Abstract
BACKGROUND
Pathogen inactivation and cold or cryopreservation of platelets (PLTs) both significantly affect PLT function. It is not known how PLTs function when both are combined.
STUDY DESIGN AND METHODS
Standard PLT concentrates (PCs) were compared to pathogen‐inactivated PCs treated with amotosalen photochemical treatment (AS‐PCT) when stored at room (RT, 22°C), cold (4°C, n = 6), or cryopreservation (−80°C, n = 8) temperatures. The impact of alternative storage methods on both arms was studied in flow cytometry, light transmittance aggregometry, and hemostasis in collagen‐coated microfluidic flow chambers.
RESULTS
Platelet aggregation of cold‐stored AS‐PCT PLTs was 44% ± 11% compared to 57% ± 14% for cold‐stored standard PLTs and 58% ± 21% for RT‐stored AS‐PCT PLTs. Integrin activation of cold‐stored AS‐PCT PLTs was 53% ± 9% compared to 77% ± 6% for cold‐stored standard PLTs and 69% ± 13% for RT‐stored AS‐PCT PLTs. Coagulation of cold‐stored AS‐PCT PLTs started faster under flow (836 ± 140 sec) compared to cold‐stored standard PLTs (960 ± 192 sec) and RT‐stored AS‐PCT PLTs (1134 ± 220 sec). Fibrin formation rate under flow was also highest for cold‐stored AS‐PCT PLTs. This was in line with thrombin generation in static conditions because cold‐stored AS‐PCT PLTs generated 297 ± 47 nmol/L thrombin compared to 159 ± 33 nmol/L for cold‐stored standard PLTs and 83 ± 25 nmol/L for RT‐stored AS‐PCT PLTs. So despite decreased PLT activation and aggregation, cold storage of AS‐PCT PLTs promoted coagulation. PLT aggregation of cryopreserved AS‐PCT PLTs (23% ± 10%) was not significantly different from cryopreserved standard PLTs (25% ± 8%).
CONCLUSION
This study shows that cold storage of AS‐PCT PLTs further affects PLT activation and aggregation but promotes (pro)coagulation. Increased procoagulation was not observed after cryopreservation.
ABBREVIATIONS
- AS‐PCT
amotosalen photochemical treatment
- ETP
endogenous thrombin potential
- MP
microparticle
- PC(s)
platelet concentrate(s)
- PS
phosphatidylserine
- RT
room temperature
- TF
tissue factor
Conventional, room temperature (RT) storage of platelets (PLTs) limits shelf life to between 4 and 7 days because of the risk of bacterial contamination and the decrease in PLT function called platelet storage lesion.1 The limited shelf life of PLT concentrates (PCs) hampers efficient inventory management and puts pressure on blood banks trying to balance risks of shortage with risks of wastage. Prolonging PC shelf life could overcome this problem and has been investigated for decades with a focus on cold storage (1‐6°C)2 and cryopreservation (−80 to −196°C).3, 4
Cold storage can potentially increase PC shelf life beyond 7 days of storage.5 Several reports indicate that cold‐stored PLTs are functionally “primed” suggesting that these are in a state of heightened responsiveness to hemostatic stimuli.5, 6, 7 The main obstacle for cold‐stored PLTs is the significantly faster clearance from circulation compared to RT storage.2, 8, 9 This may be less relevant in acutely bleeding patients who require immediate hemostasis more than extended PLT circulation times.10
Cryopreservation can increase shelf life of PLTs even longer, to at least 2 years. In this case, PLTs are hyperconcentrated in 5% to 6% dimethyl sulfoxide (DMSO) as cryoprotectant.11 Cryopreservation has a profound impact on PLT function in vitro with significantly decreased responses to agonists and a substantial increase in storage lesion markers like microparticle (MP) formation, P‐selectin, and phosphatidylserine (PS) expression.12, 13, 14 Despite this decreased responsiveness, cryopreserved PLTs may somehow retain hemostatic function particularly as procoagulants.14, 15, 16, 17 This is moreover in line with clinical experience of cryopreserved PC use in the military18 and in a recent trial with bleeding patients with thrombocytopenia.19
In recent years, pathogen inactivation treatments of PCs have been introduced in several countries to decrease the risk of transfusion‐transmitted infections.20 Three pathogen inactivation treatments have been developed for PLTs and all are based on treatment of PC with ultraviolet (UV) light with or without photosensitizer. The main biochemical principle of all pathogen inactivation methods is prevention of replication by chemical modification of genetic material in pathogens. Because PLTs are anucleate, relevant chemical modification in PLTs is believed to be minimal.21 However, all three pathogen inactivation methods impact PLT function.22, 23, 24 For instance, the amotosalen photochemical treatment (AS‐PCT) method significantly and irreversibly modifies PLT phospholipids in the PLT membrane.24
Implementation of pathogen inactivation led to further restriction of PC shelf life from 7 to 5 days in Belgium because hemovigilance data had shed doubt on transfusion efficacy of stored pathogen‐inactivated PCs.21 In surrounding EU countries longer storage times have been licensed. This decision spurred investigation to alternative storage methods of PCs in the context of pathogen inactivation. This study investigated the effect of storage at 4 or −80°C of PCs treated with AS‐PCT compared to standard untreated PCs.
MATERIALS AND METHODS
A detailed description of all materials and methods is included in Appendix S1 (available as supporting information in the online version of this paper).
Study design for cold storage
Platelet concentrates (PCs) were prepared by pooling of six buffy coats in 65% (vol/vol) additive solution (SSP+, MacoPharma) derived from whole blood donations. All donors consented in writing to the use of their products for scientific research. This study was approved by the ethical committee of the University Hospital of Antwerp (18/24/289). A pool‐and‐split design was used. Four PCs were pooled then gently mixed and divided into four equal PCs. Two of these were subjected to AS‐PCT (Intercept, Cerus Corporation), the other two were left untreated (i.e., standard). Samples were taken at this time point (Day [D]2–21°C) and PCs were immediately stored according to the following conditions. One standard and one AS‐PCT PC were stored for an additional 3 days at RT (D5–21°C) with constant agitation (PC900i, Helmer Scientific). The other standard and AS‐PCT PCs were stored for an additional 3 days at 4°C without agitation (D5–4°C). Experiments were performed on all D2 and D5 samples (n = 6 biological repeats). Cold‐stored PCs were incubated for 10 minutes on the PLT agitator at RT before sampling.
Study design for cryopreservation
A pool‐and‐split design was used. Two PCs were pooled then gently mixed and redivided into two paired PCs. One was subjected to AS‐PCT, and the other was untreated (i.e., standard). Both PCs were conditioned for cryopreservation by methods described elsewhere.14, 25, 26 In brief, a solution of 27% (vol/vol) DMSO and 0.9% NaCl (wt/vol) in water was added to all PCs yielding a final 6% (vol/vol) DMSO concentration. Next, the PCs were hyperconcentrated by centrifugation, a large volume of supernatant was taken off, and the PLT pellet was resuspended in a minimal volume of remaining supernatant. The entire conditioning regimen was never longer than 2 hours. For the 21°C arm, a small sample was taken from both the standard and the AS‐PCT bag followed by addition of ABO/D‐matched plasma to yield the original PLT concentration. The cryopreservation arm was stored at −80°C for at least 48 hours. The cryopreservation arm was thawed in a water bath for 8 minutes until reaching 30°C and then resuspended in ABO/D‐matched plasma equally prewarmed to 30°C.26 These PCs were held undisturbed for 30 minutes at RT before sampling and performing experiments. Power analysis requested a larger sample size for the cryopreservation study (n = 8) compared to the cold storage study (n = 6) because the estimated effect sizes of outcomes between standard and AS‐PCT conditions were expected to be smaller.
Microfluidic flow chambers with reconstituted blood
Fresh blood was reconstituted with PLTs from the PC aiming for an average of 40% hematocrit and 250 × 109 PLTs/L. Sample preparation and fluorescent labeling with 3,3′‐dihexyloxacarbocyanine iodide (Sigma‐Aldrich) and blue fluorescent dye (Alexa Fluor 405, Life Technologies) was as published before.22, 23, 27 Adhesion of PLTs onto collagen and formation of fibrin was studied by monitoring changes in median fluorescence of respective fluorophores as a function of time during perfusion of the reconstituted and recalcified blood at a wall shear rate of 1000/sec as described previously.27 PLT adhesion rate (/sec) indicates the rate of PLT deposition. The variables retrieved for fibrin deposition included coagulation rate (/sec), which is the linear portion of fibrin deposition kinetics and coagulation onset (sec). This is the lag time indicating the moment of coagulation onset defined as the intercept with the x‐axis of the extrapolated linear regression of the thrombus formation by fibrin fluorescence. This analysis takes into account thrombus growth in the z‐plane. The outcome variables were extracted from the raw fluorescence data using a software plugin developed in computer software (MatLab, MathWorks).
Thrombin generation assay
Generation of thrombin in vitro was analyzed with a thrombin generation assay kit (Technoclone GmbH) according to the manufacturer's instructions in a 96‐well format. PLTs were resuspended in heterologous human pooled (n = 38) plasma to final concentrations of 10 × 109, 50 × 109, or 250 × 109 PLTs/L. Each well contained a constant volume of 40% (vol/vol) of heterologous human pooled plasma, 5% (vol/vol) of saline (0.9% [wt/vol] NaCl in water), 1.0 pmol/L lipidated tissue factor (TF), 4.0 μmol/L corn trypsin inhibitor, 0.5 μmol/L fluorogenic substrate (Z‐G‐G‐RAMC, Technoclone GmbH), and 7.5 mmol/L CaCl2 (final concentrations). Samples were analyzed in a microplate reader (Infinite F200PRO, Tecan Group Ltd.) with filters for excitation (360 nm) and emission (460 nm). The signal was recorded as a function of time for a total of 120 minutes at 37°C. Raw data were converted to thrombin concentrations based on a calibration kit and a mathematical script.28 Peak thrombin measures the maximal concentration of thrombin formed during the course of the assay, lag time measures the time until a threshold concentration of thrombin is reached, and endogenous thrombin potential (ETP) corresponds to the area under the curve.
Statistical analysis
Results are reported as mean with standard deviation (SD). Normality testing was with the Shapiro‐Wilk algorithm. Comparison of RT‐ with cold‐stored PCs or RT with cryopreserved PCs, both standard and AS‐PCT, was with two‐way analysis of variance with Tukey's multiple‐comparisons correction. Data are given as means with SD. Results from statistical analysis are depicted on top of the panels and are shown as *p < 0.05, **p < 0.01, ***p < 0.005, and ****p < 0.001. All statistical analyses were performed with statistical software (Prism, Version 6, GraphPad Software Inc.).
RESULTS
Mean PLT volume, PLT recovery, and glycocalicin release
Cryopreservation but also cold storage caused significant increases in mean PLT volume (MPV) compared to RT storage and this was irrespective of AS‐PCT (Tables 1 and 2). PLT concentrations in AS‐PCT–treated products were slightly lower than standard. Storage did not cause changes in PLT concentration (Table 1). PLT recoveries after cryopreservation were not different between AS‐PCT and standard PLTs (Table 2). AS‐PCT caused a 50% increase in GPIbα ectodomain shedding (i.e., glycocalicin release) compared to standard on D2 (Table 1). Cold storage slightly attenuated GPIbα shedding caused by AS‐PCT compared to RT storage. Cryopreservation is a significant instigator of GPIbα shedding irrespective of AS‐PCT (Table 2).
Table 1.
Characteristics of standard and AS‐PCT PLTs stored at RT or 4°C
| D2‐21°C | D5‐21°C | D5‐4°C | ||||
|---|---|---|---|---|---|---|
| Standard | AS‐PCT | Standard | AS‐PCT | Standard | AS‐PCT | |
| MPV (fL) | 9.2 ± 0.2 | 9.5 ± 0.3 | 9.1 ± 0.3 | 9.4 ± 0.3 | 10.4 ± 0.2 | 10.8 ± 0.3 |
| PLT conc. (×109/L) | 1030 ± 74 | 980 ± 85 | 1036 ± 91 | 953 ± 86 | 1055 ± 68 | 991 ± 60 |
| Glycocalicin (ratio) | 1 | 1.5 ± 0.2 | 1.4 ± 0.5 | 2.4 ± 0.6 | 1.4 ± 0.3 | 2.0 ± 0.2 |
Table 2.
Characteristics of standard and AS‐PCT PLTs before and after cryopreservation
| Before cryopreservation | After cryopreservation | |||
|---|---|---|---|---|
| Standard | AS‐PCT | Standard | AS‐PCT | |
| MPV (fL) | 9.4 ± 0.3 | 9.7 ± 0.1 | 12.7 ± 0.6 | 13.1 ± 0.5 |
| PLT recovery (%) | 91.5 ± 10.2 | 89.1 ± 4.5 | ||
| Glycocalicin (ratio) | 1 | 1.1 ± 0.4 | 10.1 ± 3.9 | 9.8 ± 2.8 |
Cold storage
PLT aggregation and integrin αIIbβ3 activation
PAC‐1 binding to PLTs was significantly lower for AS‐PCT PLTs stored at 4°C (53% ± 9%) compared to RT (69% ± 13%, p = 0.0124). The decrease was smaller for standard PLTs stored at 4°C (77% ± 6%) compared to RT (85% ± 4%, p = 0.3429; Fig. 1A). Comparable results were found for PLT aggregation with collagen. Aggregation amplitude was significantly lower for AS‐PCT PLTs stored at 4°C (44% ± 11%) compared to RT storage (58% ± 21%, p = 0.0247). The decrease was smaller for standard PLTs stored at 4°C (57% ± 14%) compared to RT (62% ± 16%, p = 0.7399; Fig. 1B). No visible aggregates were detected in any PC over the course of the study.
Figure 1.
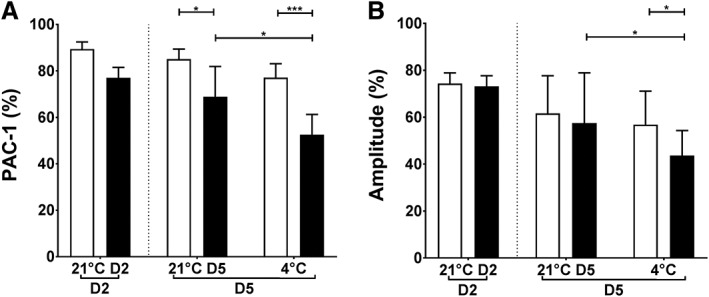
Platelet activation and aggregation of cold‐stored PLTs. (A) The percentage of PLTs expressing activated integrin αIIbβ3 in response to stimulation with 32.5 μmol/L TRAP6 determined by PAC‐1 binding in flow cytometry. (B) Maximal amplitude (%) of PLT aggregation in response to 10 μg/mL collagen. AS‐PCT PLTs ( ) were compared to standard (
) were compared to standard ( ) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
Hemostasis in microfluidic flow chambers at elevated wall shear stress
Platelet adhesion rate under flow was similar for AS‐PCT and standard PLTs after storage irrespective of its temperature although it was significantly decreased before storage in AS‐PCT compared to standard PLTs (p = 0.0196; Fig. 2A). Coagulation under shear flow measured by the rate (Fig. 2B) and the onset of coagulation (Fig. 2C) decreased slightly but not significantly for standard PLTs during storage irrespective of its temperature. However, RT‐stored AS‐PCT PLTs significantly affected coagulation under flow with a decreased coagulation rate (2.8 ± 1.3/sec vs. 4.7 ± 1.7/sec; p = 0.0383) and a delayed onset of coagulation (1135 ± 219 sec vs. 970 ± 126 sec; p = 0.0775) compared to RT‐stored standard PLTs (Figs. 2B and 2C). Of note, this functional decline was corrected when AS‐PCT PLTs were stored at 4°C with a coagulation rate of 5.0 ± 1.9/sec and onset of 836 ± 139 sec compared to 4.6 ± 1.1/sec (p = 0.9729) and 960 ± 192 sec (p = 0.3004) for cold‐stored standard PLTs.
Figure 2.
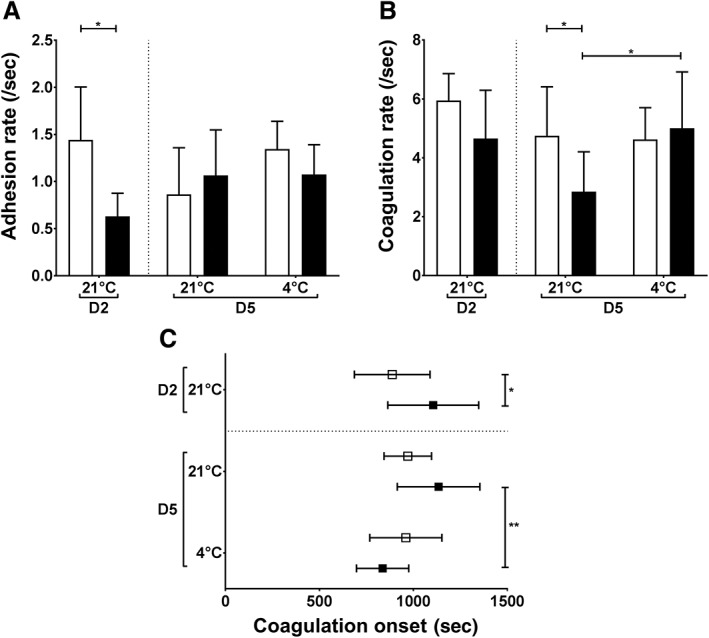
Platelet adhesion and coagulation in hydrodynamic conditions after cold storage of PLTs. Reconstituted blood containing 250 × 109 fluorescently labeled PLTs/L was perfused (1000/sec) over collagen‐coated microfluidic flow chambers in the presence of Ca2+. (A) The adhesion rate (/sec) indicates the increase in PLT fluorescent signal as a function of perfusion time. (B) The coagulation rate (/sec) indicates the accumulation of fluorescent fibrin as a function of perfusion time. (C) The coagulation onset (sec) indicates the time when fibrin formation starts. AS‐PCT PLTs ( ) were compared to standard (
) were compared to standard ( ) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
Hemostatic function in static conditions by integrated thrombin generation
Peak thrombin concentration with standard PLTs was 98 ± 30 nmol/L before storage and increased to 102 ± 13 and 160 ± 3 nmol/L after RT and cold storage, respectively. However, in the presence of cold‐stored AS‐PCT PLTs the peak thrombin concentration doubled to 297 ± 47 nmol/L, which was significantly higher than for RT‐stored AS‐PCT PLTs (83 ± 25 nmol/L; p < 0.0001; Fig. 3A). In line with increased peak thrombin, a shorter lag time (Fig. 3B) and increased ETP (Fig. 3C) were found for cold‐stored AS‐PCT PLTs compared to RT‐stored AS‐PCT PLTs. To demonstrate that the assay was dependent on PLTs, other PLT concentrations were used yielding the same results (Fig. S1, available as supporting information in the online version of this paper). Representative thrombograms of the condition with 50 × 109 PLTs/L are depicted in Fig. S2A, available as supporting information in the online version of this paper.
Figure 3.
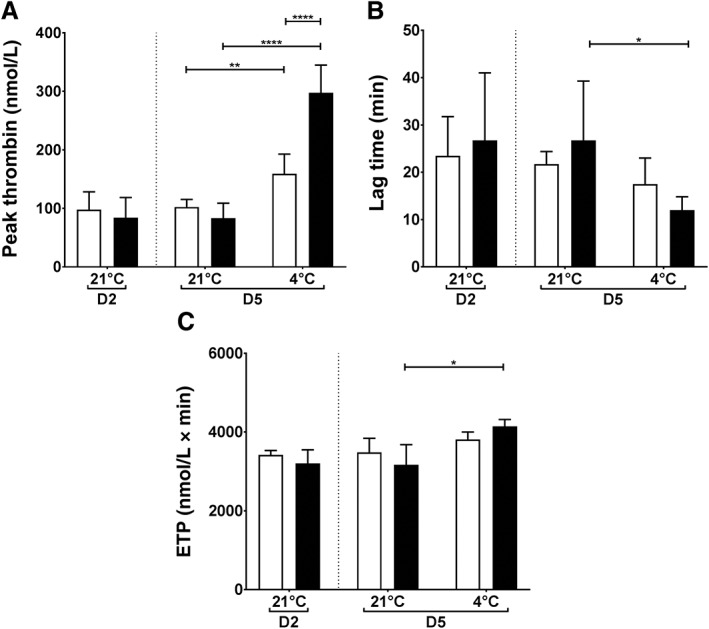
Thrombin generation in static conditions after cold storage of PLTs. Thrombin generation was measured in plasma in the presence of TF and 50 × 109 PLTs/L. (A) Peak thrombin (nmol/L), (B) lag time (min), and (C) ETP (nmol/L × min) are shown. AS‐PCT PLTs ( ) were compared to standard (
) were compared to standard ( ) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
PS expression and MP release
Molecular regulators of PLT procoagulation are aminophospholipid expression and MP release. Fig. 4 shows that cold storage caused a significant increase in annexin V binding for both AS‐PCT (33% ± 4%) and standard (16% ± 6%) PLTs compared to before storage (3% ± 1%). Yet, annexin V binding of cold‐stored AS‐PCT PLTs was double that of standard PLTs (p < 0.0001). Conversely, MP formation was more pronounced in standard (19% ± 6%) than in AS‐PCT PLTs (10% ± 2%, p < 0.0001) after cold storage.
Figure 4.
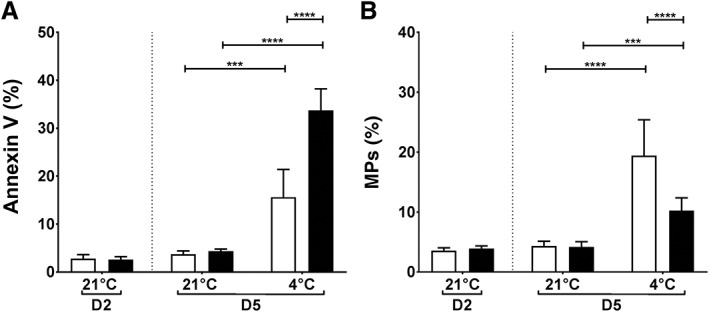
Phosphatidylserine expression and MP formation in cold‐stored PLTs. (A) The percentage of PLTs binding annexin V was used as a measure for aminophospholipid expression. (B) MPs were defined as GPIbα‐positive events smaller than 0.9 μm (detection limit, 0.5 μm). The relative number of MPs to the total number of GPIbα‐positive events is depicted. AS‐PCT PLTs ( ) were compared to standard (
) were compared to standard ( ) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
) PLTs before storage (D2) and on D5 after 3 additional storage days at RT (21°C) or cold temperature (4°C; n = 6).
Cryopreservation
PLT aggregation and integrin αIIbβ3 activation
Binding of PAC‐1 in response to PLT agonists decreased from 73% ± 6% before to 15% ± 5% after cryopreservation of AS‐PCT PLTs while it decreased from 92% ± 2% to 26% ± 10% for standard PLTs (Fig. 5A). The difference between AS‐PCT and standard PLTs was significant before and after cryopreservation (p = 0.0015 and p = 0.0208, respectively). PLT aggregation was markedly reduced after cryopreservation and required a combination of strong agonists. There were no significant differences between AS‐PCT and standard PLTs (Fig. 5B).
Figure 5.
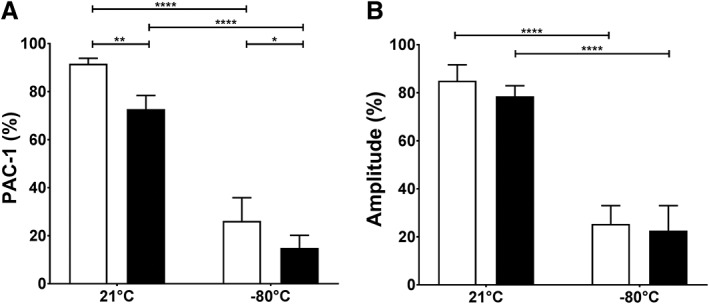
Platelet activation and aggregation of cryopreserved PLTs. (A) The percentage of PLTs expressing activated integrin αIIbβ3 in response to stimulation with 32.5 μmol/L TRAP6 was determined by PAC‐1 binding in flow cytometry. (B) Maximal amplitude (%) of PLT aggregation in response to a mix of epinephrine, MeSADP, and TRAP6 is shown. AS‐PCT PLTs ( ) were compared to standard (
) were compared to standard ( ) PLTs before storage (21°C) or after cryopreservation (−80°C; n = 8).
) PLTs before storage (21°C) or after cryopreservation (−80°C; n = 8).
Hemostatic function in static conditions by integrated thrombin generation
Thrombin generation in static conditions was significantly increased in the presence of cryopreserved PLTs. Output variables are peak thrombin concentrations (Figs. 6A, S3B, available as supporting information in the online version of this paper), lag time (Figs. 6B, S3C), and ETP (Figs. 6C, S3E). AS‐PCT treatment was not significantly different from standard except for a slight increase in peak thrombin (455 ± 37 nmol/L) compared to standard PLTs (419 ± 37 nmol/L) after cryopreservation (Fig. 6A). Representative thrombograms of the condition with 50 × 109 PLTs/L are depicted in Fig. S2B.
Figure 6.
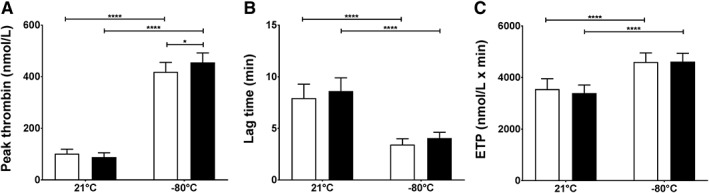
Thrombin generation in static conditions after cryopreservation of PLTs. Thrombin generation was measured in plasma in the presence of TF and 50 × 109 PLTs/L. (A) Peak thrombin (nmol/L), (B) lag time (min), and (C) ETP (nmol/L × min) of AS‐PCT PLTs ( ) were compared to standard (
) were compared to standard ( ) PLTs before storage (21°C) or after cryopreservation (−80°C; n = 8).
) PLTs before storage (21°C) or after cryopreservation (−80°C; n = 8).
DISCUSSION
An increasing number of blood banking institutions worldwide are introducing pathogen inactivation for PCs. The Belgian Red Cross Flanders provides blood products to six million inhabitants, including PCs corresponding to 25,000 PLT transfusions annually. AS‐PCT was introduced in our blood service in 2015. After AS‐PCT implementation the Belgian federal regulator mandated shortening PC shelf life from 7 to 5 days after concerns on efficacy of AS‐PCT–treated PLTs to stop bleeding.21 This was a national decision and may not apply to other countries. The short shelf life is challenging for stock management in routine blood banking and more so in remote areas or isolated communities or during military operations.29 Increasing PC shelf life therefore is an ongoing field of investigation and possible alternatives are cold storage and cryopreservation.
Several studies have demonstrated that AS‐PCT affects PLT function in vitro. Changes in gene transcription,30 metabolism,31 PLT thrombus formation under hydrodynamic flow,22 and signal transduction24 have all been reported. These studies were performed using PLTs stored at RT. Cold storage reduces the proliferation rate of pathogens adding to product safety. However, the combined effect of AS‐PCT and low‐temperature storage has not been investigated until recently.32 It is nonetheless relevant because both manipulations together may intensify or compensate each other's singular effect.33 In addition, pathogen inactivation for PCs is mandatory in a number of European countries including Belgium, France, and Switzerland. Implementation of alternative storage methods in these countries would always have to take pathogen inactivation into account.
Our study shows that cold storage affects PLT reactivity when combined with AS‐PCT as demonstrated by decreased integrin αIIbβ3 activation in response to TRAP6 and decreased aggregation in response to collagen. This was different when investigating coagulation in the presence of PLTs, which decreased significantly after RT storage of AS‐PCT PLTs but not after cold storage. This was observed in hydrodynamic as well as static conditions.
These data suggest that cold storage either slows down storage lesion in AS‐PCT PLTs or actively promotes the biochemistry of procoagulation. The former is unlikely because direct PLT activation assays were showed significantly decreased PLT function. These are archetypical in vitro hallmarks of storage lesion. Therefore, a direct change in PLT biochemistry linked to procoagulation is more likely given that twice the number of cold‐stored AS‐PCT PLTs express PS compared to cold‐stored standard PLTs. Basic PLT biology studies have shown the importance of cell‐based coagulation. This has shifted the concept of coagulation from a mere cascade of proteolytic reactions toward a cell surface–based protein complexation process.34 For instance, in the absence of PLTs or PLT membranes, coagulation rates decrease by three orders of magnitude.35 Essential to this catalytic function of PLTs are aminophospholipids like PS that support the assembly of the tenase and prothrombinase complexes on the lipid bilayer. Although the molecular basis is not entirely clear yet, conformation‐dependent lipid–protein interactions are involved.36 Consequently, the increased expression of PS on cold‐stored AS‐PCT PLTs is a possible explanation for the procoagulant character.
In addition to PS expression, MP formation is another well‐known mechanism of procoagulation. Sinauridze and colleagues37 demonstrated that a surface area of PLT‐derived MP is 50‐ to 100‐fold more procoagulant than the same surface area on activated PLTs. In our study, however, MP formation cannot explain the procoagulant character of cold‐stored AS‐PCT PLTs because significantly less MP are formed in AS‐PCT PLTs than in standard PLTs.
Several observational studies on the use of RT stored AS‐PCT PLTs suggest that these are safe to use.38, 39, 40 Whether cold‐stored AS‐PCT PLTs are safe to use in the clinic needs further study in vivo. We can only speculate on the safety of other procoagulant PLTs like those in cryopreserved PCs.14, 17 Cryopreserved standard PLTs have been used in the Dutch military and a retrospective analysis suggested these are safe.18 More recently, transfusion of cryopreserved standard PLTs in 24 bleeding thrombocytopenic patients in a prospective trial returned no serious adverse events.19
In terms of clinical efficacy of cold‐stored AS‐PCT PLTs, two major questions need to be addressed. One concerns PLT clearance and the other concerns the actual potential to prevent or stop bleeding. Cold storage significantly decreases PLT life span after transfusion2 and AS‐PCT also leads to lower 24‐hour corrected count increments (CCIs),41 which explains the increased number of PLT transfusions per patient42, 43 and which could partly explain the increased refractoriness.42 It is not clear what the combined effect of cold storage and AS‐PCT on CCIs would be. Several preclinical studies in mice have intensively examined PLT clearance mechanism but the mechanism is still incompletely understood.8, 9, 44, 45 In mice PS exposure in cold‐stored PLTs is a determinant of accelerated clearance.46, 47 This may explain the accelerated PLT clearance found in all clinical trials with cold‐stored PLTs.2, 48 Our data show that the combination of AS‐PCT and cold storage increases PS expression so requiring additional in vivo work to study this hypothesis. Another possible regulator of PLT clearance is the GPIbα receptor. Its clustering,49 deglycosylation,9, 50 or ectodomain shedding46 all have been linked to PLT clearance in mice. Our data show that AS‐PCT increases GPIbα shedding in all conditions, although it is 17% less when stored at 4°C compared to RT. This difference may contribute to longer circulation times of cold‐stored AS‐PCT compared to RT‐stored AS‐PCT PLTs but needs additional work in vivo.
Even though PLT CCI is often used as a primary endpoint in trials, it determines PLT circulation kinetics and is therefore a surrogate for transfusion success. PLT transfusions are intended to prevent or stop bleeding. In (acute) trauma, for instance, PLT circulation time may be less important than hemostatic activity, but also in other indications like autologous stem cell transplantation, therapeutic transfusion may be favored over prophylactic51 transfusion, although this may not apply in other indications.52 Cryopreserved PLTs have been suggested to serve exactly that goal as a procoagulant agent with short circulation times. If such PLTs prove to be efficient to stop bleeding, the combination of AS‐PCT and cold storage could constitute a product with very low risk of pathogen transmission in a subset of patients in acute need of treatment.
In summary, this study shows that cold storage of AS‐PCT PLTs affects PLTs in activation and aggregation studies but promotes coagulation in static and flow conditions in vitro. Investigation of PLT circulation times and clinical efficacy are essential before considering implementation of these PLTs into routine blood banking.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Appendix S1: Supplemental materials & methods.
Fig. S1. Thrombin generation in plasma spiked with cold‐stored AS‐PCT platelets (continued from Fig. 3). Thrombin generation was measured in plasma in the presence of TF and 250 × 109 (panels A, C and E) or 10 × 109 PLTs/L (panels B, D and F). Panels (A) and (B) show peak thrombin (nmol/L), panels (C) and (D) show lag time (min) and panels (E) and (F) show ETP (nmol/L × min). Baseline levels were determined on D2. AS‐PCT platelets (closed bars) were compared to standard (open bars) platelets stored until D5 at either RT (21°C) or cold temperature (4°C). Effective storage at cold temperatures was 3 days.
Fig. S2. Representative thrombograms of thrombin generation in plasma A representative thrombogram of thrombin generation measured in plasma in the presence of TF and 50 × 109 PLTs/L. AS‐PCT treated (dashed tracings) were compared to standard (full tracings) platelets. (A) The assay was performed before storage (black) and on D5 after three days of storage at RT (brown) or at 4°C (grey). (B) The assay was performed before storage (black) or after cryopreservation at −80°C (blue).
Fig. S3. Thrombin generation in plasma spiked with cryopreserved AS‐PCT platelets (continued from Fig. 6). Thrombin generation was measured in plasma in the presence of TF and 250 × 109 (panels A, C, and E) or 10 × 109 PLTs/L (panels B, D, and F). Panels (A) and (B) show peak thrombin (nmol/L), panels (C) and (D) show lag time (min) and panels (E) and (F) show ETP (nmol/L x min). AS‐PCT treated platelets (closed bars) were compared to standard (open bars) platelets before storage (21°C) or after cryopreservation (−80°C)
ACKNOWLEDGMENT
The authors thank Michelle Van den Hauwe for experimental support.
This research was supported by the Foundation for Scientific Research of the Belgian Red Cross Flanders. KRS is a fellow of the Special Research Fund of Bijzonder Onderzoeksfonds (BOFDOC2016000401).
Correction added on the 14th June 2019, after first online publication: Y‐axis legend of Figure 6 was adapted.
REFERENCES
- 1. Shrivastava M. The platelet storage lesion. Transfus Apher Sci 2009;41:105‐13. [DOI] [PubMed] [Google Scholar]
- 2. Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability‐‐deleterious effect of refrigerated storage. N Engl J Med 1969;280:1094‐8. [DOI] [PubMed] [Google Scholar]
- 3. Valeri CR, Feingold H, Marchionni LD. A simple method for freezing human platelets using 6 per cent dimethylsulfoxide and storage at −80 degrees C. Blood 1974;43:131‐6. [PubMed] [Google Scholar]
- 4. Lazarus HM, Kaniecki‐Green EA, Warm SE, et al. Therapeutic effectiveness of frozen platelet concentrates for transfusion. Blood 1981;57:243‐9. [PubMed] [Google Scholar]
- 5. Johnson L, Tan S, Wood B, et al. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: a comparison with conventional platelet storage conditions. Transfusion 2016;56:1807‐18. [DOI] [PubMed] [Google Scholar]
- 6. Reddoch KM, Pidcoke HF, Montgomery RK, et al. Hemostatic function of apheresis platelets stored at 4 degrees C and 22 degrees C. Shock 2014;41:54‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montgomery RK, Reddoch KM, Evani SJ, et al. Enhanced shear‐induced platelet aggregation due to low‐temperature storage. Transfusion 2013;53:1520‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmeister KM, Felbinger TW, Falet H, et al. The clearance mechanism of chilled blood platelets. Cell 2003;112:87‐97. [DOI] [PubMed] [Google Scholar]
- 9. Jansen AJ, Josefsson EC, Rumjantseva V, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase‐mediated cleavage in mice. Blood 2012;119:1263‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milford EM, Reade MC. Comprehensive review of platelet storage methods for use in the treatment of active hemorrhage. Transfusion 2016;56:S140‐S8. [DOI] [PubMed] [Google Scholar]
- 11. Valeri CR, Ragno G, Khuri S. Freezing human platelets with 6 percent dimethyl sulfoxide with removal of the supernatant solution before freezing and storage at −80 degrees C without postthaw processing. Transfusion 2005;45:1890‐8. [DOI] [PubMed] [Google Scholar]
- 12. Valeri CR, Giorgio A, Macgregor H, et al. Circulation and distribution of autotransfused fresh, liquid‐preserved and cryopreserved baboon platelets. Vox Sang 2002;83:347‐51. [DOI] [PubMed] [Google Scholar]
- 13. Waters L, Padula MP, Marks DC, et al. Cryopreserved platelets demonstrate reduced activation responses and impaired signaling after agonist stimulation. Transfusion 2017;57:2845‐57. [DOI] [PubMed] [Google Scholar]
- 14. Six KR, Delabie W, Devreese KMJ, et al. Comparison between manufacturing sites shows differential adhesion, activation, and GPIbalpha expression of cryopreserved platelets. Transfusion 2018;58:2645‐56. [DOI] [PubMed] [Google Scholar]
- 15. Barnard MR, MacGregor H, Ragno G, et al. Fresh, liquid‐preserved, and cryopreserved platelets: adhesive surface receptors and membrane procoagulant activity. Transfusion 1999;39:880‐8. [DOI] [PubMed] [Google Scholar]
- 16. Cid J, Escolar G, Galan A, et al. In vitro evaluation of the hemostatic effectiveness of cryopreserved platelets. Transfusion 2016;56:580‐6. [DOI] [PubMed] [Google Scholar]
- 17. Johnson L, Coorey CP, Marks DC. The hemostatic activity of cryopreserved platelets is mediated by phosphatidylserine‐expressing platelets and platelet microparticles. Transfusion 2014;54:1917‐26. [DOI] [PubMed] [Google Scholar]
- 18. Noorman F, van Dongen TT, Plat MJ, et al. Transfusion: −80 degrees C frozen blood products are safe and effective in military casualty care. PLoS One 2016;11:e0168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slichter SJ, Dumont LJ, Cancelas JA, et al. Safety and efficacy of cryopreserved platelets in bleeding patients with thrombocytopenia. Transfusion 2018;58:2129‐38. [DOI] [PubMed] [Google Scholar]
- 20. Garban F, Guyard A, Labussiere H, et al. Comparison of the hemostatic efficacy of pathogen‐reduced platelets vs untreated platelets in patients with thrombocytopenia and malignant hematologic diseases: a randomized clinical trial. JAMA Oncol 2018;4:468‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feys HB, Van Aelst B, Compernolle V. Biomolecular consequences of platelet pathogen inactivation methods. Transfus Med Rev 2019;33:29‐34. [DOI] [PubMed] [Google Scholar]
- 22. Van Aelst B, Feys HB, Devloo R, et al. Riboflavin and amotosalen photochemical treatments of platelet concentrates reduce thrombus formation kinetics in vitro. Vox Sang 2015;108:328‐39. [DOI] [PubMed] [Google Scholar]
- 23. Van Aelst B, Devloo R, Vandekerckhove P, et al. Ultraviolet C light pathogen inactivation treatment of platelet concentrates preserves integrin activation but affects thrombus formation kinetics on collagen in vitro. Transfusion 2015;55:2404‐14. [DOI] [PubMed] [Google Scholar]
- 24. Van Aelst B, Devloo R, Zachee P, et al. Psoralen and ultraviolet A light treatment directly affects phosphatidylinositol 3‐kinase signal transduction by altering plasma membrane packing. J Biol Chem 2016;291:24364‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dumont LJ, Cancelas JA, Dumont DF, et al. A randomized controlled trial evaluating recovery and survival of 6% dimethyl sulfoxide‐frozen autologous platelets in healthy volunteers. Transfusion 2013;53:128‐37. [DOI] [PubMed] [Google Scholar]
- 26. Johnson L, Reade MC, Hyland RA, et al. In vitro comparison of cryopreserved and liquid platelets: potential clinical implications. Transfusion 2015;55:838‐47. [DOI] [PubMed] [Google Scholar]
- 27. Six KR, Devloo R, Van Aelst B, et al. A microfluidic flow chamber model for platelet transfusion and hemostasis measures platelet deposition and fibrin formation in real‐time. J Vis Exp 2017;120:e55351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hemker HC, Kremers R. Data management in thrombin generation. Thromb Res 2013;131:3‐11. [DOI] [PubMed] [Google Scholar]
- 29. Lelkens CC, Koning JG, de Kort B, et al. Experiences with frozen blood products in The Netherlands military. Transfus Apher Sci 2006;34:289‐98. [DOI] [PubMed] [Google Scholar]
- 30. Osman A, Hitzler WE, Ameur A, et al. Differential expression analysis by RNA‐Seq reveals perturbations in the platelet mRNA transcriptome triggered by pathogen reduction systems. PLoS One 2015;10:e0133070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abonnenc M, Sonego G, Kaiser‐Guignard J, et al. In vitro evaluation of pathogen‐inactivated buffy coat‐derived platelet concentrates during storage: psoralen‐based photochemical treatment step‐by‐step. Blood Transfus 2015;13:255‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meinke S, Wikman A, Gryfelt G, et al. Cryopreservation of buffy coat‐derived platelet concentrates photochemically treated with amotosalen and UVA light. Transfusion 2018;58:2657‐68. [DOI] [PubMed] [Google Scholar]
- 33. Waters L, Cameron M, Padula MP, et al. Refrigeration, cryopreservation and pathogen inactivation: an updated perspective on platelet storage conditions. Vox Sang 2018;113:317‐28. [DOI] [PubMed] [Google Scholar]
- 34. Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol 2006;26:41‐8. [DOI] [PubMed] [Google Scholar]
- 35. Miletich JP, Jackson CM, Majerus PW. Interaction of coagulation factor Xa with human platelets. Proc Natl Acad Sci U S A 1977;74:4033‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Comfurius P, Smeets EF, Willems GM, et al. Assembly of the prothrombinase complex on lipid vesicles depends on the stereochemical configuration of the polar headgroup of phosphatidylserine. Biochemistry 1994;33:10319‐24. [DOI] [PubMed] [Google Scholar]
- 37. Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50‐ to 100‐fold higher specific procoagulant activity than activated platelets. Thromb Haemost 2007;97:425‐34. [PubMed] [Google Scholar]
- 38. Amato M, Schennach H, Astl M, et al. Impact of platelet pathogen inactivation on blood component utilization and patient safety in a large Austrian Regional Medical Centre. Vox Sang 2017;112:47‐55. [DOI] [PubMed] [Google Scholar]
- 39. Nussbaumer W, Amato M, Schennach H, et al. Patient outcomes and amotosalen/UVA‐treated platelet utilization in massively transfused patients. Vox Sang 2017;112:249‐56. [DOI] [PubMed] [Google Scholar]
- 40. Corash L, Benjamin RJ. The role of hemovigilance and postmarketing studies when introducing innovation into transfusion medicine practice: the amotosalen‐ultraviolet A pathogen reduction treatment model. Transfusion 2016;56:S29‐38. [DOI] [PubMed] [Google Scholar]
- 41. Estcourt LJ, Malouf R, Hopewell S, et al. Pathogen‐reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev 2017;7:CD009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Butler C, Doree C, Estcourt LJ, et al. Pathogen‐reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev 2013;3:CD009072. [DOI] [PubMed] [Google Scholar]
- 43. Estcourt LJ, Stanworth SJ, Murphy MF. Different platelet count thresholds to guide use of prophylactic platelet transfusions for patients with hematological disorders after myelosuppressive chemotherapy or stem cell transplantation. JAMA Oncol 2016;2:1091‐2. [DOI] [PubMed] [Google Scholar]
- 44. Chen W, Druzak SA, Wang Y, et al. Refrigeration‐induced binding of von willebrand factor facilitates fast clearance of refrigerated platelets. Arterioscler Thromb Vasc Biol 2017;37:2271‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bergmeier W, Burger PC, Piffath CL, et al. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro‐aged or ‐injured mouse platelets. Blood 2003;102:4229‐35. [DOI] [PubMed] [Google Scholar]
- 46. Quach ME, Chen W, Li R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018;131:1512‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med 2009;15:1273‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vostal JG, Gelderman MP, Skripchenko A, et al. Temperature cycling during platelet cold storage improves in vivo recovery and survival in healthy volunteers. Transfusion 2018;58:25‐33. [DOI] [PubMed] [Google Scholar]
- 49. Yan R, Chen M, Ma N, et al. Glycoprotein Ibalpha clustering induces macrophage‐mediated platelet clearance in the liver. Thromb Haemost 2015;113:107‐17. [DOI] [PubMed] [Google Scholar]
- 50. Josefsson EC, Gebhard HH, Stossel TP, et al. The macrophage alphaMbeta2 integrin alphaM lectin domain mediates the phagocytosis of chilled platelets. J Biol Chem 2005;280:18025‐32. [DOI] [PubMed] [Google Scholar]
- 51. Wandt H, Schaefer‐Eckart K, Wendelin K, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open‐label, multicentre, randomised study. Lancet 2012;380:1309‐16. [DOI] [PubMed] [Google Scholar]
- 52. Stanworth SJ, Estcourt LJ, Powter G, et al. A no‐prophylaxis platelet‐transfusion strategy for hematologic cancers. N Engl J Med 2013;368:1771‐80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplemental materials & methods.
Fig. S1. Thrombin generation in plasma spiked with cold‐stored AS‐PCT platelets (continued from Fig. 3). Thrombin generation was measured in plasma in the presence of TF and 250 × 109 (panels A, C and E) or 10 × 109 PLTs/L (panels B, D and F). Panels (A) and (B) show peak thrombin (nmol/L), panels (C) and (D) show lag time (min) and panels (E) and (F) show ETP (nmol/L × min). Baseline levels were determined on D2. AS‐PCT platelets (closed bars) were compared to standard (open bars) platelets stored until D5 at either RT (21°C) or cold temperature (4°C). Effective storage at cold temperatures was 3 days.
Fig. S2. Representative thrombograms of thrombin generation in plasma A representative thrombogram of thrombin generation measured in plasma in the presence of TF and 50 × 109 PLTs/L. AS‐PCT treated (dashed tracings) were compared to standard (full tracings) platelets. (A) The assay was performed before storage (black) and on D5 after three days of storage at RT (brown) or at 4°C (grey). (B) The assay was performed before storage (black) or after cryopreservation at −80°C (blue).
Fig. S3. Thrombin generation in plasma spiked with cryopreserved AS‐PCT platelets (continued from Fig. 6). Thrombin generation was measured in plasma in the presence of TF and 250 × 109 (panels A, C, and E) or 10 × 109 PLTs/L (panels B, D, and F). Panels (A) and (B) show peak thrombin (nmol/L), panels (C) and (D) show lag time (min) and panels (E) and (F) show ETP (nmol/L x min). AS‐PCT treated platelets (closed bars) were compared to standard (open bars) platelets before storage (21°C) or after cryopreservation (−80°C)


