Abstract
Objectives
This study was designed to test whether serum vitamin D levels affected Helicobacter pylori (H. pylori) infection and eradication rates.
Methods
A multicenter observational prospective cohort study was conducted. A total of 496 H. pylori − positive (H. pylori +) and 257 H. pylori‐negative (H. pylori −) patients were enrolled from four hospitals in China. Baseline serum vitamin D levels were measured and a 13C‐urea breath test (UBT) was performed for all the participants. The H. pylori + patients were divided into two subgroups based on their serum vitamin D levels (<10 or ≥10 ng/mL). A second 13C‐UBT was performed between 4 and 8 weeks after 14‐day bismuth‐containing quadruple eradication therapies. Factors potentially affecting H. pylori eradication were determined using a questionnaire survey.
Results
Serum vitamin D levels were significantly lower in the H. pylori + group than in the H. pylori − group ([17.0 ± 6.9] ng/mL vs [19.2 ± 8.0] ng/mL, P = 0.000). H. pylori eradication rate significantly differed between patients with serum vitamin D levels of <10 ng/mL and ≥10 ng/mL (71.7% vs 87.3%, P = 0.005). A multivariate analysis showed that having serum vitamin D level ≥10 ng/mL was an independent risk factor for a successful H. pylori eradication (odds ratio 0.381, 95% confidence interval 0.183‐0.791, P = 0.010).
Conclusions
Serum vitamin D level may affect H. pylori infection and its eradication. Randomized controlled trials are needed to find out whether vitamin D supplements may increase the H. pylori eradication rate.
Keywords: eradication, Helicobacter pylori, infection, vitamin D
1. INTRODUCTION
Gastric cancer (GC) generates a heavy disease burden in China. Helicobacter pylori (H. pylori) infection is known to play a role in the pathogenesis in most patients with GC.1 Accumulating evidence has shown that H. pylori infection causes almost 90% of non‐cardiac GC.2 Therefore, a successful eradication of H. pylori would significantly decrease the incidence of GC. However, both bacterial strains and host factors have been reported to impede the eradication of H. pylori infection, including the resistance to antibiotics, the virulence of the strains, and host‐related genetic disorders.3
Vitamin D regulates the calcium and phosphorus metabolism needed for bone formation, and the influence of vitamin D on H. pylori infection and eradication rates has recently been widely investigated. Several clinical studies have illustrated that vitamin D analogs may have anti‐H. pylori antimicrobial effects.4, 5, 6, 7, 8, 9, 10 Cytological research has also found that vitamin D3 decomposition product 1 (VDP1) can lyse H. pylori bacterial cells by inducing the collapse of the cell membrane.11, 12
However, the correlation between vitamin D levels and H. pylori has not been fully illustrated, and studies on the impact of serum vitamin D levels on H. pylori eradication were mostly of small sample sizes. Therefore, we conducted a multicenter prospective cohort study with a relatively large sample size to determine whether serum vitamin D levels had an impact on H. pylori infection and eradication, and whether its low level was an independent risk factor affecting H. pylori eradication.
2. PATIENTS AND METHODS
2.1. Study population
We conducted a non‐randomized, multicenter, observational and prospective cohort study with consecutively enrolled patients to estimate the influence of serum vitamin D levels on the rates of H. pylori infection and eradication. All patients were recruited between October 2017 and July 2018 from either one of the four centers: the Xijing Hospital of Digestive Diseases (Xi'an, Shaanxi Province, China), Xianyang Central Hospital (Xi'an, Shaanxi Province, China), the First Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan Province, China), and the General Hospital of the Western Theater Command (Chengdu, Sichuan Province, China). Inclusion criteria were: (a) participants aged between 18 and 75 years; (b) having undergone a 13C‐urea breath test (UBT); and (c) those in whom the serum vitamin D level had been detected. The following individuals were excluded from the study: (a) aged less than 18 years old or over 75 years old; (b) had been treated for H. pylori infection; (b) had previously undergone gastric surgery; (d) pregnant or lactating; (e) had major systemic diseases; (f) had diseases that might affect their serum vitamin D levels, such as hyperthyroidism, malabsorption, rickets, osteoma, hypercortisolism, severe liver diseases (Child‐Pugh grade B or C), renal failure (serum creatinine >177 mmol/L) and alcoholism; (g) who had administered antimicrobial agents, bismuth agents or gastric antisecretory drugs during the previous 8 weeks; (h) had refused H. pylori eradication treatment, or were allergic to any one of the drugs given in the eradication schemes; and (i) who used daily vitamin D supplements. In total, 496 patients who were tested positive (H. pylori +) and the 257 who were negative (H. pylori −) for H. pylori infection and who had had their serum vitamin D levels tested were enrolled in our study. The study was approved by the Ethics Committees of the Xijing Hospital of the Air Force Military Medical University (no. KY20173035‐1), Xianyang Central Hospital (no. KY20171011‐1), the First Affiliated Hospital of Zhengzhou University (no. KY‐2017‐33), and the General Hospital of the Western Theater Command (no. KY20171023‐1). The study was conducted according to the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration (as revised in Brazil in 2013). Written, informed consent was obtained from all participants in the study.
2.2. Data collection
The following information were extracted from the patients who were H. pylori + using a questionnaire on the factors affecting H. pylori eradication, as follows: patients’ age, sex, occupation, residential area, body mass index (BMI), marital status, educational level, family members, annual income, smoking, alcohol consumption, history of periodontal disease, hygiene of dining place, main source of drinking water, drinking of untreated water or not, 13C‐UBT results, diagnosis by gastroscopy, treatment of adequate dosage and duration, choice of proton pump inhibitors (PPIs), and serum vitamin D levels. Treatment of an adequate dosage and duration was defined as having received one of two different H. pylori eradication schemes for 14 days without forgetting to take the prescribed medication.
2.3. Treatment and follow‐up for H. pylori + patients
The H. pylori + patients were further divided into two subgroups based on their serum vitamin D levels (<10 or ≥10 ng/mL) and were given one of the two treatment options for 14 days: (a) 1000 mg amoxicillin (Kelun Pharmaceutical Company, Chengdu, Sichuan Province, China) twice daily, 500 mg clarithromycin (Livzon Pharmaceutical Group, Zhuhai, Guangdong Province, China) twice daily, 220 mg colloidal bismuth tartrate capsule (Hunan Warrant Pharmaceutical Company, Changsha, Hunan Province, China) twice daily and 40 mg esomeprazole (AstraZeneca Pharmaceutical Company, Cambridge, UK) twice daily; or (b) 1000 mg amoxicillin (Kelun Pharmaceutical Company) twice daily, 500 mg clarithromycin (Livzon Pharmaceutical Group) twice daily, 220 mg colloidal bismuth tartrate capsule (Hunan Warrant Pharmaceutical Company) twice daily and 20 mg rabeprazole (Shanghai Pharmaceuticals Company, Shanghai, China) twice daily. The choice of H. pylori eradication scheme conformed to the Fifth Chinese national consensus report.13 A cut‐off value for serum vitamin D level was set at 10 ng/mL because a study from Turkey has reported that 10 ng/mL may be the cut‐off value that affects H. pylori eradication.7 Finally, a 13C‐UBT was repeated between 4 and 8 weeks after the treatment was completed.
2.4. Statistical analysis
Statistical analyses were performed using SPSS version 22.0 software (IBM, Armonk, NY, USA). A double entry and verification method was used to record and enter the data collected from the patients. A one‐sample Kolmogorov‐Smirnov test was adopted to test the normality of continuous variables, which were expressed as medians and ranges for the variables with an abnormal distribution and mean ± standard deviation for variables with a normal distribution. Comparisons between the groups were performed using the Student's t‐test, Wilcoxon signed‐rank test, or the χ2 test, when appropriate. A two‐tailed P < 0.05 was considered statistically significant.
Binary logistic regression was applied to determine whether an independent risk factor of H. pylori eradication was the patient's serum vitamin D level. The degrees of this association were measured using the odds ratio (OR) and 95% confidence interval (CI).
3. RESULTS
3.1. Patients' characteristics
As shown in Figure 1, there were 496 H. pylori + patients and 257 H. pylori − patients who underwent a 13C‐UBT and whose serum vitamin D levels were tested in our study. Of the H. pylori + patients, 81 (16.3%) out of 496 patients were lost to follow‐up; H. pylori was successfully eradicated in 355 (eradication rate 85.5% [355/415]).
Figure 1.
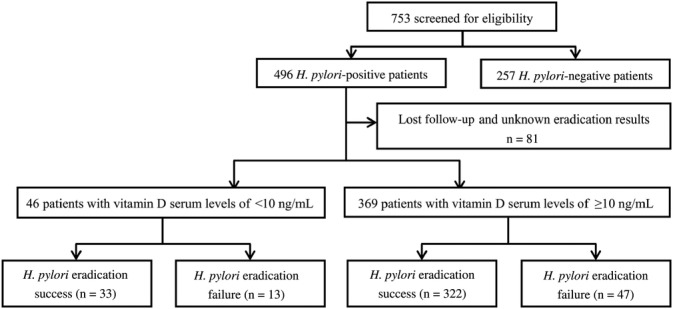
Flowchart of participant selection and grouping. H. pylori, Helicobacter pylori
3.2. Comparison of serum vitamin D levels between H. pylori + and H. pylori − patients
We found that serum vitamin D levels were significantly lower in the H. pylori + group than in the H. pylori− group ([17.0 ± 6.9] ng/mL vs [19.2 ± 8.0] ng/mL, P = 0.000); while there were no significant differences in others factors such as age, sex, season of vitamin D detection, or occupation between the two groups, as shown in Table 1. This indicates that serum vitamin D levels may affect H. pylori infection.
Table 1.
Characteristics of the Helicobacter pylori (H. pylori)‐positive and ‐negative groups
| Variables | H. pylori‐positive (N = 496) | H. pylori‐negative (N = 257) | P value |
|---|---|---|---|
| Age, y (mean ± SD) | 47.1 ± 12.6 | 48.1 ± 10.2 | 0.259 |
| Sex, n (male/female) | 236/260 | 127/130 | 0.633 |
| Season, n (spring/summer/autumn/winter) | 266/38/25/167 | 144/15/12/86 | 0.794 |
| Occupation, n (outdoor/indoor worker) | 263/233 | 147/110 | 0.275 |
| Vitamin D (25[OH]D), ng/mL (mean ± SD) | 17.0 ± 6.9 | 19.2 ± 8.0 | 0.000 |
SD, standard deviation; 25(OH)D, 25‐hydroxyvitamin D.
3.3. Differences in the H. pylori eradication rate between patients with serum vitamin D levels <10 and ≥10 ng/mL
A second 13C‐UBT was performed between 4 and 8 weeks after 14‐day bismuth‐containing quadruple eradication therapy. Significant differences were seen between the serum vitamin D levels of <10 and ≥10 ng/mL groups in terms of their H. pylori eradication rates (71.7% vs 87.3%, P = 0.005; Table 2). This result suggests that having a lower serum level of vitamin D may be related to a lower eradication rate of H. pylori.
Table 2.
Differences in Helicobacter pylori eradication rates between groups with serum vitamin D levels of <10 and ≥10 ng/mL
| Vitamin D (25[OH]D) | Success (N = 355) | Failure (N = 60) | Eradication rate (%) | P value |
|---|---|---|---|---|
| <10 ng/mL | 33 | 13 | 71.7 | ‐ |
| ≥10 ng/mL | 322 | 47 | 87.3 | 0.005 |
25(OH)D, 25‐hydroxyvitamin D.
3.4. Multivariate analyses of factors affecting the H. pylori eradication rate
Factors influencing the eradication rate of H. pylori were analyzed using a questionnaire with 20 factors. As shown in Table 3, univariate analysis showed that a treatment of adequate dosage and duration, alcohol consumption and serum levels of vitamin D differed significantly between the groups in which H. pylori was successfully eradicated and that failed, suggesting that these factors might affect the H. pylori eradication rate.
Table 3.
Factors affecting Helicobacter pylori eradication rates by univariate analysis
| Variables | Success (N = 355) | Failure (N = 60) | P value |
|---|---|---|---|
| Sex, n (male/female) | 162/193 | 32/28 | 0.269 |
| Age, y (mean ± SD) | 47.1 ± 12.7 | 48.2 ± 13.3 | 0.559 |
| Occupation, n (outdoor/indoor worker) | 164/191 | 31/29 | 0.432 |
| BMI, kg/m2 (mean ± SD) | 22.3 ± 3.2 | 21.8 ± 3.4 | 0.259 |
| Residential area, n (urban/town/rural) | 205/63/87 | 36/12/12 | 0.731 |
| Marital status, n (single/married) | 24/331 | 3/57 | 0.609 |
| Education level, n (elementary school and below/middle school/university and above) | 39/167/149 | 9/21/30 | 0.511 |
| Family size, n (<4/≥4 people) | 137/218 | 30/30 | 0.096 |
| Annual income, n (<50 000/≥50 000 CNY) | 198/157 | 35/25 | 0.712 |
| Cigarette smoking, n (yes/no) | 72/283 | 16/44 | 0.263 |
| Alcohol consumption, n (yes/no) | 23/332 | 9/51 | 0.022 |
| History of periodontal disease, n (yes/no) | 98/257 | 21/39 | 0.241 |
| Hygiene of dining place, n (good/poor) | 275/80 | 46/14 | 0.891 |
| Main source of drinking water, n (clean/probably polluted) | 307/48 | 56/4 | 0.138 |
| Drinking untreated water, n (yes/no) | 75/280 | 18/42 | 0.127 |
| 13C‐UBT value, n (Q1/Q2/Q3/Q4)* | 91/83/88/93 | 11/22/16/11 | 0.671 |
| Diagnosis by gastroscopy, n (PU/CNAG/CAG/IM/GED) | 15/180/142/16/2 | 3/27/29/1/0 | 0.623 |
| Treatment with adequate dosage and duration, n (yes/no) | 324/31 | 44/16 | 0.000 |
| Choice of PPI, n (esomeprazole/rabeprazole) | 177/178 | 35/25 | 0.225 |
| Vitamin D serum level, n (<10/≥10 ng/mL) | 33/322 | 13/47 | 0.005 |
Note: *13C‐urea breath test (UBT) values were distributed according to quartile: Q1, P0‐P25; Q2, P25‐P50; Q3, P50‐P75; and Q4, P75‐P100. P25 = 9.8; P50 = 20.3; and P75 = 35.6. Statistically significant values are bold.
Abbreviations: BMI, body mass index; CAG, chronic atrophic gastritis; CNAG, chronic non‐atrophic gastritis; GED, gastric epithelial dysplasia; IM, intestinal metaplasia; PPI, proton pump inhibitor; PU, peptic ulcer; SD, standard deviation.
A further multivariate analysis revealed that an adequate dosage and duration of treatment (OR 0.265, 95% CI 0.133‐0.530, P = 0.000) and serum vitamin D levels of ≥10 ng/mL (OR 0.381, 95% CI 0.183‐0.791, P = 0.010) were independent risk factors for a successful H. pylori eradication (Table 4).
Table 4.
Factors affecting Helicobacter pylori eradication rate by multivariate analyses
| Variables | OR (95% CI) | P value |
|---|---|---|
| Serum vitamin D level (≥10/<10 ng/mL) | 0.381 (0.183‐0.791) | 0.010 |
| Treatment with adequate dosage and duration (yes/no) | 0.265 (0.133‐0.530) | 0.000 |
| Alcohol consumption (no/yes) | 0.821 (0.292‐2.273) | 0.704 |
Note: Statistically significant values are in bold. Abbreviations: CI, confidence interval; OR, odds ratio.
4. DISCUSSION
Since the discovery of H. pylori in 1982 its related diseases have been studied worldwide. In 1994 the International Agency for Research on Cancer identified H. pylori as a class I carcinogen.14 In 1997 the Maastricht consensus produced a world agreement on H. pylori infection. Since then the Consensus has been modified four times,15, 16, 17, 18, 19 and our understanding of the role of H. pylori in GC has been constantly updated. The occurrence of GC was used to be connected to a combination of H. pylori infection, host factors and environmental factors. However, currently 90% of non‐cardiac GC are related to H. pylori infection,2, 20 the overall function of environmental factors in GC has been found to be subordinate to that of H. pylori infection, and genetic factors are considered truly decisive in only 1%‐3% of hereditary diffuse GC.21 According to the latest consensus, the eradication of H. pylori should be a primary preventative measure for GC, as H. pylori infection is currently the foremost controllable risk factor for GC.19, 22, 23
China has a high level of H. pylori infection rate, with an overall infection rate of 56.22%, and Tibet even has the highest infection rates worldwide of 84.62%.24 Bacterial and host factors that need to be taken into account in H. pylori eradication include antibiotic resistance, virulence of the strains and host‐related genetic disorders.3 However, the drug resistance rates of H. pylori to clarithromycin, metronidazole and levofloxacin (fluoroquinolones) in China have been rising. In recent years, drug resistance rates of H. pylori have been reported to be 20%‐50% to clarithromycin, 40%‐70% to metronidazole and 20%‐50% to levofloxacin.25, 26, 27, 28, 29, 30, 31, 32 H. pylori can develop double, triple, or even greater resistance to these antibiotics.25, 26, 27 The reported double resistance rate to clarithromycin and metronidazole is more than 25%.28, 29, 30 Moreover, the type of H. pylori strain also affects its eradication. H. pylori contains a variety of virulent factors, including cytotoxin‐associated gene A, vacuolating cytotoxin A, duodenal ulcer promoting gene, outer inflammatory protein A and blood group antigen‐binding adhesion, which affect gastric mucosal inflammation and injury by activating inflammatory cell infiltration. Because virulence factors play a crucial part in gastric mucosal injury and affect H. pylori therapy,33 the virulence of different strains affects the eradication of H. pylori infection. In addition, the successful treatment of H. pylori infection also depends on genetic factors of the host, such as cytochrome P450 2C19, interleukin‐1β, and multidrug resistance gene 1.
Vitamin D is responsible for regulating calcium and phosphorus metabolism, which are needed for bone formation; however, many recent studies have found that vitamin D also affects H. pylori eradication. One study showed that VDP1 had a selective bactericidal effect on H. pylori but made no difference to the viability of Enterobacteriaceae bacteria, Pseudomonas aeruginosa, or Staphylococcus aureus.11 Studies have suggested that dimyristoylphosphatidylethanolamine (DMPE) is one of the most common glycerophospholipids constituting the cell membrane of H. pylori,34, 35 and VDP1 may induce bacterial dissolution by interacting with the DMPE of the H. pylori membrane. Another study found that the alkyl of indene, a product of vitamin D decomposition, had the key conformation of interaction with the di‐14:0 DMPE in the lipid membrane component of H. pylori, which eventually induced lysis, and the absence of alkyl led to the loss of a bactericidal effect on H. pylori.12 Several clinical studies have illustrated that vitamin D analogs may have anti‐H. pylori antimicrobial effects.4, 5, 6, 7, 8, 9, 10 Some studies have also found that serum vitamin D levels may have an impact on H. pylori eradication.7, 8, 9
However, the correlation between vitamin D and H. pylori remains unknown, and studies of the impact of serum vitamin D levels on H. pylori eradication included small sample sizes. Thus, we conducted this multicenter observational prospective cohort study to determine whether serum vitamin D levels had an impact on H. pylori infection and eradication rates and whether this was an independent risk factor for H. pylori eradication. A total of 496 H. pylori + and 257 H. pylori − patients were enrolled from four centers, and the patient population were from most areas of Mainland China. The results showed that serum vitamin D levels were lower in the H. pylori + group than in the H. pylori ‐ group. Furthermore, a low level of serum vitamin D in patients was related to a reduced H. pylori eradication rate. A multivariate regression analysis of the collected factors influencing H. pylori eradication demonstrated that serum vitamin D level <10 ng/mL was an independent risk factor for the failure to eradicate H. pylori. However, the notion that vitamin D supplements might decrease the infection rate of H. pylori and increase its eradication rate has yet to be evaluated. Unfortunately, we were unable to calculate the sample size needed due to the lack of specific data about the influence of serum vitamin D levels on the H. pylori eradication rate, although our sample size was relatively large. Therefore, randomized controlled studies with large sample sizes are urgently needed to validate our findings and to clarify whether vitamin D supplements improve the eradication rate of H. pylori, in order to provide guidance for H. pylori eradication therapy and the prevention of GC.
5. CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
We thank Dr Shan Hong Tang from the General Hospital of the Western Theater Command and Dr Yong Xi Wang from Xianyang Central Hospital for their contributions to the study. This study was supported by the National Natural Science Foundation of China (nos. 81470805 and 81873554) and the Shaanxi Science and Technology Innovation Team Project (no. 2018TD‐003).
Han C, Ni Z, Yuan T, et al. Influence of serum vitamin D level on Helicobacter pylori eradication: A multi‐center, observational, prospective and cohort study. J Dig Dis. 2019;20:421–426. 10.1111/1751-2980.12793
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 81470805, 81873554; Shaanxi Science and Technology Innovation Team Project, Grant/Award Number: 2018TD‐003
REFERENCES
- 1. Loor A, Dumitraşcu DL. Helicobacter pylori infection, gastric cancer and gastropanel. Rom J Intern Med. 2016;54(3):151‐156. [DOI] [PubMed] [Google Scholar]
- 2. Herrero R, Park JY, Forman D. The fight against gastric cancer—the IARC Working Group report. Best Pract Res Clin Gastroenterol. 2014;28(6):1107‐1114. [DOI] [PubMed] [Google Scholar]
- 3. Sokwala A, Shah MV, Devani S, Yonga G. Helicobacter pylori eradication: a randomised comparative trial of 7‐day versus 14‐day triple therapy. S Afr Med J. 2012;102(6 Pt 2):368‐371. [DOI] [PubMed] [Google Scholar]
- 4. Nasri H, Baradaran A. The influence of serum 25‐hydroxy vitamin D levels on Helicobacter pylori infections in patients with end‐stage renal failure on regular hemodialysis. Saudi J Kidney Dis Transpl. 2007;18(2):215‐219. [PubMed] [Google Scholar]
- 5. Kawaura A, Takeda E, Tanida N, et al. Inhibitory effect of long term 1α‐hydroxyvitamin D3 administration on Helicobacter pylori infection. J Clin Biochem Nutr. 2006;38(2):103‐106. [Google Scholar]
- 6. Antico A, Tozzoli R, Giavarina D, Tonutti E, Bizzaro N. Hypovitaminosis D as predisposing factor for atrophic type A gastritis: a case‐control study and review of the literature on the interaction of vitamin D with the immune system. Clin Rev Allergy Immunol. 2012;42(3):355‐364. [DOI] [PubMed] [Google Scholar]
- 7. Yildirim O, Yildirim T, Seckin Y, Osanmaz P, Bilgic Y, Mete R. The influence of vitamin D deficiency on eradication rates of Helicobacter pylori . Adv Clin Exp Med. 2017;26(9):1377‐1381. [DOI] [PubMed] [Google Scholar]
- 8. Huang B, Yan S, Chen C, Ye S. Effect of 25‐hydroxyvitamin D on Helicobacter pylori eradication in patients with type 2 diabetes. Wien Klin Wochenschr. 2019;131(3‐4):75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Shahawy MS, Hemida MH, El Metwaly I, Shady ZM. The effect of vitamin D deficiency on eradication rates of Helicobacter pylori infection. JGH Open. 2018;2(6):270‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surmeli DM, Surmeli ZG, Bahsi R, et al. Vitamin D deficiency and risk of Helicobacter pylori infection in older adults: a cross‐sectional study [Published online ahead of print Septebmer 28, 2018]. Aging Clin Exp Res. 10.1007/s40520-018-1039-1. [DOI] [PubMed] [Google Scholar]
- 11. Hosoda K, Shimomura H, Wanibuchi K, et al. Identification and characterization of a vitamin D3 decomposition product bactericidal against Helicobacter pylori . Sci Rep. 2015;5:8860 10.1038/srep08860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wanibuchi K, Hosoda K, Ihara M, et al. Indene compounds synthetically derived from vitamin D have selective antibacterial action on Helicobacter pylori . Lipids. 2018;53(4):393‐401. [DOI] [PubMed] [Google Scholar]
- 13. Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23(2):e12475. 10.1111/hel.12475. [DOI] [PubMed] [Google Scholar]
- 14. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (Vol. 61): Schistosomes, Liver Flukes and Helicobacter pylori. Lyon, France: International Agency for Research on Cancer; 1994. [PMC free article] [PubMed] [Google Scholar]
- 15. European Helicobacter pylori Study Group . Current European concepts in the management of Helicobacter pylori infection. The Maastricht consensus report. Gut. 1997;41(1):8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malfertheiner P, Mégraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection—the Maastricht 2‐2000 consensus report. Aliment Pharmacol Ther. 2002;16(2):167‐180. [DOI] [PubMed] [Google Scholar]
- 17. Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Gut. 2007;56(6):772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence consensus report. Gut. 2012;61(5):646‐664. [DOI] [PubMed] [Google Scholar]
- 19. Malfertheiner P, Megraud F, O'morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66(1):6‐30. [DOI] [PubMed] [Google Scholar]
- 20. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609‐e616. [DOI] [PubMed] [Google Scholar]
- 21. Tan RYC, Ngeow J. Hereditary diffuse gastric cancer: what the clinician should know. World J Gastrointest Oncol. 2015;7(9):153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol. 2013;23(6 Pt B):492‐501. [DOI] [PubMed] [Google Scholar]
- 24. Zhang WD, Hu FL, Xiao SD, et al. Prevalence of Helicobacter pylori infection in China [in Chinese]. Mod Dig Intervent. 2010;15(5):265‐270. [Google Scholar]
- 25. Zhang YX, Zhou LY, Song ZQ, Zhang JZ, He LH, Ding Y. Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: a prospective serial study. World J Gastroenterol. 2015;21(9):2786‐2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su P, Li Y, Li H, et al. Antibiotic resistance of Helicobacter pylori isolated in the southeast coastal region of China. Helicobacter. 2013;18(4):274‐279. [DOI] [PubMed] [Google Scholar]
- 27. Bai P, Zhou LY, Xiao XM, Luo Y, Ding Y. Susceptibility of Helicobacter pylori to antibiotics in Chinese patients. J Dig Dis. 2015;16(8):464‐470. [DOI] [PubMed] [Google Scholar]
- 28. Zhou L, Zhang J, Chen M, et al. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. Am J Gastroenterol. 2014;109(4):535‐541. [DOI] [PubMed] [Google Scholar]
- 29. Zhou L, Zhang J, Song Z, et al. Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment: a randomized trial. Helicobacter. 2016;21(2):91‐99. [DOI] [PubMed] [Google Scholar]
- 30. Song Z, Zhou L, Zhang J, He L, Bai P, Xue Y. Hybrid therapy as first‐line regimen for Helicobacter pylori eradication in populations with high antibiotic resistance rates. Helicobacter. 2016;21(5):382‐388. [DOI] [PubMed] [Google Scholar]
- 31. Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first‐line Helicobacter pylori therapy. Gut. 2015;64(11):1715‐1720. [DOI] [PubMed] [Google Scholar]
- 32. Xie C, Lu NH. Review: clinical management of Helicobacter pylori infection in China. Helicobacter. 2015;20(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 33. Broutet N, Tchamgoué S, Pereira E, Lamouliatte H, Salamon R, Mégraud F. Risk factors for failure of Helicobacter pylori therapy—results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther. 2003;17(1):99‐109. [DOI] [PubMed] [Google Scholar]
- 34. Shimomura H, Hosoda K, Hayashi S, Yokota K, Oguma K, Hirai Y. Steroids mediate resistance to the bactericidal effect of phosphatidylcholines against Helicobacter pylori . FEMS Microbiol Lett. 2009;301(1):84‐94. [DOI] [PubMed] [Google Scholar]
- 35. Shimomura H, Hosoda K, Hayashi S, Yokota K, Hirai Y. Phosphatidylethanolamine of Helicobacter pylori: function as a steroid‐binding lipid in the assimilation of free‐cholesterol and 3β‐hydroxl steroids into the bacterial cell membrane. J Bacteriol. 2012;194(10):2658‐2667. [DOI] [PMC free article] [PubMed] [Google Scholar]


