Abstract
Industrial sensitizing agents (allergens) in living and working environments play an important role in eliciting type 1 allergic disorders including asthma and allergic rhinitis. Successful management of allergic diseases necessitates identifying their specific causes (ie, identify the causative agent(s) and the route of contact to allergen: airborne, or skin contact) to avoid further exposure. Identification of sensitization by a sensitive and validated measurement of specific IgE is an important step in the diagnosis. However, only a limited number of environmental and occupational allergens are available on the market for use in sIgE testing. Accordingly, specific in‐house testing by individual diagnostic and laboratory centers is often required. Currently, different immunological tests are in use at various diagnostic centers that often produce considerably divergent results, mostly due to lack of standardized allergen preparation and standardized procedures as well as inadequate quality control. Our review and meta‐analysis exhibited satisfactory performance of sIgE detection test for most high molecular weight (HMW) allergens with a pooled sensitivity of 0.74 and specificity of 0.71. However, for low molecular weight (LMW) allergens, pooled sensitivity is generally lower (0.28) and specificity higher (0.89) than for HMW tests. Major recommendations based on the presented data include diagnostic use of sIgE to HMW allergens. A negative sIgE result for LMW agents does not exclude sensitization. In addition, the requirements for full transparency of the content of allergen preparations with details on standardization and quality control are underlined. Development of standard operating procedures for in‐house sIgE assays, and clinical validation, centralized quality control and audits are emphasized. There is also a need for specialized laboratories to provide a custom service for the development of tests for the measurement of putative novel occupational allergens that are not commercially available.
Keywords: asthma, IgE, occupational allergies, rhinitis, urticaria
Abbreviations
- HMW
high molecular weight
- LMW
low molecular weight
- OA
occupational asthma
- OR
occupational rhinitis
- RADS
reactive airways dysfunction syndrome
- RUDS
reactive upper airwas dysfunction syndrome
- SIC
specific inhalation challenge
- sIgE
specific IgE
- WRA
work‐related asthma
1. BACKGROUND AND OBJECTIVES
Airborne allergens in living and working environments play an important role in eliciting type 1 allergic disorders, including asthma and hay fever. Allergies belong to the so‐called noncommunicable diseases which have dramatically risen with industrialization and urbanization worldwide.1, 2 Nearly 600 asthma‐causing occupational agents have been identified, of which two thirds are airway sensitizers.3 Every year new allergic agents are identified at changing industrial or agricultural worksites, for example.4, 5 Some of these agents (chemical/biological) are also general environmental pollutants which can be passively transported by air (sometimes over long distances), and often found in homes. Other industrial sensitizers are used by consumers, for example, genetically engineered enzymes in home detergents, perfume sprays or used for food processing in home kitchens. All these substances contribute to a long list of new sensitizing agents of relevance for both occupational and general environmental medicine and public health.
Asthma, the disorder this guideline focuses on represents an increasing global health problem and is now affecting between 8%‐10% of the population.6 The World Health Organization estimates that around 235 million people suffer from asthma worldwide.7 This implicates a high socio‐economic cost in terms of work and school absenteeism, consumption of resources (drugs, consultations, hospitalizations), and deaths.8 The increase in asthma figures is partly due to an improvement in diagnostic techniques, but other factors such as environmental pollution (Figure 1) are also relevant in industrialized countries.9
Figure 1.
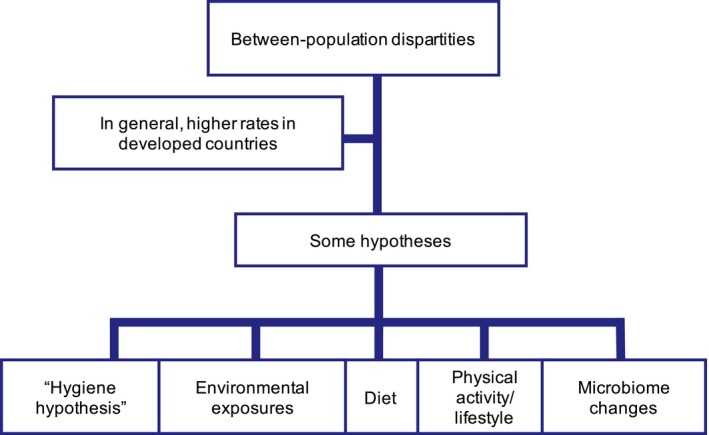
Asthma between population discrepancies. Figure adapted from: ISAAC (1998), Lancet 351:1225‐32
Environmental pollution is a growing public health problem although the exact mechanisms by which it can aggravate asthma are presently not known. Also less exposure to microbial load and diversity in both childhood and adulthood due to the global increase in urbanization has been suggested to contribute to the increase in asthma.10 Rhinitis is approximately 3 times more prevalent than asthma in the adult population; although symptoms and impairment are generally less life threatening than those in asthma, this disorder also represents a highly relevant socio‐economic issue. It is known that there exists a relationship between asthma and rhinitis as well as with respiratory infections.
Current research is aimed at establishing the immunological pathways that determine, not only the phenotypes and endotypes of these diseases,11, 12 but also the presence of certain biomarkers that allow precision medicine to be performed.13, 14 Although there is agreement to distinguish three possible immune responses in the development of respiratory allergies, such as the type 2 immune response, the non–type 2 immune response, and the mixed Th2/Th17 immune response, the intimate mechanisms that generate them are still largely unknown. Most of the aforementioned aspects also refer to the less frequent type 1 allergic dermatitis, such as contact urticaria including protein contact urticaria which have to be differentiated from type 4 allergic contact dermatitis, not covered by this guideline.
The diagnostic measurement of specific IgE (sIgE) is often a major step in identifying the precise cause(s) of respiratory allergy. However, only a limited number of allergen‐sIgE are commercially available, especially in the occupational field. This necessitates the development and use of in‐house tests in specialized laboratories. A further limitation of sIgE measurement is the lack of validated preparations for many allergenic agents. Currently, different immunological tests are available on the market and are in use. The manufacturers of such tests, rarely, if at all, provide detailed data on the allergen preparations used, standardization of the method, and quality controls applied. The various tests often differ considerably and their results are hard to compare. So, a major current problem is lack of transparency in commercial sIgE tests and a lack of generally accepted and applied procedures to assess whether the sensitivity and specificity of these tests are appropriate.
There is an urgent need for an international consensus on technical standards and quality control for allergen preparation to serve as an important resource for physicians and laboratories in diagnostics of exposure‐related type 1 respiratory allergic diseases. This document represents international practical statements with the primary objective to provide an overview on available methods required for state‐of‐the‐art clinical diagnostic purposes and for making recommendations for clinical practice. This document focuses on diagnosis of respiratory allergic disorders caused by occupational and/or environmental industrial agents, but may also be applied to related skin allergic disorders. The recommendations include exposure assessment and in‐house in vitro testing with commercially and noncommercially available allergens. A further aim is to present current evidence on sIgE testing in the diagnostics of type 1 allergic disorders with special regard to allergic work‐related asthma (WRA) including occupational asthma (OA, where nonoccupational agents do not play a causative role) and allergic work‐aggravated (also called allergic work‐exaggerated or work‐exacerbated) asthma. Recommendations for clinical practice are formulated. This practical statement is intended to provide guidance to clinicians, public health professionals as well as others who interpret the scientific evidence, when new outcomes are reported (eg, policy makers).
Methodological details of this practical guideline are given in the Supporting information (see Supplementary Methods, page S2).
2. CLINICAL PICTURES OF TYPE I ALLERGY DUE TO AIRBORNE OR SKIN EXPOSURES
Type 1 allergy due to airborne or skin exposures is defined clinically as an immediate allergic response caused by a product or raw material (eg, flour) that is present in the living or working environments. For occupational allergy, the agent should be specific to the workplace. Such allergies can affect many target organs, including lower airways, nose, eyes, and skin. The corresponding diseases are allergic asthma, allergic rhinitis, allergic conjunctivitis, allergic contact urticaria/protein contact dermatitis (details are given in the Supporting information, clinical pictures).
There is evidence that type 1 allergies caused by industrial allergens (by either a product or a raw material) are frequently not documented in the literature. Since these cases are left either unrecognized or are not published, the number of causative agents may be higher than that currently found in the list of sensitizers. See example in Supporting information.
Industrial products can also cause allergic reactions in consumers (see Allergen exposure assessment section) but these cases are rather seldom described and presumably rare. Ingredients such as enzymes in fragrances, cosmetic products, detergents, or food (the latter includes processing at home) are some of the examples.4, 15, 16
3. CAUSAL AGENTS
Agents that cause allergic WRA and/or allergic occupational rhinitis encompass more than 400 natural agents and synthetic chemicals. They are listed in textbooks,17 review articles3, 18 and websites (eg, http://www.eaaci.org; http://www.csst.qc.ca/en/prevention/reptox/occupational-asthma ; http://www.aoecdata.org/ExpCodeLookup.aspx).
Individual publications on allergens causing occupational asthma are listed in the Supporting information (see Table S1).
These agents are categorized into high molecular weight (HMW) and low molecular weight (LMW; <5 kDa) agents. High molecular weight agents are proteins of animal, vegetable, or microbial origin acting through an IgE‐mediated mechanism. Low molecular weight agents include organic and inorganic compounds that, may function as haptens and with a few exceptions, are associated with an IgE mechanism in a proportion of affected subjects. Commonly identified agents of OA characterized by a latency period between initial exposure and asthmatic symptoms are diisocyanates, flour, allergens from laboratory animals and insects, enzymes, colophony fluxes, solders, wood dusts, natural rubber latex, acrylates, and glutaraldehyde. Following sensitization, workers with OA may develop an asthmatic attack to very low exposures to the sensitizer. The extent of airway responsiveness may diminish away from exposure, but usually increases with re‐exposure to the sensitizer.
4. DIAGNOSTIC MEASUREMENT OF sIgE AS THE MAJOR STEP IN IDENTIFYING THE PRECISE CAUSE OF RESPIRATORY ALLERGY
The practical division into HMW and those of LMW allergens is based on the fact that LMW substances cannot induce an IgE‐mediated response by themselves; rather they act as incomplete antigens (or haptens), either by binding to host proteins or as reactive agents forming new antigenic sites upon reaction with host molecules.19, 20 High molecular weight allergens are generally polypeptides, proteins, or glycoproteins from animals, plants, bacteria, or fungi, and often have a molecular weight between 20 and 50 kDa. Low molecular weight agents are usually chemical substances with mass <5 kDa.
The distinction between HMW and LMW allergens is important when performing an immunological investigation. It is relatively easy to demonstrate an immune response mediated by IgE in cases of HMW agents and more difficult for most LMW agents. This fact has important repercussions in terms of commercially available tests.
The presence of allergen‐sIgE can be investigated by in vivo skin tests or by in vitro laboratory tests. The procedures are briefly discussed in the Supporting information (see Diagnostic measurement, page S11).
Currently, laboratory methods for the detection of serum antibodies are routine. The most commonly applied techniques are the quantification of total and allergen‐specific serum IgE (as a classical biomarker for a given allergen). These procedures have in spite of some limitation a number of advantages over the skin tests because there is no risk to the patient, the results are not influenced by drugs and are more diagnostic for certain groups of patients such as patients with dermographism or atopic dermatitis.
Reaginic antibodies were identified in the 1960s as belonging to a new immunoglobulin class, namely IgE21 and shortly afterward the radioallergosorbent test (RAST) was developed.22 The modern sIgE assay is a modification of the RAST assay; using the same basic three‐steps, solid phase, noncompetitive binding, immune‐metric (labeled antibody) chemistry, combined with a third step, that is, enzyme‐substrate color, florescence, or luminescence development. Allergen‐sIgE antibodies are bound in the first incubation to a solid phase allergen and then bound IgE is detected in a second reaction with labeled anti‐human IgE. The magnitude of the response after the final buffer wash is proportional to the quantity of allergen‐sIgE antibody in the original test serum. A multipoint total IgE serum calibration curve is used that ranges from 0.1 to 100 kUA/l traceable to the World Health Organization human IgE reference preparations (note WHO recently depleted 75/502 preparation is replaced with third standard: 11/234). This allows interpolation of IgE antibody results from any of the hundreds of allergen specificities as long as the allergen‐sIgE portions of the assay dilute out in parallel with each other. Recently, an international guidance document has been published on the analytical performance and clinical utility of human IgE.23 See also Supporting information (page S18).
Currently, there are three singleplex auto analyzers that dominate the clinical market for the measurement of sIgE.23 These are ImmunoCAP (Thermo Fisher Scientific/ Phadia), Immulite (Siemens Healthcare Diagnostics) and HyTEC88 (Hycor Biomedical, which is being replaced with Falcon). Other IgE assays with a limited market share include Thabest IgE (Visual Diagnostics), Optigen (Hitachi Chemical Diagnostics), and ALLERG‐O‐LIQ (Fooke laboratories).
Each of the principal auto analyzers has comparable performance parameters, which have to be described and cleared by regulatory agencies. These include excellent intra‐assay precision (typically < 15% coefficient of variance), inter‐assay reproducibility (generally < 20% coefficient of variance), a limit of quantification of 0.1 kUA/l and a reportable assay range of 0.1‐100 kUA/l.23
Studies have compared the three main sIgE assays and all reported different levels of IgE antibody for any given specificity.24, 25, 26 This indicates that it is not currently possible to compare sIgE levels measured using different sIgE assays. This is probably due to the allergen used in the assay, the orientation and structural modifications of the allergen, and differing assay protocols including the solid phase material, slightly different assay calibration, and data computation procedures.27
Comparing the analytical precision and accuracy of sIgE assays, performed in different laboratories using various methods, despite standardization to the WHO human IgE reference preparation, revealed that there were disparate results in four of the six laboratories.28 This highlights the need for laboratories to participate in quality assurance schemes (eg, CLIA, ISO 17025) and to make those results available to their clients.
Historically, 0.35 kUA/l of allergen‐sIgE was considered to be the cutoff point where an individual was considered as a threshold for positivity of the immunoassay, demonstrating sensitization.29 The current recommendation to clinical laboratories, by a consensus guidance document,23 is that sIgE levels must be reported as analytical measurements down to the cleared limit of quantitation of the assays, which is usually 0.1 kUA/l. The limit of quantitation within an assay is set to ensure that reported low quantitative results are comfortably above background signal (noise—chemical, instrument and from non–specific IgE binding). Thus, all test results above the limit of quantitation should be regarded as true allergen‐sIgE antibodies. Historically, sIgE ≥ 0.35 kUA/l was considered to be clinically relevant, however, it is now considered that clinicians should interpret IgE levels in the context of the clinical history, rather than have a defined cutoff point.29 See Supporting information (page S18) for more details.
It is important to understand that the presence of sIgE is an indicator of sensitization, it is not proof of allergic disease and is only clinically relevant when there are objectively defined allergic symptoms that are temporally associated with a known allergen exposure.27 A positive IgE test that is concordant with the patient's history and/or a challenge test confirms “allergy” to a particular allergen and can be used as confirmatory evidence of sensitization to manage the patient appropriately and encourage improvement of environmental control measures within the workplace.
4.1. What is commercially available and suitable?
New allergens are continually being identified within the workplace, for example, horticultural nematode, Steinernema feltiae.30 The list of commercially available occupational allergens is very restricted, for example, Thermo Fisher (Phadia) currently has only 36 ImmunoCAPs for occupational allergens, although they do also provide others such as animal allergens, for example, rat and mouse. The limited availability of commercial assays for the measurement of sIgE is a significant problem within the field of occupational allergy and asthma that necessitates the use of “in‐house” assays to measure sIgE to noncommercially available allergens.
There are many allergens and some components available for CAP tests; however, there are only a limited number of occupational HMW allergen components, for ImmunoCAP, for example, there are nine recombinant latex allergens. Availability of allergen components has led to improved diagnosis of sensitization in some cases.31, 32, 33, 34
4.2. Occupational allergens are categorized as either high or low molecular weight
sIgE assays are useful to demonstrate IgE‐mediated sensitization to HMW allergens (animal and plant proteins) but less so with LMW allergens, which are predominantly synthetic chemicals. Low molecular weight allergens are too small to be recognized by the immune system without conjugation to a protein, for example, human serum albumin (Table 1; Figure 2; Figure 3).
Table 1.
Statements
| Diagnostics |
| The occupational type 1 allergy of greatest concern is occupational asthma; exposure‐related type 1 allergic rhinitis, conjunctivitis, and protein contact dermatitis also play a role |
| The diagnoses of occupational type 1 respiratory allergies follows an algorithm approach, starting with clinical and qualified occupational history, followed by confirmation of the disease with objective methods and allergy testing (SPT test or sIgE measurement), and, if needed, spirometry monitoring during work shifts or specific nasal challenge or SIC |
| Commercial sIgE tests generally lack transparent information on the allergen preparation used, applied standardization and quality control |
| In‐house tests mostly do not follow appropriate standardization and quality control; they differ from place to place considerably and do not allow comparison of the results definitively |
| There are limited commercial occupational allergens available on CAP. This necessitates specialized laboratories to provide bespoke “in‐house” assays for novel putative and allergens which are not available on CAP |
| Negative results from a bespoke in‐house assay for putative novel allergens do not necessarily imply a negative test, if there are no positive controls. sIgE tests are only as good as the composition of the allergens used in the assay. Thus, we need to ensure that for any sIgE measurement, we use the appropriate allergens |
| The laboratory should always assess the performance of sIgE assay by carrying out a clinical audit. The performance of any sIgE test, whether it is RAST or CAP, is very dependent on allergen used and the positive cut off value |
| CAP, RAST, and other methods for measurement of sIgE can give similar sensitivity to skin prick test when the same allergen is used for both test, for example, 95% for protease and cellulase, 98% for amylase, 99% rat urine |
| sIgE to HMW allergens provide acceptable sensitivity and specificity and are very useful as a diagnostic test |
| Specific IgE assays to LMW allergens are more problematic and dependent on the allergen investigated. sIgE measurement to isocyanates is specific but not sensitive, whereas sIgE to acid anhydride could have acceptable sensitivity and specificity, which needs to be assessed with larger cohorts. To date, we are unable to measure sIgE for platinum salts. Thus each low molecular weight allergen must be considered case by case |
| By use of sIgE tests HMW allergens provide acceptable sensitivity and specificity mostly above 0.7 kUA/l. This is especially shown for extracts from cereals, latex, enzymes, bovine epithelium and bovine dander, molds, insects, and for particular approaches with recombinant allergen components |
| Some wood extracts and LMW agents such as diisocyanates, acid anhydrides provide much lower sensitivity than confirmed HMW allergens; obviously, this is at least in part due to heterogenous patho‐mechanisms including irritant effects |
| Prevention |
| Early diagnosis of respiratory allergies combined with avoidance of the causative allergen is important because it prevents the allergy march from rhinitis to asthma, as well as chronification and deterioration of the disorders |
| Exposure assessment |
| Standard chemical air sampling and analysis methods exist for many of the low molecular weight allergens, however, in many cases exposure to these electrophilic chemicals is not just to the monomeric form. Exposure can be to a mixture of monomers, polymers and prepolymeric forms, but analytical methods are mainly only for monitoring the monomeric forms |
| Exposure assessment for characterizing levels leading to immunological sensitization (vs. asthma elicitation) is extremely difficult and in general lacking. This includes both respiratory and dermal sensitizing events |
| Biomarkers of exposure have been reported (especially for LMW allergens) in the literature, (eg, allergen metabolites or adducts), but they have not always been used as an exposure monitoring tool |
| Exposure monitoring for high molecular weight allergens may entail measurement of multiple allergenic proteins, especially from natural products. The specific aeroallergen(s) responsible for disease may vary with the life cycle of that product |
| Quantitative dermal exposure assessment methods are lacking, which hinders the assessment of the level of dermal sensitization in subsequent asthma development |
| Early biomarkers of allergic sensitization, in addition to specific IgE are needed to prevent subsequent asthma development |
| Direct reading instruments with sufficient sensitivity are needed to monitor relevant worker exposure to agents known to cause occupational asthma |
Figure 2.
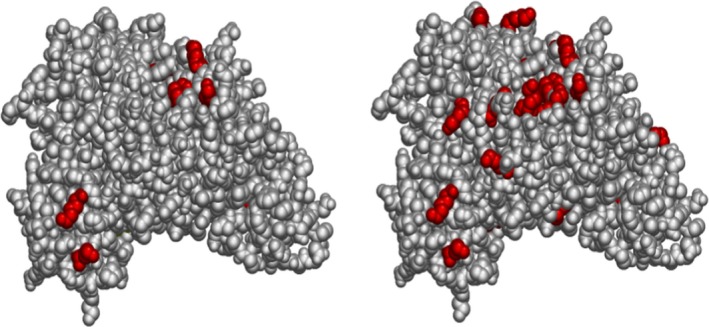
Chemical haptenation of albumin upon dermal exposure to diisocyanate (from Hettick and Spiegel, International Journal of Mass Spectrometry 309, 168‐75, 2017) . The figure displays diisocyanate haptenation sites on human serum albumin (left = MDI, right = TDI). Electrophilic chemical allergens can bind to multiple nucleophilic sites on self‐proteins. Diisocyanates can also self‐polymerize, thus multiple potential neo‐antigens may be produced following chemical exposure. Left: serum albumin Lys residues haptenated by MDI. Right: serum albumin Lys residues haptenated by TDI. Serum albumin and skin keratins have been identified by mass spectrometry as targets of haptenation upon dermal exposure. Which residues are haptenated depends on: Identity of the isocyanate (electrophilic reactivity and size); Accessibility of the site (sterics); Chemical composition of the residue (primary amines); pH of the microenvironment and/or pKa of the sidechain (‐NH2 reactive, not); Concentration of the isocyanate (less kinetically favorable observerd at higher concentration). “Dilysine” motifs are preferred conjugation sites in vitro and in vivo: Lys413‐Lys414 and Lys524‐525 of serum albumin are conjugated in skin and lung of mouse model; These sequence motifs are conserved in humans; Enhanced reactivity most likely due to suppressed pKa of second Lys residue. For more information, see: Hettick, J et al Xenobiotica. 2017 Jul 21:1‐11; Nayak, A et al Toxicol Sci. 2014 Aug 1;140(2):327‐37; Hettick, J et al Anal Biochem. 2012 Feb 15;421(2):706‐11; Wisnewski, A et al Anal Biochem. 2010 May 15;400(2):251‐8
Figure 3.
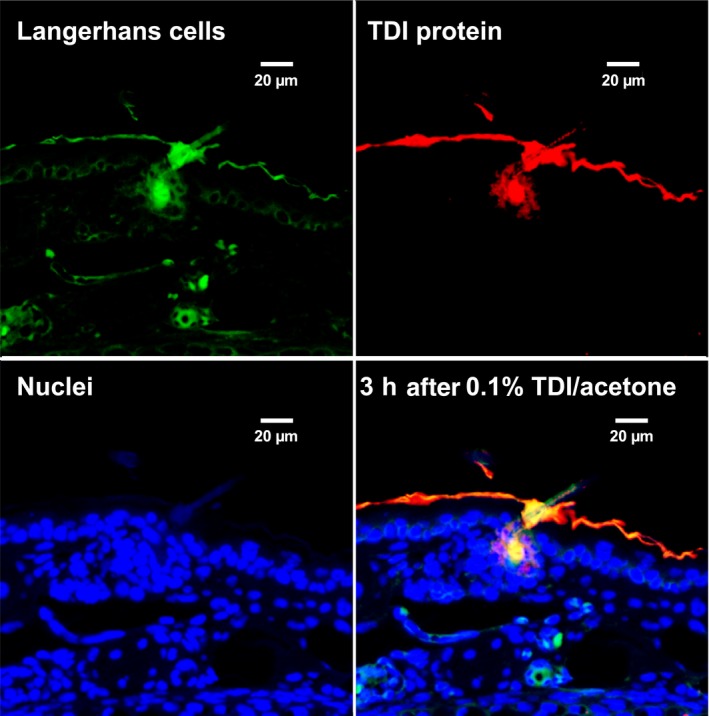
Immunochemical co‐localization in mouse skin of 2,4‐TDI haptenated proteins (albumin, and cuticular and cytoskeletal keratins) along with antigen presenting cells (from: Nayak et al Tox Sci: 140(2) 327‐337, 2014). Dermal LMW chemical sensitization is often used with subsequent respiratory challenge to model LMW chemical asthma. TDI was observed to rapidly haptenate dermal proteins, especially in the outer root sheath of the hair follicle, and recruit antigen‐presenting cells (CD11b APCs, CD207 Langerhans cells and CD103+CD207+ Langerhans cells) with subsequent transport to local draining lymph nodes. Confocal microscopic images of Langerhans cells (top left), TDI haptenated tissue (top right), cell nuclei (bottom left) and overlay of Langerhans cells, nuclei, and TDI haptenated tissue (bottom right)
There is a wide spectrum in the ability to detect sIgE for LMW allergens ranging from the possible exception of acid anhydrides with relatively high detection rates (sensitivity 81%) to isocyanates (sensitivity 21%; specificity 94%) and plicatic acid (sensitivity 9.6%).33
Recent studies highlighted the difference in sIgE binding according to the conjugate preparation conditions.35 , 36 One of the difficulties with measuring sIgE to LMW allergens (including products acting as haptens37, 38) is that there are no standardized protocols for the preparation of protein‐LMW allergen conjugates and, characterization of the resultant protein hapten complex can be technically challenging and complex. There were various approaches to bind LMW agents such as acid anhydrides,7, 39 glutaraldehyde,40 or isocyanates (for more details see Table S2). The diisocyanates are emblematic of difficulties of elucidating underlying antigenic forms of diisocyanate haptenated proteins. Albumin is the preferred protein carrier, but multiple proteins are potentially haptenated in vivo (Figure 2). Diisocyanates have been reported to covalently bind to albumin, hemoglobin, lung epithelial proteins, tubulin, and keratins.41, 42, 43, 44 Proteomic mass spectrometric studies of in vitro conjugation of diisocyanate to albumin and hemoglobin have demonstrated that diisocyanates can form a variety of complexes with proteins including inter‐ and intramolecular cross‐linked species (see Figure 3).
There has been a debate as to whether the low sensitivity of sIgE assay to diisocyanates may in part be due to heterogeneous patho‐mechanisms. In a small study of patients with a clinical diagnosis of occupational asthma based on a positive bronchial challenge, there was a striking absence of IL‐4 and Cε mRNA observed locally within bronchial mucosa following an active challenge, which is in contrast to patients with asthma to high molecular weight allergens such as grass pollen.45 This suggests that in some patients, isocyanate induced asthma may be a non‐IgE‐mediated disease. Confirming this observation in serum of isocyanate patients with occupational asthma is not feasible due to lack of a reliable validated IL‐4 assay.46 Studies also suggest that sIgE against plicatic acid‐human serum albumin‐conjugate is unlikely to be a causative factor in the patho‐mechanism of western red cedar asthma, suggesting that this may also be a non‐IgE‐mediated disease, at least in some patients. Animal models of chemical‐induced asthma also suggest that IgE mechanisms are not necessary to elicit respiratory reactions in previously dermally exposed animals.47, 48 Finding sIgE to LMW allergens is confirmatory of sensitization, but a negative sIgE does not rule out that agent as the cause of a WRA.
4.3. In‐house sIgE tests
In‐house assays are required, if no commercial tests, are available (ie, in case of a new allergenic protein). In many cases, commercial tests show inconsistent results, though clinical data clearly points to allergen‐related symptoms (showing inconsistency of in vivo and in vitro diagnostic tests).49 sIgE antibodies are generally measured with either in‐house fluorescence enzyme labeled immunoassay using biotinylated protein coupled to streptavidin CAPs, in‐house ELISAs, or in‐house immunoblots.
There is a need for establishment of a publicly funded laboratory working with this sophisticated in‐house sIgE issue. For more details see additional Supporting information.
4.4. The need for assay standardization
At present there are no standard operating procedures for conducting “in‐house” assays.33 Current FDA guidance regulations, regarding fluorescence enzyme labeled immune‐assay based methods, state that the source and stability of allergen‐specific control sera should be specified. It is, however, to be emphasized that all immunological methods (in‐house and commercial) have to be validated routinely with control serum samples and additional standard set points (two analytic standards, one with low concentration and the other with high concentration, are used as set points).4 For validation of the assays, a minimum of the following controls need to be included: tests with pooled sera from sensitized and nonsensitized subjects, biotin and HSA‐control samples. The measured day‐to‐day precision and variability in series measurements should follow good laboratory practice rules.
For further details, techniques, and analyses of mediators see Supporting information.
4.5. Early biomarkers of allergic sensitization, mediators, and cellular tests used in diagnostics
Allergy is an immune mediated, inflammatory response involving different types of cells that release a multitude of inflammatory mediators.50 The mast cells act as effector cells releasing mediators such as histamine and tryptase among others upon antigen‐ and IgE‐dependent activation, but also by several other mechanisms. During cell activation, eosinophils secrete eosinophil cationic protein (ECP), while basophils release the content of their granules after a process of activation dependent on the antigen.
For diagnostic details see the Supporting information.
5. OUTCOME OF THE META‐ANALYSIS AND REVIEW PERTINENT QUESTIONS
5.1. What are the sIgE test performances for high molecular weight allergens?
The aforementioned meta‐analysis of studies including asthmatic subjects exposed to various occupational HMW agents found that specific IgE determination provided a sensitivity of 74% [95% CI 66%‐80%], as compared with specific inhalation challenge or serial peak flow measurements, while the specificity of these tests was 71% [95% CI 63%‐77%].33 The results are in the range of a previous analysis based on fewer studies.51
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for a cohort of bakers were calculated at a cutoff value of 0.35 kUA/l for wheat and rye flour sIgE. Specificity was 68% and 62%, PPV 74% and 82% and NPV was 82% and 71%, respectively, whereas sensitivity was 87% for each of these flours.52
For natural rubber latex (NRL), the sensitivity, specificity, PPV, and NPV of a sIgE level ≥ 0.35 kUA/l as compared to the result of SICs were 94%, 48%, 86%, and 71%, respectively.31
Results of our new meta‐analysis for sensitivities of single HMW allergens ranged from 72% [95% CI 42%‐90%] for cellulase, 0.79 [95% CI 65%‐88%] for wheat, 0.84 [95% CI 65%‐94%] for rye to 0.88 [95% CI 42%‐99%] for latex.33
5.2. What are the test performances for allergenic components?
Using commercial ImmunoCAP analysis, sensitization to green coffee beans was found in 2/18 (11%) of workers with symptoms of allergic rhinitis and/or conjunctivitis under coffee dust exposure. ELISA screening showed sIgE reactivity to rCof a 1, rCof a 2, and rCof a 3 in a total of 8 out of 18 (44%) sera of symptomatic coffee workers.53
The specificity of measuring sIgE to a panel of Triticum aestivum components, (Tri a) 27, 28, 29.02, 32, and 39 was 97% with a sensitivity of 70% for wheat flour allergy among bakers.54
Twenty‐one (48.8%) of the 43 sera with negative results to commercial wheat flour ImmunoCAP (f4) yielded positive results, with at least 1 of 6 newly identified wheat recombinant proteins.55
However, for routine diagnosis of baker's allergy, allergen‐sIgE tests with whole wheat and rye flour extracts were shown to be of superior diagnostic sensitivity, when compared to components.54
Vandenplas et al31 found that the sum of sIgE concentrations against the recombinant allergens of Hevea brasiliensis (rHev b5) and r Hev b 6.01 or 6.02 yielded a higher predictive value (>95%) for positive SIC similar to that provided by the level of sIgE against the whole latex extract, but with higher sensitivity (79%) and diagnostic efficiency (0.67) as compared with whole latex‐sIgE (49% and 0.41, respectively). Combining positive sIgE results for the NRL extract and the recombinant allergen components rHev b 5, 6.01, 6.02, and 11 provided similar AUC (0.84‐0.85) and Youden index (0.61‐0.65) values (data not detailed), but did not improve the diagnostic performance. Nevertheless, measurement of sIgE antibodies against the tested panel of recombinant latex allergen components did not improve the negative predictive value of immunological testing as none of the subjects with positive SIC and a negative IgE against the whole latex extract showed IgE reactivity to allergen components.
Supplementation of natural extracts with recombinant components (“spiking”) has already entered daily practice in the field of occupational allergy laboratory analyses. Latex component Hev b 5 added to the natural extract raised sensitivity from 76% to 90% without lowering specificity. The method became commercially available with a CAP test.56
5.3. What are the sIgE test performances for low molecular weight allergens?
For LMW the preparation conditions can greatly affect the degree of haptenation and test performance. There are no well accepted standard methods for reacting LMW agents to albumin, or for characterizing the resultant haptenated protein.
Lux et al33 reported a sensitivity of 28% [95% CI 18%‐40%] with a specificity of 89% [95% CI 77%‐95%] for occupational asthma caused by various LMW allergens. In a previous publication with a smaller study group, similar data with sensitivity of 31% and specificity of 97% were reported.51
Elevated sIgE by the ELISA method to diisocyanate conjugated with human serum albumin had a sensitivity of 31% and a specificity of 97%, in bronchial provocation test positive patients.57 Tee et al58 reported a sensitivity of 28% and a specificity of 92% with a RAST ratio cutoff point of 2.0 in patients who had a positive bronchial challenge test to isocyanates. Baur found a sensitivity of 14%,59 and later 20% with comparable specificity (personal communication). Meta‐analysis provided 21% [95% CI 14%‐31%] and 94% [95% CI 88%‐97%]) for pooled pairs of sensitivity and specificity.33 Pooled sensitivities of single diisocyanates HDI, TDI, and HDI ranged from 21%‐42%. Sensitivity for acid anhydrides was exceptionally high with 81% [95% CI 46%‐95%], but the estimation was based on few results33.
6. ALLERGEN EXPOSURE ASSESSMENT
An important aspect in the management and diagnosis of type 1 allergic disorders is the knowledge of the environmental levels of antigens. This involves an understanding of the total environment including both the external environment and also the interior of the houses or work areas. The quantification of substances in the environment has various applications that can be of help in the diagnosis of these disorders. Quantitative and qualitative measures of past and present allergen exposure are especially important in identifying the sensitizing agent(s), to estimate dose response relationships and for preventing the elicitation of asthmatic episodes. Exposure assessment can be multifaceted including the use of questionnaires, job exposure matrices, direct sampling of the air and on skin, and biomonitoring.
6.1. Air sampling methods and databases
Heederik et al60 thoroughly addressed the topic of workplace exposure sampling, analyses, assessment strategies, and data interpretation. For asthma, inhalable dust sampling is most commonly measured and recommended in order to collect the portion of the aerosol that can be inhaled by the worker and deposited in any part of the respiratory tract (see Supporting information, Figure S1).
For air monitoring to allergens, it is important to consider that generally HMW allergen and many LMW exposures occur in the workplace as dust or aerosols (the latter may also occur as vapors) which dictates the type of corresponding sampling procedures. Established and validated procedures for the sampling of dust are available from NIOSH, the MAK commission, and other working groups (NIOSH Manual of Analytical Methods 5th edition61; the MAK‐Collection for Occupational Health and Safety Part III: Air Monitoring Methods (DFG).62 Further collection of air monitoring methods is provided by the Health and Safety Executive (UK)63: In the United States, the National Institute for Occupational Safety and Health's Manual of Analytical Methods (NMAM; 4th and 5th addition) is available on line https://www.cdc.gov/niosh/docs/2003-154/default.html; https://www.cdc.gov/niosh/nmam/default.html.61 In addition to specific methods, guidance chapters are available in the NMAM to assist in developing sampling strategies and methods development. Particularly, the following chapters of the NIOSH Manual of Analytical Methods may be helpful as guiding documents: Purpose and Scope, Development and Evaluation of Methods, Measurement Uncertainty and NIOSH Method Accuracy Range, General Considerations for Sampling Airborne Contaminants, Factors Affecting Aerosol Sampling, Sampling and Characterization of Bioaerosols, Filter Pore Size and Aerosol Sample Collection, Measurement of Fibers, and Sampling and Analysis of Soluble Metal Compounds. The Occupational Safety and Health Administration (OHSA) has a similar database at https://www.osha.gov/dts/sltc/methods/index.html. While these databases are not specific for chemicals that cause asthma, they contain many methods for the more common ones. Methods harmonization for occupational exposure monitoring among stakeholders including government entities and consensus standard organizations is recognized as important in “leveraging current and future applied research, as well as technology transfer endeavors, within the discipline of occupational hygiene chemical and biochemical sampling and analysis.”64 Assessing exposure to low molecular weight agents is mostly performed with help of ambient‐ and biomonitoring methods. 60
For details see60 and Supporting information (page S23).
7. INTEGRATED DIAGNOSTIC APPROACH FOR OCCUPATIONAL AND ENVIRONMENTAL INDUSTRIAL ALLERGENS
Details are given in Figure 4 and in the Figure S2.
Figure 4.
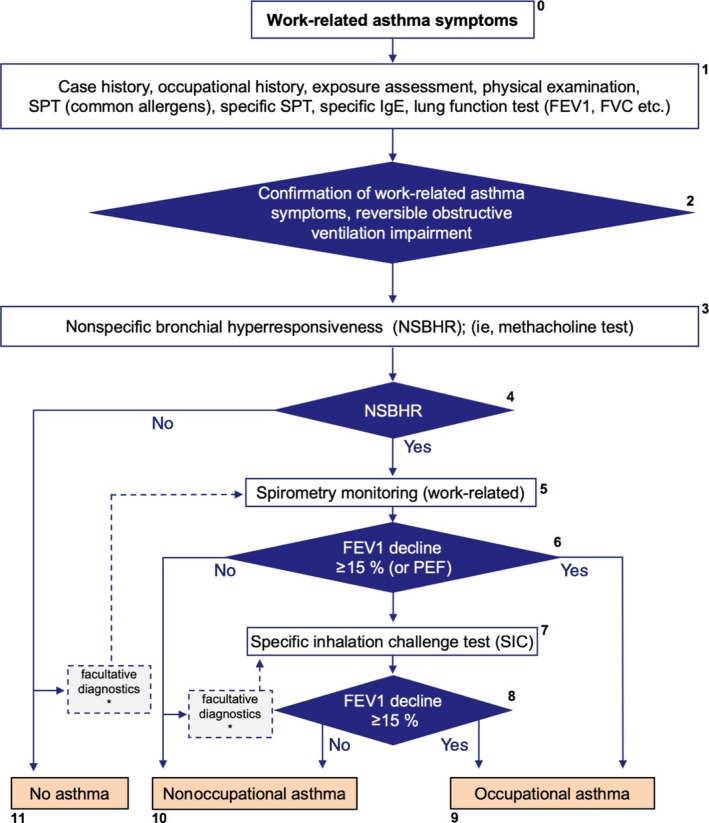
Diagnostic approach in case of suspected occupational (environmental‐related) asthma. For details see text in the Supporting information. *Please note that there are rare cases of work‐related asthma without NSBHR or absence of an obstructive ventilation pattern during work. If the case history is supportive of work‐related asthma additional (facultative) diagnostics is recommended as indicated in Figure 4. It should be taken into consideration that false‐negative outcomes of the NSBHR test, spirometric monitoring and the SIC test may occur due to medication or latency periods of several weeks or more since last exposure. The SIC may also be false negative if not perfored with the correct agent. If this has to be assumed in a case whose history is strongly supportive of asthma, repetition of the individual diagnostic tests has to be considered
8. STATEMENTS AND RECOMMENDATIONS
Based on the aforementioned data and results, we make the following statements and recommendations (Table 1, Table 2).
Table 2.
Recommendations
| General diagnostic aspects |
| Diagnostics of respiratory and skin type 1 disorders should start with a qualified clinical and occupational case history, followed by non‐ or less‐invasive methods, that is, clinical status, functional, and allergy tests (prick and/or sIgE) and finally, if needed for prevention or therapy, by serial lung function measurement and/or specific nasal challenge or SIC |
| In case of suspected allergic OA, but unclear diagnostic findings, serial lung function measurement according to standardized protocol (2 weeks work, 2 weeks off work) are recommended. SIC is only recommended in suspected occupational asthma cases if serial lung function measurement is not possible and all diagnostic tests do not provide a clear diagnosis but diagnosis is needed for far‐reaching preventive or therapeutic measures |
| Measurement of sIgE to suspected causative environmental allergens by use of a standardized specific and sensitive method is recommended within the diagnostic setup in order to identify the causative agent |
| The manufacturers of commercial sIgE are requested to provide full transparency of the allergen preparations they use along with details of standardization and quality control |
| For in‐house sIgE assays there should be standard operating procedures which should include batch to characterization of allergen for in‐house s IgE assays (including measurement of protein content, electrophoresis and immunoblotting). Development of assay should include testing of at least 20 negative sera of subjects not exposed to individual allergen (however, their total IgE should cover a range up to 1000 IU/ml) and at least three sera of subjects suffering from IgE sensitization. The sIgE assay should be standardized against the gold standard 3rd World Health Organization (WHO) International Reference Reagent (IRR) for serum IgE (75/502;5000 IU/ampoule). (Note: this may be difficult for putative novel allergens). http://www.who.int/biologicals/BS_2220_Candidate_Preparation.pdf |
| There is a need for specialized laboratories to provide a bespoke service for the measurement of putative novel occupational allergens and also those allergens not available commercially |
| Determination of sIgE to high molecular weight allergens is recommended as a valuable diagnostic tool within the diagnostic setup algorithm in bronchial asthma |
| The presence of sIgE to low molecular weight agents such as diisocyanates and acid anhydrides should be regarded as strong evidence for relevant sensitization, whereas negative sIgE must be regarded with caution due to frequent false negative results |
| Laboratories should always carry out clinical audits (systematic and independent examination whether processes, requirements and rules meet required standards) on their sIgE assays which will determine whether commercially available assays or “in‐house” assay provides the best diagnostic specific IgE assay |
| Commercially available sIgE assays can be recommended for most allergens (although there are exceptions eg, acid anhydride, isocyanates) as a standardized method to measure sIgE; however, its relatively high costs have to be considered and limit its broad application |
| Nasal diagnostic aspects |
| The diagnosis of allergic rhinitis including occupational rhinitis is based on history, clinical findings and confirmation by sIgE to relevant allergens |
| The nasal endoscopy is indicated to evaluate anatomical or infectious diseases |
| The nasal challenge test is recommended to diagnose allergic rhinitis, local allergic rhinitis, nasal hyperreactivity, or occupational rhinitis |
| Specific nasal allergen challenges should be performed according to the standardized protocol of the recently published EAACI Position paper 65Diagnostic tests such as assessment of nonspecific nasal hyperresponsiveness or specific nasal allergen challenges are a key aspect of specialized clinical centers |
| Skin testing |
| Patients suspected to suffer from immediate‐type allergy to occupational/environmental allergens should be appropriately screened for possibly causative agents, and, where possible, discontinued on medications that interfere with test results, accentuate systemic allergic reactions or render patients less responsive to treatment with epinephrine. |
| Even though SPT is safe with no reported fatalities, a physician or other healthcare professional and emergency equipment should be immediately available when such tests are performed |
| SPT should be performed with extra caution during the respective allergy season when the patient has allergic symptoms, or when baseline tryptase levels are elevated.66, 67 The latter is a risk factor for anaphylaxis and mastocytosis |
| Relative contraindications for SPT include pregnancy, in view of a remote possibility of inducing a systemic allergic reaction that could induce uterine contractions or necessitate the use of epinephrine (thought to cause constriction of the umbilical artery |
| SPTs are difficult to perform or to interpret in patients with severe eczema, dermographism, or who are taking antihistamines or other medications such as certain antidepressants or calcineurin inhibitors |
| The degree of skin test reactivity can be decreased in subjects with chronic illnesses such as renal failure, or cancer. Furthermore, chronic or acute UV‐B radiation of the skin in the test area may reduce the wheal size from SPT |
| It is difficult or impossible to develop stable test extracts for certain allergens, in particular, certain foods, for example, for skin testing to uncooked fruits and vegetables. A prick‐to‐prick technique is utilized, that is, first pricking the fresh food with the lancet and then pricking the skin, to test for sensitization to such allergens when clinical allergy is suspected, in particular, oral allergy syndrome. Dry foods, for example, nuts or cereal, can be tested in saline and also utilized using the prick‐to‐prick technique |
| Exposure assessment |
| “At‐risk” worksites exposure monitoring of the specific allergenic components should be routinely conducted and employ direct reading continuous (personal) monitors whenever possible |
| Biomarkers of exposure should be standardized and used to supplement environmental monitoring |
It is important that manufacturers of commercially available sIgE tests communicate their data on diagnostic allergen preparation, standardization, quality control, including background definition, and inform whether their method provides quantitative results by referring to the WHO IgE standard. Development of in‐house methods for sIgE testing necessitates expertise with such methods and is rather expensive and laborious. It would be helpful if in‐house assays could aspire to aforementioned prerequisites for commercially available tests, although in reality it is difficult to be able to include a WHO IgE standard reference. Regarding diagnostics we also stress exposure assessment by a questionnaire combined with measurement of the allergen load in the specific environment; this allows the risk of allergic disorders to be estimated. Surveillance of high risk subjects (eg, those with high level of sIgE antibodies) and individuals in “at‐risk” sites, especially workplaces, are recommended in order to take appropriate preventive measures and to optimize medication.
For a broader view of this items see Supporting information, section Points to be emphasized and prospective.
CONFLICT OF INTEREST
None of the authors has a financial or any other conflict of interest to disclose. The findings and conclusions shown in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention (NIOSH/CDC). Mention of any company or product does not constitute endorsement by either NIOSH/CDC or any other participating institution.
Supporting information
Baur X, Akdis CA, Budnik LT, et al. Immunological methods for diagnosis and monitoring of IgE‐mediated allergy caused by industrial sensitizing agents (IMExAllergy). Allergy. 2019;74:1885–1897. 10.1111/all.13809
Practical guideline from the international DiMoPEx task force, with European Respiratory Society Task Force, European Society for Environmental and Occupational Medicine and the Global Allergy Forum
Funding information
All authors are salaried staff members of their respective institutions (XB is retired University Professor and chair for occupational medicine, University of Hamburg). The guideline was prepared with financial support from the EU‐COST‐Action (CA‐15129, DiMoPEx), which is supported by the EU Framework Program Horizon 2020.
REFERENCES
- 1. Ring J, Akdis C, Behrendt H, et al. Davos declaration: allergy as a global problem. Allergy. 2012;67(2):141‐143. [DOI] [PubMed] [Google Scholar]
- 2. Weisel CP. Assessing exposure to air toxics relative to asthma. Environ Health Perspect. 2002;110(Suppl 4):527‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baur X. A compendium of causative agents of occupational asthma. J Occup Med Toxicol. 2013;8(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Budnik LT, Scheer E, Burge PS, Baur X. Sensitising effects of genetically modified enzymes used in flavour, fragrance, detergence and pharmaceutical production: cross‐sectional study. Occup Environ Med. 2017;74(1):39‐45. [DOI] [PubMed] [Google Scholar]
- 5. Hilger C, Swiontek K, Hentges F, Donnay C, de Blay F, Pauli G. Occupational inhalant allergy to pork followed by food allergy to pork and chicken: sensitization to hemoglobin and serum albumin. Int Arch Allergy Immunol. 2010;151(2):173‐178. [DOI] [PubMed] [Google Scholar]
- 6. Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. In: http://ginasthma.org/; 2018.
- 7. Ghosh D, Clay C, Bernstein JA. The utility of monitoring trimellitic anhydride (TMA)‐specific IgG to predict IgE‐mediated sensitization in an immunosurveillance program. Allergy. 2017;73(5):1075‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masoli M, Fabian D, Holt S, Beasley R. Global initiative for asthma P. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59(5):469‐478. [DOI] [PubMed] [Google Scholar]
- 9. Thurston GD, Kipen H, Annesi‐Maesano I, et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J. 2017;49(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haahtela T, Laatikainen T, Alenius H, et al. Hunt for the origin of allergy ‐ comparing the Finnish and Russian Karelia. Clin Exp Allergy. 2015;45(5):891‐901. [DOI] [PubMed] [Google Scholar]
- 11. Terl M, Sedlák V, Cap P, et al. Asthma management: A new phenotype‐based approach using presence of eosinophilia and allergy. Allergy. 2017;72(9):1279‐1287. [DOI] [PubMed] [Google Scholar]
- 12. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343‐373. [DOI] [PubMed] [Google Scholar]
- 13. Muraro A, Lemanske RF Jr, Hellings PW, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis‐PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137(5):1347‐1358. [DOI] [PubMed] [Google Scholar]
- 14. Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72(8):1156‐1173. [DOI] [PubMed] [Google Scholar]
- 15. Elberling J, Linneberg A, Dirksen A, et al. Mucosal symptoms elicited by fragrance products in a population‐based sample in relation to atopy and bronchial hyper‐reactivity. Clin Exp Allergy. 2005;35(1):75‐81. [DOI] [PubMed] [Google Scholar]
- 16. Hannu T, Riihimäki VE, Piirilä PL. Reactive airways dysfunction syndrome from acute inhalation of a dishwasher detergent powder. Can Respir J. 2012;19:25‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malo JL, Chan‐Yeung M. Agents causing occupational asthma with key references In: Bernstein IL, Chan‐Yeung M, Malo JL, Bernstein DI, editors. Asthma in the workplace. New York, NY: Taylor & Francis; 2006: pp 825‐849. [Google Scholar]
- 18. Baur X, Bakehe P. Allergens causing occupational asthma: an evidence‐based evaluation of the literature. Int Arch Occup Environ Health. 2014;87(4):339‐363. [DOI] [PubMed] [Google Scholar]
- 19. Aasen TB, Burge PS, Henneberger PK, Schlunssen V, Baur X. Diagnostic approach in cases with suspected work‐related asthma. J Occup Med Toxicol. 2013;8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baur X. Immunologic cross‐reactivity between different albumin‐bound isocyanates. J Allergy Clin Immunol. 1983;71(2):197‐205. [DOI] [PubMed] [Google Scholar]
- 21. Ishizaka K, Ishizaka T, Lee EH. Physiochemical properties of reaginic antibody. II. Characteristic properties of reaginic antibody different from human gamma‐A‐isohemagglutinin and gamma‐D‐globulin. J Allergy. 1966;37(6):336‐349. [DOI] [PubMed] [Google Scholar]
- 22. Wide L, Bennich H, Johansson S. Diagnosis by in vitro test for allergen antibodies. Lancet 1967;290:1105–1107. [DOI] [PubMed] [Google Scholar]
- 23. Hamilton RG, Oppenheimer J. Serological IgE analyses in the diagnostic algorithm for allergic disease. J Allergy Clin Immunol Pract. 2015;3(6): 833–840; quiz 841–832. [DOI] [PubMed] [Google Scholar]
- 24. Hamilton RG. Proficiency survey‐based evaluation of clinical total and allergen‐specific IgE assay performance. Arch Pathol Lab Med. 2010;134(7):975–982. [DOI] [PubMed] [Google Scholar]
- 25. Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol. 2007;99(1):34–41. [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121(5):1219–1224. [DOI] [PubMed] [Google Scholar]
- 27. Matricardi PM, Kleine‐Tebbe J, Hoffmann HJ, et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250. [DOI] [PubMed] [Google Scholar]
- 28. Williams PB, Barnes JH, Szeinbach SL, Sullivan TJ. Analytic precision and accuracy of commercial immunoassays for specific IgE: establishing a standard. J Allergy Clin Immunol. 2000;105(6 Pt 1):1221–1230. [DOI] [PubMed] [Google Scholar]
- 29. Hamilton RG. Clinical laboratories worldwide need to report IgE antibody results on clinical specimens as analytical results and not use differential positive thresholds. J Allergy Clin Immunol. 2015;136(3):811–812. [DOI] [PubMed] [Google Scholar]
- 30. Feary J, Cannon J, Tarzi M, Wincell S, Welch J, Cullinan P. Occupational asthma from a horticultural nematode, Steinernema feltiae. Lancet Respir Med. 2015;3(8):e28–e29. [DOI] [PubMed] [Google Scholar]
- 31. Vandenplas O, Froidure A, Meurer U, et al. The role of allergen components for the diagnosis of latex‐induced occupational asthma. Allergy. 2016;71(6):840–849. [DOI] [PubMed] [Google Scholar]
- 32. Raulf M. Allergen component analysis as a tool in the diagnosis and management of occupational allergy. Mol Immunol. 2018;100:21–27. [DOI] [PubMed] [Google Scholar]
- 33. Lux H, Lenz K, Budnik LT, Baur X. Performance of specific immunoglobulin E tests for diagnosing occupational asthma: A systematic review and meta‐analysis. Occup Environ Med.. 2019;76(4):269–278. [DOI] [PubMed] [Google Scholar]
- 34. Roberts G, Ollert M, Aalberse R, et al. A new framework for the interpretation of IgE sensitization tests. Allergy. 2016;71(11):1540–1551. [DOI] [PubMed] [Google Scholar]
- 35. Hagerman LM, Law BF, Bledsoe TA, et al. The influence of diisocyanate antigen preparation methodology on monoclonal and serum antibody recognition. J Occup Environ Hyg. 2016;13(11):829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Budnik LT, Preisser AM, Permentier H, Baur X. Is specific IgE antibody analysis feasible for the diagnosis of methylenediphenyl diisocyanate‐induced occupational asthma? Int Arch Occup Environ Health. 2013;86(4):417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torres MJ, Montañez MI, Ariza A, et al. The role of IgE recognition in allergic reactions to amoxicillin and clavulanic acid. Clin Exp Allergy. 2016;46(2):264–274. [DOI] [PubMed] [Google Scholar]
- 38. Ariza A, Garzon D, Abánades DR, et al. Protein haptenation by amoxicillin: high resolution mass spectrometry analysis and identification of target proteins in serum. J Proteomics. 2012;77:504–520. [DOI] [PubMed] [Google Scholar]
- 39. Rosqvist S, Nielsen J, Welinder H, Rylander L, Lindh CH, Jonsson BA. Exposure‐response relationships for hexahydrophthalic and methylhexahydrophthalic anhydrides with total plasma protein adducts as biomarkers. Scand J Work Environ Health. 2003;29(4):297–303. [DOI] [PubMed] [Google Scholar]
- 40. Curran AD, Burge PS, Wiley K. Clinical and immunologic evaluation of workers exposed to glutaraldehyde. Allergy. 1996;51(11):826–832. [DOI] [PubMed] [Google Scholar]
- 41. Sabbioni G, Dongari N, Sepai O, Kumar A. Determination of albumin adducts of 4,4'‐methylenediphenyl diisocyanate in workers of a 4,4'‐methylenedianiline factory. Biomarkers. 2016. ;21(8):731‐738. [DOI] [PubMed] [Google Scholar]
- 42. Redlich CA. Skin exposure and asthma: is there a connection? Proc Am Thorac Soc. 2010;7(2):134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sabbioni G, Hartley R, Henschler D, Hollrigl‐Rosta A, Koeber R, Schneider S. Isocyanate‐specific hemoglobin adduct in rats exposed to 4, 4'‐methylenediphenyl diisocyanate. Chem Res Toxicol. 2000;13(2):82–89. [DOI] [PubMed] [Google Scholar]
- 44. Nayak AP, Hettick JM, Siegel PD, et al. Toluene diisocyanate (TDI) disposition and co‐localization of immune cells in hair follicles. Toxicol Sci. 2014;140(2):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones M, Floyd A, Nouri‐Aria KT, et al. Is occupational asthma to diisocyanates a non–IgE‐mediated disease? J Allergy Clin Immunol. 2006;117:663–669. [DOI] [PubMed] [Google Scholar]
- 46. Blindow S, Preisser AM, Baur X, Budnik LT. Is the analysis of histamine and/or interleukin‐4 release after isocyanate challenge useful in the identification of patients with IgE‐mediated isocyanate asthma? J Immunol Methods 2015; 422:35‐50. [DOI] [PubMed] [Google Scholar]
- 47. Vanoirbeek JA, Tarkowski M, Ceuppens JL, Verbeken EK, Nemery B, Hoet PH. Respiratory response to toluene diisocyanate depends on prior frequency and concentration of dermal sensitization in mice. Toxicol Sci. 2004;80(2):310–321. [DOI] [PubMed] [Google Scholar]
- 48. Cruz MJ, Olle‐Monge M, Vanoirbeek JA, Assialioui A, Gomez‐Olles S, Munoz X. Persistence of respiratory and inflammatory responses after dermal sensitization to persulfate salts in a mouse model of non‐atopic asthma. Allergy Asthma Clin Immunol. 2016;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heutelbeck AR, Schulz T, Bergmann KC, Hallier E. Environmental exposure to allergens of different dog breeds and relevance in allergological diagnostics. Journal of toxicology and environmental health. Part A 2008;71(11–12):751–758. [DOI] [PubMed] [Google Scholar]
- 50. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beach J, Russell K, Blitz S, et al. A systematic review of the diagnosis of occupational asthma. Chest. 2007;131(2):569–578. [DOI] [PubMed] [Google Scholar]
- 52. Van Kampen V, Rabstein S, Sander I, et al. Prediction of challenge test results by flour‐specific IgE and skin prick test in symptomatic bakers. Allergy. 2008;63(7):897–902. [DOI] [PubMed] [Google Scholar]
- 53. Peters U, Frenzel K, Brettschneider R, Oldenburg M, Bittner C. Identification of two metallothioneins as novel inhalative coffee allergens cof a 2 and cof a 3. PLoS ONE. 2015;10(5):e0126455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sander I, Rihs H‐P, Doekes G, et al. Component‐resolved diagnosis of baker's allergy based on specific IgE to recombinant wheat flour proteins. J Allergy Clin Immunol. 2015;135(6):1529–1537. [DOI] [PubMed] [Google Scholar]
- 55. Bittner C, Peters U, Frenzel K, Musken H, Brettschneider R. New wheat allergens related to baker's asthma. J Allergy Clin Immunol. 2015;136(5):1416–1418. [DOI] [PubMed] [Google Scholar]
- 56. Huss‐Marp J, Raulf M, Jakob T. Spiking with recombinant allergens to improve allergen extracts: benefits and limitations for the use in routine diagnostics: Part 19 of the Series Molecular Allergology. Allergo J Int. 2015;24:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cartier A, Grammer L, Malo JL, et al. Specific serum antibodies against isocyanates: association with occupational asthma. J Allergy Clin Immunol. 1989;84(4 Pt 1):507–514. [DOI] [PubMed] [Google Scholar]
- 58. Tee RD, Cullinan P, Welch J, Burge PS, Newman‐Taylor AJ. Specific IgE to isocyanates: a useful diagnostic role in occupational asthma. J Allergy Clin Immunol. 1998;101(5):709–715. [DOI] [PubMed] [Google Scholar]
- 59. Baur X. Occupational asthma due to isocyanates. Lung. 1996;174(1):23–30. [DOI] [PubMed] [Google Scholar]
- 60. Heederik D, Budnik L, Roberge B, Goyer N. How to assess exposure in the workplace: Sampling, analysis, exposure assessment strategy, and interpretation of exposure data. New York, NY: CRC Press Taylor & Francis Group; 2013. [Google Scholar]
- 61. Hettick JM, Siegel PD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. Int J Mass Spectrom. 2012;309:168–175. [Google Scholar]
- 62. The MAK‐Collection for Occupational Health and Safety‐Part I‐III. https://www.onlinelibrary.wiley.com: Wiley; 2017.
- 63. HSE, Health and Safety Executive (UK): Methods for the determination of hazardous substances in air. http://www.hsl.gov.uk/resources/publications/mdhs/mdhs-revisions. 2017.
- 64. Ashley K. NIOSH Manual of Analytical Methods 5(th) Edition and Harmonization of Occupational Exposure Monitoring. Gefahrst Reinhalt Luft. 2015;2015(1–2):7–16. [PMC free article] [PubMed] [Google Scholar]
- 65. Augé J, Vent J, Agache I, et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy. 2018;73(8):1597–1608. [DOI] [PubMed] [Google Scholar]
- 66. Heinzerling L, Mari A, Bergmann K‐C, et al. The skin prick test ‐ European standards. Clin Transl Allergy 2013;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ruëff F, Przybilla B, Biló MB, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase‐a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J Allergy Clin Immunol. 2009;124(5):1047–1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


