Abstract
Cache Valley virus (CVV) is a mosquito‐borne RNA virus detected throughout North America, Central America and parts of South America. A limited number of human case reports have described severe illness. CVV infection has been associated with outbreaks of congenital defects in small ruminants in Canada and the United States. A scoping review was conducted to identify, characterize and summarize research on CVV, and to identify research gaps. A structured search was conducted in eight electronic databases, with additional search verification and grey literature investigation. All captured studies were independently appraised by two reviewers for relevance and data characterization. The review captured 143 relevant studies investigating CVV epidemiology (n = 104), pathogenesis (n = 37), viral characteristics (n = 24), transmission (n = 14), diagnostic test performance (n = 8) and mitigation strategies (n = 2). Evidence of CVV infection was found in mosquito studies (n = 47), and serological evidence of exposure was demonstrated in animals (n = 41), as well as human (n = 20) studies. In sheep, five outbreaks of birth defects following asymptomatic dam CVV infection during the first 50 days of pregnancy were reported. Only six human cases of CVV‐associated illness were captured, with case symptoms described as initially non‐specific, progressing to more severe clinical signs (e.g., meningitis). No research was identified investigating treatment, societal knowledge and risk perception, economic burden or predictive models related to the impact of climate change on CVV. CVV circulates in mosquito and animal species across a large area of the Americas. Small ruminants are the only animals in which CVV‐associated clinical disease has been extensively studied. It is likely that human cases are under‐reported or misdiagnosed. Future research should focus on the impact of CVV infection in human and animal populations.
Keywords: vector‐borne diseases, viral pathogens, zoonoses
Impacts.
Cache Valley virus (CVV) circulates in mosquito and animal species across a large area of North, Central and South America.
The impact of CVV infection has only been extensively studied in small ruminants in the United States of America (USA) and Canada; foetal death or severe malformations may occur whether exposure occurs early in pregnancy.
There are only six published records of human CVV‐associated illness; however, serological studies indicate exposure may be common in some areas, in both humans and small ruminants.
1. INTRODUCTION
Cache Valley virus (CVV) is a mosquito‐borne virus belonging to the Bunyamwera serogroup of the Orthobunyavirus genus. The virus was first isolated from Culiseta inornata mosquitoes in Cache Valley, Utah, in 1956 (Holden & Hess, 1959). Since then, other closely related viruses have been identified. The Bunyamwera serogroup was created in 1960 by grouping together related viruses demonstrating some level of cross‐reactivity (Casals & Whitman, 1960). Currently, recognized CVV subtypes include Tlacotalpan and Playas, identified in Mexico and Ecuador, respectively (Armstrong, Andreadis, & Anderson, 2015; Blitvich, Lorono‐Pino, Garcia‐Rejon, Farfan‐Ale, & Dorman, 2012; Yang, Chan, et al., 2018). Similar viruses, such as Maguari and Xingu, have been categorized as genomically distinct from CVV (Armstrong et al., 2015; Blitvich, Lorono‐Pino, et al., 2012; Dunn, Pritlove, & Elliott, 1994; Groseth et al., 2017). Reassortant Bunyamwera viruses have been isolated, including Cholul virus and Potosi virus; both of these reassortants are also categorized as distinct from CVV (Armstrong et al., 2015; Groseth et al., 2017).
Cache Valley virus circulates in mosquitoes and mammals throughout North America, Central America and parts of South America (Armstrong et al., 2015). However, there have only been a small number of cases of human illness attributed to CVV infection (Wilson et al., 2017). Most of the reports of mammalian pathology attributable to CVV infection in mammals describe infection in pregnant sheep and goats (McConnell, Livingston, Calisher, & Crandell, 1985; Rodrigues, 2011; Shapiro et al., 2012). While CVV has been detected in multiple mosquito species, little research has investigated the epizootic cycle of CVV, or the relative importance of the major vectors responsible for transmission (Andreadis, Armstrong, Anderson, & Main, 2014; Ayers et al., 2018; Yang, Chan, et al., 2018).
A scoping review (ScR) on CVV was prioritized to identify, characterize and summarize the available primary research on CVV, and to identify knowledge gaps. The ScR is a research synthesis methodology that aims to describe and map the research underpinning a broad research question or emerging research area in a reproducible and updateable manner (Peters et al., 2017). Bias is minimized through a rigorous process and the use of a study protocol developed a priori. To the best of our knowledge, a ScR on CVV has not previously been conducted.
2. METHODS
2.1. ScR protocol and team
A study protocol was drafted a priori to document the rationale for the ScR and the search strategy, as suggested by Peters et al. (2017). The protocol also includes the appraisal tools and the analysis plan, which are provided in Appendix S1.
This manuscript conforms to the requirements of the PRISMA extension for ScRs (Tricco et al., 2018). The ScR team included seven individuals with expertise in knowledge synthesis, vector‐borne disease, zoonotic disease and library science.
2.2. Research question
The research question was “What is the global evidence on CVV?” The relevant context for the research question included the identification of all primary research from any settings ranging from insects and animals sampled in their natural environments, to species studied in laboratory settings, to in vitro studies of CVV. All populations which might potentially be sampled for demonstration of CVV or exposure to CVV were deemed relevant, including potential vectors, animal populations and human populations.
2.3. Search strategy
The pretested search algorithm was implemented on 16 May 2017 and updated 7 January 2019 in eight electronic bibliographic databases to capture publications relevant to the ScR: PubMed/MEDLINE, CAB, Agricola, EMBASE, Scopus, ProQuest Public Health, ProQuest Theses and Dissertations, and the COCHRANE library: (“cache valley” OR playas OR tlacotalpan) AND (virus).
Complete descriptions of each electronic search are presented in Appendix S1. In addition, a grey literature search was conducted (May 2017 and January 2019) in the Centers for Disease Control and Prevention (CDC) Stacks Public Health Database using the same search algorithm. Two Google searches were conducted, one using the original algorithm and one using (“cache valley” OR playas OR tlacotalpan) AND (virus) AND (outbreak); sequential hits were screened until hits were deemed irrelevant, in order to identify any additional grey literature reports. All potentially relevant citations not captured by the database search were added to the ScR.
A search verification strategy was executed to ensure that all relevant publications were identified. Reference lists from 15 selected CVV literature reviews were hand searched for potentially relevant references missed by the electronic search. Additionally, 10 CVV primary research studies were identified as “key studies,” and a search for studies citing these key studies was conducted in Google Scholar. Results were then cross‐referenced with those captured by the database search, and any potentially relevant citation was added to the ScR. A complete list of the literature reviews and key studies included in the search verification is presented in the study protocol (Appendix S1). Search verification was stopped at the point of saturation, when no additional new references were identified.
2.4. Relevance screening
Relevance screening, performed on the abstracts, titles and keywords of citations captured by the search, consisted of assessment of each citation with one question (“Does this citation describe primary research on CVV, or a predictive model examining the impacts of climate change on CVV”) to rapidly identify potentially relevant research (Appendix S1). All citations describing primary research on CVV or CVV subtypes Tlacotalpan virus and Playas virus were deemed relevant and promoted to the next review level of data characterization, and the full article was procured.
2.5. Study characterization
The second level of assessment, study characterization, was performed on full articles and captured data pertaining to the study focus, design, populations/samples and outcomes of the study. Exclusion criteria included publication in a language other than English or French, or study outcomes not pertaining to CVV or a subtype of CVV. A complete list of the characteristics captured at the data characterization level is presented in the data characterization tool (Appendix S1).
2.6. Data management and analysis
Citations captured by the search were uploaded and managed in Endnote X7© (Thomson Reuters). Duplicate citations were removed from the database prior to uploading the list of unique citations to DistillerSR (Evidence Partners, Ottawa, ON, Canada), a web‐based systematic review management platform. Each article was screened and characterized within DistillerSR independently by two reviewers; any conflicts between reviewers were resolved by consensus. The final data set was exported from DistillerSR to an Excel spreadsheet (Microsoft 365, Microsoft Corp.) where the descriptive analysis was conducted.
3. RESULTS
3.1. General characteristics
Two hundred and seventy‐four unique citations were screened at level one of the ScR for relevance, with 160 relevant studies promoted to second‐level study characterization (Figure 1). General characteristics of the 143 included relevant studies that were characterized in this ScR are described in Table 1. Most of the research originated from North America (n = 126 studies) and was published in English (n = 142) in journals (n = 123). Only one Spanish study was excluded due to language of publication. Eight reports were classified as grey literature containing primary data and included government surveillance reports (n = 6) and laboratory reports (n = 2). Overall, a median of two (range 0–7) studies on CVV have been published annually since the first CVV investigation was published in 1959 (Table 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram of the citations and articles throughout the scoping review process
Table 1.
General characteristics of 143 included publications
| Category | Count |
|---|---|
| Type of citation | |
| Primary peer‐reviewed research | 124 |
| Thesis | 8 |
| Grey literature with primary data | 8 |
| Conference proceeding | 3 |
| Language | |
| English | 142 |
| French | 1 |
| Continenta | |
| North America | 125 |
| USA | 104 |
| Canada | 12 |
| Mexico | 11 |
| Central America/South America/Caribbean | 16 |
| Europe | 2 |
| Australasia | 1 |
| Date of publication | |
| 1950–1959 | 1 |
| 1960–1969 | 20 |
| 1970–1979 | 17 |
| 1980–1989 | 25 |
| 1990–1999 | 30 |
| 2000–2009 | 17 |
| 2010‐Present | 33 |
| Study designa | |
| Observational | 110 |
| Prevalence | 74 |
| Cross‐sectional | 12 |
| Surveillance or monitoring programme | 12 |
| Case‐series or case report | 7 |
| Outbreak report | 6 |
| Case–control | 2 |
| Other | 7 |
| Experimental | 48 |
| Challenge trial | 31 |
| Molecular characterization | 13 |
| Controlled trial | 1 |
| Other | 9 |
| Evaluation of diagnostic tests | 8 |
Total may sum to more than 143 studies as some studies contributed to more than one category.
The most common research focus was epidemiology (n = 104 studies), of which nine studies reported surveillance activities; multiple studies investigated more than one population category (e.g., animals and mosquitoes from same location). Pathogenesis or case reports of CVV in human (n = 8 reports describing a total of six cases) and non‐human hosts (n = 29), in vitro studies of the virus (n = 24) and CVV transmission (n = 14) were also common foci for investigation. Fewer studies evaluated the performance of diagnostic tests for CVV (n = 8) or described the evaluation of mitigation strategies for CVV (n = 2) (Figure 2). No studies were captured addressing the treatment of clinical cases of CVV infection, societal knowledge and risk perceptions, economic burden of CVV or predictive models examining the impact of climate change on CVV. Forty‐one of 143 included studies reported more than one focus of investigation (e.g., CVV prevalence survey with characterization of isolates recovered).
Figure 2.
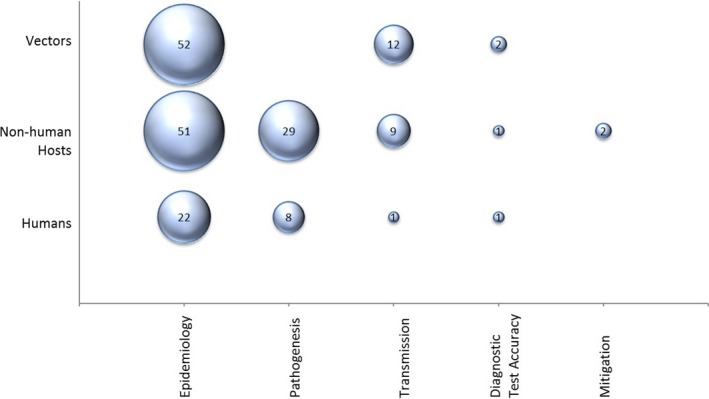
Bubble plot of 126 studies sampling humans, non‐human hosts and vectors, categorized by study focus. The sum of studies across categories may be larger than the category total as some studies cover more than one population or focus
3.2. Viral characteristics
Across 82 studies reporting on viral characteristics including genome and antigenicity of CVV, or CVV isolation, 108 different isolates of CVV were identified, as well as seven isolates of Tlacotalpan virus, and three isolates of Playas virus, with additional banked CVV isolates included in phylogenetic analyses (Appendix S3). Of 24 in vitro studies of CVV, the majority focused on viral characterization through immunological and molecular testing (n = 21/24 studies) of various CVV strains and other Bunyamwera viruses. Studies (n = 14) also focused on CVV transmission characteristics. Phylogenetic analysis was conducted on CVV sequences in 11 studies investigating the relationship among CVV isolates and between CVV and other closely related Bunyamwera viruses (Appendix S3).
Based upon more recent phylogenetic analyses, some of the early isolates collected from South America have been reclassified primarily from CVV to Maguari; the original studies of these virus isolates indicated that they seemed antigenically indistinguishable (Casals & Whitman, 1960; Downs, Spence, Aitken, & Whitman, 1961). These included the first isolates from Brazil (BeAr 7272) and Trinidad (Tr20659) and described around the same time as the first isolate reported in the USA (6V‐633) (Causey, Causey, Maroja, & Macedo, 1961; Holden & Hess, 1959). CVV isolates BeAr 7272 and 6V‐633 formed the basis of most antigenic testing in studies performed during the 1960s, with five studies from this period exclusively applying the Brazilian isolate (BeAr 7272) to analysis of samples from the USA (n = 3), Panama (n = 1) and Argentina (n = 1) (Buescher et al., 1970; Collins, 1965; Peralta, Shelokov, Vogel, & Longfellow, 1966; Sabattini, Shope, & Vanella, 1965; Work, 1964). More recent phylogenetic analyses have identified genetic mixing occurring among the related viruses; however, to date, Maguari or Maguari‐like viruses have been identified in Brazil, Argentina and Peru, whereas CVV isolates were identified in the USA, Canada, Mexico and Colombia, as well as CVV subtypes in Ecuador (Playas virus) and Mexico (Talactopan virus) (Groseth et al., 2017). Thus, the range of CVV and its subtypes may include all of North and Central America as well as some northern areas of South America.
3.3. Vectors of CVV
Findings of 52 observational studies sampling various mosquito species and 12 laboratory‐based experiments of transmission and vector competence of selected mosquito species are presented in Table 2. Investigators sampled 146 wild‐caught mosquito species (Appendix S4), with 47 of 52 studies reporting CVV detection in at least one sample. Eight laboratory‐based studies examined mosquito species for competence as a vector of CVV (defined as the ability of a vector to be infected with, replicate and transmit CVV). Natural infection with CVV and confirmed competence in experimental studies were reported for several species from the Aedes, Anopheles, Coquillettidia and Culiseta genuses including Ae. japonicus, Ae. scapularis, Ae. sollicitans, Ae. taeniorhynchus, Ae. vexans, An. punctipennis, An. quadrimaculatus, Co. perturbans and Cu. inornata (Table 2). Five additional mosquito species reportedly maintained experimental CVV infection although their ability to transmit CVV was not studied or demonstrated (Aedes albopictus, Coquillettidia venezuelensis, Culex corniger, Culex quinefasciatus and Mansonia arribalzaggi). One study reported maintenance of experimental CVV infection in Culex nigripalpus; however, no reports of CVV detection in wild‐caught Cu. nigripalpus were identified in this ScR. CVV was also detected in mosquito species from three other studies; however, vector competence was not an evaluated outcome in these studies (Table 2).
Table 2.
Summary of the 44 mosquito species from which Cache Valley virus (CVV) was detected in at least one sample (47 studies) across 52 observational studies or which were evaluated in 12 experimental studies
| Mosquito species | Total | Observational studies | Experimental studies | ||
|---|---|---|---|---|---|
| No. of Studies | No. of Studies (# reporting CVV) |
CVV Strains isolated |
No. of Studies | Competence description | |
| Aedes aegypti | 5 | 4 (0) | 1 | ||
| Aedes albopictus | 9 | 7 (3) | IL95−1704 | 2 | Disseminated infection in 1.2% of mosquitoes. No transovarial transmission |
| Aedes cantator | 7 | 7 (1) | 0 | ||
| Aedes cinereus | 12 | 11 (2) | 0 | ||
| Aedes japonicus | 5 | 4 (3) | 1 | Able to transmit the virus. After a 14‐day incubation, CVV was present in 28% of saliva samples | |
| Aedes scapularis | 6 | 5 (2) | TR 20659a | 1 | Able to transmit CVV to mice1 |
| BeAr 7272b | |||||
| Aedes sollicitans | 17 | 16 (6) | M273/61 | 1 | Able to transmit CVV to mice |
| M291/61 | |||||
| M303/61 | |||||
| M424/61 | |||||
| M515/61 | |||||
| M1724/63 | |||||
| Aedes taeniorhynchus | 14 | 13 (8) | 63B10 | 1 | Able to transmit CVV to mice |
| 63B54 | |||||
| 63B84 | |||||
| 63B141 | |||||
| CVV−478 | |||||
| CVV−390 | |||||
| CVV−213 | |||||
| CVV−078 | |||||
| CVV−002 | |||||
| M517/61 | |||||
| M1727/63 | |||||
| M1728/63 | |||||
| M2410/64 | |||||
| M2522/64 | |||||
| Tr48736 | |||||
| Aedes trivittatus | 1 | 1 | 0 | ||
| Aedes vexans | 28 | 27 (6) | SMIS 708 | 1 | Able to transmit CVV to mice |
| Anopheles albimanus | 4 | 4 (1) | 61C1 | 0 | |
| Anopheles crucians complex | 2 | 2 (1) | M600/61 | 0 | |
| Anopheles freeborni | 2 | 2 (1) | 0 | ||
| Anopheles grabhami | 1 | 1 (1) | J 7998 | 0 | |
| Anopheles punctipennis | 19 | 18 (6) | AR 4381 | 1 | Able to transmit CVV to mice |
| Anopheles quadrimaculatus | 21 | 17 (6) | AR 531 | 3 | Able to transmit CVV to mice |
| Anopheles walkeri | 10 | 10 (2) | 0 | ||
| Coquillettidia perturbans | 20 | 18 (4) | 2 | Able to transmit to mice and rabbits | |
| Coquillettidia venezuelensis c | 4d | 3 (0) | 1 | Maintained virus for at least 10 daysa | |
| Culex corniger | 3 | 2 (0) | 1 | Maintained virus for at least 22 daysa | |
| Culex fatigans | 2 | 1 (0) | 1 | No transovarial transmission | |
| Culex nigripalpus | 8d | 7 (0) | 1 | Able to transmit CVV to micea | |
| Culex pilosus | 1 | 1 (0) | 1 | ||
| Culex pipiens | 13 | 12 (1) | 1 | ||
| Culex quinquefasciatus | 7 | 6 (0) | 1 | Able to transmit CVV to mice after the 4th passagea | |
| Culex restuans | 15 | 15 (1) | 0 | ||
| Culex salinarius | 15 | 15 (2) | 0 | ||
| Culex tarsalis | 16 | 16 (3) | WMC 72 WMC 216 | 0 | |
| Culiseta inornata | 22 | 21 (8) | 6V−633 | Able to transmit CVV to mice. Demonstrated a low level of transovarial transmission | |
| OM 123 | |||||
| SMIS 381 | |||||
| SM 43 | |||||
| SMIS 436 | |||||
| SMIS 483 | |||||
| SMIS 833 | |||||
| SMIS 1035 | |||||
| SMIS 976 | |||||
| SMIS 1057 | |||||
| SMIS 1049 | |||||
| SMIS 160 | |||||
| SMIS 679 | |||||
| SMIS 735 | |||||
| SMIS 662 | |||||
| WM165 | |||||
| WM‐247‐71 | |||||
| Culiseta melanura | 9 | 9 (1) | 0 | ||
| Mansonia arribalzagai | 1 | 0 (0) | 1 | Maintained virus for at least 10 daysa | |
| Mansonia perturbans | 1 | 1 (1) | 0 | ||
| Mansonia titillans | 4 | 4 (1) | 61D240 | 0 | |
| Ochlerotatus canadensis | 17 | 17 (3) | 0 | ||
| Ochlerotatus communis | 8 | 7 (0) | 1 | No infection and did not transmit to mice | |
| Ochlerotatus dorsalis | 14 | 14 (3) | 0 | ||
| Ochlerotatus fitchii | 6 | 6 (1) | 85‐0708 | 0 | |
| Ochlerotatus serratus | 3 | 2 (0) | 1 | The virus had difficulty propagating. Low level of transmission to micea | |
| Ochlerotatus sticticus | 9 | 8 (0) | 1 | Low level of infection demonstrated and did not transmit to mice | |
| Ochlerotatus stimulans | 10 | 10 (1) | 0 | ||
| Ochlerotatus triseriatus | 20 | 18 (4) | Ar‐560‐79 | 2 | No infection demonstrated and did not transmit to mice |
| Ochlerotatus trivittatus | 17 | 17 (5) | 0 | ||
| Psorophora cingulata | 2d | 2 (2) | BT‐2368 | 0 | |
| Psorophora ferox | 14d | 14 (2) | AR 4249 | 0 | |
| Aedes/Ochlerotatus genus, species not specified | 14d | 14 (1) | 0 | ||
| Anopheles genus, species not specified | 6d | 6 (1) | 0 | ||
| Genus and species not specified | 13 | 13 (4) | 0 | ||
Isolate TR 20659 was isolated in Trinidad 1958 and was originally classified as CVV, but is now considered indistinguishable from BeAR7272 which is a Maguari virus isolate (Downs et al., 1961; Groseth et al., 2017).
Isolate BeAr 7272 was isolated in Brazil in 1954 and was originally classified as antigenically indistinguishable from CVV isolate 6V‐633 {634 Casals}, but has since been reclassified as Maguari virus (Groseth et al., 2017). This isolate came from a pool of mixed species: Aedes scapularis, Ae. serratus, Ae. sexlineatus, Mansonia sp., P. ferox.
Mosquito Coquillettidia venezuelensis has also been referred to as Mansonia venezuelensis in studies from the 1960s (Aitken & Spence, 1963; Peralta et al., 1966; Galindo, Srihongse, De Rodaniche, & Grayson,1966).
The isolate in one study included in this data set (Peralta et al., 1966) was confirmed as CVV using two isolates TR20659 and BeAr 7272 now categorized as Maguari virus.
Reports of North American mosquito surveillance, describing programmes operating between 1964 and 2016, were captured; sampling included Iowa, New York and across the USA and in the Canadian provinces of Manitoba and Saskatchewan (Appendix S3). One study demonstrated a correlation between quantity or load of circulating CVV, and the number of different mosquito species from which CVV could be isolated (Andreadis et al., 2014).
Other arthropods from which CVV was not detected include midges (Culicoides sonorensis, Culicoides variipennis and other species), sandflies (Lutzomyia diabolica and other species), ticks (Amblyomma americanum and Dermacentor variabilis), horseflies (Chrysops species, Diachlorus species, Hybomitra species and Tabanus species) and stable flies (Stomoxys calcitrans) (Appendix S3). One experimental transmission study concluded that Cu. sonorensis is not a competent vector of CVV (Reeves & Miller, 2013).
3.4. Non‐human hosts of CVV
Fifty‐one observational studies and 26 challenge trials examined the epidemiology, pathogenesis and mitigation strategies for CVV in infected animal hosts (Appendix S3). The hosts studied included the following: sheep, goats, cattle, horses, chickens, pigs, dogs, cats, rats, mice, squirrels, deer, rabbits, foxes, reptiles, primates and birds, as well as other rodents and wild animals. Challenge studies investigated CVV pathogenesis and transmission in sheep, goats, mice, cows, chickens, hamsters, deer, guinea pigs, opossums, donkeys, rabbits, raccoons and pigs (Appendix S3).
3.4.1. CVV studies in sheep and goats
The impact of CVV on small ruminants has been the focus of most of the non‐human host CVV literature (n = 31 studies), as CVV infection during pregnancy results in a range of negative birth outcomes from reproductive failure to major congenital defects. Eleven studies describing twelve challenge trials were conducted in the USA in sheep (n = 8 trials) and goats (n = 4) from 1969 to 2013, studying CVV pathogenesis (Appendix S3). Sixteen studies described the adverse foetal outcomes associated with CVV infection in small ruminants during early pregnancy (days 1–50 of gestation), the hallmark of which is a range of congenital defects of the skeletal and central nervous systems (Table 3). Six studies reported pathology in sheep naturally infected with CVV (Appendix S3). One study estimated the interval of time between CVV exposure and development of viremia in sheep (Chung, Livingston, Jones, & Collisson, 1991), with another describing the duration of viremia in CVV‐infected goats (Kokernot, Radivojevic, & Anderson, 1969).
Table 3.
Clinical signs and pathology reported in offspring of Cache Valley virus‐infected ewes and does across nine observational and eight experimental studies
| Outcomes of CVV infection | Number of studies reporting the sign | |||
|---|---|---|---|---|
| No. of observational studies | Proportion (%) subjects affected | No. of experimental studies | Proportion (%) subjects affected | |
| Clinical signs | ||||
| Appetite loss | 1 | 100.0 | ||
| Depression | 1 | 100.0 | ||
| Disorientation | 1 | NRa | ||
| Dystocia | 1 | NRa | 1 | 5.6 |
| Fever | 2 | NRa | ||
| Muscle spasms | 1 | NRa | ||
| Seizure | 1 | NRa | ||
| Suckling difficulties | 1 | NRa | ||
| Tremors | 1 | NRa | ||
| Congenital defects | ||||
| Ankylosis | 1 | 15.0 | ||
| Ataxia | 1 | NRa | ||
| Arthrogryposis | 5 | 50.0–80.0 | 5 | 12.5–41.6 |
| Concave spinal flexion | 1 | 6.3 | ||
| Encephalopathy | 3 | 93.3–100.0 | 1 | NRa |
| Hydranencephaly | 1 | NRa | 2 | 2.8 |
| Hydrocephalus | 1 | NRa | 1 | NRa |
| Inability to walk | 1 | 5.0 | ||
| Incomplete diaphragm | 1 | NRa | ||
| Kyphosis | 2 | NRa | 2 | 5.6–12.5 |
| Limb stiffness | 1 | NRa | ||
| Micromyelia | 1 | NRa | ||
| Mummification | 2 | NRa | 3 | 11.1–27.8 |
| Muscular abnormalities | 5 | NRa | 1 | NRa |
| Scoliosis | 5 | NRa | 2 | 5.6 |
| Skeletal abnormalities | 3 | 5.0–19.2 | ||
| Low birthweight | 1 | NRa | ||
| Stillbirths | 5 | 5.0–50.0 | ||
| Torticollis | 5 | NRa | 3 | 11.1–12.5 |
| Vertebral malformations | 1 | NRa | 1 | NRa |
| Weak/small trachea | 1 | 5.0 | ||
| Generalized weakness | 1 | 50.0 | ||
NR = proportion affected by symptom not reported in one or more articles.
Five outbreaks of clinical disease caused by CVV infection in sheep populations were described in six papers, occurring in the USA (in Texas, 1986–1988 and North Dakota, 1990) and in Canada (in Ontario, during 2011 and 2016, and Québec, 2013) (Appendix S3). These outbreaks were reported between the months of December and February, during lambing season, presenting with an unusually high prevalence of stillbirths and foetal deformities.
Sixteen studies reported the seroprevalence of CVV in sheep (Ovis aries 12/16, Ovis ammon 1/16, Ovis musimon 1/16) and in goats (Capra aegagrus 3/16), sampling animals during an outbreak (n = 3 studies), dams of lambs with congenital defects (n = 1) or selected healthy populations (n = 14) (Table 4, Appendix S3). The single study quantitatively investigating the association between housing and CVV exposure in sheep described a significant (p < 0.05) positive association between seroprevalence of CVV and sheep kept outside, relative to sheep kept in housing with four walls, a roof and a door closed most of the time (Meyers et al., 2015). This finding was consistent with a field experiment conducted in Texas, where sheep housed indoors remained CVV seronegative despite high seroconversion in pastured sheep (Crandell, Livingstone, & Shelton, 1989). Other potential risk factors identified for CVV seropositivity included: various flock management factors; increasing age, sex and breed of animals; year of sampling and location in Eastern USA states (Meyers et al., 2015; Shelton, de la Bermejillo, Willingham, & Mock, 1994).
Table 4.
Seroprevalence of Cache Valley virus (CVV) reported in populations of sheep and goats in 16 studies from Argentina, Canada, Mexico and the USA
| Location | Study date | Host species |
Seroprevalence +/N (%) |
Screening type | References |
|---|---|---|---|---|---|
| Argentina | |||||
| Córdoba | 1962–1963 | Capra aegagrus | 1/34 (2.9%)a | General Population | Sabattini et al. (1965) |
| Mexico | |||||
| Coahuila, Nuevo Leon & Tamaulipas | 1989 | Ovis musimon | 1/6 (16.7%) | General Population | Aguirre, McLean, Cook, and Quan (1992) |
| Nuevo Leon | 1988 | Ovis aries | 5/44 (11.4%) | Ewes with adverse birth outcomes | Chung (1992) |
| Yucatan | 2007–2008 | Ovis aries | 3/31 (9.7%) | General Population | Blitvich, Saiyasombat, Da Rosa, et al. (2012), Blitvich, Saiyasombat, DaRosa, et al. (2012) |
| USA | |||||
| California, Oregon and Washington | 2011 | Ovis aries | 12/686 (1.7%)b | General population | Meyers et al. (2015) |
| Colorado | 1981 | Ovis aries | 1/8 (12.5%) | General population | Chung (1992) |
| Colorado, Idaho, Kansas, Montana, New Mexico, South Dakota, Texas, Utah & Wyoming | 2011 | Ovis aries | 130/2459 (5.3%)b | General population | Meyers et al. (2015) |
| Illinois | 1988 | Ovis aries | 6/6 (100%) | Ewes with adverse birth outcomes | Chung (1992) |
| Indiana | 1992 | Capra aegagrus | 3/4 (75.0%) | General population | Blackmore (1996) |
| Iowa, Kentucky, Michigan, Minnesota, Missouri, New York, Ohio, Pennsylvania, Virginia and Wisconsin | 2011 | Ovis aries | 319/2005 (15.9%)b | General population | Meyers et al. (2015) |
| Kansas | 1981 | Ovis aries | 3/21 (14.3%) | General population | Chung (1992) |
| Kansas and Texas | 1991–1992 | Ovis aries | 336/1100 (30.5%) | General population | Shelton et al.. (1994) |
| Maryland and Virginia | 1962 | Capra aegagrus | 4/13 (30.8%)b | General population | Buescher et al. (1970) |
| Maryland and Virginia | 1962 | Ovis aries | 6/22 (27.3%)b | General population | Buescher et al. (1970) |
| Maryland | 1990 | Ovis aries | 9/9 (100%) | Ewes with adverse birth outcomes | Chung (1992) |
| Michigan | 1986 | Ovis aries | 9/10 (90%) | Ewes with adverse birth outcomes | Chung (1992) |
| Montana | 2013–2014 | Ovis aries | 1/104 (1.0%) | General population | Johnson et al. (2014) |
| Nebraska | 1987 | Ovis aries | 15/19 (78.9%) | Ewes with adverse birth outcomes | Chung (1992) |
| New York | 1989 | Ovis aries | 2/5 (40%) | Ewes with adverse birth outcomes | Chung (1992) |
| Oklahoma | 1988 | Ovis aries | 5/17 (29.4%) | Ewes with adverse birth outcomes | Chung (1992) |
| Texas | 1981 | Ovis aries | 7/20 (35.0%) | General population | Chung et al. (1991), Chung (1992) |
| Texas | 1981 | Ovis aries | 1/61 (1.6%) | General population | McConnell et al. (1985) |
| Texas | 1981 | Ovis aries | 1/1 (100%) | General population | McConnell et al. (1985), McConnell, Livingston, Calisher and Crandell (1987) |
| Texas | 1983 | Ovis ammon | 2/2 (100%) | General population | McConnell et al. (1987) |
| Texas | 1986 | Ovis aries | 9/104 (8.7%) | Outbreak population | Chung et al. (1991), Chung (1992) |
| Texas | 1986 | Ovis aries | (5%)c | Outbreak population | Edwards et al. (1988) |
| Texas | 1987 | Ovis aries | (78%)c | Outbreak population | Edwards et al. (1988) |
| Texas | 1987 | Ovis aries | 3/5 (60%)d | Outbreak population | Edwards et al. (1988) |
| Texas | 1988 | Ovis aries | 5/44 (11.4%) | Outbreak population | Chung et al. (1991), Chung (1992) |
| Texas | 1989 | Ovis aries | 64/89 (71.9%) | Outbreak population | Chung et al. (1991), Chung (1992) |
| Wyoming | 1981 | Ovis aries | 1/3 (33.3%) | General population | Chung et al. (1991) Chung (1992) |
| Canada | |||||
| Saskatchewan | 2013–2014 | Ovis aries |
84/130sheep (64.6%) 47/50 flocks (94.0%) |
General population | Uehlinger et al. (2018) |
The isolate used to make the antigenic test in this study was BeAr 7272, which has been reclassified as Maguari virus.
Number of positive cases estimated from data provided in the study.
N value not reported.
CVV was isolated from all three seropositive ewes.
3.5. CVV studies in other non‐human hosts
Seroprevalence of CVV was investigated in 33 studies sampling deer, rabbits, horses, swine, cows, foxes, dogs, raccoons, woodchucks, rats, voles, mules, wild birds, chickens, mongooses and monkeys (Appendix S5). Researchers reported isolation of CVV in Michigan from naturally infected horses in two studies conducted during a 1980 epizootic of eastern equine encephalitis (McLean et al., 1985; McLean, Calisher, & Parham, 1987). A study conducted in Maryland reported 45% of cattle sampled developed CVV neutralizing antibodies over a 6‐month study period (Yuill, Gochenour, Lucas, Collins, & Buescher, 1970). An association between increasing age and odds of CVV seropositivity was reported in studies sampling white‐tailed deer (n = 2 studies), horses (n = 1) and swine (n = 1) (Blackmore, 1996; Blackmore & Grimstad, 1998; McLean et al., 1987; Neitzel & Grimstad, 1991).
Challenge experiments (n = 15) were conducted in cows, guinea pigs, opossums, horses, chickens, hamsters, mice, deer, rabbits, raccoons and swine (Appendix S6). Most of these studies investigated the pathogenesis of CVV in animals (12/15) and/or the transmission of CVV to and from competent vectors (9/15). Researchers also evaluated the influence of vitamin A on the immune response to CVV in deer (Blackmore, 1996). Only one study reported pathology (failure to litter in mice) resulting from CVV infection in an animal host other than sheep or goats (Nowicki, 1996). Several aspects of experimentally induced CVV infection were described, including time of onset of CVV viremia post‐exposure in rabbits, swine, goats and calves (Blackmore & Grimstad, 2008; Kokernot et al., 1969) and duration of viremia in deer, hamsters, mice, guinea pigs, rabbits and calves (Blackmore & Grimstad, 1998; Edwards, Higgs, & Beaty, 1998; Kokernot et al., 1969). White‐tailed deer were identified as a potential reservoir for CVV, with reservoir defined as a population in which CVV naturally persists (Blackmore & Grimstad, 1998).
3.6. Transmission of CVV
Fourteen studies reported investigation of one or more broad aspects of CVV transmission, including vector‐to‐host transmission (n = 6 studies), host‐to‐vector transmission (n = 3), vector‐to‐vector transovarial only transmission (n = 3) and host‐to‐host transmission (n = 2). Four studies investigated more than one mode of transmission (Blackmore, Blackmore, & Grimstad, 1998; Blackmore & Grimstad, 2008; Corner, Robertson, Hayles, & Iversen, 1980; Saliba, DeFoliart, Yuill, & Hanson, 1973). Successful transmission of CVV to mice was demonstrated from eight species of mosquito: Ae. scapularis, Ae. sollicitans, Ae. taeniorhynchus, Ae. vexans, An. punctipennis, An. quadrimaculatus, Co. perturbans and Cu. inornata (Table 5). In one study, eastern cottontails (Sylvilagus floridanus) were successfully infected by Co. perturbans but did not experience sufficient viremia to transmit CVV back to the mosquito (Blackmore & Grimstad, 2008). In contrast, CVV transmission from hosts to mosquitoes was successful in studies using Syrian hamsters (n = 1) and mice (n = 3) (Table 5). One study demonstrated an interaction between detectable CVV infection in mice following a challenge and exposure to mosquitoes (Edwards et al., 1998). Eight studies investigated determinants of vector competence such as dose, extrinsic incubation period, infection rate, dissemination rate and transmission rate under experimental conditions (Aitken & Spence, 1963; Ayers et al., 2018; Blackmore et al., 1998; Miller, 1997; Reeves & Miller, 2013; Saliba et al., 1973; Yang, Chan, et al., 2018; Yuill & Thompson, 1970).
Table 5.
Transmission of Cache Valley virus reported in vectors and hosts across 10 studies
| Transmission type | Original CVV host | Recipient | Transmission successful? | References |
|---|---|---|---|---|
| Ae | Micea | Yesb | Aitken and Spence (1963) | |
| Vector to host | Aedes sollicitans | Mice: Charles River albino | Yes | Yuill & Thompson, (1970) |
| Aedes taeniorhynchus | Mice: Charles River albino | Yes | Yuill & Thompson (1970) | |
| Aedes vexans | Mice: Charles River CD1 and HA/ICR strain | Yes | Saliba et al. (1973) | |
| Anopheles punctipennis | Mice: Charles River CD1 and HA/ICR strain | Yes | Saliba et al. (1)973 | |
| Anopheles quadrimaculatus | Mice: ICR strain | Yes | Blackmore et al. (1998) | |
| Anopheles quadrimaculatus | Mice: Charles River CD1 and HA/ICR strain | Yes | Saliba et al. (1973) | |
| Coquillettidia perturbans | Mice: ICR strain | Yes | Blackmore et al. (1998) | |
| Coquillettidia perturbans | Rabbit: Sylvilagus floridanus | Yes | Blackmore and Grimstad (2008) | |
| Culiseta inornata | Micea | Yes | Corner et al. (1980) | |
| Culex nigripalpus | Micea | Yesb | Aitken and Spence (1963) | |
| Culex quinquefasciatus | Micea | Yesb | Aitken and Spence (1963) | |
| Ochlerotatus communis | Mice: Charles River CD1 and HA/ICR strain | No | Saliba et al. (1973) | |
| Ochlerotatus serratus | Micea | Yesb | Aitken and Spence (1963) | |
| Ochlerotatus triseriatus | Mice: Charles River CD1 and HA/ICR strain | No | Saliba et al. (1973) | |
| Host to vector | Syrian hamster: Mesocricetus auratus | Coquillettidia perturbans | Yes | Saliba et al. (1973) |
| Micea | Aedes scapularis | Yesb | Aitken and Spence (1963) | |
| Micea | Culiseta inornata | Yes | Corner et al. (1980) | |
| Mice and a feeding devicea | Aedes vexans | Yes | Saliba et al. (1973) | |
| Mice and a feeding devicea | Anopheles punctipennis | Yes | Saliba et al. (1973) | |
| Mice and a feeding devicea | Anopheles quadrimaculatus | Yes | Saliba et al. (1973) | |
| Mice and a feeding devicea | Ochlerotatus communis | No | Saliba et al. (1973) | |
| Mice and a feeding devicea | Ochlerotatus sticticus | Yes | Saliba et al. (1973) | |
| Mice and a feeding devicea | Ochlerotatus triseriatus | No | Saliba et al. (1973) | |
| Rabbit: Sylvilagus floridanus | Coquillettidia perturbans | No | Blackmore and Grimstad (2008) | |
| Vector to vector: transovarial | Aedes albopictus | Aedes albopictus (offspring) | No | Miller (1997) |
| Culex fatigans | Culex fatigans (offspring) | No | Tesh and Gubler (1975) | |
| Culiseta inornata | Culiseta inornata (offspring) | Ye | Corner et al. (1980) | |
| Host to host | Mice: ICR strain | Mice: ICR strain | No | Blackmore (1996) |
| Micea | Mice (offspring) a | Yes | Nowicki (1996) | |
| Humans | Humans (offspring)c | No | Nowicki (1996) |
Some studies investigated more than one mode of transmission (Blackmore et al., 1998; Blackmore & Grimstad, 2008; Corner et al., 1980; Saliba et al., 1973).
Species or strain name not specified.
Isolate TR 20659 was isolated in Trinidad 1958 and was originally classified as CVV but is now considered to be indistinguishable from BeAR7272 which is a Maguari virus isolate (Downs et al., 1961; Groseth et al., 2017).
Prenatal transmission determined by the presence of IgM antibodies to CVV in cord blood.
Successful transovarial transmission of CVV in Cu. inornata was reported in one study (Corner et al., 1980). However, unsuccessful transovarial CVV transmission attempts were reported in Ae. albopictus and Culex fatigans species, two species categorized to have limited competence as vectors of CVV (Table 5). In hosts, vertical transmission of CVV was demonstrated in mice, but adults did not become infected from the consumption of viremic pups and other contaminated bodily fluids (Blackmore, 1996; Edwards & Hendricks, 1997; Nowicki, 1996).
3.7. CVV studies in humans
Six human clinical cases of CVV were captured by this ScR; all attributed to CVV exposure occurring in the USA. The first case was reported in 1995 in North Carolina (CDC, 1996; Sexton et al., 1997), followed by single cases occurring in Wisconsin in 2003 (Campbell et al., 2006), New York in 2011 (CDC, 2012; Nguyen et al., 2013) and Missouri 2015 (CDC, 2017). More recently, in 2016 two immune‐compromised men from New York and Australia, the latter with travel history to the USA, were diagnosed with CVV‐associated meningoencephalitis (Wilson et al., 2017; Yang, Qiu, et al., 2018b). Researchers hypothesized that the Australian patient's exposure likely occurred in 2013, but the diagnosis of CVV infection did not occur until 2016 after several years of chronic neurological deficits, eventually diagnosed as chronic meningoencephalitis (Wilson et al., 2017). The clinical pathology of these severe human CVV cases was reported in journal articles, except for the Missouri case, which was only described in a surveillance report with limited case information (CDC, 2017).
Symptoms of CVV infection reported from five of the six published cases include non‐specific clinical signs: fever (reported in 5/5 cases), headaches (3/5), nausea (3/5), vomiting (3/5), appearance of rash (2/5), body aches (1/5) and confusion (1/5). Three patients experienced long‐term symptoms including persistent headaches (reported in 2/3 cases), difficulty in word finding (1/3), memory loss (1/3) and motor control deficits (1/3) (Campbell et al., 2006; Nguyen et al., 2013; Sexton et al., 1997). The Australian patient eventually diagnosed with CVV chronic meningoencephalitis displayed 6 months of progressive memory decline, slowing of speech and mood disturbance, predominantly anxiety; the diagnosis was made via metagenomic next‐generation sequencing of the patient's cerebrospinal fluid and brain biopsy tissue (Wilson et al., 2017). CVV illness was ultimately fatal, either directly or due to other complications, in three of five CVV cases for which outcome data were reported (Sexton et al., 1997; Wilson et al., 2017; Yang, Qiu, et al., 2018b).
Twenty human CVV serosurveys conducted in North and South America, reported human CVV seroprevalence ranging from 0% to 50%, in studies published from 1956 to 2010 (Table 6). As with non‐human animal hosts, increasing age was associated with increasing odds of seropositivity in some studies (Blitvich, Saiyasombat, Talavera‐Aguilar, et al., 2012; Heard, 1997). Two case–control studies investigated the potential association between the presence of maternal CVV antibodies and congenital malformations in their babies. While one study did not detect any CVV antibodies in the sample population (Edwards & Hendricks, 1997), the second case–control study had a very small sample size, but did report a significant association between CVV antibodies in mothers and the occurrence of macrocephaly or microcephaly in their newborns (Calisher & Sever, 1995).
Table 6.
Seroprevalence of Cache Valley virus reported in human populations between 1956 and 2009 in 20 studies
| Country/Regiona | Sampling date | Study design | Population | Positive/N | Proportion positive (%) | References |
|---|---|---|---|---|---|---|
| North America | ||||||
| Canada | ||||||
| Manitoba | 2009 | Prevalence Survey | Suspected West Nile cases. | 9/55 | 16.4 | Dimitrova et al. (2011) |
| Saskatchewan | 2009 | Prevalence Survey | Suspected West Nile cases. | 9/216 | 4.2 | Dimitrova et al. (2011) |
| Mexico | ||||||
| Chiapas & Tabasco | 1969 | Prevalence Survey | Outpatients; Men in factories, prisons, and the army | 1/46 | 2.2 | Goldsmith et al. (1979) |
| Coatetelco | 1961 | Prevalence Survey | Humans age 41–75 | 0/20 | 0 | Scherer, Campillo‐Sainz, Dickerman, Diaz‐Najera, and Madalengoitia (1967) |
| Oaxaca | 1969 | Prevalence Survey | Outpatients; Men in factories, prisons, and the army | 13/493 | 2.6 | Goldsmith et al. (1979) |
| Tlacotalpan | 1961 | Prevalence Survey | Humans age 20–41 | 0/20 | 0 | Scherer et al. (1967) |
| Tlacotalpan | 1961 | Prevalence Survey | Humans age 41–75 | 7/18 | 38.9 | Scherer et al. (1967) |
| Campeche, Quintana Roo & Yucatan | 2007 | Prevalence Survey | Febrile patients | 18/146b | 12.3 | Blitvich, Lorono‐Pino, et al. (2012) |
| USA | ||||||
| Alaska | 1983–1986 | Prevalence Survey | General population | 0/90 | 0 | Walters, Tirrell, and Shope (1999) |
| California | 1987 | Prevalence Survey | Outpatients; Outdoor workers | 0/235 | 0 | Campbell, Reeves, Hardy, and Eldridge (1992) |
| California | 1988 | Prevalence Survey | Outpatients; Outdoor workers | 0/118 | 0 | Campbell et al. (1992) |
| Colorado | 2008–2009 | Prevalence Survey | Rocky Mountain National Park Employees | 2/60 | 3.3 | Kosoy et al. (2016) |
| Florida | 1960 | Cross‐sectional | Humans aged 1–15 living a reservation | 2/5 | 40.0 | Work (1964)c |
| Florida | 1960 | Cross‐sectional | Humans age 16 + living in a reservation | 12/60 | 20.0 | Work (1964)c |
| Illinois | 1964 | Prevalence Survey | General population | 1/59 | 1.7 | Kokernot et al. (1969) |
| Indiana | 1989–1990 | Prevalence Survey | Hospital patients | 36/2696 | 1.3 | Heard (1997) |
| Indiana | 1990–1993 | Prevalence Survey | Individuals with CNS infections | 3/244 | 1.2 | Heard (1997) |
| Indiana | 1993–1994 | Cross‐sectional | Live birth cord samples | 32/1088 | 2.9 | Nowicki (1996) |
| Kentucky | 1965 | Prevalence Survey | General population | 3/55 | 5.5 | Kokernot et al. (1969) |
| Kentucky | 1966 | Prevalence Survey | General population | 2/72 | 2.8 | Kokernot et al. (1969) |
| Maryland | 1962–1963 | Prevalence Survey | General population | 9/180 | 5.0 | Buescher et al. (1970)c |
| Tennessee | 2008–2009 | Prevalence Survey | Great Smoky Mountain National Park Employees | 2/75 | 2.7 | Kosoy et al. (2016) |
| Utah | 1956 | Prevalence Survey | Humans in the vicinity of the first CVV isolations | 0/5 | 0 | Holden and Hess (1959) |
| Virginia | 1961 | Prevalence Survey | General population | 33/176 | 18.7 | Buescher et al. (1970)c |
| Wyoming | 2010 | Prevalence Survey | Grand Teton National Park seasonal employees | 5/160 | 3.1 | Kosoy et al. (2016) |
| South America | ||||||
| Argentina | ||||||
| Buenos Aires, Mendoza & Tucumán | 1961 | Prevalence Survey | Healthy males 20–40 years | 4/175 | 2.3 | Mettler, Parodi, and Casals (1963) |
| Córdoba & Litoral | 1961–1963 | Prevalence Survey | General population | 6/213 | 2.8 | Sabattini et al. (1965)c |
| Córdoba | 2004–2005 | Prevalence Survey | Outpatients | 40/638 | 6.3 | Tauro, Almeida, and Contigiani (2009)c |
| Guyana | ||||||
| Upper Takutu‐Upper Essequibo | 1956 | Prevalence Survey | General population | 4/18 | 22.2 | Downs et al. (1961)c |
| Upper Takutu‐Upper Essequibo | 1959 | Prevalence Survey | General population | 4/8 | 50.0 | Downs et al. (1961)c |
| Jamaica | ||||||
| Kingston, Portland, St. Andrew, St. James & St. Thomas | 1963–1964 | Prevalence Survey | Children and adults; positive results were mainly children under 5 | 29/531 | 5.5 | Belle, Grant and Griffiths (1966) |
| Portland, St. Andrew & St. Thomas | 1963–1967 | Prevalence Survey | Humans age 5–15 | 0/25 | 0 | Belle, King, Griffiths, and Grant (1980) |
| Portland, St. Andrew & St. Thomas | 1963–1967 | Prevalence Survey | Humans age 15+ | 5/77 | 6.5 | Belle et al. (1980) |
| NRd | 1989e | Prevalence Survey | Individuals with and without HTLV−1 antibodies | 16/200 | 8.0 | Murphy, Calisher, Figueroa, Gibbs, and Blattner (1989) |
| Trinidad | ||||||
| Port of Spain | 1961e | Prevalence Survey | Field staff members of the Trinidad Regional Virus Laboratory | 4/11 | 36.4 | Downs et al. (1961)c |
| Sangre Grande | 1961e | Prevalence Survey | General population | 15/46 | 32.6 | Downs et al. (1961)c |
Region is the state or province studied as described by the author.
Number of positive cases estimated from data provided in the study.
The antigenic test employed was developed using isolate BeAr 7272 (Buescher et al., 1970; Work, 1964), TR 20659 (Downs et al., 1961) and CbaAr‐ 426 (Sabattini et al., 1965), which have been reclassified as Maguari virus (Groseth et al., 2017). CVV and Maguari virus isolates were shown to be indistinguishable antigenically at the time of these studies (Casals & Whitman, 1960).
NR = data not recorded.
Publication date reported as study date was not available.
3.8. Diagnostic test performance
Eight studies assessed the performance of diagnostic tests for the detection of viral RNA or serological evidence of prior CVV infection. The majority of these papers described initial proof‐of‐accuracy studies, using CVV‐spiked samples at various dilutions and in various media to evaluate the sensitivity of the test; they also employed an array of other viruses to assess test specificity. Among these eight studies, samples used included pooled mosquitoes (n = 2/8 studies), and animal (n = 1/8) or human (n = 1/8) sera. We also captured in vitro experiments (n = 5/8). The captured diagnostic test studies investigated the performance of molecular (n = 5/8) and immunological (n = 3/8) assays, as well as virus isolation techniques (n = 1/8). All five molecular assays were reverse‐transcription polymerase chain reaction tests (Appendix S3). Reported performance parameters included analytical sensitivity (n = 2 studies), specificity (n = 3) and limits of detection (n = 2). One study investigated both immunological and molecular tests (Brockus,2000).
4. DISCUSSION
The literature captured in this ScR describes a relatively small, but ongoing area of viral research. Serosurveys of mammals have yielded evidence of CVV exposure in domestic and wild animals across the Americas. The interpretation of the chronologically older surveys is complicated by the relative lack of diagnostic specificity of earlier assays, which could have led to some misclassification of samples (Drebot, 2015). The relatively widespread evidence of CVV exposure in mammals, and detection in a variety of mosquito species across the Americas, makes the very small number of reported cases of clinical illness attributable to CVV infection striking.
Outbreaks of congenital anomalies in lambs born to CVV‐infected dams were reported in the USA in the late 1980s (Chung et al., 1991; Edwards et al., 1988). More recently, three outbreaks of CVV‐associated reproductive failure in sheep have been reported by diagnostic laboratories in the Canadian provinces of Ontario and Québec (Leboef & Cotê, 2013; Shapiro et al., 2012; Spinato et al., 2016). A serosurvey of domestic animals in the western Canadian province of Saskatchewan found widespread evidence of CVV exposure in sheep, cattle, goats, horses and mule deer (Uehlinger, Wilkins, Godson, & Drebot, 2018). Thus, it is plausible that CVV infection may have been under‐diagnosed as a potential cause of reproductive failure and congenital defects in small ruminants in Canada, at least in regions where evidence of CVV exposure has been reported in domestic or wild animals. Given that two of the recent Canadian outbreaks were only reported in a non‐indexed producer resource, it is also likely that CVV outbreaks are under‐reported or are reported in grey literature media that are difficult to identify in a systematic search. Thus, despite our best efforts, there may be reports of CVV outbreaks in small ruminants that were missed by our search.
There have been a very small number of human clinical cases of CVV‐associated illness recorded to date. The scarcity of human clinical cases contrasts with the much larger proportion of humans with serological markers of CVV exposure reported from various populations in the Americas. As with the CVV situation in animals, CVV exposure seems to occur much more frequently than clinical disease. However, there is insufficient evidence to identify the full spectrum of clinical signs during CVV‐associated illness, or the degree of under‐diagnosis of CVV occurring, or potentially at‐risk populations (e.g., 2/6 CVV cases occurred in immune‐compromised subjects) (Wilson et al., 2017; Yang, Qiu, et al., 2018b).
In Canada, ongoing “lookbacks” at suspect West Nile virus patients have revealed that some of these subjects have serological markers of CVV exposure, further supporting the possibility that under‐diagnosis of CVV in human encephalitis or meningitis patients may occur (Dimitrova et al., 2011). Similarly, the potential impact of CVV infection during pregnancy, currently recognized only in small ruminants, merits further research in other species, given the very small number of studies investigating CVV during pregnancy in humans and mice (Blackmore, 1996; Calisher & Sever, 1995; Edwards & Hendricks, 1997; Nowicki, 1996).
Subgroups, or complexes, within the Bunyamwera serogroup have been proposed, and these subgroups are correlated with the geographic location where the viruses were isolated (Hunt & Calisher, 1979). Based on the most recent phylogenetic analyses, CVV has been identified as being most closely related to Maguari and Fort Sherman viruses, with Tlacotalpan and Playas classified as subtypes of CVV (Armstrong et al., 2015; Blitvich, Lorono‐Pino, et al., 2012; Yang, Chan, et al., 2018). The relationship between CVV and these closely related viruses is complex, and overtime has resulted in the reclassification of several isolates; for example, BeAr 7272 and CbaAr‐426, originally categorized as CVV, have been reclassified as Maguari or Bunyamwera virus isolates (Groseth et al., 2017).
Reports of recent phylogenetic analyses conclude that some genetic diversity has been reported across CVV strains, but overall CVV is a well‐conserved virus across geography and several decades (Armstrong et al., 2015; Blitvich, Lorono‐Pino, et al., 2012; Groseth et al., 2017). The emergence of a new CVV lineage in Connecticut, more closely related genomically to CVV strains from Mexico than to other locations in the United States, suggests a possible importation event of a novel CVV strain to a new location (Armstrong et al., 2015). Therefore, the potential exists for future introductions of relatively novel CVV strains into new geographic areas, whether fortuitously in association with human activity, or as the geographic range of vectors expands.
Recent genomic analyses identified that different segments of the viral genome are conserved across similar, but distinct viruses, which explains the antigenic similarities and differences identified between viruses using different antigen‐based methods of viral characterization (Groseth et al., 2017). One implication of this finding is that distinguishing between viruses within the Orthobunyavirus genus may be difficult with antigenic tests, and even molecular tests need to be comprehensive enough (e.g., target several discerning segments of the viral genome) to distinguish between closely related viruses and any reassortments that may occur (Blitvich, Lorono‐Pino, et al., 2012; Groseth et al., 2017). Thus, CVV serological testing across studies may not have distinguished between some distinct, but related viruses, particularly in the early CVV studies from the 1950s and 1960s. Given the evidence for the current geographic distribution of CVV, it is likely that some CVV‐positive serological results in the southern areas of South America were detecting exposure to closely related viruses such as Maguari virus (Casals & Whitman, 1960; Peralta et al., 1966; Sabattini et al., 1965). Also, considering the virus’ geography, studies conducted in North America in which the antigenic test was developed with a Maguari virus isolate likely represent exposure to CVV rather than Maguari (Buescher et al., 1970; Work, 1964). Across studies presenting serological results, some misclassification of CVV exposure may have occurred with an unknown direction and magnitude, and this contributes some uncertainty about the estimates from these studies.
While we tried to conduct an exhaustive literature search, it is possible that some relevant research may have been missed, especially if it were only reported in grey literature such as producer updates. However, we feel that the potential for language bias in this ScR was low since only one potentially relevant Spanish paper was excluded due to language of publication.
Several knowledge gaps were identified by our ScR, including evidence characterizing the CVV epizootic cycle through studies that identified important vectors and mammalian reservoirs. The factors associated with increased prevalence of circulating CVV in major vectors or reservoirs are unclear. The prevalence of CVV exposure in humans in the Americas in the general population, or within targeted patient groups such as encephalitis patients, lacks representative evidence and therefore also remains somewhat unclear.
Future directions for CVV research therefore include several topic areas. Continued improvement in assays for evidence of CVV exposure, for example, in the area of specificity, that is the correct categorization of samples truly not exposed to CVV, would be useful in the ongoing surveillance of mammalian and human populations. The use of representative sampling frames for serosurveillance could help to identify potential reservoir species and guide additional monitoring for viral detection in reservoir populations. Regular lookbacks for CVV case finding in human populations, such as suspect cases for other viral encephalitides, would help to improve our understanding of the true burden of clinical disease associated with human CVV infection. Similarly, knowledge translation to improve awareness among food animal veterinarians of CVV as a potential cause of reproductive failure and congenital defects in small ruminants could help provide a more accurate understanding of the true burden of CVV infection in these populations.
5. CONCLUSIONS
Centers for Disease Control circulates in numerous mosquito and animal species across a large area from North to South America. However, clinical disease caused by CVV infection has only been extensively studied in small ruminants in the USA and Canada. Published reports of human disease attributable to CVV infection describe six severe cases; however, CVV‐associated illness is likely under‐reported or misdiagnosed. Future research should focus on characterizing the impact of CVV infection in human and animal populations.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGEMENT
We would like to acknowledge the PHAC library for their help with procuring articles.
Waddell L, Pachal N, Mascarenhas M, et al. Cache Valley virus: A scoping review of the global evidence. Zoonoses Public Health. 2019;66:739–758. 10.1111/zph.12621
REFERENCES
- Aguirre, A. A. , McLean, R. G. , Cook, R. S. , & Quan, T. J. (1992). Serologic survey for selected arboviruses and other potential pathogens in wildlife from Mexico. Journal of Wildlife Diseases, 28, 435–442. 10.7589/0090-3558-28.3.435 [DOI] [PubMed] [Google Scholar]
- Aitken, T. H. , & Spence, L. (1963). Virus transmission studies with Trinidadian mosquitoes. III. Cache Valley virus. West Indian Medical Journal, 12, 128–132. [PubMed] [Google Scholar]
- Andreadis, T. G. , Armstrong, P. M. , Anderson, J. F. , & Main, A. J. (2014). Spatial‐temporal analysis of Cache Valley virus (Bunyaviridae: Orthobunyavirus) infection in anopheline and culicine mosquitoes (diptera: Culicidae) in the northeastern United States, 1997–2012. Vector‐Borne and Zoonotic Diseases, 14, 763–773. 10.1089/vbz.2014.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, P. M. , Andreadis, T. G. , & Anderson, J. F. (2015). Emergence of a new lineage of Cache Valley virus (Bunyaviridae: Orthobunyavirus) in the northeastern United States. American Journal of Tropical Medicine and Hygiene, 93, 11–17. 10.4269/ajtmh.15-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers, V. B. , Huang, Y. J. S. , Lyons, A. C. , Park, S. L. , Higgs, S. , Dunlop, J. I. , … Vanlandingham, D. L. (2018). Culex tarsalis is a competent vector species for Cache Valley virus. Parasites and Vectors, 11, 519 10.1186/s13071-018-3103- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle, E. A. , Grant, L. S. , & Griffiths, B. B. (1966). The isolation of Cache Valley virus from mosquitoes in Jamaica. West Indian Medical Journal, 15, 217–220. [PubMed] [Google Scholar]
- Belle, E. A. , King, S. D. , Griffiths, B. B. , & Grant, L. S. (1980). Epidemiological investigation for arboviruses in Jamaica, West Indies. American Journal of Tropical Medicine and Hygiene, 29, 667–675. [DOI] [PubMed] [Google Scholar]
- Blackmore, C. G. M. (1996). The epizootiology of Cache Valley and Potosi viruses (Bunyaviridae) in northern Indiana. Available from ProQuest Dissertations & Theses A&I; The epizootiology of Cache Valley and Potosi viruses (Bunyaviridae) in northern Indiana. (304282973). [Google Scholar]
- Blackmore, C. G. M. , Blackmore, M. S. , & Grimstad, P. R. (1998). Role of Anopheles quadrimaculatus and (diptera: Culicidae) in the transmission cycle of Cache Valley virus (Bunyaviridae: Bunyavirus) in the midwest, U.S.A. Journal of Medical Entomology, 35, 660–664, 197. [DOI] [PubMed] [Google Scholar]
- Blackmore, C. G. M. , & Grimstad, P. R. (1998). Cache Valley and Potosi viruses (Bunyaviridae) in white‐tailed deer (Odocoileus virginianus): Experimental infections and antibody prevalence in natural populations. American Journal of Tropical Medicine and Hygiene, 59, 704–709, 196. 10.4269/ajtmh.1998.59.704 [DOI] [PubMed] [Google Scholar]
- Blackmore, C. G. , & Grimstad, P. R. (2008). Evaluation of the eastern cottontail Sylvilagus floridanus as an amplifying vertebrate host for Cache Valley virus (Bunyaviridae) in Indiana. Journal of Wildlife Diseases, 44, 188–192. 10.7589/0090-3558-44.1.188 [DOI] [PubMed] [Google Scholar]
- Blitvich, B. J. , Lorono‐Pino, M. , Garcia‐Rejon, J. , Farfan‐Ale, J. , & Dorman, K. S. (2012). Nucleotide sequencing and serologic analysis of Cache Valley virus isolates from the Yucatan peninsula of Mexico. Virus Genes, 45, 176–180. 10.1007/s11262-012-0741-x [DOI] [PubMed] [Google Scholar]
- Blitvich, B. J. , Saiyasombat, R. , Da Rosa, A. T. , Tesh, R. , Calisher, C. , Dorman, K. , … Maria, L. P. (2012). Cholul virus is a novel orthobunyavirus reassortant created between Cache Valley and Potosi viruses and a cause of infection of humans and livestock in the Yucatan peninsula of Mexico. American Journal of Tropical Medicine and Hygiene, 1, 170. [Google Scholar]
- Blitvich, B. J. , Saiyasombat, R. , DaRosa, A. T. , Tesh, R. B. , Calisher, C. H. , Garcia‐Rejon, J. , … Loroño‐Pino, M. A. (2012). Orthobunyaviruses, a common cause of infection of livestock in the Yucatan peninsula of Mexico. American Journal of Tropical Medicine and Hygiene, 87, 1132–1139. 10.4269/ajtmh.2012.12-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich, B. J. , Saiyasombat, R. , Talavera‐Aguilar, L. G. , Garcia‐Rejon, J. E. , Farfan‐Ale, J. A. , Carlos Machain‐Williams, C. , & Loroño‐Pino, M. A. (2012). Orthobunyavirus antibodies in humans, Yucatan peninsula, Mexico. Emerging Infectious Diseases, 18, 1629–1632. 10.3201/eid1810.120492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockus, C. L. (2000). The medium genomic segment of Cache Valley virus: Molecular and immunological characteristics of the G1 glycoprotein among virus isolates. Ann Arbor: University of Notre Dame. [Google Scholar]
- Buescher, E. L. , Byrne, R. J. , Clarke, G. C. , Gould, D. J. , Russell, P. K. , Scheider, F. G. , & Yuill, T. M. (1970). Cache Valley virus in the Delmarva peninsula. I. virologic and serologic evidence of infection. American Journal of Tropical Medicine and Hygiene, 19, 493–502. [DOI] [PubMed] [Google Scholar]
- Calisher, C. H. , & Sever, J. L. (1995). Are North American Bunyamwera serogroup viruses etiologic agents of human congenital defects of the central nervous system? Emerging Infectious Diseases, 1, 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, G. L. , Mataczynski, J. D. , Reisdorf, E. S. , Powell, J. W. , Martin, D. A. , Lambert, A. J. , … Lanciotti, R. S. (2006). Second human case of Cache Valley virus disease. Emerging Infectious Diseases, 12, 854–856. 10.3201/eid1205.051625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, G. L. , Reeves, W. C. , Hardy, J. L. , & Eldridge, B. F. (1992). Seroepidemiology of California and Bunyamwera serogroup bunyavirus infections in humans in California. American Journal of Epidemiology, 136, 308–319. 10.1093/oxfordjournals.aje.a116496 [DOI] [PubMed] [Google Scholar]
- Casals, J. , & Whitman, L. (1960). A new antigenic group of arthropod‐borne viruses. The American Journal of Tropical Medicine and Hygiene, 9, 73–77. 10.4269/ajtmh.1960.9.73 [DOI] [PubMed] [Google Scholar]
- Causey, O. R. , Causey, C. E. , Maroja, O. M. , & Macedo, D. G. (1961). The isolation of arthropod‐borne viruses, including members of two hitherto undescribed serological groups, in the Amazon region of Brazil. American Journal of Tropical Medicine and Hygiene, 10, 227–249. 10.4269/ajtmh.1961.10.227 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (1996). Summary of notifiable disease, United States 1995. Morbidity and Mortality Weekly Report, 44 Retrieved from https://stacks.cdc.gov/view/cdc/5644 [PubMed] [Google Scholar]
- Centers for Disease Control (2012). West Nile virus disease and other arboviral diseases ‐ United States, 2011. Morbidity and Mortality Weekly Report, 61, 510–514. [PubMed] [Google Scholar]
- Centers for Disease Control (2017). West Nile virus disease and other arboviral diseases ‐ United States, 2015. Morbidity and Mortality Weekly Report, 66, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S. (1992). Molecular and pathogenesis studies of cache valley virus: A common teratogenic agent in sheep. Available from ProQuest Dissertations & Theses A&I; Molecular and pathogenesis studies of Cache Valley virus: A common teratogenic agent in sheep. (304025564). [Google Scholar]
- Chung, S. I. , Livingston, C. W. Jr , Jones, C. W. , & Collisson, E. W. (1991). Cache Valley virus infection in Texas sheep flocks. Journal of the American Veterinary Medical Association, 199, 337–340. [PubMed] [Google Scholar]
- Collins, M. J. Jr (1965). Studies on physical, chemical, and biological properties of Cache Valley (BeAr 7272) virus. Available from ProQuest Dissertations & Theses A&I (6601346). [Google Scholar]
- Corner, L. C. , Robertson, A. K. , Hayles, L. B. , & Iversen, J. O. (1980). Cache Valley virus: Experimental infection in Culiseta inornata . Canadian Journal of Microbiology, 26, 287–290. [PubMed] [Google Scholar]
- Crandell, R. A. , Livingston, C. W. Jr , & Shelton, M. J. (1989). Laboratory Investigation of a Naturally Occurring Outbreak of Arthrogryposis‐Hydranencephaly in Texas Sheep. Journal of Veterinary Diagnostic Investigation, 1, 62–65. 10.1177/104063878900100117 [DOI] [PubMed] [Google Scholar]
- Dimitrova, K. , Andonova, M. , Makowski, K. , Holloway, K. , Levett, P. N. , Kadkhoda, K. , … Drebot, M. A. (2011). Preliminary evidence of Cache Valley virus infections and associated human illness in western Canada in 2009. Canadian Journal of Infectious Diseases and Medical Microbiology, 22, 15A.22379483 [Google Scholar]
- Downs, W. G. , Spence, L. , Aitken, T. H. , & Whitman, L. (1961). Cache Valley virus, isolated from a Trinidadian mosquito, Aedes scapularis . The West Indian Medical Journal, 10, 13–15. [PubMed] [Google Scholar]
- Drebot, M. A. (2015). Emerging mosquito‐borne bunyaviruses in Canada. Canada Communicable Disease Report, 41(6), 117–123. 10.14745/ccdr.v41i06a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, E. F. , Pritlove, D. C. , & Elliott, R. M. (1994). The S RNA genome segments of Batai, Cache Valley, Guaroa, Kairi, Lumbo, Main drain and Northway bunyaviruses: Sequence determination and analysis. Journal of General Virology, 75(Pt 3), 597–608. 10.1099/0022-1317-75-3-597 [DOI] [PubMed] [Google Scholar]
- Edwards, J. F. , & Hendricks, K. (1997). Lack of serologic evidence for an association between Cache Valley virus infection and anencephaly and other neural tube defects in Texas. Emerging Infectious Diseases, 3, 195–197. 10.3201/eid0302.970215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, J. F. , Higgs, S. , & Beaty, B. J. (1998). Mosquito feeding‐induced enhancement of Cache Valley virus (bunyaviridae) infection in mice. Journal of Medical Entomology, 35, 261–265. 10.1093/jmedent/35.3.261 [DOI] [PubMed] [Google Scholar]
- Edwards, J. F. , Livingston, C. W. Jr , Collisson, E. W. , Chung, S. I. , Olson, J. K. , & Norman, J. L. (1988). An outbreak of arthrogryposis and hydrocephalus in lambs due to Cache Valley virus. Texas Agricultural Experimental Station, 4573, 22–23. [Google Scholar]
- Galindo, P. , Srihongse, S. , De Rodaniche, E. , & Grayson, M. A. (1966). An ecological survey for arboviruses in Almirante, Panama, 1959–1962. Am J Trop Med Hyg, 15(3), 385–400. [DOI] [PubMed] [Google Scholar]
- Groseth, A. , Vine, V. , Weisend, C. , Guevara, C. , Watts, D. , Russell, B. , … Ebihara, H. (1979). Seroepidemiologic studies in Oaxaca, Mexico. II. Survey for arbovirus antibody. Archivos De Investigacion Medica (Mexico), 10, 239–259. [PubMed] [Google Scholar]
- Groseth, A. , Vine, V. , Weisend, C. , Guevara, C. , Watts, D. , Russell, B. , … Ebihara, H. (2017). Maguari Virus Associated with Human Disease. Emerging Infectious Diseases, 8, 1325–1331. 10.3201/eid2308.161254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard, P. B. (1997). The epidemiology of California and Bunyamwera serogroup viruses in Indiana. Available from ProQuest Dissertations & Theses A&I; The epidemiology of California and Bunyamwera serogroup viruses in Indiana. (304380232). [Google Scholar]
- Holden, P. , & Hess, A. D. (1959). Cache Valley virus, a previously undescribed mosquito‐borne agent. Science, 130, 1187–1188. 10.1126/science.130.3383.1187 [DOI] [PubMed] [Google Scholar]
- Hunt, A. R. , & Calisher, C. H. (1979). Relationships of Bunyamwera group viruses by neutralization. American Journal of Tropical Medicine and Hygiene, 28, 740–749. 10.4269/ajtmh.1979.28.740 [DOI] [PubMed] [Google Scholar]
- Johnson, G. D. , Bahnson, C. S. , Ishii, P. , Cochrane, Z. N. , Hokit, D. G. , Plummer, P. J. , … Blitvich, B. J. (2014). Monitoring sheep and culicoides midges in Montana for evidence of Bunyamwera serogroup virus infection. Veterinary Record Open, 1, e000071 10.1136/vetreco-2014-000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschner, J. , Therrien, A. , Frier, G. , McLean, R. G. , Campos, E. G. , Monath, T. P. , … Parham, G. L. (1985). Investigations of the vertebrate hosts of eastern equine encephalitis during an epizootic in Michigan, 1980. American Journal of Tropical Medicine and Hygiene, 34, 1190–1202. 10.4269/ajtmh.1985.34.1190 [DOI] [PubMed] [Google Scholar]
- Kokernot, R. H. , Radivojevic, B. , & Anderson, R. J. (1969). Susceptibility of wild and domesticated mammals to four arboviruses. American Journal of Veterinary Research, 30, 2197. [PubMed] [Google Scholar]
- Kosoy, O. , Rabe, I. , Geissler, A. , Adjemian, J. , Panella, A. , Laven, J. , … Lanciotti, R. (2016). Serological survey for antibodies to mosquito‐borne bunyaviruses among US National Park Service and US Forest Service Employees. Vector‐borne and Zoonotic Diseases, 6, 191–198. 10.1089/vbz.2015.1865 [DOI] [PubMed] [Google Scholar]
- Leboeuf, G. C. , Cote, A. (2013). Cas de malformations congenitales chez des ovins causes par le virus de la valle cache. Info‐RAIZO, Reseau d’alerte d’information zoosanitaire, Direction de la santé animale, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec. [Google Scholar]
- McConnell, S. , Livingston, C. Jr , Calisher, C. , & Crandell, R. (1985). Isolation of Cache Valley virus from livestock in Texas, 1981 In Della‐Porta A. J. (Ed.), Veterinary viral diseases: their significance in south‐east Asia and the western Pacific. Sydney, Australia: Academic Press; 434-438. [Google Scholar]
- McConnell, S. , Livingston, C. Jr , Calisher, C. H. , & Crandell, R. A. (1987). Isolations of cache valley virus in Texas, 1981. Vet Microbiol, 13(1), 11–18. [DOI] [PubMed] [Google Scholar]
- McLean, R. G. , Calisher, C. H. , & Parham, G. L. (1987). Isolation of Cache Valley virus and detection of antibody for selected arboviruses in Michigan horses in 1980. American Journal of Veterinary Research, 48, 1039–1041. [PubMed] [Google Scholar]
- Mettler, N. E. , Parodi, A. S. , & Casals, J. (1963). Survey for antibodies against arthropod‐borne viruses in man in Argentina. The American Journal of Tropical Medicine and Hygiene, 12, 653–656. 10.4269/ajtmh.1963.12.653 [DOI] [Google Scholar]
- Meyers, M. T. , Bahnson, C. S. , Hanlon, M. , Kopral, C. , Srisinlapaudom, S. , Cochrane, Z. N. , … Blitvich, B. J. (2015). Management factors associated with operation‐level prevalence of antibodies to Cache Valley virus and other Bunyamwera serogroup viruses in sheep in the United States. Vector‐Borne and Zoonotic Diseases, 15, 683–693. 1089/vbz.2015.1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W. B. (1997). The vector competence of Aedes albopictus (skuse) (diptera: Culicidae) for Cache Valley virus (bunyaviridae: Bunyavirus) and a survey for vectors of the Cache Valley and reticuloendotheliosis viruses in Brazos County, Texas. Available from ProQuest Dissertations & Theses A&I; The vector competence of Aedes albopictus (Skuse) (Diptera: Culicidae) for Cache Valley virus (Bunyaviridae: Bunyavirus) and a survey for vectors of the Cache Valley and reticuloendotheliosis viruses in Brazos County, Texas (304403006). [Google Scholar]
- Murphy, E. L. , Calisher, C. H. , Figueroa, J. P. , Gibbs, W. N. , & Blattner, W. A. (1989). HTLV‐I infection and arthropod vectors. New England Journal of Medicine, 320, 1146. [DOI] [PubMed] [Google Scholar]
- Neitzel, D. F. , & Grimstad, P. R. (1991). Serological evidence of California group and Cache Valley virus infection in Minnesota white‐tailed deer. Journal of Wildlife Diseases, 27, 230–237. 10.7589/0090-3558-27.2.230 [DOI] [PubMed] [Google Scholar]
- Nguyen, N. L. , Zhao, G. , Hull, R. , Shelly, M. A. , Wong, S. J. , Wu, G. , … Menegus, M. A. (2013). Cache Valley virus in a patient diagnosed with aseptic meningitis. Journal of Clinical Microbiology, 51, 1966–1969. 10.1128/JCM.00252-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki, W. L. (1996). Bunyavirus infections and the associated humoral immune response and birth defects. Available from ProQuest Dissertations & Theses A&I; Bunyavirus infections and the associated humoral immune response and birth defects. (304279571). [Google Scholar]
- Peralta, P. H. , Shelokov, A. , Vogel, J. E. , & Longfellow, D. (1966). Isolation and characterization of arboviruses from Almirante, Republic of Panama. The American Journal of Tropical Medicine and Hygiene, 15, 369–378. 10.4269/ajtmh.1966.15.369 [DOI] [PubMed] [Google Scholar]
- Peters, M. D. J. , Godfrey, C. , McInerney, P. , Baldini Soares, C. , Khalil, H. , & Parker, D. C. , (2017). Chapter 11: Scoping reviews In Aromataris E., & Munn Z. (Eds.), Joanna Briggs Institute Reviewer's Manual. Adelaide, Australia: The Joanna Briggs Institute; Available from https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- Reeves, W. K. , & Miller, M. M. (2013). Culicoides sonorensis (diptera: Ceratopogonidae) is not a competent vector of Cache Valley virus (family bunyaviridae, genus orthobunyavirus). Archives of Virology, 158, 2175–2177. 10.1007/s00705-013-1715-6 [DOI] [PubMed] [Google Scholar]
- Rodrigues, A. (2011). The pathogenesis of Cache Valley virus in the ovine fetus. Available from ProQuest Dissertations & Theses A&I; The pathogenesis of Cache Valley virus in the ovine fetus. (926203217). [Google Scholar]
- Sabattini, M. S. , Shope, R. E. , & Vanella, J. M. (1965). Serological survey for arboviruses in Cordoba province, Argentina. American Journal of Tropical Medicine and Hygiene, 14, 1073–1078. [DOI] [PubMed] [Google Scholar]
- Saliba, E. K. , DeFoliart, G. R. , Yuill, T. M. , & Hanson, R. P. (1973). Laboratory transmission of Wisconsin isolates of a Cache Valley‐like virus by mosquitoes. Journal of Medical Entomology, 10, 470–476. [DOI] [PubMed] [Google Scholar]
- Scherer, W. F. , Campillo‐Sainz, C. , Dickerman, R. W. , Diaz‐Najera, A. , & Madalengoitia, J. (1967). Isolation of Tlacotalpan virus, a new Bunyamwera‐group virus from Mexican mosquitoes. American Journal of Tropical Medicine and Hygiene, 16, 79–91. 10.4269/ajtmh.1967.16.79 [DOI] [PubMed] [Google Scholar]
- Sexton, D. J. , Rollin, P. E. , Breitschwerdt, E. B. , Corey, G. R. , Myers, S. A. , Dumais, M. R. , … Ksiazek, T. G. (1997). Life‐threatening Cache Valley virus infection. New England Journal of Medicine, 336, 547–549. 10.1056/NEJM199702203360804 [DOI] [PubMed] [Google Scholar]
- Shapiro, J. , Brooks, A. , Menzies, P. , Rau, J. , Drebot, M. , Andonova, M. , … Carman, S. (2012). Cache Valley virus identified as a cause of malformed lambs in Ontario. Animal Health Laboratory Newsletter, 16, 15. [Google Scholar]
- Shelton, M. , de la Bermejillo, A. , Willingham, T. , & Mock, R. (1994). An update on the problem of Cache Valley virus in sheep. Sheep and Goat Research Journal, 10, 124–128. [Google Scholar]
- Spinato, M. , Stalker, M. , DeLay, J. , Shapiro, J. , Fairles, J. , Menzies, P. , & Jansen, J. (2016). Cache Valley virus – An outbreak of congenital malformations in Ontario lambs. Animal Health Laboratory Newsletter; Retrieved from https://www.uoguelph.ca/ahl/content/ruminants-1. Accessed 8 January 2019. [Google Scholar]
- Tauro, L. B. , Almeida, F. L. , & Contigiani, M. S. (2009). First detection of human infection by Cache Valley and Kairi viruses (Orthobunyavirus) in Argentina. Transactions of the Royal Society of Tropical Medicine and Hygiene, 103, 197–199. 10.1016/j.trstmh.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Tesh, R. B. , & Gubler, D. J. (1975). Laboratory studies of transovarial transmission of La Crosse and other arboviruses by Aedes albopictus and Culex fatigans . American Journal of Tropical Medicine and Hygiene, 24, 876–880. 10.4269/ajtmh.1975.24.876 [DOI] [PubMed] [Google Scholar]
- Tricco, A. C. , Lillie, E. , Zarin, W. , O'Brien, K. K. , Colquhoun, H. , Levac, D. , … Straus, S. E. (2018). PRISMA Extension for Scoping Reviews (PRISMA‐ScR): Checklist and Explanation. Annals of Internal Medicine, 169, 467–473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- Uehlinger, F. D. , Wilkins, W. , Godson, D. L. , & Drebot, M. A. (2018). Seroprevalence of Cache Valley virus and related viruses in sheep and other livestock from Saskatchewan, Canada. Canadian Veterinary Journal, 4, 413–418. [PMC free article] [PubMed] [Google Scholar]
- Walters, L. L. , Tirrell, S. J. , & Shope, R. E. (1999). Seroepidemiology of california and bunyamwera serogroup (bunyaviridae) virus infections in native populations of Alaska. American Journal of Tropical Medicine and Hygiene, 60, 806–821. 10.4269/ajtmh.1999.60.806 [DOI] [PubMed] [Google Scholar]
- Wilson, M. R. , Suan, D. , Duggins, A. , Schubert, R. D. , Khan, L. M. , Sample, H. A. , … DeRisi, J. L. (2017). A novel cause of chronic viral meningoencephalitis: Cache Valley virus. Annals of Neurology, 82, 105–114. 10.1002/ana.24982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work, T. H. (1964). Serological evidence of Arbovirus infection in the Seminole Indians of southern Florida. Science, 145, 270–272. 10.1126/science.145.3629.270 [DOI] [PubMed] [Google Scholar]
- Yang, F. , Chan, K. , Marek, P. E. , Armstrong, P. M. , Liu, P. , Bova, J. E. , … Paulson, S. L. (2018). Cache Valley Virus in Aedes japonicus japonicus Mosquitoes, Appalachian Region, United States. Emerging Infectious Diseases, 24, 553–557. 10.3201/eid2403.161275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Qiu, J. , Snyder‐Keller, A. , Wu, Y. , Sun, S. , Sui, H. , … Hernandez‐Ilizaliturri, F. (2018b). Fatal Cache Valley virus meningoencephalitis associated with rituximab maintenance therapy. American Journal of Hematology, 93, 590–594. 10.1002/ajh.25024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuill, T. M. , Gochenour, W. S. Jr , Lucas, F. R. , Collins, M. J. Jr , & Buescher, E. L. (1970). Cache Valley virus in the Delmarva peninsula. 3. Serologic evidence for natural infection of dairy cattle. American Journal of Tropical Medicine and Hygiene, 19, 506–512. 10.4269/ajtmh.1970.19.506 [DOI] [PubMed] [Google Scholar]
- Yuill, T. M. , & Thompson, P. H. (1970). Cache Valley virus in the Del Mar Va Peninsula. IV. Biological transmission of the virus by Aedes sollicitans and Aedes taeniorhynchus. American Journal of Tropical Medicine and Hygiene, 19(3), 513–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


