Abstract
Large genetic variations in starvation tolerance in animals indicate that there are multiple strategies to cope with low‐nutrient conditions. Fruit flies (Drosophila melanogaster) typically respond to starvation by suppressing sleep and enhancing locomotor activity presumably to search for food. However, we hypothesized that in a natural population, there are costs and benefits to sleep suppression under low‐nutrient conditions and that conserving energy through sleep could be a better strategy depending on food availability. In this study, we quantified the variation in sleep‐related traits in 21 wild‐derived inbred lines from Katsunuma, Japan, under fed and starved conditions and analysed the relationship between those traits and starvation tolerance. Although most of the lines responded to starvation by suppressing the total time in sleep, there were indeed two lines that responded by significantly increasing the sleep‐bout durations and thus not reducing the total time in sleep. These genotypes survived longer in acute starvation conditions compared to genotypes that responded by the immediate suppression of sleep, which could be due to the reduced metabolic rate during the long uninterrupted sleep bouts. The coexistence of the enhanced foraging and resting strategies upon starvation within a single population is consistent with the presence of a behavioural trade‐off between food search and energy conservation due to unpredictable food availability in nature. These results provide insights into the evolutionary mechanisms that contribute to the maintenance of genetic variations underlying environmental stress resistance.
Keywords: Drosophila, evolutionary physiology, sleep, starvation, trade‐offs
Trade‐off between food search and energy conservation upon food shortage. While most of the genotypes sampled from a natural population responded to starvation by suppressing sleep, some responded by significantly increasing the sleep‐bout durations. The coexistence of both strategies within a single population is consistent with the presence of such trade‐off due to unpredictable food availability in nature.
1. INTRODUCTION
Coping with starvation is essential in all animals. However, the large genetic variation in starvation tolerance indicates that high tolerance does not always maximize fitness because of the cost involved in achieving tolerance (Gergs & Jager, 2014; Harbison, McCoy, & Mackay, 2013; Hoffmann & Harshman, 1999; Matzkin, Watts, & Markow, 2009; Pijpe, Brakefield, & Zwaan, 2007). For example, storing energy can kill energy allocated to growth, immunity and reproduction (reviewed in McCue, 2010; Rion & Kawecki, 2007). Such trade‐offs (benefits and costs) are likely to exist at the behavioural level as well (Holm, Rodríguez‐Torres, Hansen, & Almeda, 2019; Keene et al., 2010; Skora, Mende, & Zimmer, 2018). In order to conserve energy when the food is scarce, it would be better to sleep or reduce activity, but then the chances of finding food would be lost. On the other hand, foraging may result in food location, but carries the risk of energy exhaustion or predation before success.
The adult fruit fly (Drosophila melanogaster) shows a sleep‐like resting state, which can be quantified by monitoring locomotion (Hendricks et al., 2000; Shaw, Cirelli, Greenspan, & Tononi, 2000). It has been shown that starvation induces sleep suppression and enhances locomotor activity (Keene et al., 2010; Linford, Chan, & Pletcher, 2012; Linford, Ro, Chung, & Pletcher, 2015; Murakami et al., 2016), which suggests that adult flies generally respond to starvation with enhanced foraging behaviour. Despite this general tendency, flies with little sleep suppression upon starvation were selected after 60–80 generations of artificial selection for starvation resistance (Masek et al., 2014) and the sleep phenotype was further shown to be associated with starvation resistance (Slocumb et al., 2015). In addition, a single population among 24 worldwide natural populations showed increased sleep upon starvation and a positive correlation between the difference between total sleep time under the fed and starved conditions and the survival time under the starved condition was observed among those populations excluding an outlier (Brown et al., 2018). These results suggest that conserving energy through sleep might be a better strategy under acute starvation than to suppressing sleep, even though flies potently suppress sleep upon nutrient deprivation.
We hypothesize that in a natural population, there are costs and benefits to sleep suppression under low‐nutrient conditions and that both strategies can be adaptive depending on the environmental conditions of food availability. The enhanced foraging strategy can be adaptive if the success rate for food location is sufficiently high, whereas, if it is very low, resting should be a better solution in order to conserve energy. It is important to consider that, in laboratory studies, starvation resistance is typically measured under complete food deprivation in which foraging never results in a reward.
One prediction from the above hypothesis is that the trade‐offs involved in enhanced foraging or resting strategies should result in the segregation of both strategies within a single natural population. Neither strategy is likely to go to fixation since food availability fluctuates drastically in wild populations. Additionally, a recent study showed that metabolic rates are reduced in uninterrupted sleep bouts of approximately 30 min or greater in flies (Stahl, Slocumb, Chaitin, DiAngelo, & Keene, 2017), which suggests that longer sleep bouts may be a preferred phenotype in flies that do not suppress sleep upon starvation.
In this study, we investigated the variation in sleep‐related traits under fed and starved conditions and analysed the relationship between sleep and starvation tolerance in 21 wild‐derived inbred lines from Katsunuma, Japan. We showed that there are genotypes segregating in a wild‐derived population that respond to starvation by increased sleep bouts. These genotypes survived longer during acute starvation compared to genotypes that responded by immediate sleep suppression. Taken together, our results provide insights into the underlying evolutionary mechanisms for the maintenance of genetic variation in environmental stress resistance.
2. MATERIALS AND METHODS
2.1. Fly strains
Twenty‐one Katsunuma (KA) lines of D. melanogaster, KA13001, KA13006, KA13010, KA13017, KA13021, KA13022, KA13023, KA13024, KA13025, KA13026, KA13027, KA13028, KA13029, KA13030, KA13032, KA13033, KA13035, KA13037, KA13038, KA13067 and KA13088 were used for the experiments. Katsunuma lines are wild‐derived inbred lines (sib‐mated for 20–21 generations, except KA13033, which was sib‐mated for 12 generations) established from flies collected in 2013 from Katsunuma, Koshu City, in Yamanashi Prefecture (N 35°65′ E 138°71′). Flies were maintained under a 12‐hr:12‐hr light–dark cycle at 25°C and fed with standard cornmeal food with dry yeast, glucose and agar.
2.2. Monitoring locomotor activity
Virgin females at 7 days post‐eclosion were anesthetized with CO2 and then individually placed into glass tubes (length, 65 mm; diameter, 5 mm) containing 5% sucrose and 2% agar food (fed condition). Females were used because of the wider range in duration of sleep across lines in the Drosophila melanogaster Genetic Reference Panel compared to males (Harbison et al., 2009, 2013). Sixteen flies were used for each measurement. Fly activity was monitored for 6 days by a Drosophila Activity Monitor (DAM2, Trikinetics, Waltham, MA) following the manufacturer's protocol. DAM2 measures the locomotor activity of each fly by counting the number of times the fly crosses an infrared beam. These data were used to quantify sleep‐related traits. The measurements were replicated three times using different sets of individuals.
2.3. Quantification of activity and sleep‐related traits
The total activity was calculated as the sum of the total number of times flies crossed the infrared beam during a given time period. Total time in sleep was calculated by extracting inactive bouts of 5 min or longer (Shaw et al., 2000). Wake‐time activity was calculated as follows: [total activity (counts)]/[total time – total time in sleep (min)]. Sleep‐bout number was calculated as the number of inactive bouts of 5 min or longer, and sleep‐bout length was calculated as follows: [the sum of length of all inactive bouts of 5 min or longer (min)]/[the total number of sleep bouts]. These traits were quantified by running a custom R script (R Core Team, 2018). Broad‐sense heritability was estimated from the proportion of variance component between lines by restricted maximum likelihood using lmerTest package in R (Kuznetsova, Brockhoff, & Christensen, 2017).
2.4. Calculation of starvation‐induced changes in sleep‐related traits and 50% lethal time (LT50)
For the starved condition, virgin females at 7 days post‐eclosion were individually placed into glass tubes containing 2% agar food and monitored by DAM2 together with flies under the fed condition (5% sucrose and 2% agar food). Starvation‐induced changes (%) in sleep‐related traits were calculated as follows: [(starved measurement – fed measurement)/fed measurement] × 100. Survival time under the starved condition was defined as the last activity time point in min from the final recorded activity bout for each fly. Fifty per cent lethal time (LT50) was calculated as the median survival time for each replication. Sleep‐related traits under fed and starved conditions and survival time under starved conditions were quantified by running a custom R script (R core team, 2018).
2.5. Data exclusion
Data from individuals that were abnormal (i.e. more than 256 activities in 1 min, more than a 12‐hr inactive bout and/or less than a 3‐min survival time under starved conditions), died during measurement under fed conditions, was wounded or was placed in a glass tube contaminated with mould were excluded.
3. RESULTS AND DISCUSSION
Within‐population variations in daily locomotor activity rhythmicity and sleep were investigated by obtaining locomotor activity and sleep profiles across a 24‐hr period under fed conditions (Appendix S1). Most of the Katsunuma flies showed typical locomotor activity and sleep patterns with small differences among replicates. Broad‐sense heritability estimates were between 0.23 and 0.53 for these sleep‐related phenotypes (Appendix S2). Individual measurements are provided in Appendix S3. Next, in order to investigate variations in the response to nutritional stress, sleep‐related traits under the starved and fed (control) conditions were analysed. These traits were calculated from the activity data of the first dark period (D0; 12 hr) beginning approximately 6 hr after the start of the measurements. Data from flies that showed their last activity (died) during this period were excluded from all the analyses, and those from flies that did not sleep at all during this period were excluded from the analysis of sleep‐bout length. Individual measurements of sleep‐related traits in D0 period and LT50 are listed in Appendix S4.
The relationship between starvation tolerance and sleep‐related traits is shown in Appendix S5. No significant correlation was detected between the LT50 under starved conditions and the total time in sleep, wake‐time activity, sleep‐bout number and sleep‐bout length under fed conditions (Appendix S5A–D). However, the total time in sleep under the starved condition showed a significant positive correlation with the LT50 suggesting that modulation of sleep upon starvation may be associated with survival more strongly than sleep characteristics under normal food conditions (Appendix S5 A′). Indeed, when the LT50 under the starved condition was compared to the changes in sleep‐related traits upon starvation (Figure 1), a stronger positive correlation was detected between the changes in total time in sleep and the LT50 under the starved condition (Figure 1a). Interestingly, an even stronger positive correlation was observed between changes in sleep‐bout length and the LT50 under the starved condition, and two lines with the longest LT50s under starvation, KA13022 and KA13035, showed significantly larger sleep‐bout lengths upon starvation compared to the fed condition (Figure 1d, Appendix S6). In these lines, the sleep‐bout number was significantly smaller in the starved flies than the fed flies (Figure 1c, Appendix S6), but no difference in total time in sleep was observed (Figure 1a, Appendix S6) because of longer sleep‐bout duration.
Figure 1.
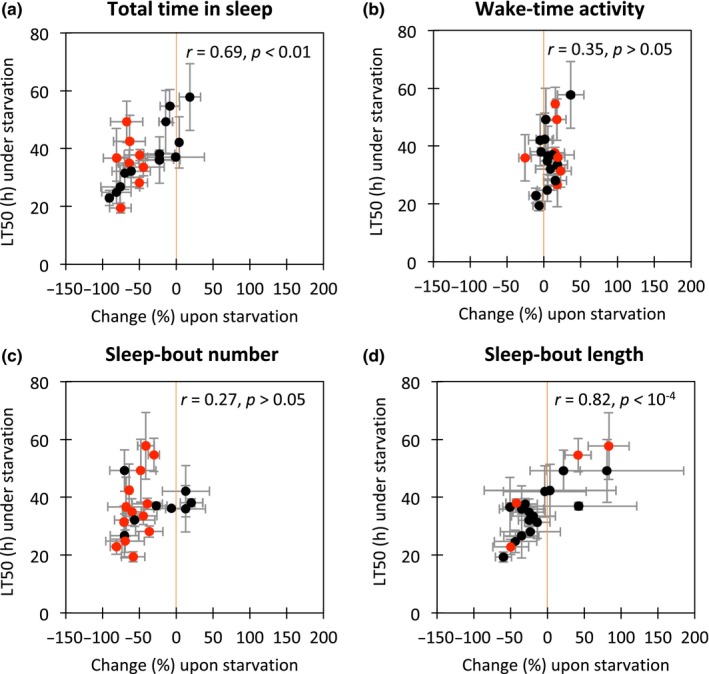
The relationships between changes upon starvation in sleep‐related traits and survival time (LT50) under starved conditions among 21 lines from Katsunuma, Japan. (a) The relationship between change (%) in total time in sleep and the LT50 under starvation. (b) The relationship between change (%) in wake‐time activity and the LT50 under starvation. (c) The relationship between change (%) in sleep‐bout number and the LT50 under starvation. (d) The relationship between change (%) in sleep‐bout length and the LT50 under starvation. Pearson's correlation coefficient (r) is indicated in each panel. p‐values are adjusted for multiple testing (four tests) using the Bonferroni correction. All sleep‐related traits were analysed in the first dark period (D0, 12‐hr period beginning from approximately 6 h after starting the experiment). Error bars indicate standard deviations. Red circles indicate significant differences between starved and fed conditions (p < .05 by two‐way ANOVA after applying the Bonferroni correction for multiple testing (21 tests), Appendix S6). Orange lines indicate x = 0
A recent study by Stahl et al. (2017) showed that metabolic rate is reduced during sleep, and longer sleep bouts, typically > approximately 30 min, are associated with an additional reduction, although to a smaller degree in starved flies. Therefore, the increased length of uninterrupted sleep in the KA13022 and KA13035 lines may be an adaptive response to effectively reduce energy consumption upon starvation. To further investigate this possibility, the LT50 under starvation was plotted against the frequencies of sleep bouts of different lengths in flies under starved and fed conditions (Figure 2). The strengths of correlation should indicate which sleep‐bout‐length categories are more strongly associated with starvation tolerance. The results indicate that survival (LT50) under starvation was positively associated with the frequency of sleep‐bout lengths of greater than 60 min under the starved condition (Figure 2d) but not under the fed condition (Figure 2h). This is consistent with the prediction that longer uninterrupted sleep contributes to survival under acute starvation.
Figure 2.
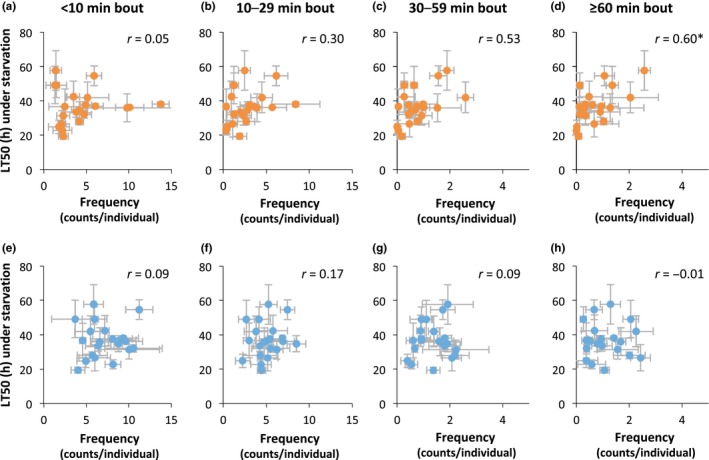
The relationships between the frequencies of sleep bouts of different lengths and the survival time (LT50) under starved condition. (a–d) Survival time (LT50) under starvation plotted against sleep‐bout frequencies from D0 (12‐hr dark period beginning from approximately 6 hr after starting the experiment) under the starved condition. (e–h) Survival time (LT50) under starvation plotted against sleep‐bout frequencies from D0 under the fed condition. (a, e) Sleep bouts of 5–9 min. (b, f) Sleep bouts of 10–29 min. (c, g) Sleep bouts of 30–59 min. (d, h) Sleep bouts of ≥ 60 min. Pearson's correlation coefficient (r) is indicated in each panel. * p < .05 after applying the Bonferroni correction for multiple testing (eight tests)
Further investigation of the two lines, KA13022 and KA13035, revealed that the lengths of sleep bouts under acute starvation were reduced in later hours as the flies approached death (Figure 3a–d). Therefore, as the physiological conditions of the flies become severely worsened, they may have abandoned the strategy of saving energy to allocate more time to foraging. This trend is consistent with the results from Brown et al. (2018) who showed that among 24 lines originating from worldwide populations, the number of lines that showed sleep suppression increased after a 48‐hr starvation compared to a 24‐hr starvation implying that sleep suppression is enhanced in more disparate flies. However, individual fly vigour within lines estimated from the survival time (either died before or after the LT50) did not affect the D0 sleep‐bout length (Figure 3e,f). This suggests that the initial response to acute starvation is determined by the genotype rather than the physiological condition of the fly.
Figure 3.
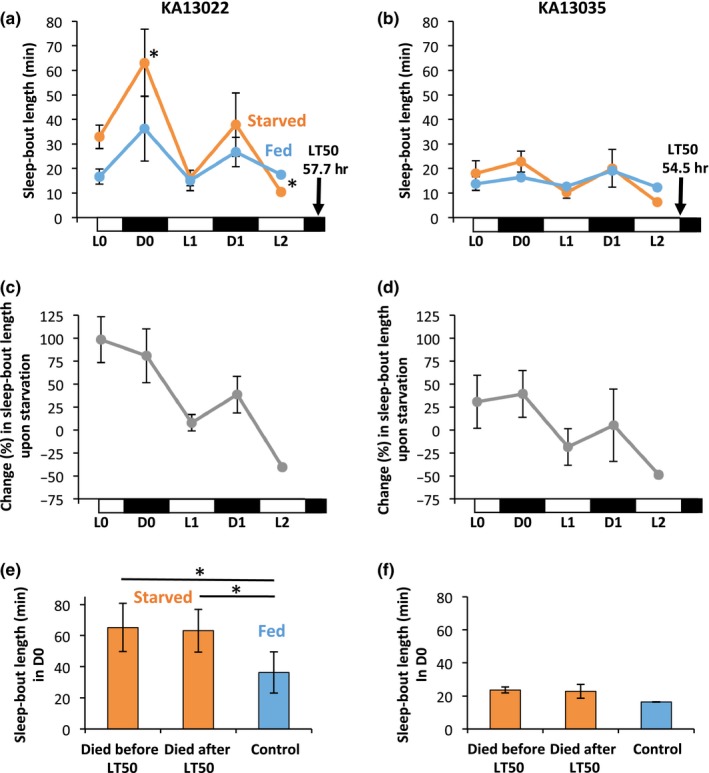
Sleep‐bout lengths of two lines that showed significant increases in D0 (12‐hr dark period beginning from approximately 6 hr after starting the experiment) sleep‐bout length during starvation. (a) Sleep‐bout lengths under starved (orange) and fed (blue) conditions in dark (D0 and D1) and light (L0, L1 and L2) periods up to the LT50 in KA13022. (b) Sleep‐bout lengths under starved (orange) and fed (blue) conditions in dark (D0 and D1) and light (L0, L1 and L2) periods up to the LT50 in KA13035. (c) Changes (%) in sleep‐bout lengths upon starvation in dark (D0 and D1) and light (L0, L1 and L2) periods up to the LT50 in KA13022. (d) Changes (%) in sleep‐bout lengths upon starvation in dark (D0 and D1) and light (L0, L1 and L2) periods up to the LT50 in KA13035. (e) Comparisons of sleep‐bout length in individuals dying before and after the LT50 under starved conditions in KA13022. (f) Comparisons of sleep‐bout length in individuals dying before and after the LT50 under starved conditions in KA13035. *p < .05 by paired t‐test after applying the Bonferroni correction for multiple testing
Studies have shown that the perception of reduced sweetness from the gustatory receptors plays an important role in initiating sleep suppression (Linford et al., 2012, 2015). It would be interesting to further investigate whether the variation between lines in sleep modulation upon starvation is due to variations in sensitivity of sweetness perception. Alternatively, the two genotypes that responded to starvation by prolonged sleep bouts might have neuronal networks (i.e. Chung et al., 2017; Keene et al., 2010; Murakami et al., 2016) that differentially transduce gustatory and/or metabolic information to sleep centres in the brain to modulate sleep.
The majority of the lines reduced total time in sleep by primarily reducing the number of sleep bouts (Figure 1). This implies that enhancing foraging activity when encountering food shortages is generally a better survival strategy than reducing energy consumption by resting. Aside from energy consumption, sleep loss in itself can be deleterious (Shaw, Tortoni, Greenspan, & Robinson, 2002). Therefore, the latter strategy could become more adaptive depending on the availability of food sources. Indeed, we found that two of the 21 lines responded to starvation by increasing sleep‐bout lengths consistent with the view that such a strategy remained segregating in this natural population, although it is possible that part of the genotypic effects may come from rare recessive mutations uncovered by inbreeding. The flies from these two lines did not show increases in total sleep time, but may have reduced energy consumption by sleeping deeply during longer bouts, which may be a preferred sleep adaptation in flies taking the strategy of resting to save energy.
Collectively, our results showing the coexistence of enhanced foraging and resting strategies is consistent with the trade‐offs between the probability of finding food and saving energy during starvation. In particular, the enhanced resting strategy by increasing the length of uninterrupted sleep has not been reported previously. The trade‐off is likely to stem from unpredictable food availability in nature, which may be the fundamental factor influencing the large genetic variations in starvation resistance and in sleep patterns in various animal species.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
MM and AT designed the study. MM conducted the experiments and analyzed the data. MM and AT wrote the manuscript.
Supporting information
ACKNOWLEDGMENTS
We would like to thank Nancy Lindford and Kazuhiko Kume for sharing their scripts, Marufuji winery for the help in collecting samples, and Kaito Ogiyama for constructing inbred strains. This work was partly supported by JSPS KAKENHI JP19H03276 to AT.
Miura M, Takahashi A. Starvation tolerance associated with prolonged sleep bouts upon starvation in a single natural population of Drosophila melanogaster . J Evol Biol. 2019;32:1117–1123. 10.1111/jeb.13514
REFERENCES
- Brown, E. B. , Torres, J. , Bennick, R. A. , Rozzo, V. , Kerbs, A. , DiAngelo, J. R. , & Keene, A. C. (2018). Variation in sleep and metabolic function is associated with latitude and average temperature in Drosophila melanogaster . Ecology and Evolution, 8(8), 4084–4097. 10.1002/ece3.3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, B. Y. , Ro, J. , Hutter, S. A. , Miller, K. M. , Guduguntla, L. S. , Kondo, S. , & Pletcher, S. D. (2017). Drosophila Neuropeptide F signaling independently regulates feeding and sleep‐wake behavior. Cell Reports, 19(12), 2441–2450. 10.1016/j.celrep.2017.05.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergs, A. , & Jager, T. (2014). Body size‐mediated starvation resistance in an insect predator. Journal of Animal Ecology, 83(4), 758–768. 10.1111/1365-2656.12195 [DOI] [PubMed] [Google Scholar]
- Harbison, S. T. , Carbone, M. A. , Ayroles, J. F. , Stone, E. A. , Lyman, R. F. , & Mackay, T. F. (2009). Co‐regulated transcriptional networks contribute to natural genetic variation in Drosophila sleep. Nature Genetics, 41(3), 371–375. 10.1038/ng.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison, S. T. , McCoy, L. J. , & Mackay, T. F. (2013). Genome‐wide association study of sleep in Drosophila melanogaster . BMC Genomics, 14, 281 10.1186/1471-2164-14-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks, J. C. , Finn, S. M. , Panckeri, K. A. , Chavkin, J. , Williams, J. A. , Sehgal, A. , & Pack, A. I. (2000). Rest in Drosophila is a sleep‐like state. Neuron, 25(1), 129–138. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , & Harshman, L. G. (1999). Desiccation and starvation resistance in Drosophila: Patterns of variation at the species, population and intrapopulation levels. Heredity, 83(6), 637–643. 10.1046/j.1365-2540.1999.00649.x [DOI] [PubMed] [Google Scholar]
- Holm, M. W. , Rodríguez‐Torres, R. , Hansen, B. W. , & Almeda, R. (2019). Influence of behavioral plasticity and foraging strategy on starvation tolerance of planktonic copepods. Journal of Experimental Marine Biology and Ecology, 511, 19–27. 10.1016/j.jembe.2018.11.002 [DOI] [Google Scholar]
- Keene, A. C. , Duboué, E. R. , McDonald, D. M. , Dus, M. , Suh, G. S. B. , Waddell, S. , & Blau, J. (2010). Clock and cycle limit starvation‐induced sleep loss in Drosophila . Current Biology, 20(13), 1209–1215. 10.1016/j.cub.2010.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Linford, N. J. , Chan, T. P. , & Pletcher, S. D. (2012). Re‐patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genetics, 8(5), e1002668 10.1371/journal.pgen.1002668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford, N. J. , Ro, J. , Chung, B. Y. , & Pletcher, S. D. (2015). Gustatory and metabolic perception of nutrient stress in Drosophila . Proceedings of the National Academy of Sciences of the United States of America, 112(8), 2587–2592. 10.1073/pnas.1401501112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek, P. , Reynolds, L. A. , Bollinger, W. L. , Moody, C. , Mehta, A. , Murakami, K. , … Keene, A. C. (2014). Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster . Journal of Experimental Biology, 217(17), 3122–3132. 10.1242/jeb.103309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzkin, L. M. , Watts, T. D. , & Markow, T. A. (2009). Evolution of stress resistance in Drosophila: Interspecific variation in tolerance to desiccation and starvation. Functional Ecology, 23(3), 521–527. 10.1111/j.1365-2435.2008.01533.x [DOI] [Google Scholar]
- McCue, M. D. (2010). Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comparative Biochemistry and Physiology ‐ A Molecular and Integrative Physiology, 156(1), 1–18. 10.1016/j.cbpa.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Murakami, K. , Yurgel, M. E. , Stahl, B. A. , Masek, P. , Mehta, A. , Heidker, R. , … Keene, A. C. (2016). translin is required for metabolic regulation of sleep. Current Biology, 26(7), 972–980. 10.1016/j.cub.2016.02.01324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpe, J. , Brakefield, P. M. , & Zwaan, B. J. (2007). Phenotypic plasticity of starvation resistance in the butterfly Bicyclus anynana . Evolutionary Ecology, 21(5), 589–600. 10.1007/s10682-006-9137-5 [DOI] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. Vienna, Austria: R Core Team; Retrieved from http://www.r-project.org/ [Google Scholar]
- Rion, S. , & Kawecki, T. J. (2007). Evolutionary biology of starvation resistance: What we have learned from Drosophila . Journal of Evolutionary Biology, 20(5), 1655–1664. 10.1111/j.1420-9101.2007.01405.x [DOI] [PubMed] [Google Scholar]
- Shaw, P. J. , Cirelli, C. , Greenspan, R. J. , & Tononi, G. (2000). Correlates of sleep and waking in Drosophila melanogaster . Science, 287(5459), 1834–1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- Shaw, P. J. , Tortoni, G. , Greenspan, R. J. , & Robinson, D. F. (2002). Stress response genes protect against lethal effects of sleep deprivation in Drosophila . Nature, 417(6886), 287–291. 10.1038/417287a [DOI] [PubMed] [Google Scholar]
- Skora, S. , Mende, F. , & Zimmer, M. (2018). Energy scarcity promotes a brain‐wide sleep state modulated by insulin signaling in C. elegans . Cell Reports, 22(4), 953–966. 10.1016/j.celrep.2017.12.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocumb, M. E. , Regalado, J. M. , Yoshizawa, M. , Neely, G. G. , Masek, P. , Gibbs, A. G. , & Keene, A. C. (2015). Enhanced sleep is an evolutionarily adaptive response to starvation stress in Drosophila . PLoS ONE, 10(7), 1–16. 10.1371/journal.pone.0131275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, B. A. , Slocumb, M. E. , Chaitin, H. , DiAngelo, J. R. , & Keene, A. C. (2017). Sleep‐dependent modulation of metabolic rate in Drosophila . Sleep, 40(8), zsx084. 10.1093/sleep/zsx084 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


