Summary
This review aimed to investigate the impact of obesity treatment, with a dietary component, on eating disorder (ED) prevalence, ED risk, and related symptoms in children and adolescents with overweight or obesity. Four databases were searched to identify pediatric obesity treatment interventions, with a dietary component, and validated pre‐post intervention assessment of related outcomes. Of 3078 articles screened, 36 met inclusion criteria, with a combined sample of 2589 participants aged 7.8 to 16.9 years. Intervention duration ranged from 1 week to 13 months, with follow‐up of 6 months to 6 years from baseline. Prevalence of ED was reported in five studies and was reduced post‐intervention. Meta‐analyses showed a reduction in bulimic symptoms (eight studies, standardized mean difference [SE], −0.326 [0.09], P < 0.001), emotional eating (six studies, −0.149 [0.06], P = 0.008), binge eating (three studies, −0.588 [0.10], P < 0.001), and drive for thinness (three studies, −0.167 [0.06], P = 0.005) post‐intervention. At follow‐up, a reduction in ED risk (six studies, −0.313 [0.13], P = 0.012), emotional eating (five studies, −0.259 [0.05], P < 0.001), eating concern (three studies, −0.501 [0.06], P < 0.001), and drive for thinness (two studies, −0.375 [0.07], P < 0.001) was found. Structured and professionally run obesity treatment was associated with reduced ED prevalence, ED risk, and symptoms.
Keywords: dieting, disordered eating, pediatrics, weight loss
Abbreviations
- BED
binge eating disorder
- BN
bulimia nervosa
- ED
eating disorder
1. INTRODUCTION
Over the past 40 years, the worldwide prevalence of childhood obesity has increased.1 In 2016, it was estimated that 337 million children and adolescents (5‐19 y) had overweight or obesity, with 5.6% of girls and 7.8% of boys with obesity.1 Childhood obesity is associated with a range of comorbidities including cardiovascular, metabolic, and orthopaedic complications.2 Of particular concern is the predicted increase in early onset type 2 diabetes, which when diagnosed during childhood is associated with earlier complications and has a higher risk of mortality than type 1 diabetes.3, 4 In contrast, eating disorders (ED) are generally rare5 but carry one of the highest mortality risks of any psychiatric disorder.6 The prevalence of any ED was reported to be 1.4% in a US sample of 4524 children aged 9 to 10 years.7 Similarly, a lifetime prevalence of 1.7% for anorexia nervosa, 0.8% for bulimia nervosa (BN), and 2.3% for binge eating disorder (BED) was reported in a Dutch sample of 1584 adolescents,8 on the basis of the most recent Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) criteria.9
Lifestyle intervention, often with dietary, physical activity, and/or behavioral components, is the first‐line treatment for childhood obesity. However, dieting, as a self‐reported practice to control weight, has been associated with an increased risk of disordered eating and the development of ED in observational studies. For example, Project EAT, a longitudinal cohort of 2516 adolescents in the United States, reported up to an eight times increased risk of binge eating following reported dieting behaviors.10 Similarly, an Australian cohort of 888 adolescents followed for 3 years found that adolescents who reported dieting had a five‐ to 18‐fold increased risk of developing an ED.11 However, these studies were in predominantly healthy weight adolescents and may not be generalizable to a population of young people seeking treatment for obesity or where weight management is medically indicated.
Considering the cornerstone component of childhood obesity treatment involves dietary change, often coupled with energy restriction,2 it is important to examine the safety implications of these interventions and the impact on ED risk and the development of clinical ED. A 2005 narrative review of five studies assessing the relationship between pediatric obesity treatment and ED12 concluded that professionally administered weight loss programs for pediatric overweight or obesity have minimal risk of precipitating ED and that significant improvements in psychological status were observed.12 However, to our knowledge, this relationship has not been systematically examined. Therefore, the aim of this systematic review with meta‐analysis was to assess the impact of obesity treatment, with a dietary component, on the prevalence of ED, ED risk, and related symptoms in children and adolescents with overweight or obesity.
2. METHODS
The protocol13 for this review was registered with PROSPERO (CRD42017069496), accessible at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=69496, and has been reported according to Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA)14 guidelines.
2.1. Eligibility criteria
Eligible studies recruited treatment‐seeking children and/or adolescents (≤18 y), with overweight or obesity (defined as body mass index [BMI] above a healthy weight range, BMI z‐score > 1, clinical diagnosis, or study population reported as having overweight or obesity). Intervention studies were eligible for inclusion including randomized controlled trials, nonrandomized controlled trials, noncontrolled trials (pre‐post studies), and retrospective medical record reviews. Interventions included a nutrition education or prescriptive dietary component and excluded case reports, bariatric surgery, pharmacotherapy, and online interventions. Interventions conducted in residential camps, community, and hospital settings were included, as were individual‐, family‐, and group‐based programs. Studies were required to report on the prevalence of clinical ED, ED risk, and/or ED‐related symptoms including bulimic symptoms, binge eating, emotional eating, drive for thinness, and/or eating concern, using a validated assessment tool. Outcome data needed to be reported at pre‐intervention and post‐intervention or follow‐up or as a mean change across the intervention period. Explicit weight maintenance periods were included within the intervention period if there was ongoing contact with study personnel. Data at the latest follow‐up were collected when reported, this being defined as a period within which there was no contact with study personnel and no intervention delivery.
Excluded studies focused on prevention of overweight or included healthy weight participants. Interventions relating to the treatment of ED or psychological morbidity were also excluded, as were studies treating secondary or syndromic causes of obesity. No limitation was placed on intervention duration, length of follow‐up, or date, but this review was limited to studies published in the English language.
2.2. Search strategy
A systematic search of published literature up to August 2018 was undertaken using the electronic databases MEDLINE, EMBASE, Cochrane Library, and PsychINFO. Keywords were searched as both Medical Subject Headings (MeSH) of the National Library of Medicine and as independent search terms, for example, overweight, obesity, weight loss, diet therapy, calorie restriction, ED, disordered eating, binge eating, and bulimia (Table S1). Relevant truncations and adjacencies were used to enhance results by allowing variations of search terms. The search was limited to studies in children and adolescents. Hand searching of reference lists was conducted to identify studies that may have been missed.
Removal of duplicates and screening of eligible studies were conducted using Covidence online software (Veritas Health Innovation Ltd, Australia). Records were first assessed by title and abstract and then full text. All records were independently assessed for inclusion in duplicate on the basis of the defined criteria. Discrepancies were resolved through discussion by H. J and M. L. G. or N. B. L.
2.3. Data extraction
Data were independently extracted from eligible studies by one reviewer and cross‐checked for accuracy by a second reviewer. Extracted data included sample characteristics, BMI classification, and baseline psychopathology; intervention setting, intensity, and design; tools used to assess outcome measures; and pre‐, post‐intervention, and follow‐up data for both ED‐related and weight‐related outcomes (BMI and BMI z‐score were primarily extracted where available; if not reported, other relevant weight‐related outcomes were extracted). If data pertinent to the review were not reported, authors were contacted to obtain this. Studies were excluded if the author could not be contacted after two attempts or the required data could not be obtained.
2.4. Risk of bias
Studies were assessed for quality using the US Academy of Nutrition and Dietetics Quality Criteria Checklist: Primary Research15 by two reviewers, with discrepancies resolved through discussion (H. J and M. L. G.). The checklist allows an objective rating (positive, neutral, or negative) to be given to each study. Publication bias was assessed by visual inspection of a funnel plot and use of the classic fail‐safe N statistic16 with interpretation based on the tolerance level suggested by Rosenthal.17
2.5. Data synthesis
Meta‐analyses were performed to determine the difference in means of outcome measures from baseline to post‐intervention and follow‐up using the Comprehensive Meta‐analysis (CMA) package, version 3.0 (Biostat, Englewood, New Jersey), and presented in the form of forest plots. As a range of tools were used to report outcome measures, a standardized mean difference (SMD) was used to calculate an effect size (where 0.2 = small effect, 0.5 = medium effect, and 0.8 = large effect),18 allowing outcomes to be combined in meta‐analysis. All intervention arms of a study were included in the meta‐analysis of outcome measures. Where included studies reported data that were unable to be included in meta‐analysis, a narrative synthesis of results was conducted. A meta‐analysis of randomized controlled trials including a no‐treatment control group was conducted to assess intervention effect where at least two studies reported data. Moderator analysis was conducted to identify intervention characteristics that may impact on ED risk and ED‐related symptoms. Categorical moderators were used where at least two studies were available in each group to allow comparison, including setting (inpatient/camps, tertiary hospital outpatient, community, and school), intensity (daily, weekly/biweekly, monthly, and >monthly contact with the study team), physical activity component (no physical activity component reported, education only, and structured exercise program), energy prescription (no, nutrition education only; yes, prescribed energy target), inclusion of ED‐related content, and involvement of a psychologist, counsellor, or therapist in the intervention delivery. Meta‐regression was used to assess the association between change in ED risk with continuous variables including mean age at baseline, duration of intervention (wk), and the effect size for change in weight‐related outcomes. Heterogeneity between studies was assessed using the I 2 statistic. A random‐effect model was used because of assumed heterogeneity between studies. P values of ≤0.05 were considered statistically significant.
3. RESULTS
3.1. Included studies
Of 3078 articles identified during the literature search, 36 articles representing 29 studies, published between 2000 and 2018, were included (Figure 1). Study characteristics are summarized in Table S2. In brief, 18 articles reported on noncontrolled trials (pre‐post),19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 17 on randomized controlled trials,37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 and one on a nonrandomized controlled trial.54 Studies were conducted in the United States,19, 32, 33, 42, 44, 45, 46, 47, 52, 53 Belgium,20, 21, 22, 35, 36, 38 Brazil,23, 24, 25, 26, 27 Australia,37, 39, 48, 49 the United Kingdom,29, 34, 41 the Netherlands,31, 43, 50 Canada,40 Iran,51 Israel,30 Romania,54 and Spain.28 Interventions were conducted in tertiary hospital outpatient19, 23, 24, 25, 26, 27, 28, 29, 32, 40, 43, 44, 45, 47, 48, 49, 52, 54 or hospital inpatient treatment programs,20, 21, 22, 35, 36 community settings,33, 34, 37, 39, 46, 50, 51 primary care facilities,41, 42 a combination of a residential component and tertiary hospital outpatient program,30, 31, 38 or within a school‐based health clinic.53 The participant sample size of each study ranged from 17 to 220 children or adolescents with overweight or obesity, with a combined sample of 2589 participants. At baseline, the mean age ranged from 7.8 to 16.9 years, mean BMI from 27.4 to 44.8 kg/m2, and a mean BMI z‐score from 2.00 to 3.42. Intervention duration ranged from 1 week to 13 months, with a follow‐up of 6 months to 6 years from baseline reported in 19 studies.19, 20, 21, 22, 29, 31, 33, 36, 37, 38, 40, 41, 42, 44, 45, 46, 47, 48, 49, 50, 52, 54
Figure 1.
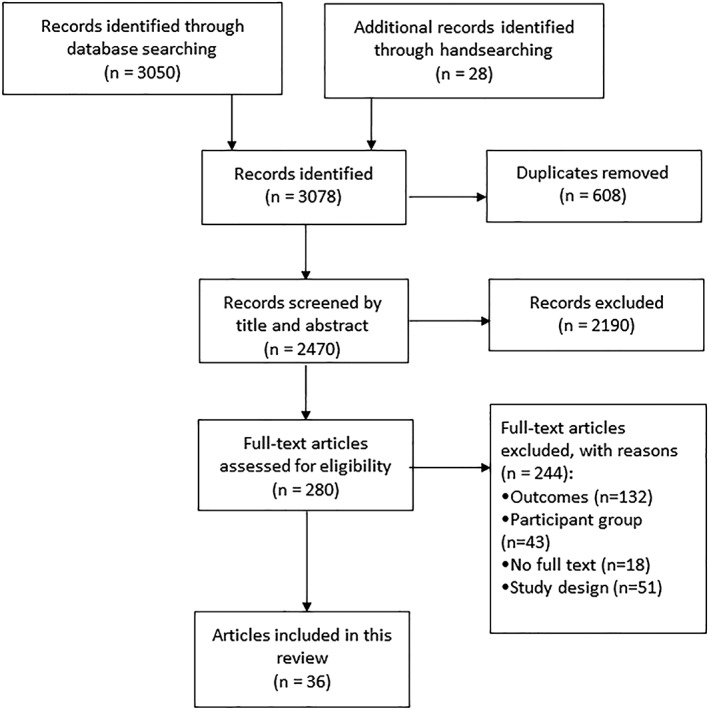
Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) flow diagram of the literature search and screening process
3.2. Intervention design
Interventions were led by multidisciplinary teams,20, 21, 22, 23, 24, 25, 26, 27, 30, 31, 34, 35, 36, 38, 41, 42, 43, 48, 49, 51 defined as two or more health professionals (physician, dieticians/nutritionists, exercise physiologists, personal trainers, and psychologists/therapist and nurse) in 14 studies. Personnel involved in delivering the intervention were not reported in five studies,29, 32, 33, 46, 47 with the remaining studies delivered by a single health professional or trained consultant.
Nutrition education included a range of topics relating to following a balanced diet, including fruit and vegetable intake, healthy snacks, reducing intake of sugar‐sweetened beverages and/or fat, and portions sizes. A prescriptive energy target ranging from 1000 to 1900 kcal/d was reported in 10 studies.20, 21, 22, 30, 33, 35, 36, 38, 42, 44, 47, 48, 49 A moderate calorie restriction of 10% to 40% was prescribed for 3 weeks in the study by De Miguel‐Etayo et al, followed by transition to a flexible meal plan.28 Physical activity education focused on goal setting for increased activity each week and a reduction in sedentary behaviors. Supervised exercise classes were provided in 14 studies,20, 21, 22, 23, 24, 25, 26, 27, 30, 31, 32, 33, 35, 36, 38, 43, 46, 48, 49, 51 and one study50 did not report the inclusion of a physical activity component. All interventions involved behavior modification strategies, including problem solving, goal setting, self‐monitoring, and stimulus control, with some studies addressing ED specifically,23, 24, 25, 26, 27 eating behaviors,28, 30, 31, 39, 42, 50, 54 and/or body image23, 24, 25, 26, 27, 30, 37, 42, 46, 47 during these sessions. A psychologist, counsellor, or therapist was reported to be involved in intervention delivery in 17 studies.20, 21, 22, 23, 24, 25, 26, 27, 30, 31, 34, 35, 36, 38, 39, 41, 42, 43, 44, 50, 52, 54
Outcomes were reported using 20 validated assessment tools55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 and included the prevalence of ED, ED risk, bulimic symptoms, binge eating, emotional eating, drive for thinness, and eating concern.
3.3. Risk of bias assessment
Of the 36 studies that met the inclusion criteria, 11 studies obtained a positive quality rating and 25 a neutral rating (Table S2). Studies received a neutral quality rating if the intervention did not have a comparator group or if participant selection processes were poorly described.
3.4. Prevalence of diagnosed eating disorders
The change in prevalence of BN or BED between pre‐ and post‐intervention was reported in five studies.22, 23, 35, 36, 42 All studies reported a resolution of BN or BED for some participants. No studies reported on anorexia nervosa.
Diagnosis of BN and BED was assessed following a 10‐month hospital inpatient treatment program in two studies.35, 36 Van Vlierberghe et al35 reported that of 76 participants at baseline, one had BN and five had BED, all of which had resolved post‐intervention. Similarly, from 110 participants who completed the 24‐month follow‐up measures in the study by Braet et al,36 a reduction in BN from 3% at baseline to 1% at both post‐intervention and 24‐month follow‐up was reported, as was a reduction in BED from 32% at baseline to 5% at post‐treatment, and 1% at 24‐month follow‐up. DeBar et al42 reported a reduction in the prevalence of binge‐related ED in study completers in both the intervention (12.4% to 2.2% to 0%, n~100) and usual care control group (12.8% to 4.8% to 1.3%, n~100) from baseline to post‐intervention and 12‐month follow‐up. Carnier et al23 reported an 89% decreased prevalence of adolescents with obesity and ED following a 12‐month interdisciplinary intervention but did not report data by disorder. The development of BED was reported in one study. Goossens et al22 reported that of 56 participants assessed at the 6‐year follow‐up timepoint, following a 10‐month inpatient treatment program, no participants had BED at baseline, and three participants had developed BED at the 6‐year follow‐up.
3.5. Eating disorder risk
The total score on tools measuring ED risk, ED pathology, or disordered eating attitudes/behaviors was used to report on overall ED risk. Seven different assessment tools60, 61, 65, 66, 67, 68, 74 were used across 13 studies.20, 29, 30, 32, 33, 37, 41, 44, 45, 46, 47, 52, 53 A significant reduction in ED risk was reported in three studies20, 41, 53 and no change in six studies30, 32, 37, 46, 47, 53 post‐intervention. The work of Edwards et al29 was the only study to report a significant increase in ED risk. The authors attributed this increase to the scores on the dieting behavior subscale, specifically, the items “I stay away from foods with sugar in them,” “I eat diet foods,” and “I have been dieting,” all of which increased (all P < 0.05). Items assessing abnormal eating behaviors and attitudes (food preoccupation, anorexia, or bulimia) were not changed significantly during treatment.29 Four studies reported follow‐up data only, reporting no change33, 44, 45 or a significant reduction in ED risk.52
Meta‐analysis of the intervention arms from nine studies,20, 29, 30, 32, 37, 41, 46, 47, 53 with a combined sample of 491 participants, found no change in ED risk post‐intervention (Figure 2A; SMD [SE], −0.10 [0.10], P = 0.317, I 2 86%). A follow‐up measure of up to 4.6 years from baseline was reported in six studies,20, 33, 44, 45, 46, 52 with a combined effect of −0.31 [0.13], P = 0.012, I 2 88% (Figure 2B) representing a reduction in ED risk. Funnel plots appeared symmetrical, and the classic fail‐safe N statistic estimated that 91 unpublished studies would be required for P > 0.05 at follow‐up, and therefore, publication bias is unlikely. Moderator analysis found that intervention intensity (P < 0.001) and inclusion of ED‐related content (P = 0.021) had a significant effect on ED risk. Studies with daily contact had a larger effect on ED risk post‐intervention (two studies, −0.47 [0.10], P < 0.001), compared with studies with weekly/biweekly contact (six studies, 0.08 [0.07], P = 0.30). Studies that reported inclusion of ED‐related content (four studies, 0.08 [0.05], P = 0.096) had a smaller effect on ED risk than studies without ED‐related content (six studies, −0.26 [0.14], P = 0.062), although neither group showed a significant change. There was no effect based on use of energy prescription, inclusion of a physical activity component, or involvement of a psychologist. Meta‐regression found a small correlation between intervention duration and change in ED risk (R 2 = 0.29, P = 0.05), where longer interventions had a greater reduction in ED risk. There was no effect based on participant age at baseline.
Figure 2.
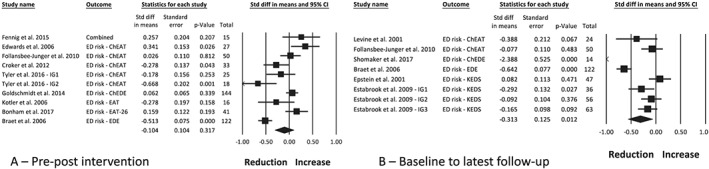
Meta‐analysis of the change in eating disorder risk between pre‐ and post‐intervention (A) and between baseline and the latest follow‐up timepoint (B), following obesity treatment with a dietary component in children and adolescents with overweight and obesity. ChEAT, Children's Eating Attitudes Test; ChEDE, Child Eating Disorder Examination; EAT, Eating Attitudes Test; EAT‐26, Eating Attitudes Test‐26 items; EDE, Eating Disorder Examination; IG, intervention group; KEDS, Kids Eating Disorder Survey
Two studies reported on participants with scores above a clinical cut‐point for ED risk. Epstein et al44 reported a reduction to below clinical cut‐points at the 24‐month follow‐up from an elevated score at baseline in six children. Scores remained elevated from baseline in six children and increased to above the clinical cut‐points in seven children. Follansbee‐Junger et al46 reported a downward trend in the number of youth with scores in the clinical range from baseline to 10‐month follow‐up in both the intervention (12.5% to 8.6%) and waitlist groups (31.6% to 13.3%).
3.6. Eating disorder–related symptoms
3.6.1. Bulimic symptoms
The change in bulimic symptoms was measured using six different assessment tools56, 66, 67, 70, 71, 74 reported in eight studies24, 25, 28, 30, 34, 37, 39, 41 at post‐intervention only, two studies22, 44 at follow‐up only, and one study36 at both timepoints. Eight studies22, 24, 25, 28, 34, 36, 41, 44 reported a significant reduction in bulimic symptoms, and three studies30, 37, 39 reported no change post‐intervention. Meta‐analysis of the intervention arm from eight studies,25, 28, 30, 34, 36, 37, 39, 41 with a combined sample of 375 participants, found a reduction in bulimic symptoms post‐intervention (Figure 3A; −0.33 [0.09], P < 0.001, I 2 72%). This change was no longer statistically significant at follow‐up of up to 6 years from baseline (Figure 3B; −0.25 [0.24], P = 0.30, I 2 94%), as reported in three studies.22, 36, 44 Publication bias is unlikely (funnel plot symmetry, fail‐safe N = 105 studies). Moderator analysis and meta‐regression found no effect by participant age, duration of the intervention, inclusion of ED‐related content, use of energy prescription, inclusion of a physical activity component, or involvement of a psychologist.
Figure 3.
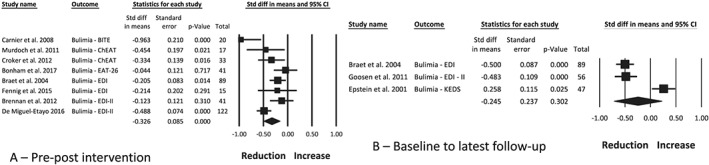
Meta‐analysis of the change in bulimic symptoms between pre‐ and post‐intervention (A) and between baseline and the latest follow‐up timepoint (B), following obesity treatment with a dietary component in children and adolescents with overweight and obesity. BITE, Bulimic Investigatory Test; ChEAT, Children's Eating Attitudes Test; EAT‐26, Eating Attitudes Test‐26 items; EDI, Eating Disorder Inventory; EDI‐II, Eating Disorder Inventory‐second edition; KEDS, Kids Eating Disorder Survey
Two studies reported on participants with scores above a clinical cut‐point for bulimic symptoms.21, 25 Braet et al21 identified seven children at baseline with elevated scores, five of which had returned to normal at post‐treatment. Similarly, Carnier et al25 reported the presence of some bulimic symptoms in all enrolled adolescents at baseline, with a significant reduction post‐intervention to 63% in boys and 70% in girls.
3.6.2. Binge eating
The prevalence of binge eating was reported in five studies.22, 25, 26, 35, 36 A reduction in the prevalence of binge eating between pre‐ and post‐intervention was reported by Braet et al36 (54% to 19%) and Van Vlierberghe et al35 (30.3% to 12.9%) following a 10‐month inpatient treatment program and by Damaso et al26 (6% to 2%) following a 24‐week multidisciplinary tertiary hospital outpatient program. Goossens et al22 reported an overall reduction in objective (eight to four participants) and subjective binge eating (nine to one participants) but also the development of these behaviors in four participants of 47 enrolled in the study. Carnier et al25 reported the change in BED symptoms by sex. At baseline, 63% of boys and 25% of girls presented with BED symptoms, and following a 12‐month interdisciplinary intervention, these values decreased significantly to 25% and 10%, respectively, with a reported decrease in symptom severity.
The severity or frequency of binge eating was measured using three different assessment tools55, 60, 61 in four studies.20, 24, 27, 35 All studies reported a reduction in binge eating. Meta‐analysis of the intervention arm from three studies,20, 27, 35 with a combined sample of 198 participants, found an overall combined effect representing a significant reduction in binge eating post‐intervention (Figure 4; −0.59 [0.10], P < 0.001, I 2 41%). Publication bias is unlikely (funnel plot symmetry, fail‐safe N = 74 studies). Braet20 reported a sustained significant reduction in binge eating at follow‐up of 36 months from baseline (−0.49 [0.07], P < 0.001).
Figure 4.
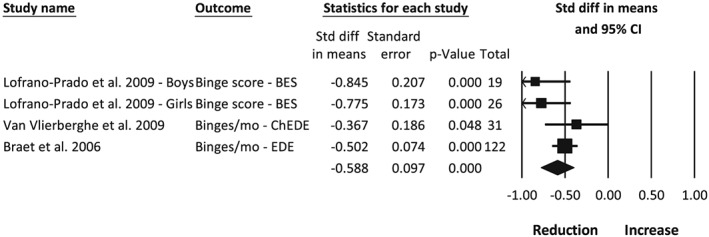
Meta‐analysis of the change in binge eating between pre‐ and post‐intervention following obesity treatment with a dietary component in children and adolescents with overweight and obesity. BES, binge eating scale; ChEDE, Child Eating Disorder Examination; EDE, Eating Disorder Examination
3.6.3. Emotional eating
The change in emotional eating was measured using five different assessment tools59, 63, 64, 72, 73, 75 and reported in two studies43, 51 at post‐intervention only, two studies38, 54 at follow‐up only, and five studies19, 31, 36, 40, 48, 49 at both timepoints. Three studies reported a reduction19, 40, 43 and four studies reported no change36, 38, 51, 54 in emotional eating, and one study31 reported a reduction in boys but not girls. One study48, 49 reported no change in emotional eating post‐intervention and a reduction at follow‐up. Meta‐analysis of the intervention arm from six studies,19, 31, 36, 43, 48, 49, 51 with a combined sample of 508 participants, found a small combined effect indicating a reduction in emotional eating (Figure 5A; −0.15 [0.06], P = 0.008, I 2 47%). This was maintained at follow‐up of up to 36 months from baseline in six studies19, 31, 36, 38, 48, 49, 54 (Figure 5B; −0.21 [0.06], P = 0.001, I 2 47%). Publication bias is possible post‐intervention (funnel plot symmetry, fail‐safe N = 22 studies) but unlikely at follow‐up (funnel plot symmetry, fail‐safe N = 43 studies). Moderator analysis found a significant effect for use of an energy prescription (P = 0.05), whereby studies without an energy prescription, providing nutrition education only, had a small reduction in emotional eating (four studies, −0.22 [0.07], P = 0.003), compared with studies with an energy prescription that did not have an effect on emotional eating (two studies, −0.04 [0.06], P = 0.067). There was no effect based on age of participants at baseline, duration of the intervention, inclusion of ED‐related content or a physical activity component, or involvement of a psychologist.
Figure 5.
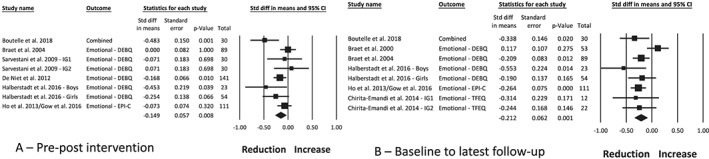
Meta‐analysis of the change in emotional eating between pre‐ and post‐intervention (A) and between baseline and the latest follow‐up timepoint (B), following obesity treatment with a dietary component in children and adolescents with overweight and obesity. Combined, combined subscales of the emotional eating scale; DEBQ, Dutch Eating Behaviour Questionnaire; EPI‐C, Eating Pattern Inventory for Children; IG, intervention group; TFEQ, Three‐Factor Eating Questionnaire
3.6.4. Drive for thinness
Drive for thinness was measured using two versions of the Eating Disorder Inventory70, 71 in four studies,22, 28, 36, 39 two28, 39 reporting no change, and two22, 36 a significant reduction. Braet et al36 reported that those who withdrew from the study had significantly higher baseline scores on drive for thinness. Meta‐analysis of the intervention arm from three studies28, 36, 39 with a combined sample of 252 participants found a small effect on drive for thinness post‐intervention (Figure 6A; −0.17 [0.06], P = 0.005, I 2 27%), representing a reduction in this outcome. This was maintained at follow‐up in two studies22, 36 of the same 10‐month inpatient treatment program (Figure 6B; −0.38 [0.07], P < 0.001, I 2 0%). Publication bias is possible post‐intervention (funnel plot symmetry, fail‐safe N = 6 studies) and could not be calculated at follow‐up because of the small number of included studies.
Figure 6.
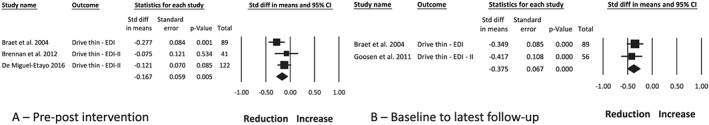
Meta‐analysis of the change in drive for thinness between pre‐ and post‐intervention (A) and between baseline and the latest follow‐up timepoint (B), following obesity treatment with a dietary component in children and adolescents with overweight and obesity. EDI, Eating Disorder Inventory; EDI‐II, Eating Disorder Inventory‐second edition
3.6.5. Eating concern
The change in eating concern was measured using four different assessment tools60, 61, 68, 69 and reported in five studies.22, 30, 35, 36, 50 Two studies30, 50 reported no change and two studies35, 36 reported a significant reduction in eating concern post‐intervention. One study22 reported follow‐up data only, reporting a reduction in eating concern at 6 years from baseline. Meta‐analysis of the intervention arm from four studies,30, 35, 36, 50 with a combined sample of 194 participants, found no change post‐intervention (Figure 7A; −0.19 [0.13], P = 0.13, I 2 72%). However, the change became statistically significant at follow‐up of up to 6 years from baseline in three studies22, 36, 50 (Figure 7B; −0.50 [0.06], P < 0.001, I 2 0%). Publication bias at follow‐up is unlikely (funnel plot symmetry, fail‐safe N = 55 studies). Moderator analysis found that intervention intensity and inclusion of ED‐related content (P = 0.021) were associated with eating concern. Two studies35, 36 with daily contact and which did not include ED‐related content had a reduction in eating concern (−0.39 [0.08], P < 0.001), compared with two studies30, 50 with weekly/biweekly contact and addressed ED‐related content (0.014 [0.16], P = 0.931) which had no change. Meta‐regression found that a longer intervention duration was associated with a greater reduction in eating concern (R 2 = 0.96, P = 0.007), but no association was found on the basis of participant age.
Figure 7.
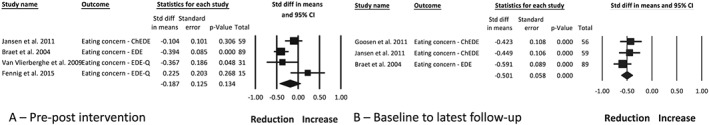
Meta‐analysis of the change in eating concern between pre‐ and post‐intervention (A) and between baseline and the latest follow‐up timepoint (B), following obesity treatment with a dietary component in children and adolescents with overweight and obesity. ChEDE, Child Eating Disorder Examination; EDE, Eating Disorder Examination; EDE‐Q, Eating Disorder Examination Questionnaire
3.7. Randomized controlled trials
Six randomized controlled trials included a no‐treatment control group.37, 39, 40, 41, 46, 50 Meta‐analysis of three studies each reporting ED risk37, 41, 46 and bulimic symptoms37, 39, 41 found no difference between intervention and control groups post‐intervention (data not shown). Three studies reported no significant difference for the change in drive for thinness,39 emotional eating,40 and eating concern50 between intervention and control groups post‐intervention.
3.8. Weight‐related outcomes
BMI and BMI z‐score were the most commonly reported weight‐related outcomes by included studies. Of those studies not reporting BMI or BMI z‐score, two studies reported change in percentage overweight,38, 44 and one study each reported percent weight loss,35 BMI expressed as a percent of the 95th percentile48, 49 and BMI percentiles.50 Meta‐analysis of the intervention arm from 22 studies,19, 20, 21, 26, 27, 28, 29, 30, 31, 33, 34, 36, 37, 38, 39, 41, 42, 43, 46, 47, 48, 49, 50, 52, 53 with a combined sample of 1562 participants, found a significant reduction in weight‐related outcomes post‐intervention (Figure S1A; −0.49 [0.06], P < 0.001, I 2 87%). This was maintained up to 6 years from baseline (Figure S1B; −0.40 [0.06], P < 0.001, I 2 80%) in 14 studies.19, 20, 21, 22, 31, 36, 37, 38, 42, 44, 45, 46, 47, 48, 49, 50, 52 The combined mean difference [SE] for change in BMI z‐score in 13 studies19, 20, 22, 29, 31, 34, 36, 37, 39, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 52, 53 was −0.16 [0.03], P < 0.001, I 2 86%, and BMI in 11 studies19, 20, 21, 26, 27, 28, 30, 31, 33, 36, 37, 41, 52 was −2.21 [0.92] kg/m2, P = 0.016, I 2 98%, post‐intervention. The change in BMI z‐score was maintained at follow‐up of up to 29 months from baseline (−0.13 [0.02], P < 0.001, I 2 73%) in seven studies.31, 37, 42, 45, 46, 47, 52
A relationship was found between the effect size for change in ED risk and the effect size for change in weight‐related outcomes post‐intervention, where a larger decrease in weight was associated with a larger reduction in ED risk (R 2 = 0.43, P = 0.02) (Figure S2). This should be interpreted with caution as the association appears to be skewed by one study and is no longer statistically significant with this study removed (P = 0.24). No relationship was found between bulimic symptoms or emotional eating and weight‐related outcomes.
Five studies were not included in meta‐analysis because of insufficient data being reported. Two studies reported a reduction in BMI z‐score at 6 months54 and 12 months40 from baseline. Sarvestani et al51 reported a reduction in BMI, and Kotler et al32 reported no change, post‐intervention. Van Vlierberghe et al35 reported a mean percentage weight loss of 52.5% following a 10‐month inpatient treatment program.
4. DISCUSSION
This is the first systematic review with meta‐analysis to examine the change in ED‐related outcomes following treatment for child and adolescent obesity. This review demonstrates a reduction in the prevalence of ED, ED risk, and in a range of ED‐related symptoms including bulimic symptoms, binge eating, emotional eating, drive for thinness, and eating concern, post‐intervention and/or follow‐up of up to 6 years from baseline. In line with previous work,12 findings from our review provide evidence that structured and professionally run obesity treatment interventions, with a dietary component, may reduce the risk of ED development in the short and longer term.
Concern over adolescent dieting has arisen from a number of observational studies which show that dieting in any form is associated with an increased risk of undertaking disordered eating behaviors10, 77 and the development of ED.11 The results of our review are in contrast to these data, and we offer two possible explanations. First, there are likely to be variations in the nature of dieting being undertaken, and second, there may be differing cohorts of adolescents represented in observational studies compared with intervention trials.
It is possible that there is disparity between the types of dieting being undertaken by adolescents independently, compared with the intervention trials represented in this review. It is well documented that adolescent engagement with health services is poor,78 and little is known about the self‐reported dieting practices of adolescents. Project EAT10 identified healthy (increased fruit and vegetables and ate less high‐fat foods), unhealthy (fasted, use of food substitutes, and skipped meals), and extreme (use of laxatives or diet pills and self‐induced vomiting) dieting behaviors as increasing the risk for binge eating in adolescent boys and girls. However, this same study10, 77 also identified increased nutrition knowledge, availability of healthy food, regular meals, self‐esteem, and self‐efficacy towards healthy eating as protective against binge eating. Many of these protective factors are incorporated into professionally run obesity treatment interventions in combination with dietary change. Considering this review has shown mild improvements in a range of ED‐related risk factors following treatment, for most people, it is possible that these protective factors negate the potential risk from dieting. This suggests that a structured and well‐supported environment is vital in the treatment process, highlighting the importance of increasing access to obesity treatment services for young people. It may also be worth considering changes in psychological wellbeing as markers of intervention success independent of weight change and physiological health.
It is also possible that differing cohorts of adolescents may be presenting for obesity treatment interventions, compared with those undertaking self‐reported dieting. In the general population, adolescents with higher psychiatric morbidity are at highest risk of using extreme dieting behaviors79 and of developing ED independent of dieting status.11 However, these young people are also unlikely to be accessing health services,80, 81 may not be participating in clinical trials, and therefore, may not be represented within this review. In addition, data are not captured on those who withdrew from the included interventions, and it is possible that some young people may be more vulnerable to worsening psychopathology.82 The meta‐regression conducted within this review highlighted that interventions of longer duration had a greater reduction in ED risk. This indicates that extended contact with and the support provided by obesity treatment programs is beneficial. Novel and cost‐effective strategies to allow long‐term engagement with young people are required.
Despite an overall reduction in ED risk, some participants were identified to have developed an ED at follow‐up measures. Within this review, one study reported the development of BED in three of 56 participants.22 Similarly, Epstein et al83 reported on 10‐year outcomes following four randomized controlled trials for the treatment of childhood obesity. Although this study did not meet the inclusion criteria for this review, the authors identified six girls, from 137 who completed follow‐up measures, who were undergoing treatment for an ED at the 10‐year follow‐up timepoint.83 It is uncertain if these differ from the rate of development of ED that may be seen within a population of non–treatment‐seeking adolescents with obesity. However, the early signs and symptoms of ED in adolescents with obesity attempting to lose weight may be missed, particularly when the focus is on weight loss or the young person remains within or above a healthy weight range.84, 85 These findings support recommendations by Goldschmidt et al86 that treatment providers monitor for the development of or exacerbation of ED symptoms during obesity treatment.
It is interesting that meta‐analyses of ED risk and eating concern showed no change post‐intervention but a significant reduction at the follow‐up timepoint. Edwards et al29 highlight some limitations in the use of ED risk questionnaires during weight management interventions. Behaviors such as “I stay away from foods with sugar in them” and “I eat diet foods” would increase the ED risk score on these questionnaires; however, these practices are seen as helpful in the context of obesity treatment and the related dietary change. In addition, measures of dietary restraint60, 61, 68, 69 and dieting behavior65, 66, 67 are included within questionnaires that assess overall ED risk. However, these are often encouraged or are a necessary component of obesity treatment. It is important to note that dietary restraint, often used as a measure of dieting practice in prospective cohort studies, may not reflect actual energy restriction.87, 88 Consequently, the energy restriction itself may not increase eating pathology.87, 88 Further research is required to better define dietary restriction,87 including specific tools encompassing the needs of a treatment‐seeking population to accurately measure ED risk in children and adolescents with obesity.
4.1. Strengths and limitations
This review includes a broad range of search terms and extensive hand searching to provide a detailed assessment of the change in ED risk. Inclusion of meta‐analysis allows quantitative synthesis of results. This review addresses concerns over longer term ED risk, with the inclusion of follow‐up data for 18 studies with follow‐up timepoints of up to 6 years from baseline, including seven studies with a follow‐up of ≥2 years. This review also has a number of limitations. Publication bias is always possible when assessing secondary outcomes; however, the use of the fail‐safe N statistic provided a more rigorous assessment of bias than use of funnel plots alone. Although validated to assess ED‐related outcomes, to our knowledge, the assessment tools used in the included studies have not been validated in treatment‐seeking samples of children and adolescents with overweight and obesity. Considering that only a small proportion of participants may experience a worsening of eating pathology, these outliers are not well represented when using sample means in analysis. In addition, many studies also reported completer analysis rather than intention‐to‐treat analysis, so participants who withdrew from treatment may not be well represented in these data.
4.2. Recommendations for clinical practice
For the majority of participants who engage with and complete weight management interventions, disordered eating symptomology improves. A small proportion of participants are likely to be at risk, and measures such as monitoring should be in place to identify these young people. Little is known about ED risk in those who withdraw from treatment; these young people may be particularly vulnerable and require additional support. Where possible, contact should be maintained with these young people and appropriate referrals provided. The results of this review indicate that a longer duration of contact is associated with a greater reduction in ED risk. To encourage longer term reduction of ED risk, cost‐effective ways of extending intervention duration and maintaining longer term contact with young people following intervention completion, eg, via text message or group sessions, should be considered.
4.3. Recommendations for future research
This review highlights a number of avenues for future research on the relationship between pediatric obesity treatment and ED risk. First, the development of appropriate tools for the assessment of ED risk in treatment‐seeking populations is required. Where dietary modification is being encouraged or prescribed, ED‐related risk could focus on cognitive distortions related to food and eating, obsessive or compensatory behaviors, internalized weight bias, and elevated body disparagement. Findings from our review also indicate that obesity treatment interventions should include measurement of ED risk factors at pre‐ and post‐intervention and follow‐up. The identification of predictors for those at higher risk for ED, as well as protective factors, within the context of obesity treatment is important to inform intervention design. It would be of benefit to utilize individual patient data in future analyses, if possible, as this may be useful in providing a more accurate representation of change in ED risk for those with outlying pathology following obesity treatment.
5. CONCLUSION
This review demonstrates that structured and professionally run obesity treatment leads to a reduction in the prevalence of ED, ED risk, and ED‐related symptoms for most participants. Maintained longer term engagement with the program appears to be an important contributor to the reduction in ED risk. However, further research is required to better understand the relationship between dieting and ED risk in the context of obesity treatment for children and adolescents and in those who withdraw from treatment.
CONFLICT OF INTEREST
Authors have no conflict of interest.
SOURCES OF SUPPORT
H. J is supported by a Research Program Stipend (The University of Sydney); N. B. L. and M. L. G. are in receipt of NHMRC Peter Doherty Early Career Fellowships.
Supporting information
Table S1. Search strategy used on the Ovid platform with the Medline database. Search terms contained reference to all three criteria: overweight/obesity, dietary treatment interventions, and eating disorder related outcome measures. Medical Subject Headings (MeSH) are bolded, and key word searches are italicised.
Table S2: Characteristics of included studies reporting change in ED prevalence, ED risk and related symptoms following obesity treatment, with a dietary component, in children and adolescents with overweight or obesity
Figure S1: Meta‐analysis of the change in weight‐related outcomes between pre‐ and post‐intervention (A) and between baseline and the latest follow‐up timepoint (B), following obesity treatment with a dietary component in children and adolescents with overweight and obesity. Results are presented as Standardised Mean Difference (SE) using a random effects model Abbreviations: %overweight, percentage overweight calculated based on normative data; BMI, body mass index; BMI95, BMI expressed as a percentage of the 95th percentile; IG, intervention group
Figure S2: Meta‐regression of the effect size for change in eating disorder risk and the effect size for change in weight‐related outcomes between pre‐ and post‐intervention following professionally administered obesity treatment with a dietary component in children and adolescents with overweight and obesity (R2=0.43, p=0.02)
ACKNOWLEDGEMENTS
The authors would like to thank Ms Katharine Aldwell, Ms Sarah Thomas, and Ms Julia Elise King for assistance with screening and data extraction and the EVAYSON study group (Ascensiόn Marcos, Luis Moreno, Pilar De Miguel‐Etayo) for provision of data for meta‐analysis.28
Jebeile H, Gow ML, Baur LA, Garnett SP, Paxton SJ, Lister NB. Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: A systematic review with meta‐analysis. Obesity Reviews. 2019;20:1287–1298. 10.1111/obr.12866
REFERENCES
- 1. Abarca‐Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steinbeck KS, Lister NB, Gow ML, Baur LA. Treatment of adolescent obesity. Nat Rev Endocrinol. 2018;14(6):331‐344. [DOI] [PubMed] [Google Scholar]
- 3. Pinhas‐Hamiel OD, Zeitler PMD. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369(9575):1823‐1831. [DOI] [PubMed] [Google Scholar]
- 4. Constantino MI, Molyneaux L, Limacher‐Gisler F, et al. Long‐term complications and mortality in young‐onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36(12):3863‐3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smink FRE, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14(4):406‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173(1):11‐53. [DOI] [PubMed] [Google Scholar]
- 7. Rozzell K, Moon D, Klimek P, Brown T, Blashill AJ. Prevalence of eating disorders among us children aged 9 to 10 years: data from the Adolescent Brain Cognitive Development (ABCD) study. JAMA Pediatr. 2019;173(1):100‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smink FRE, van Hoeken D, Oldehinkel AJ, Hoek HW. Prevalence and severity of DSM‐5 eating disorders in a community cohort of adolescents. Int J Eat Disord. 2014;47(6):610‐619. [DOI] [PubMed] [Google Scholar]
- 9. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. Fifth ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 10. Neumark‐Sztainer DR, Wall MM, Haines JI, Story MT, Sherwood NE, van den Berg PA. Shared risk and protective factors for overweight and disordered eating in adolescents. Am J Prev Med. 2007;33(5):359‐369. [DOI] [PubMed] [Google Scholar]
- 11. Patton GC, Selzer R, Coffey C, Carlin JB, Wolfe R. Onset of adolescent eating disorders: population based cohort study over 3 years. BMJ. 1999;318(7186):765‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butryn ML, Wadden TA. Treatment of overweight in children and adolescents: does dieting increase the risk of eating disorders? Int J Eat Disord. 2005;37(4):285‐293. [DOI] [PubMed] [Google Scholar]
- 13. Jebeile H, Paxton SJ, Garnett SP, Baur LA, Lister NB. The impact of weight management interventions on psychological wellbeing in children and adolescents with overweight/obesity: a systematic review. PROSPERO: International prospective register of systematic reviews. CRD42017069496;2017.
- 14. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Handu DPRDN, Moloney LMSRDN, Wolfram TMSRDN, Ziegler PPRDN, Acosta A, Steiber APRDN. Academy of Nutrition and Dietetics methodology for conducting systematic reviews for the evidence analysis library. J Acad Nutr Diet. 2016;116(2):311‐318. [DOI] [PubMed] [Google Scholar]
- 16. Card NA. Applied Meta‐Analysis for Social Science Research. New York: Guilford Press; 2012. [Google Scholar]
- 17. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638‐641. [Google Scholar]
- 18. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Saint Louis: Elsevier Science & Technology; 2013. [Google Scholar]
- 19. Boutelle KN, Braden A, Knatz‐Peck S, Anderson LK, Rhee KE. An open trial targeting emotional eating among adolescents with overweight or obesity. Eat Disord. 2018;26(1):79‐91. [DOI] [PubMed] [Google Scholar]
- 20. Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity. 2006;14(1):148‐155. [DOI] [PubMed] [Google Scholar]
- 21. Braet C, Tanghe A, Bode PD, Franckx H, Winckel MV. Inpatient treatment of obese children: a multicomponent programme without stringent calorie restriction. Eur J Pediatr. 2003;162(6):391‐396. [DOI] [PubMed] [Google Scholar]
- 22. Goossens L, Braet C, Verbeken S, Decaluwe V, Bosmans G. Long‐term outcome of pediatric eating pathology and predictors for the onset of loss of control over eating following weight‐loss treatment. Int J Eat Disord. 2011;44(5):397‐405. [DOI] [PubMed] [Google Scholar]
- 23. Carnier J, de Lima Sanches P, da Silva PL, et al. Obese adolescents with eating disorders: analysis of metabolic and inflammatory states. Physiol Behav. 2012;105(2):175‐180. [DOI] [PubMed] [Google Scholar]
- 24. Carnier J, de Piano A, de Lima Sanches P, et al. The role of orexigenic and anorexigenic factors in an interdisciplinary weight loss therapy for obese adolescents with symptoms of eating disorders. Int J Clin Pract. 2010;64(6):784‐790. [DOI] [PubMed] [Google Scholar]
- 25. Carnier J, Lofrano MC, Prado WL, et al. Hormonal alteration in obese adolescents with eating disorder: effects of multidisciplinary therapy. Horm Res. 2008;70(2):79‐84. [DOI] [PubMed] [Google Scholar]
- 26. Damaso AR, De Piano A, Campos RMDS, et al. Multidisciplinary approach to the treatment of obese adolescents: effects on cardiovascular risk factors, inflammatory profile, and neuroendocrine regulation of energy balance. Int J Endocrinol. 2013;2013 (no pagination)(541032):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lofrano‐Prado MC, Antunes HK, do Prado WL, et al. Quality of life in Brazilian obese adolescents: effects of a long‐term multidisciplinary lifestyle therapy. Health Qual Life Outcomes. 2009;7(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Miguel‐Etayo P, Muro C, Santabarbara J, et al. Behavioral predictors of attrition in adolescents participating in a multidisciplinary obesity treatment program: EVASYON study. Int J Obes (Lond). 2016;40(1):84‐87. [DOI] [PubMed] [Google Scholar]
- 29. Edwards C, Nicholls D, Croker H, Van Zyl S, Viner R, Wardle J. Family‐based behavioural treatment of obesity: acceptability and effectiveness in the UK. Eur J Clin Nutr. 2006;60(5):587‐592. [DOI] [PubMed] [Google Scholar]
- 30. Fennig S, Brunstein‐Klomek A, Sasson A, Halifa Kurtzman I, Hadas A. Feasibility of a dual evaluation/intervention program for morbidly obese adolescents. Isr J Psychiatry Relat Sci. 2015;52(2):107‐112. [PubMed] [Google Scholar]
- 31. Halberstadt J, van Strien T, de Vet E, Eekhout I, Braet C, Seidell JC. The association of eating styles with weight change after an intensive combined lifestyle intervention for children and adolescents with severe obesity. Appetite. 2016;99:82‐90. [DOI] [PubMed] [Google Scholar]
- 32. Kotler LA, Etu SF, Davies M, Devlin MJ, Attia E, Walsh BT. An open trial of an intensive summer day treatment program for severely overweight adolescents. Eat Weight Disord. 2006;11(4):e119‐e122. [DOI] [PubMed] [Google Scholar]
- 33. Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family‐based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30(3):318‐328. [DOI] [PubMed] [Google Scholar]
- 34. Murdoch M, Payne N, Samani‐Radia D, et al. Family‐based behavioural management of childhood obesity: service evaluation of a group programme run in a community setting in the United Kingdom. Eur J Clin Nutr. 2011;65(6):764‐767. [DOI] [PubMed] [Google Scholar]
- 35. Van Vlierberghe L, Braet C, Goossens L, Rosseel Y, Mels S. Psychological disorder, symptom severity and weight loss in inpatient adolescent obesity treatment. Int J Pediatr Obes. 2009;4(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 36. Braet C, Tanghe A, Decaluwe V, Moens E, Rosseel Y. Inpatient treatment for children with obesity: weight loss, psychological well‐being, and eating behavior. J Pediatr Psychol. 2004;29(7):519‐529. [DOI] [PubMed] [Google Scholar]
- 37. Bonham MP, Dordevic AL, Ware RS, Brennan L, Truby H. Evaluation of a commercially delivered weight management program for adolescents. J Pediatr. 2017;03;185:73‐80. [DOI] [PubMed] [Google Scholar]
- 38. Braet C, Van Winckel M. Long‐term follow‐up of a cognitive behavioral treatment program for obese children. Behav Ther. 2000;31(1):55‐74. [Google Scholar]
- 39. Brennan L, Wilks R, Walkley J, Fraser SF, Greenway K. Treatment acceptability and psychosocial outcomes of a randomised controlled trial of a cognitive behavioural lifestyle intervention for overweight and obese adolescents. Behav Chang. 2012;29(1):36‐62. [DOI] [PubMed] [Google Scholar]
- 40. Cohen TR, Hazell TJ, Vanstone CA, Rodd C, Weiler HA. Changes in eating behavior and plasma leptin in children with obesity participating in a family‐centered lifestyle intervention. Appetite. 2018;125:81‐89. [DOI] [PubMed] [Google Scholar]
- 41. Croker H, Viner RM, Nicholls D, et al. Family‐based behavioural treatment of childhood obesity in a UK National Health Service setting: randomized controlled trial. Int J Obes (Lond). 2012;36(1):16‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeBar LL, Stevens VJ, Perrin N, et al. A primary care‐based, multicomponent lifestyle intervention for overweight adolescent females. Pediatrics. 2012;129(3):e611‐e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Niet J, Timman R, Bauer S, et al. The effect of a short message service maintenance treatment on body mass index and psychological well‐being in overweight and obese children: a randomized controlled trial. Pediatr Obes. 2012;7(3):205‐219. [DOI] [PubMed] [Google Scholar]
- 44. Epstein LH, Paluch RA, Saelens BE, Ernst MM, Wilfley DE. Changes in eating disorder symptoms with pediatric obesity treatment. J Pediatr. 2001;139(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 45. Estabrooks PA, Shoup JA, Gattshall M, Dandamudi P, Shetterly S, Xu S. Automated telephone counseling for parents of overweight children: a randomized controlled trial. Am J Prev Med. 2009;36(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 46. Follansbee‐Junger K, Janicke DM, Sallinen BJ. The influence of a behavioral weight management program on disordered eating attitudes and behaviors in children with overweight. J Am Diet Assoc. 2010;110(11):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goldschmidt AB, Best JR, Stein RI, Saelens BE, Epstein LH, Wilfley DE. Predictors of child weight loss and maintenance among family‐based treatment completers. J Consult Clin Psychol. 2014;82(6):1140‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ho M, Gow M, Halim J, et al. Effect of a prescriptive dietary intervention on psychological dimensions of eating behavior in obese adolescents. Int J Behav Nutr Phys Act. 2013;10(119):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gow ML, Baur LA, Ho M, et al. Can early weight loss, eating behaviors and socioeconomic factors predict successful weight loss at 12‐ and 24‐months in adolescents with obesity and insulin resistance participating in a randomised controlled trial? Int J Behav Nutr Phys Act. 2016;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jansen E, Mulkens S, Jansen A. Tackling childhood overweight: treating parents exclusively is effective. Int J Obes (Lond). 2011;35(4):501‐509. [DOI] [PubMed] [Google Scholar]
- 51. Sarvestani RS, Jamalfard MH, Kargar M, Kaveh MH, Tabatabaee HR. Effect of dietary behaviour modification on anthropometric indices and eating behaviour in obese adolescent girls. J Adv Nurs. 2009;65(8):1670‐1675. [DOI] [PubMed] [Google Scholar]
- 52. Shomaker LB, Tanofsky‐Kraff M, Matherne CE, et al. A randomized, comparative pilot trial of family‐based interpersonal psychotherapy for reducing psychosocial symptoms, disordered‐eating, and excess weight gain in at‐risk preadolescents with loss‐of‐control‐eating. Int J Eat Disord. 2017;50(9):1084‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tyler DO, Horner SD. A primary care intervention to improve weight in obese children: a feasibility study. J Am Assoc Nurse Pract. 2016;28(2):98‐106. [DOI] [PubMed] [Google Scholar]
- 54. Chirita‐Emandi A, Puiu M. Outcomes of neurofeedback training in childhood obesity management: a pilot study. J Altern Complement Med. 2014;20(11):831‐837. [DOI] [PubMed] [Google Scholar]
- 55. Freitas S, Lopes CS, Coutinho W, Appolinario JC. Tradução e adaptação para o português da Escala de Compulsão Alimentar Periódica. Braz J Psychiatry. 2001;23(4):215‐220. [Google Scholar]
- 56. Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47‐55. [DOI] [PubMed] [Google Scholar]
- 57. Cordás T, Hochgraf P. The “BITE”: tool for evaluation of bulimia nervosa—Portuguese version. J Bras Psiquiatr. 1993;42(1):141‐144. [Google Scholar]
- 58. Henderson M, Freeman C. A self‐rating scale for bulimia the ‘BITE. Br J Psychiatry. 1987;150(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 59. Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the children's eating behaviour questionnaire. J Child Psychol Psychiatry. 2001;42(7):963‐970. [DOI] [PubMed] [Google Scholar]
- 60. Bryant‐Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the Eating Disorder Examination with children: a pilot study. Int J Eat Disord. 1996;19(4):391‐397. [DOI] [PubMed] [Google Scholar]
- 61. Cooper Z, Fairburn C. The Eating Disorder Examination: a semi‐structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord. 1987;6(1):1‐6. [Google Scholar]
- 62. Hien D, Matzner F, First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM‐IV‐Child Edition (Version 1.0). New York: Columbia University; 1994. [Google Scholar]
- 63. Van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5(2):295‐315. [Google Scholar]
- 64. Van Strien T, Oosterveld P. The children's DEBQ for assessment of restrained, emotional, and external eating in 7‐ to 12‐year‐old children. Int J Eat Disord. 2008;41(1):72‐81. [DOI] [PubMed] [Google Scholar]
- 65. Garner DM, Garfinkel PE. The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol Med. 1979;9(2):273‐279. [DOI] [PubMed] [Google Scholar]
- 66. Maïano C, Morin AJ, Lanfranchi M‐C, Therme P. The Eating Attitudes Test‐26 revisited using exploratory structural equation modeling. J Abnorm Child Psychol. 2013;41(5):775‐788. [DOI] [PubMed] [Google Scholar]
- 67. Maloney MJ, McGuire JB, Daniels SR. Reliability testing of a children's version of the Eating Attitude Test. J Am Acad Child Adolesc Psychiatry. 1988;27(5):541‐543. [DOI] [PubMed] [Google Scholar]
- 68. Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self‐report questionnaire? Int J Eat Disord. 1994;16(4):363‐370. [PubMed] [Google Scholar]
- 69. Decaluwé V, Braet C. Assessment of eating disorder psychopathology in obese children and adolescents: interview versus self‐report questionnaire. Behav Res Ther. 2004;42(7):799‐811. [DOI] [PubMed] [Google Scholar]
- 70. Garner DM. Eating Disorder Inventory‐2: Professional Kit. Lutz, Florida: Psychological Assessment Resources; 1991. [Google Scholar]
- 71. Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int J Eat Disord. 1983;2(2):15‐34. [Google Scholar]
- 72. Schacht M, Richter‐Appelt H, Schulte‐Markwort M, Hebebrand J, Schimmelmann BG. Eating Pattern Inventory for Children: a new self‐rating questionnaire for preadolescents. J Clin Psychol. 2006;62(10):1259‐1273. [DOI] [PubMed] [Google Scholar]
- 73. Arnow B, Kenardy J, Agras WS. The emotional eating scale: the development of a measure to assess coping with negative affect by eating. Int J Eat Disord. 1995;18(1):79‐90. [DOI] [PubMed] [Google Scholar]
- 74. Childress AC, Jarrell MP, Brewerton TD. The kids' eating disorders survey (Keds): internal consistency, component analysis, and reliability. Eat Disord. 1993;1(2):123‐133. [Google Scholar]
- 75. de Lauzon B, Romon M, Deschamps V, et al. The threefactor eating questionnaire‐R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134(9):2372‐2380. [DOI] [PubMed] [Google Scholar]
- 76. Johnson WG, Grieve FG, Adams CD, Sandy J. Measuring binge eating in adolescents: adolescent and parent versions of the questionnaire of eating and weight patterns. Int J Eat Disord. 1999;26(3):301‐314. [DOI] [PubMed] [Google Scholar]
- 77. Neumark‐Sztainer D, Wall M, Story M, Sherwood NE. Five‐year longitudinal predictive factors for disordered eating in a population‐based sample of overweight adolescents: implications for prevention and treatment. Int J Eat Disord. 2009;42(7):664‐672. [DOI] [PubMed] [Google Scholar]
- 78. Patton GC, Sawyer SM, Santelli JS, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet. 2016;387(10036):2423‐2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Patton GC, Carlin JB, Shao Q, et al. Adolescent dieting: healthy weight control or borderline eating disorder? J Child Psychol Psychiatry. 1997;38(3):299‐306. [DOI] [PubMed] [Google Scholar]
- 80. Collishaw S. Annual research review: secular trends in child and adolescent mental health. In Vol 562015:370–393. [DOI] [PubMed]
- 81. Patel V, Flisher AJ, Hetrick S, McGorry P. Mental health of young people: a global public‐health challenge. Lancet. 2007;369(9569):1302‐1313. [DOI] [PubMed] [Google Scholar]
- 82. Hill A. Obesity and eating disorders. Obes Rev. 2007;8(s1):151‐155. [DOI] [PubMed] [Google Scholar]
- 83. Epstein LH, Valoski A, Wing RR, McCurley J. Ten‐year outcomes of behavioral family‐based treatment for childhood obesity. Health Psychol. 1994;13(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 84. Golden NH, Schneider M, Wood C. Preventing obesity and eating disorders in adolescents. Pediatrics. 2016;138(3):e20161649. [DOI] [PubMed] [Google Scholar]
- 85. Sim LA, Lebow J, Billings M. Eating disorders in adolescents with a history of obesity. Pediatrics. 2013;132(4):e1026‐e1030. [DOI] [PubMed] [Google Scholar]
- 86. Goldschmidt AB, Aspen VP, Sinton MM, Tanofsky‐Kraff M, Wilfley DE. Disordered eating attitudes and behaviors in overweight youth. Obesity. 2008;16(2):257‐264. [DOI] [PubMed] [Google Scholar]
- 87. Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychol Assess. 2004;16(1):51‐59. [DOI] [PubMed] [Google Scholar]
- 88. Schaumberg K, Anderson DA, Anderson LM, Reilly EE, Gorrell S. Dietary restraint: what's the harm? A review of the relationship between dietary restraint, weight trajectory and the development of eating pathology. Clin Obes. 2016;6(2):89‐100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy used on the Ovid platform with the Medline database. Search terms contained reference to all three criteria: overweight/obesity, dietary treatment interventions, and eating disorder related outcome measures. Medical Subject Headings (MeSH) are bolded, and key word searches are italicised.
Table S2: Characteristics of included studies reporting change in ED prevalence, ED risk and related symptoms following obesity treatment, with a dietary component, in children and adolescents with overweight or obesity
Figure S1: Meta‐analysis of the change in weight‐related outcomes between pre‐ and post‐intervention (A) and between baseline and the latest follow‐up timepoint (B), following obesity treatment with a dietary component in children and adolescents with overweight and obesity. Results are presented as Standardised Mean Difference (SE) using a random effects model Abbreviations: %overweight, percentage overweight calculated based on normative data; BMI, body mass index; BMI95, BMI expressed as a percentage of the 95th percentile; IG, intervention group
Figure S2: Meta‐regression of the effect size for change in eating disorder risk and the effect size for change in weight‐related outcomes between pre‐ and post‐intervention following professionally administered obesity treatment with a dietary component in children and adolescents with overweight and obesity (R2=0.43, p=0.02)


