicted time to events is assigned based on the relationship of clinical prognostic factors (e.g. prior MI) to event rates from EMPA‐REG OUTCOME data. The model compares the event times to determine which event happens first. Following the event, two options are possible: (i) the participant exits the model if a fatal event occurs or the time horizon ends; or (ii) the participant remains in the model and treatment history, risk of future events and time to next event are updated. Once all participants have been simulated on both treatments, individual participant outcomes are aggregated to compute the mean population outcomes.
Figure 1.
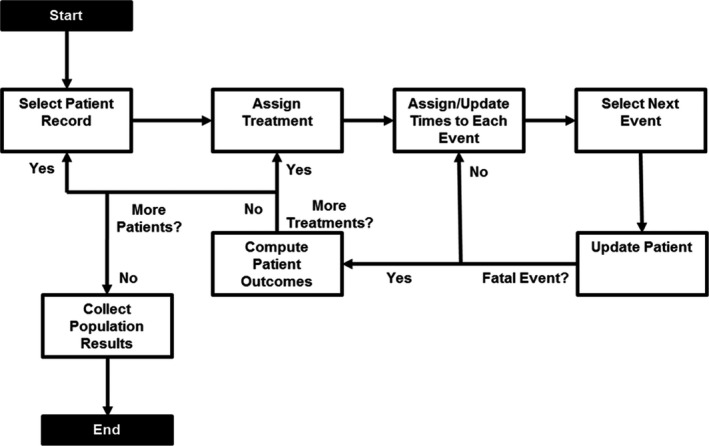
Schematic of the simulation flow.
Participant population
The simulated population was created by randomly sampling complete individual profiles with replacement from the observed subject‐level data describing characteristics of participants collected at baseline in the EMPA‐REG OUTCOME trial (N = 7020), which considers natural correlations among risk factors and medical histories. Each participant record included demographic attributes such as age, sex, health condition (BMI and HbA1c), history of cardiovascular and renal events, and region (Table 1). All participants with Type 2 diabetes had established cardiovascular disease. Assessment of effect modification suggested that treatment effect was homogeneous in patients of different region, ethnicity and race (P‐values for interaction did not meet a significance level of 0.05), thus the overall trial population was used to evaluate the impact of treatment on costs and QoL 6.
Table 1.
Baseline characteristics and model inputs for the cost‐effectiveness model
| Parameters | Base‐case value (95% CI) | Distribution | Data source [Ref.] |
|---|---|---|---|
| Baseline population characteristics | |||
| Demographic characteristics | |||
| Mean age, years | 63 | EMPA‐REG OUTCOME 6 | |
| Women, % | 29 | EMPA‐REG OUTCOME 6 | |
| BMI ≥ 30, % | 52 | EMPA‐REG OUTCOME 6 | |
| HbA1c ≥ 69 mmol/mol (8.5%) | 31 | EMPA‐REG OUTCOME 6 | |
| CV history, % | |||
| Non‐fatal stroke | 23 | EMPA‐REG OUTCOME 6 | |
| Non‐fatal MI | 47 | EMPA‐REG OUTCOME 6 | |
| CABG | 25 | EMPA‐REG OUTCOME 6 | |
| MCAD | 47 | EMPA‐REG OUTCOME 6 | |
| SVCAD | 10 | EMPA‐REG OUTCOME 6 | |
| PAD | 21 | EMPA‐REG OUTCOME 6 | |
| eGFR, % | |||
| ≥ 90 ml/min/1.73 m2 | 22 | EMPA‐REG OUTCOME 6 | |
| 60 to < 90 ml/min/1.73 m2 | 52 | EMPA‐REG OUTCOME 6 | |
| <60 ml/min/1.73 m2 | 26 | EMPA‐REG OUTCOME 6 | |
| Geographical region, % | |||
| Africa | 4 | EMPA‐REG OUTCOME6 | |
| Asia | 19 | EMPA‐REG OUTCOME6 | |
| Europe | 41 | EMPA‐REG OUTCOME6 | |
| Latin America | 15 | EMPA‐REG OUTCOME6 | |
| North America | 20 | EMPA‐REG OUTCOME6 | |
| Empagliflozin daily drug cost, £ | 1.31 | MIMS Drug Database8 | |
| Cost per episode of clinical events, £ | |||
| Non‐fatal MI | 8120 | Gamma | Alva et al., 20159 |
| Non‐fatal stroke | 11 921 | Gamma | Alva et al., 20159 |
| Unstable angina | 5186 | Gamma | Clarke et al., 200310 |
| Heart failure | 5001 | Gamma | Alva et al., 20159 |
| Transient ischemic attack | 5654 | Gamma | Ward et al., 201211 |
| Revascularization | 6192 | Gamma | Cassar, 200612 |
| CV death | 3684 | Gamma | Alva et al., 20159 |
| Macro‐albuminuria | 8896 | Gamma | Gordios et al., 200413 |
| Renal injury | 676 | Gamma | Kent et al., 201514 |
| Renal failure | 44,876 | Gamma | NICE technology appraisal 33615 |
| Baseline utility value | 0.785 | Beta | Sullivan et al., 2015 16 |
| Utility decrements for clinical events | |||
| Non‐fatal MI | −0.047 (−0.057, −0.036) | Beta | Sullivan et al., 2015 16 |
| Non‐fatal stroke | −0.060 (−0.074, −0.046) | Beta | Sullivan et al., 2015 16 |
| Unstable angina | −0.047 (−0.057, −0.036) | Beta | Sullivan et al., 2015 16 |
| Heart failure | −0.050 (−0.064, −0.036) | Beta | Sullivan et al., 2015 16 |
| Transient ischemic attack | −0.070 (−0.131, −0.008) | Beta | Sullivan et al., 2015 16 |
| Revascularization | −0.030 (−0.036, −0.024) | Beta | Lindgren et al., 200717 |
| Macro‐albuminuria | −0.038 (−0.059, −0.016) | Beta | Sullivan et al., 2015 16 |
| Renal injury | −0.038 (−0.059, −0.016) | Beta | Sullivan et al., 2015 16 |
| Renal failure | −0.038 (−0.059, −0.016) | Beta | Sullivan et al., 2015 16 |
| Utility effect of multiple events (additive to utility) | |||
| 2 events | 0.017 | Sullivan et al., 2015 16 | |
| 3 events | 0.042 | Sullivan et al., 2015 16 | |
| 4 events | 0.070 | Sullivan et al., 2015 16 | |
| ≥ 5 events | 0.087 | Sullivan et al., 2015 16 | |
CABG, coronary artery bypass grafting; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio; MCAD, multivessel coronary artery disease; MI, myocardial infarction; NICE, National Institute for Health and Care Excellence; PAD, peripheral artery disease; SVCAD, single vessel coronary artery disease
We used deidentified data from a clinical trial involving human participants, but did not deal with or report on any specific participants, so we did not seek ethics committee approval. However, the EMPA‐REG OUTCOME trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines and was approved by local authorities. An independent ethics committee or institutional review board approved the clinical protocol for each participating centre. Participants provided written informed consent before entering the trial.
Clinical and treatment inputs
The model captured treatment effectiveness by tracking 10 clinical events specified in EMPA‐REG OUTCOME: cardiovascular death, non‐fatal MI, non‐fatal stroke, unstable angina hospitalization, heart failure hospitalization, transient ischaemic attack, revascularization, new onset of macro‐albuminuria, renal injury (doubling of serum creatinine, with eGFR <45 ml/min/1.73 m2), and renal failure (initiation of continuous renal replacement therapy). The model captures death through non‐cardiovascular causes using United Kingdom (UK) mortality data 18. Non‐fatal cardiovascular events were permitted to recur; renal events were considered non‐recurring. Rare complications of diabetes (e.g. blindness) were not included in the model because the occurrence rates were low. Similarly, adverse events (e.g. genital infection) were not included, because these are usually transient events not associated with hospitalization or inpatient costs.
Occurrence of cardiovascular and renal events was obtained from statistical analysis of EMPA‐REG OUTCOME data. Parametric models were used to extrapolate the observed trends in the hazard of each event to allow prediction of individual participant histories in the simulation model over a lifetime horizon. This is a conventional approach to long‐term estimation of outcomes in cost‐effectiveness modelling, when only short‐term clinical trial evidence is available.
For modelled events, a two‐stage analysis was performed. First, a population‐level, event‐free survival function was fit to the trial data using candidate parametric distributions. Second, Cox regression analysis was used to generate individual‐level risk estimates based on participant characteristics and event history. The population‐level occurrence of each diabetes‐related complication in EMPA‐REG OUTCOME was captured as an event‐free survival curve. Individual‐level data from EMPA‐REG OUTCOME was fit using parametric survival models (Weibull, exponential, log‐normal and Gompertz were tested) to describe the distribution of time to each first event in the clinical trial. The best‐fit survival model was chosen based on numerical fit (Akaike's Information Criterion and Bayesian Information Criterion), realistic extrapolation beyond the trial time horizon and parsimony (preferring the simplest functional form) 19. Survival analyses were performed in Statistical Analysis System version 9.4.
The individual‐level risk equations were developed by testing baseline and time‐dependent characteristics as potential predictors of the outcomes in Cox regression analyses. Candidate characteristics for predictors were selected based on clinical relevance, and included basic demographic information (age, sex, geographic region), baseline biomarkers (HbA1c, BMI, eGFR), baseline event history (cardiovascular, cerebrovascular or peripheral arterial disease), and cardiovascular and renal events experienced during trial follow‐up, along with treatment arm. Candidate predictors were included in time‐dependent multivariate parametric equations (using R, version 3.2.2). 20 The multivariate equations were developed by first considering all candidate predictors that trended towards significance in univariate analyses (P < 0.20). The final multivariate equations were then reduced by eliminating terms in order of highest P‐value until all terms had P < 0.20 level. Treatment effect was assumed to apply across all participants and to be constant over time. The assumption of common treatment effect was supported by subgroup analyses of trial data in which only baseline age and HbA1c indicated significant interactions (P ≤ 0.05) with treatment effect for the primary outcome, but not for cardiovascular mortality; meanwhile only BMI indicated an interaction with treatment for cardiovascular mortality, but not for the primary outcome. The assumption of constant treatment effect over time was justified by proportional hazards testing.
Based on the clinical relationships, renal events were permitted as predictors of the risk of future cardiovascular events and mortality, but cardiovascular events were not predictors of renal events.
Costs and perspective
A UK National Health Service (NHS) third‐party healthcare payer perspective was taken; thus, only direct costs were included in the model (Table 1). All costs are expressed in UK pounds (£), with inflation to 2019 values using the health component of the UK Harmonized Index of Consumer Prices 21. The model captures costs associated with empagliflozin drug use and the acute costs of managing each analysed clinical event. Events indirectly imposed long‐term costs by increasing the risk of future costly events. Thus, to avoid any double counting, the model did not include any long‐term costs associated with each clinical event. This approach captured most relevant inpatient costs, but excludes long‐term outpatient costs.
Daily drug acquisition cost of empagliflozin (£1.31) was extracted from the MIMS Drug Database 22. Because EMPA‐REG OUTCOME compared empagliflozin plus standard of care, no other pharmacy costs were considered 23.
All other regular disease management and monitoring costs were assumed to be the same for both the empagliflozin and placebo regimens, and were therefore not included in the model.
Quality of life
Health outcomes were expressed in quality‐adjusted life‐years (QALYs) to measure the length of life adjusted for QoL (Table 1). QALYs were calculated using a baseline value and a permanent decrement associated with history of each event from published literature 16, 17, 23. As participants accumulate multiple diabetes‐related conditions, utility decrements from each event were summed and adjusted using an additive utility effect based on the number of events.
Model analyses
In the base‐case, a lifetime horizon was chosen to fully capture costs and QoL benefits of empagliflozin. The same cohort (5000 participants) was used for all analyses to ensure comparability. Model outputs include cumulative events per 100 patient‐years, life expectancy, QALYs, costs and incremental cost‐effectiveness ratios (ICERs). Costs and utilities were discounted at 3.5% annually 24.
Univariate sensitivity analyses were carried out to assess: (i) what components of the treatment effect are most influential, (ii) how predictions vary over time, (iii) participant population histories and (iv) the impact of uncertainty in cost and utility inputs. Specifically, the impact of no treatment effect on cardiovascular mortality, heart failure hospitalization, non‐fatal MI, and non‐fatal stroke was assessed. The baseline risk of clinical events was varied by applying a hazard ratio (ranging from 0.90 to 1.10) to the survival function of each cardiovascular and renal outcome. The model was run using a reduced 10‐year time horizon. Analyses in people with history of MI, stroke and peripheral artery disease at baseline was conducted. Discount rates on costs and QALYs ranging from 0% to 5% were applied. Higher (+20%) and lower (−20%) costs (drug acquisition and event management) and utilities (no event history and decrements) were used. Probabilistic sensitivity analysis was performed using distributions (beta distributions for probabilities and utilities and gamma distributions for costs) reflecting parameter uncertainties, producing 1000 pairs of incremental effectiveness and cost estimates.
Results
Table S1 shows the parameters included in the final risk equations that trended towards a significant effect (at P < 0.20) or are important prognostic factors that show a non‐negligible effect size. The regression coefficients can be interpreted as the log of the HRs, with a negative value indicating that a characteristic is associated with lower risk and a positive value indicating association with higher risk. As expected, several linkages between events were observed. For example, a history of MI increases the risk of a stroke, i.e. experiencing an MI during follow‐up has a positive coefficient in the stroke risk equation.
Validation of the derived equations reproduced the overall event rates in EMPA‐REG OUTCOME when treated as competing events. A large number of participants (10 000) were simulated for the 3‐year mean trial follow‐up duration for the validation, because the short time horizon and relatively low rate of events make the validation results sensitive to random variation. The largest deviations are in rates of revascularization (model HR 0.92) and cardiovascular death (model HR 0.70), both of which show rate ratios that are slightly less favourable to empagliflozin than the trial data (HR 0.86 and 0.62, respectively).
The composite of cardiovascular mortality, non‐fatal MI, and non‐fatal stroke rates were 7.0 events/100 patient‐years with empagliflozin vs. 8.6 events/100 patient‐years with standard of care only (Table 2). This translated to improvements in survival, because participants who received empagliflozin plus standard of care lived on average 2 years longer than participants treated with standard of care alone. Modelled participants receiving empagliflozin plus standard of care were estimated to have a higher rate of non‐cardiovascular‐related mortality than those on standard of care alone, although participants receiving empagliflozin in the trial had a lower rate of death from any cause. In the model, every participant dies, because we are running lifetime analyses. Given the magnitude of reductions in cardiovascular death, the population receiving empagliflozin survives longer and their increased age results in an increase in the predicted non‐cardiovascular death rate. In net, longer overall survival and reduced rates of clinical events translate to an incremental 1.0 QALY. Clinical event costs were reduced by £1177/participant despite the longer survival, partially off‐setting the total lifetime costs of empagliflozin (£4914/participant) to yield an incremental cost of £3737/participant. This results in an ICER of £4083/QALY, well below the commonly accepted £30 000/QALY UK cost‐effectiveness threshold.
Table 2.
Simulation model results comparing treatment with empagliflozin to standard of care
| Results | Base‐case* | Probabilistic sensitivity analysis† | |||
|---|---|---|---|---|---|
| Standard of care plus empagliflozin | Standard of care alone | Incremental | Standard of care plus empagliflozin | Standard of care alone | |
| Non‐fatal MI | 2.0 | 2.2 | −0.2 | 2.0 (1.7, 2.5) | 2.4 (1.9, 3.0) |
| Non‐fatal stroke | 1.4 | 1.1 | 0.3 | 1.3 (1.0, 1.8) | 1.1 (0.8, 1.5) |
| Unstable angina | 1.3 | 1.2 | 0.1 | 1.4 (1.1, 1.9) | 1.4 (0.9, 1.9) |
| Heart failure | 2.0 | 3.0 | −1.0 | 2.1 (1.6, 2.8) | 3.1 (2.2, 4.2) |
| Transient ischemic attack | 0.3 | 0.3 | 0.0 | 0.2 (0.2, 0.4) | 0.3 (0.2, 0.4) |
| Revascularization | 2.7 | 2.9 | −0.2 | 2.6 (2.4, 2.8) | 2.8 (2.5, 3.1) |
| Macro‐albuminuria | 5.4 | 6.5 | −1.1 | 5.6 (5.1, 6.1) | 6.7 (6.1, 7.3) |
| Renal injury | 1.0 | 1.6 | −0.6 | 0.9 (0.7, 1.3) | 1.5 (1.1, 2.1) |
| Renal failure | 0.3 | 0.6 | −0.3 | 0.3 (0.1, 0.5) | 0.5 (0.2, 0.8) |
| CV death | 3.9 | 5.6 | −1.7 | 4.2 (3.6, 5.0) | 5.9 (5.1, 6.8) |
| Non‐CV death | 3.2 | 2.8 | 0.4 | 3.3 (3.1, 3.5) | 2.9 (2.7, 3.1) |
| Undiscounted life expectancy (years) | 14.0 | 11.9 | 2.1 | ||
| Discounted QALYs | 7.8 | 6.8 | 1.0 | ||
| Discounted costs over participant lifetimes | |||||
| Total costs (£) | 19 776 | 16 040 | 3737 | ||
| Drug acquisition cost (£) | 4914 | 0 | 4914 | ||
| Event management cost (£) | 14 862 | 16 040 | −1177 | ||
| ICER (£/QALY) | – | – | 4083 | ||
Cumulative events per 100 patient‐years.
Event rates per patient‐years (95% confidence intervals).
CV, cardiovascular; ICER, incremental cost‐effectiveness ratio; MI, myocardial infarction; QALY, quality‐adjusted life years.
Results of one‐way sensitivity analyses are largely stable relative to variation in most model parameters (Fig. 2). The ICER ranges from £1755 to £10 531/QALY, with all scenarios yielding ICERs below the £30 000/QALY UK threshold for cost‐effectiveness. The predicted benefits of empagliflozin accumulate steadily over time, with the ICER dropping as the time horizon increased from 5 years (£10 531/QALY) to 10 years (£5618/QALY) to the base‐case lifetime horizon (£4083/QALY). Varying the discount rate on QALYs (£1755 to £5052/QALY) and costs (£3418 to £6508/QALY) has an impact on cost‐effectiveness results, as does varying the acquisition cost of empagliflozin (£3020 to £5173/QALY). A key driver of cost‐effectiveness results was empagliflozin's effect on cardiovascular mortality (ICER of £5978/QALY). Variation in the utility parameter with no event history results in ICERs from £3378/QALY to £5160/QALY. Discount rate on both costs and QALYs (£2796 to £4229/QALY), changes in event rates (except cardiovascular mortality; £3663 to £5320/QALY), baseline disease history (£3104 to £4556/QALY), baseline risk of events (£3895 to £4372/QALY), event management costs (£3826 to £4341/QALY) and utility decrements (£4035 to £4132/QALY), had smaller effects on the ICER.
Figure 2.
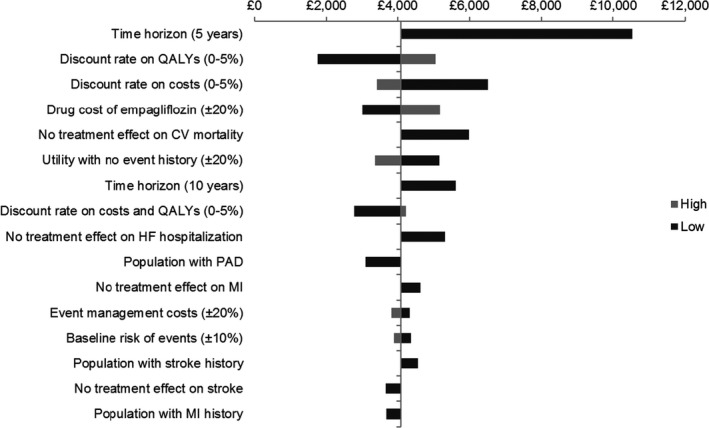
Tornado diagram. CV, cardiovascular; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; QALY, quality‐adjusted life years.
Probabilistic sensitivity analyses found relatively broad 95% CIs around mean event rates for both the empagliflozin and placebo regimens (Table 2). Given that event rates in both treatment arms tended to vary together by similar magnitude as reflected in the covariance results from the statistical analysis, the resulting range of ICERs was relatively narrow, from £2497 to £7228/QALY (mean £4404/QALY). In all instances, empagliflozin plus standard of care provides a QALY benefit over standard of care alone, and the values simulated fall well below the UK's £30 000/QALY cost‐effectiveness threshold (see Fig. S1), showing that empagliflozin is consistently cost‐effective.
Discussion
Results of this cost‐effectiveness model, based on EMPA‐REG OUTCOME, suggest that empagliflozin is highly cost‐effective in the UK for treatment of people with diabetes and high cardiovascular risk. The model closely reproduces the 3‐year, within‐trial outcomes and was used to extrapolate those results beyond the trial follow‐up period to a lifetime horizon. Improvements in cardiovascular outcomes from the trial translated to long‐term clinical benefits at acceptable costs. Model analyses showed that empagliflozin use in people with diabetes and high cardiovascular risk reduces cardiovascular mortality, events and their associated costs. These savings from management of fewer clinical events partially offset the additional cost of empagliflozin, resulting in a highly cost‐effective use of NHS resources (£4083/QALY, well below the UK threshold of £30 000/QALY). The benefit was relatively, broadly distributed among the clinical endpoints (cardiovascular mortality, vascular outcomes), where no single endpoint dominated, as shown by the stability of the ICER in both deterministic and probabilistic sensitivity analyses.
This study uses only hard endpoint data from a cardiovascular outcomes trial to estimate the cost‐effectiveness of a treatment for Type 2 diabetes. More commonly, diabetes models assume a cluster of major cardiovascular risk factors (e.g. high HbA1c, elevated blood pressure) and predisposing determinants (e.g. obesity, sex) to predict cardiovascular risk in Type 2 diabetes. Treatment effects are then represented as changes in these surrogate markers with a set of risk equations, as in the UKPDS model 23, 25, that is then used to predict treatment effect on cardiovascular events. This approach, however, is not adequate for an analysis of cardiovascular event risk based on EMPA‐REG OUTCOME. Specifically, an approach based on surrogate markers and UKPDS equations will not be able to capture the change in cardiovascular event rates observed in EMPA‐REG OUTCOME and thus would yield model outcomes that could not be validated against the observed data.
The ICER for empagliflozin plus standard of care vs. standard of care alone may be influenced by modelling assumptions. First, we assume that clinical event rates observed in clinical practice will mirror those observed in EMPA‐REG OUTCOME. This assumption is a typical limitation of interpreting any trial outcomes. However, the EMPA‐REG OUTCOME trial design calls for usual standards of care in controlling HbA1c – improving the likelihood of direct relevance to clinical practice. Additionally, risk factors, including HbA1c, were similar between the arms in the trial, limiting the impact on net treatment effect of any potential deviation in control from clinical practice. Second, changes in risk due to changes in treatment are implicitly captured in event rate trajectories, and regardless of changes in event or treatment history, a constant treatment effect for the same event type is assumed. Third, there may be unmodelled comorbidities that would influence the shapes of the statistical extrapolations or the role of specific risk predictors. The role of any baseline confounders not influenced by empagliflozin is minimized by the randomization process in the trial, which insures balance between treatment arms. Fourth, heterogeneity of event rates over time is assumed to be adequately captured by event history. Other evolving risk factors, such as the ongoing progression of diabetes pathophysiology, were assumed to be implicitly captured in the shape of the parametric survival functions.
The greatest strength of this study is that the model directly predicts lifetime clinical event rates in a high‐risk population using data exclusively from EMPA‐REG OUTCOME, requiring no extrapolated changes in surrogate biomarkers. Additionally, the model was conservative in modelling treatment, assuming no difference in the treatment costs between arms other than the presence of empagliflozin, and participants were assumed to remain on the same treatment regimen for the duration of the simulation. We know that ~ 16% more study participants in the placebo group (39.9%) had intensification of background glucose‐lowering therapy compared with those in the empagliflozin groups (23.7% and 22.9% in the empagliflozin 10 and 25 mg arms, respectively) 26.
There are limitations of this study to acknowledge. First, analyses are based on data from the EMPA‐REG OUTCOME trial that involve a selected patient population (inclusion/exclusion criteria were previously published 6) treated in a specific practice environment, and may not be applicable to all people with cardiovascular disease or clinical practice settings. Second, the trial data and the modelling approach are not easily used to capture evolving treatment sequencing over time, as this cannot be simply extrapolated from the trial period; thus, conservative treatment assumptions are used. Third, the model does not capture some rare, but severe, diabetes‐related complications, such as blindness and amputation, which were not observed in sufficient numbers in the trial data. Fourth, model outcomes are sensitive to the impact of subsequent events on future event rates (e.g. survivors of acute MI are at an elevated risk of a recurrent MI and other cardiovascular events), but there are relatively few data from the trial to estimate the change in risk associated with recurrent events. Nor did we incorporate the costs of well‐recognized but relatively mild adverse events associated with sodium–glucose co‐transporter 2 (SGLT‐2) inhibitor therapy (e.g. genital infections, polyuria). These would have only minimal impacts on modelled overall healthcare costs, so would not materially affect the model findings. Fifth, we did not account for diabetic ketoacidosis episodes, an increasingly recognized rare complication of SGLT‐2 inhibition, because in EMPA‐REG OUTCOME there were few cases and no imbalance between the groups.
As is typically done in cost‐effectiveness analyses, short‐term clinical trial data with follow‐up of ~ 3 years was used to make long‐term predictions, assuming the risks of clinical events remain constant beyond the trial length. For example, as duration of diabetes lengthens, incidence of hypertension and development of renal dysfunction increases. However, in the absence of long‐term clinical follow‐up data, simulation modelling is an efficient tool to integrate and synthesize short‐term trial results with data from multiple sources to forecast long‐term clinical outcomes and costs of healthcare strategies.
Overall, extrapolation of EMPA‐REG OUTCOME trial data using an individual‐level simulation model suggests that empagliflozin is highly cost‐effective, contributing to QoL improvement at a reasonable increase in costs and thus has the potential to have a positive benefit for both payers and people with diabetes.
Funding sources
This analysis and manuscript were sponsored and funded by Boehringer Ingelheim Pharma GmbH & Co KG, Binger Strasse 173, 55216, Ingelheim am Rhein, Germany. Boehringer Ingelheim contracted with Evidera for services on this project and manuscript.
Competing interests
A.K., O.S.R. and I.P. are current employees, and at the time of this project and manuscript development, Y.Z. was an employee, of Evidera, a research and consulting firm for the biopharmaceutical industry. As salaried employees, they do not accept remuneration of any kind directly from clients of Evidera for their services. E.P., J.T.G., P.K. and A.R. are employees of Boehringer Ingelheim.
Author contributions
A.K. and O.S.R. wrote the manuscript and contributed to the model development. E.P. and J.T.G. researched data and reviewed/edited the manuscript. I.P. performed statistical analyses, and reviewed/edited the manuscript. Y.Z. contributed to the model development. P.K. and A.R. contributed to the model concept and reviewed/edited the manuscript.
Ethical approval
The study was approved by the Medical Ethics Committee of [INSTITUTION], and informed consent was obtained from all participants. This research study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Supporting information
Figure S1. Scatterplot of incremental loss vs. incremental QALYs.
Table S1. Parameters included in risk equations.
Acknowledgements
The authors gratefully acknowledge discussions with Brigitta Monz and Roberto Palencia, formerly of Boehringer Ingelheim, and Sonja Sorensen of Evidera.
Diabet. Med. 36: 1494–1502(2019)
Parts of this work were presented at the ISPOR 21st Annual International Meeting, 21–25 May 2016, Washington, DC, USA and American Diabetes Association 76th Scientific Sessions, 10–14 June 2016, New Orleans, LA, USA.
References
- 1. Diabetes mellitus: a major risk factor for cardiovascular disease . A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation 1999; 100: 1132–1133. [DOI] [PubMed] [Google Scholar]
- 2. American Heart Association . Cardiovascular Disease and Diabetes, 2015. Available at http://www.heart.org/HEARTORG/Conditions/Diabetes/WhyDiabetesMatters/Cardiovascular-Disease-Diabetes_UCM_313865_Article.jsp/#.V63g5PkrKCi Last accessed 12 August 2016.
- 3. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV et al Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999; 100: 1134–1146. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Standards of medical care in diabetes – 2016. Diabetes Care 2016; 39: S1–S112.26696671 [Google Scholar]
- 5. Tandon N, Ali MK, Narayan KM. Pharmacologic prevention of microvascular and macrovascular complications in diabetes mellitus: implications of the results of recent clinical trials in type 2 diabetes. Am J Cardiovasc Drugs 2012; 12: 7–22. [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 7. Kansal AR, Zheng Y, Palencia R, Ruffolo A, Hass B, Sorensen SV. Modeling hard clinical end‐point data in economic analyses. J Med Econ 2013; 16: 1327–1343. [DOI] [PubMed] [Google Scholar]
- 8. Monthly Index of Medical Specialities (MIMS) . MIMS Drug Database. Available at https://www.mims.co.uk/ Last accessed 5 April 2019.
- 9. Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes‐related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015; 32: 459–466. [DOI] [PubMed] [Google Scholar]
- 10. Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes‐related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 2003; 20: 442–450. [DOI] [PubMed] [Google Scholar]
- 11. Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event‐year and annual state costs (USD 2012). J Med Econ 2014; 17: 176–183. [DOI] [PubMed] [Google Scholar]
- 12. Cassar K. Intermittent claudication. BMJ 2006; 333: 1002–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordois A, Scuffham P, Shearer A, Oglesby A. The health care costs of diabetic nephropathy in the United States and the United Kingdom. J Diabetes Complicat 2004; 18: 18–26. [DOI] [PubMed] [Google Scholar]
- 14. Kent S, Schlackow I, Lozano‐Kuhne J, Reith C, Emberson J, Haynes R et al What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate‐to‐severe kidney disease? BMC Nephrol 2015; 16: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence (NICE) . Empagliflozin in Combination Therapy for Treating Type 2 Diabetes. Technology appraisal guidance 336. Available at https://www.nice.org.uk/guidance/ta336 Last accessed 5 April 2019.
- 16. Sullivan PW, Ghushchyan VH. EQ‐5D scores for diabetes‐related comorbidities. Value Health 2016; 19: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 17. Lindgren P, Graff J, Olsson AG, Pedersen TJ, Jonsson B; Ideal Trial Investigators . Cost‐effectiveness of high‐dose atorvastatin compared with regular dose simvastatin. Eur Heart J 2007; 28: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 18. Office for National Statistics . National Life Tables, United Kingdom: 2012–2014, 2015. Available at https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2015-09-23 Last accessed 15 May 2017.
- 19. Ishak KJ, Kreif N, Benedict A, Muszbek N. Overview of parametric survival analysis for health‐economic applications. Pharmacoeconomics 2013; 31: 663–675. [DOI] [PubMed] [Google Scholar]
- 20. R Core Team . R: A Language and Environment for Statistical Computing. Available at https://www.r-project.org/ Last accessed 12 August 2016.
- 21. Eurostat . Harmonised Index of Consumer Prices (HICP) – Health Component – United Kingdom. Available at https://ec.europa.eu/eurostat/web/hicp Last accessed 5 April 2019.
- 22. Lee DW, Schernthaner G, Scheen A, Johansen OE, Zinman B. LBPS 02‐49 impact of changes in glucose‐lowering therapy on analyses of glycemic control and weight in EMPA‐REG OUTCOME. J Hypertens 2016; 34: e519. [Google Scholar]
- 23. Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ et al A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004; 47: 1747–1759. [DOI] [PubMed] [Google Scholar]
- 24. National Institute for Health and Care Excellence . 5.6. Discounting. In Guide to the Methods of Technology Appraisal 2013 Process and Methods 9. Available at https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#discounting Last accessed 12 August 2016. [PubMed]
- 25. Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia 2013; 56: 1925–1933. [DOI] [PubMed] [Google Scholar]
- 26. Schernthaner G, Scheen A, Johansen OE, Mattheus M, Zinman B. Impact of changes in glucose‐lowering therapy on analyses of glycemic control and weight in EMPA‐REG OUTCOME® . Diabetes 2016; 65: 1127‐P.27208025 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scatterplot of incremental loss vs. incremental QALYs.
Table S1. Parameters included in risk equations.


