Abstract
Respiratory syncytial virus (RSV) causes significant mortality in hospitalized adults. Prediction of poor outcomes improves targeted management and clinical outcomes. We externally validated and updated existing models to predict poor outcome in hospitalized RSV‐infected adults. In this single center, retrospective, observational cohort study, we included hospitalized adults with respiratory tract infections (RTIs) and a positive polymerase chain reaction for RSV (A/B) on respiratory tract samples (2005‐2018). We validated existing prediction models and updated the best discriminating model by revision, recalibration, and incremental value testing. We included 192 RSV‐infected patients (median age 60.7 years, 57% male, 65% immunocompromised, and 43% with lower RTI). Sixteen patients (8%) died within 30 days. During hospitalization, 16 (8%) died, 30 (16%) were admitted to intensive care unit, 21 (11%) needed invasive mechanical ventilation, and 5 (3%) noninvasive positive pressure ventilation. Existing models performed moderately at external validation, with C‐statistics 0.6 to 0.7 and moderate calibration. Updating to a model including lower RTI, chronic pulmonary disease, temperature, confusion and urea, increased the C‐statistic to 0.76 (95% confidence interval, 0.61‐0.91) to predict in‐hospital mortality. In conclusion, existing models to predict poor prognosis among hospitalized RSV‐infected adults perform moderately at external validation. A prognostic model may help to identify and treat RSV‐infected adults at high‐risk of death.
Keywords: adults, external validation, mortality, prognostic, respiratory infection, RSV
Highlight
Prediction of poor outcomes among RSV‐infected adults may improve targeted management and clinical outcomes.
Existing models to predict poor prognosis among hospitalized RSV‐infected adults perform moderately at external validation.
A model including lower RTI, chronic pulmonary disease, temperature, confusion and urea, had a C‐statistic of 0.76 (95%CI 0.61‐0.91) to predict in‐hospital mortality.
1. INTRODUCTION
There is increasing evidence that respiratory syncytial virus (RSV) is a common cause of respiratory tract infections (RTI) in adult patients,1 often with a complicated course of disease.2, 3, 4, 5 Among hospitalized elderly ≥65 years of age mortality is as high as 8%,2 but among high‐risk groups as patients with chronic heart or lung disease, long‐term care facility residents and immunocompromised patients as lung or hematopoietic cell transplant (HCT) recipients, RSV may even lead to mortality rates over 50%.2, 3, 6, 7 With the widespread implementation of rapid tests for respiratory viruses in‐hospital care settings, early detection of RSV enables early treatment with either aerosolized or oral ribavirin6, 8, 9, 10, 11, 12, 13, 14 and future medicaments as fusion protein inhibitors (eg, presatovir), nucleoside inhibitors (eg, lumicitabine),15 and viral replication lowering immunoglobulins (eg, palivizumab), which might have an additional positive effect to ribavirin.11, 16, 17, 18 Ideally, in light of effectivity and potential side effects, treatment should be targeted to patients at the highest risk of a life‐threatening infection. Identification of RSV‐infected patients at high‐risk of death is therefore necessary to improve targeted therapy and clinical outcomes. In addition, the prediction of individual prognosis improves decision making on the necessity to apply supportive in‐hospital management as intensive care unit (ICU) admission and strict isolation procedures.3 However, a validated prognostic model to identify adult patients with a high mortality risk is not available. Therefore, we aimed to establish factors associated with poor prognosis and externally validate and update existing models to predict mortality in hospitalized RSV‐infected adults.
2. METHODS
2.1. Study population
We performed a single center cohort study to validate prognostic models for poor outcomes in hospitalized adults with RSV. In the validation cohort we included adult patients (≥18 years) with a laboratory confirmed community acquired RSV‐infection between January 2005 and April 2018 who were admitted to the University Medical Center Utrecht (UMCU), a 1042‐bedded tertiary care hospital in the central region of The Netherlands. We excluded patients with hospital acquired RSV‐infection (RSV result >48 hours after admission). When patients had more than one hospitalized RSV‐infection episode during the study period, only the first episode was included. RSV positive patients were identified retrospectively using the microbiology laboratory database of the UMCU. During the inclusion period, in‐house reverse transcription polymerase chain reaction (RT‐PCR) was used for detection of RSV and other respiratory viral pathogens19, 20 in respiratory tract specimens. A positive RSV result was defined as having a cycle time (Ct) value les than 40.21 For immunocompromised patients, the conventional in‐house RT‐PCR was replaced by a qualitative RT‐PCR‐the FilmArray respiratory viral panel version 1.7 (BioFire Diagnostics, Salt Lake City)22 from November 2016 onwards. Collection of predictor and outcome variables was performed retrospectively from the electronic patient files. This study was assessed by the medical ethics committee of the UMCU (METC protocol no 18‐410/C). Due to the retrospective nature of the study, informed consent was not required. Results were reported to conform with the transparent reporting of a multivariable prediction model for individual prognosis or Diagnosis (TRIPOD) statement (Table S1).23
2.2. External validation
We searched available literature on predictive models for RSV prognosis in the MEDLINE. We aimed to validate models predicting mortality, but also included studies using a composite outcome including mortality. For the external validation, we applied the included original prognostic models to our study cohort exactly as they were published, with similar definitions of predictor variables and outcomes (Table S2).24, 25, 26, 27 If the intercept from the original model was not reported, we calculated a new intercept by recalibration. We compared the discriminative ability of the models using the Harrell's C‐statistic. Calibration of the models was assessed in calibration plots.28, 29, 30
2.3. Model update
We selected the model with the best discrimination and calibration for further updating.24 In view of increasingly shorter turnaround times of molecular diagnostics and increased effectiveness of antiviral treatment when given at an early stage,6 we first removed any eventual predictors that could not be assessed at the time of presentation/RSV diagnosis, eg, bacterial coinfection. Furthermore, we replaced binary predictors with continuous to avoid loss of information, eg, temperature instead of fever. Next, we recalibrated the calibration slope and intercept by refitting this adapted model in the validation cohort. Consequently, we tested the incremental value of the model by adding objectively assessable predefined predictor variables (age, gender, urea, confusion, cardiovascular comorbidities, immunocompromised status, and the number of other comorbidities), based on the existing prognostic models for poor outcomes in patients with positive influenza virus.26, 27, 31 We performed backward variable selection based on the Akaike information criterion and Occam's razor principle. Finally, we performed internal validation with optimism correction by bootstrap.32 Discrimination and calibration of this final updated and extended model was assessed for in‐hospital mortality, 30‐day mortality and a composite outcome consisting of in‐hospital death, ICU‐admission and/or need for mechanical ventilation separately. Furthermore, we performed a decision curve analysis to provide insight into the range of predicted risks for which the final model results in better clinical decision making, eg, is better than either classifying all or none of the patients as having the outcome.33
2.4. Statistical analysis
For the validation cohort, we accounted for missing values of predictors using a multiple imputation model including baseline characteristics, predictors, and outcome variables. Results shown are pooled from the 10 multiple imputed datasets.34 Calibration plots were derived from all 10 multiple imputed datasets combined. Analyses were performed by the SPSS version 25 (IBM Corp) and the rms, mice, survival, and rmda packages of R‐3.1 for Windows (http://cran.r-project.org).
3. RESULTS
3.1. Validation cohort
We included 192 hospitalized, RSV‐infected adult patients. Demographics and characteristics of the included patients are displayed in Table 1. The median age was 60.7 (interquartile range [IQR], 50.8‐69.2) years. In total, 125 patients (65.1%) were immunocompromised, of whom 42 patients were HCT and 34 solid organ transplant recipients. At presentation, 83 patients (43.2%) were diagnosed with a lower RTI. After hospitalization, 16 patients (8.3%) died during their hospital stay. In‐hospital mortality was not different between immunocompromised (n = 9) and immunocompetent (n = 7) patients (odds ratio [OR], 0.62 [95% confidence intervals [CI], 0.22‐1.73]) or between HCT (n = 2) and solid organ transplant (n = 3) recipients (OR, 0.52 [95%CI, 0.08‐3.29]). At 30 days, 16 patients had died (Figure 1). During hospitalization, 30 patients (15.6%) were admitted to the ICU, of whom 21 patients needed invasive mechanical ventilation, five needed noninvasive positive pressure ventilation and four needed no ventilator support. Of all ICU‐admitted patients, 23 were admitted to the ICU within the first 48 hours of admission. The median length of hospital stay was 5 days (IQR, 3‐10) and 77 patients (40.1%) had a hospital stay ≥7 days. In total, 147 patients (76.6%) were treated with antibiotics empirically and 25 patients (13.0%) were treated with oral ribavirin, of whom 18 for ≥7 days. Over the years, the annual number of included patients increased, with no clear changes in in‐hospital mortality rate (Figure S1).
Table 1.
Demographics and characteristics of included patients in validation cohort (n = 192)
| Characteristics | Validation cohort (n = 192), n (%) or median (IQR) |
|---|---|
| Demographics | |
| Age, y | 60.7 (50.8‐69.2) |
| Male gender | 110 (57.3%) |
| Immunocompromised a | 125 (65.1%) |
| Smoking a | 100 (52.1%) |
| Chronic pulmonary disease a | 67 (34.9%) |
| Disease characteristics at presentation | |
| Symptom duration before presentation, d | 3.4 (2.0‐7.0) |
| Confusion a | 17 (8.9%) |
| Heart rate, beats per minute | 100 (88‐115) |
| Ear‐based temperature, °C | 37.8 (37.1‐38.9) |
| Systolic blood pressure, mmHg | 130 (115‐145) |
| Diastolic blood pressure, mmHg | 75 (65‐85) |
| Breathing frequency, breaths per minute | 20 (16‐26) |
| Saturation, % b | 95 (92‐97) |
| Meeting sepsis criteria, qSOFA score ≥2 c | 15 (7.8%) |
| Laboratory findings at presentation | |
| pO2 arterial blood gas, mmHg b | 71 (58‐94) |
| pH arterial blood gas | 7.46 (7.39‐7.50) |
| Hemoglobin, mmol/L | 7.9 (6.8‐8.6) |
| Thrombocytes (×109/L) | 203 (128‐257) |
| Leukocytes (×109/L) | 8.3 (4.7‐12.1) |
| Lymphocytes (×109/L) | 1.3 (0.6‐2.5) |
| Neutrophils (×109/L) | 5.5 (2.5‐9.5) |
| C‐reactive protein (mg/L) | 60 (21‐136) |
| Sodium, mmol/L | 135 (133‐138) |
| Urea, mmol/L | 7.1 (4.8‐10.7) |
| Results from other diagnostics at presentation | |
| Ct value RSV, quantitative RT‐PCR | 29.1 (25.2‐33.8) |
| Lower RTI a | 83 (43.2%) |
| Bacterial coinfection a | 81 (42.2%) |
Abbreviations: Ct, cycle time; IQR, interquartile range; pO2, partial pressure of oxygen; RSV, respiratory syncytial virus; RTI, respiratory tract infection; RT‐PCR, reverse transcription polymerase chain reaction.
Table S2 for definitions.
Not always clear if taken with or without oxygen replacement therapy.
qSOFA criteria: altered mental status (Glasgow Coma Scale <15), respiratory rate ≥22, systolic blood pressure ≤100.
Figure 1.
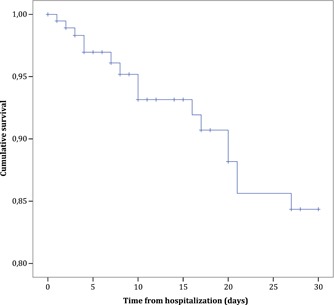
Kaplan‐Meier survival curve of 192 adults hospitalized with RSV‐infection. RSV, respiratory syncytial virus
3.2. External validation
We found five studies that developed a prognostic model for hospitalized RSV‐infected adult patients, of which two to predict mortality24, 25 and three to predict disease progression to a lower RTI34, 35, 36, 37 (Figure S2). The two models to predict mortality were included for external validation. A detailed overview of these two models is shown in Table 2. The first study, of Park et al24, developed a logistic regression model to predict in‐hospital death, ICU‐admission and/or the need for mechanical ventilation. In our validation cohort, 36 patients (18.8%) met this composite outcome (vs 15.0% in the original study; P = .300). When applying the original logistic regression model of Park et al24 (with a recalibrated intercept) to our validation cohort, the C‐statistic was 0.65 (95% CI, 0.55‐0.76) for this composite outcome. The model showed good calibration when plotting predicted against observed poor outcomes (Figure 2A). The second study, of Lee et al25, developed a survival model to predict 30‐day mortality. In our validation cohort, 16 patients (8.3%) died within 30 days (vs 9.1% in the original study; P = .735). When applying the original Cox proportional hazards model of Lee et al25 with 30‐day mortality as outcome, the C‐statistic was 0.61 (95% CI, 0.49‐0.73). The calibration plot of this model plotting predicted against observed survival at 30 days, showed reasonable calibration (Figure 2B).
Table 2.
Characteristics of included models
| Study characteristics | Park et al24 | Lee et al25 |
|---|---|---|
| Study population | Hospitalized adults with an RSV RTI presenting at the emergency department (n = 227); 133 (59%) community acquired, 94 (41%) healthcare‐associated. In total, 84 (37%) patients were immunocompromised (25 solid organ recipients, 9 patients, with HCT 50 using immunosuppressants/ corticosteroids) and 42 (19%) had a chronic pulmonary disease | Hospitalized adults with an RSV RTI (n = 607). In total, 83 (13.7%) patients were immunocompromised and 216 (36%) had a chronic pulmonary disease |
| Exclusion: ≤18 y, outpatient treatment, RSV diagnosis >48 h after admission, concurrent infections at other sites | Exclusion: none | |
| Primary outcome | Life‐threatening RSV‐infection (admission to ICU, need for ventilator care or in‐hospital death; n = 34, 15.0%) | 30‐d mortality (n = 55, 9.1%), 60‐d mortality (n = 72, 11.9%) |
| Patient identification and data collection | Identification using RSV positive PCR assays; retrospective data collection | Identification of RSV positive viral antigen immunofluorescence assay tests; retrospective data collection |
| Inclusion location | ED of a 2700‐bed tertiary care hospital in Seoul, South Korea | Three acute care, general public hospitals in Hong Kong, China |
| Inclusion period | October 2013‐September 2015 | January 2009‐December 2011 |
| Modeling technique | Multivariable logistic regression analysis with stepwise backward variable selection | Multivariable Cox proportional hazards analysis with stepwise backward variable selection |
| Variable selection for multivariable analysis | Variables with P ≤ .05 in univariate analyses of association with life‐threatening infection. Exclusion of variables in causal pathway (confusion, saturation); subjective symptoms (dyspnea); correlated variables (smoking history, correlated with chronic pulmonary disease) | Variables with P ≤ .1 in univariate analyses of association with mortality. Inclusion of demographics, comorbidities, cardiorespiratory complications, ventilation requirement, bacterial superinfection, and corticosteroid use |
| Variables included in multivariable analysis a | Lower RTI, chronic pulmonary disease, bacterial coinfection, fever ≥38°C, rhinorrhoea, CRP, procalcitonin, RSV type A and B, antimicrobial use b , ribavirin use b | Age, gender, major systemic comorbidity, chronic pulmonary disease exacerbation, cardiovascular complications, pneumonia, need for ventilatory support, bacterial coinfection, urea, total white cell count, systemic corticosteroid use |
| Variables in final model a | Lower RTI, chronic pulmonary disease, bacterial coinfection, fever ≥38⁰C | Age >75 y, male gender, pneumonia, need for ventilatory support, bacterial coinfection, urea |
| Missing data handling | Not described | Not described |
Abbreviations: CRP, C‐reactive protein; DFA, direct fluorescent antibody; ED, emergency department; HCT, hematopoietic cell transplant; ICU, intensive care unit; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; RTI, respiratory tract infection.
Table S2 for definitions.
No further definition or details given.
Figure 2.
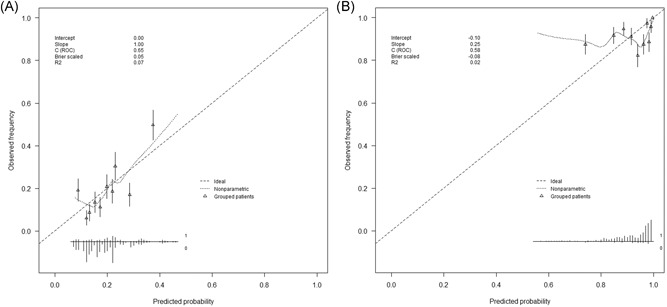
Calibration plots of original prognostic models. A, Predicted probabilities determined by the original model of Park et al24 (chronic pulmonary disease, lower RTI, temperature ≥38°C, bacterial coinfection)—with a recalibrated intercept—plotted against the observed frequency of the primary outcome (ICU‐admission, need for mechanical ventilation, and/or in‐hospital death) divided in 10‐deciles of predicted probabilities. B, Predicted probability of 30‐day survival determined by the original model by Lee et al25 (age >75, male gender, pneumonia, need for ventilatory support, bacterial coinfection, and urea) plotted against the actually observed 30‐day survival. ICU, intensive care unit; RTI, respiratory tract infection
3.3. Model update
We updated and extended the model of Park et al,24 which was the best performing model in terms of discrimination and calibration, by performing variable revision, recalibration of the regression coefficients and incremental value testing. The final model included three predictors from the original model of Park et al24, eg, lower RTI, chronic pulmonary disease and temperature, and two newly added predictors, eg, urea and confusion. The final updated, optimism corrected model had a C‐statistic of 0.76 (95% CI, 0.61‐0.91) for the prediction of in‐hospital mortality, a C‐statistic of 0.73 (95% CI, 0.59‐0.88) for prediction of 30‐day mortality and a C‐statistic of 0.74 (95% CI, 0.64‐0.84) for prediction of in‐hospital mortality and/or ICU‐admission and/or need for mechanical ventilation. The updated model showed good calibration for the composite outcome (Figure 3). Results of the decision curve analysis of the updated model is shown in Figure 4. For the whole range of predicted risks, the updated prognostic model showed a positive net benefit. However, only with a risk threshold—eg, a predicted risk threshold that can be used for decision‐making regarding therapy—above 40%, the updated model improved the net benefit as compared to the original model of Park et al.24
Figure 3.
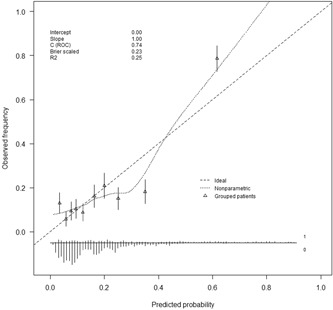
Calibration plot of updated and extended prognostic model of Park et al24 (with predictors chronic pulmonary disease, lower RTI, temperature, confusion and urea) for the prediction of ICU‐admission, need for mechanical ventilation and/or in‐hospital death. ICU, intensive care unit; RTI, respiratory tract infections
Figure 4.
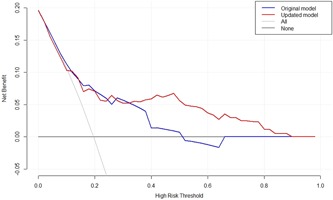
Decision curve analysis showing the net benefit curve of the original model of Park et al24 (in blue) and of the final updated prognostic model (in red) for the composite poor outcome (ICU‐admission, need for mechanical ventilation and/or in‐hospital death). The horizontal gray line is the net benefit when all RSV‐infected hospitalized adults are considered as not having the poor outcome; vertical gray line is the net benefit when all RSV‐infected hospitalized adults are considered as having the poor outcome. The higher the net benefit (blue line) at any given threshold, the better the model performs. Example: with a risk threshold of 25% (threshold above which we would treat), the net benefit (derived from the true positives and true negatives) is 5.33 per 100 patients when using the original model of Park et al24 and 5.95 when using the updated model. RSV, respiratory syncytial virus; ICU, intensive care unit
4. DISCUSSION
We showed that hospitalized, RSV‐infected adults had an 8% in‐hospital and 8% 30‐day mortality rate. We validated and updated models to predict poor outcome in these patients at the time of RSV diagnosis. This model can be used to develop a risk score or decision tool to guide decisions on treatment with ribavirin, immune globulins, and other antivirals and on site‐of‐care and strict isolation procedure decisions, as is already common practice for influenza virus.38 These interventions might improve clinical outcomes for patients with life‐threatening disease.
To our knowledge, this is one of the largest studies in RSV‐infected adult patients in a hospital care setting. We found a high percentage of 8% in‐hospital mortality, which is in line with 8% to 9% mortality rates reported in former publications.2, 6, 24, 25 This high mortality rate underlines the great importance of targeted treatment for these patients. Also, this is the first study to externally validate existing models to predict poor prognosis in RSV‐infected hospitalized adult patients, and allows for a head‐to‐head comparison of two published models. Unfortunately, model performance in the development cohorts was not described,30 but the poor to moderate discriminative abilities of both models in our validation cohort with C‐statistics under 0.7 with CI close to or overlapping 0.5, indicate that both models are not suitable for use in daily practice, at least not in our Dutch tertiary care setting. To some extent, the poor predictions in our validation cohort might be caused by differences in average values of various predictors and administered treatments as compared to the development studies.30 Geographical validation is also very likely to have played a role and affected the performance of these models in our validation cohort,30 since both development studies were performed in Asia. Temporal and domain validation—with 37%24 and 14%25 vs 65% immunocompromised patients for example‐might also have resulted in lower prediction accuracy of the two models, although the proportions of our patients who met the outcomes were quite similar to the development studies.30 Another, maybe the most important factor that might have caused the moderate performance of both models at external validation, was the relatively small cohort in which these models were developed, with a rather low number of events causing overfitted estimations of predictor effects.32 If internal validation methods as bootstrap would have been performed after development of these models, poor external validation might have been foreseen.32, 39
During the model update, the viral load (eg, Ct value) of RSV was not considered a useful predictor. First, the interpretation of single viral load measurements is difficult. Not only are viral loads of respiratory viruses highly dependent on variation in sampling timing, location and technique, they also rise and drop rapidly and it is known that symptoms mostly follow the highest peak in viral load.40, 41 Second, since more and more rapid qualitative molecular methods are implemented, viral loads will not always be available.
The updated model of Park showed good discrimination and calibration and the net benefit of this updated model was positive for the whole range of predicted risks. For clinical practice, to be able to use this prediction model as decision tool for RSV treatment, a new external validation and a well‐considered harm‐benefit based treatment threshold are needed. The more convincing the benefits of RSV treatment on improved clinical patient outcomes and hospital management, and the lower the potential harms—serious side effects, complications, and increased costs, the lower the appropriate treatment threshold. When a consensus based threshold is determined, the positive predictive value of the model determines the positive effect of implementing such a model in clinical practice, eg, the benefit of implementing the model over treating all or none of the patients.
In addition to the fact that we had a large cohort and performed external validation according to current guidelines, we performed a model update according to the TRIPOD statement,23 including internal validation procedures.30 However, some limitations of our study need to be addressed. First, we had a limited amount of patients with the primary outcome. For studies validating prognostic models, there is no solid sample size recommendation, but it is recommended to consider at least the number of predictors, the total sample size and the event fraction.42, 43, 44 The low number of events in our study might have resulted in biased and less precise performance measures, which is also indicated by the broad CI of the reported C‐statistics. Second, the performance of routine clinical care diagnostic RSV tests was non‐standardized and subjected to change during the 14‐year study period, bearing the risk of selective patient inclusion with more severely ill patients and the risk of missed RSV diagnoses. Third, over the years, increased awareness for the disease burden of RSV in adult patients might have led to and more targeted treatment with a positive effect on the prognosis of RSV‐infected patients. Increased awareness might also have resulted in more frequent testing for RSV. Unfortunately, due to the absence of the number of adults tested for RSV, we cannot confirm this hypothesis based on our data. Finally, we included relatively many immunocompromised patients, making results potentially less generalizable to other settings as nonacademic hospitals.
In conclusion, hospitalized RSV‐infected adults have a very poor prognosis with 8% in‐hospital and 8% 30‐day mortality. This poor prognosis could be improved by targeting RSV treatment with ribavirin, immune globulins, future antiviral treatment options, site‐of‐care decisions, and strict isolation procedures for patients at highest risk of serious complications. Existing models to predict mortality in these patients perform moderately or poor at external validation. An updated model including chronic pulmonary disease, lower RTI, confusion, temperature, and urea, however, reasonably predicts which RSV‐infected patients are at highest risk of poor prognosis. Implementation of this prediction model in clinical practice could improve clinical outcomes of high‐risk patients, without putting low‐risk patients at an unnecessary treatment risk.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LV, CN, and JO designed the study. LV collected the clinical data of included patients and performed all formal analyses with close involvement of CA. All authors contributed to reviewing and editing of the manuscript. Dr. Bont has regular interaction with pharmaceutical and other industrial partners (AbbVie, MedImmune, Janssen, the Bill and Melinda Gates Foundation, MeMed Diagnostics, Regeneron, Ablynx, Bavaria Nordic, MabXience and Novavax). He has not received personal fees or other personal benefits.
Supporting information
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
Results of this study will be presented at the ECCMID 2019, the 29th European Congress of Clinical Microbiology and Infectious Diseases, taking place in Amsterdam, the Netherlands, from 13 to 16 April 2019. We thank Dr. R.A. Coutinho for his valuable comments on the draft version of the manuscript.
Vos LM, Oosterheert JJ, Hoepelman AIM, Bont LJ, Coenjaerts FEJ, Naaktgeboren CA. External validation and update of a prognostic model to predict mortality in hospitalized adults with RSV: A retrospective Dutch cohort study. J Med Virol. 2019;91:2117–2124. 10.1002/jmv.25568
References
REFERENCES
- 1. Ieven M, Coenen S, Loens K, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;24(11):1158‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falsey AR, Hennessey Pa, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high‐risk adults. N Engl J Med. 2005;352:1749‐1759. [DOI] [PubMed] [Google Scholar]
- 3. Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 5. Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trang TP, Whalen M, Hilts‐Horeczko A, Doernberg SB, Liu C. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: a single‐center retrospective cohort study and review of the literature. Transpl Infect Dis. 2018;20(2):e12844. [DOI] [PubMed] [Google Scholar]
- 7. Ebbert JO, Limper AH. Respiratory syncytial virus pneumonitis in immunocompromised adults: clinical features and outcome. Respiration. 2005;72(3):263‐269. [DOI] [PubMed] [Google Scholar]
- 8. Marcelin JR, Wilson JW, Razonable RR. Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transpl Infect Dis. 2014;16(2):242‐250. [DOI] [PubMed] [Google Scholar]
- 9. Burrows FS, Carlos LM, Benzimra M, et al. Oral ribavirin for respiratory syncytial virus infection after lung transplantation: efficacy and cost‐efficiency. J Heart Lung Transplant. 2015;34(7):958‐962. [DOI] [PubMed] [Google Scholar]
- 10. Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68(8):1872‐1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh S, Champlin R, Englund J, et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: Combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. 2000;25(7):751‐755. [DOI] [PubMed] [Google Scholar]
- 12. McCurdy LH, Milstone A, Dummer S. Clinical features and outcomes of paramyxoviral infection in lung transplant recipients treated with ribavirin. J Heart Lung Transplant. 2003;22(7):745‐753. [DOI] [PubMed] [Google Scholar]
- 13. Schiffer JT, Kirby K, Sandmaier B, Storb R, Corey L, Boeckh M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non‐myeloablative hematopoietic stem cell transplantation. Haematologica. 2009;94(8):1101‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hynicka LM, Ensor CR. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother. 2012;46(4):558‐566. [DOI] [PubMed] [Google Scholar]
- 15. Brendish NJ, Clark TW. Antiviral treatment of severe non‐influenza respiratory virus infection. Curr Opin Infect Dis. 2017;30(6):573‐578. [DOI] [PubMed] [Google Scholar]
- 16. Null DM, Weisman LE. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high‐risk infants. Pediatrics. 1998;102(3):531‐537. [PubMed] [Google Scholar]
- 17. Bagga B, Cehelsky JE, Vaishnaw A, et al. Effect of preexisting serum and mucosal antibody on experimental respiratory syncytial virus (RSV) challenge and infection of adults. J Infect Dis. 2015;212(11):1719‐1725. [DOI] [PubMed] [Google Scholar]
- 18. Whimbey E, Champlin RE, Englund JA, et al. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16(3):393‐399. [PubMed] [Google Scholar]
- 19. Van de Pol AC, Wolfs TF, Jansen NJ, van Loon AM, Rossen JW. Diagnostic value of real‐time polymerase chain reaction to detect viruses in young children admitted to the paediatric intensive care unit with lower respiratory tract infection. Crit Care. 2006;10(2):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houben ML, Coenjaerts FEJ, Rossen JWA, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82(7):1266‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Do LAH, van Doorn HR, Bryant JE, et al. A sensitive real‐time PCR for detection and subgrouping of human respiratory syncytial virus. J Virol Methods. 2012 Jan;179(1):250‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammond SP, Gagne LS, Stock SR, et al. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J Clin Microbiol. 2012;50(10):3216‐3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. Eur Urol. 2015;67(6):1142‐1151. [DOI] [PubMed] [Google Scholar]
- 24. Park SY, Kim T, Jang YR, et al. Factors predicting life‐threatening infections with respiratory syncytial virus in adult patients. Infect Dis. 2017;49:333‐340. [DOI] [PubMed] [Google Scholar]
- 25. Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069‐1077. [DOI] [PubMed] [Google Scholar]
- 26. Huang WT, Chang CH, Hsu YF, Chuang JH. Prognostic factors for mortality in patients hospitalized with influenza complications, in Taiwan. Int Health. 2014;7(1):73‐75. [DOI] [PubMed] [Google Scholar]
- 27. Capelastegui A, Quintana JM, Bilbao A, et al. Score to identify the severity of adult patients with influenza A (H1N1) 2009 virus infection at hospital admission. Eur J Clin Microbiol Infect Dis. 2012;31(10):2693‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: From utopia to empirical data. J Clin Epidemiol. 2016;74:167‐176. [DOI] [PubMed] [Google Scholar]
- 29. Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moons KGM, Kengne AP, Grobbee DE, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;98(9):691‐698. [DOI] [PubMed] [Google Scholar]
- 31. Cvetanovska M, Milenkovik Z, Uroshevik VK, Demiri I, Cvetanovski V. Factors associated with lethal outcome in patients with severe form of influenza. Pril Makedon Akad Nauk Umet Odd Med Nauk. 2016;37(37):63‐72. [DOI] [PubMed] [Google Scholar]
- 32. Steyerberg EW, Harrell FE. Prediction models need appropriate internal, internal‐external, and external validation. J Clin Epidemiol. 2016;69:245‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2016;26(6):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim Y‐J, Guthrie Ka, Waghmare A, et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis. 2014;209(8):1195‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah DP, Ghantoji SS, Ariza‐Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263‐3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boeckh MJ, Gooley T, Englund J, et al. Respiratory synctial virus (rsv) infection in hematopoietic stem cell transplant (HCT) recipients: risk factors for acquisition and lower respiratory tract disease, and impact on mortality. Blood. 2004;104(11):187. [Google Scholar]
- 38. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. CID. 2019;68(6):e1‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Ney York: Springer; 2009:500. [Google Scholar]
- 40. Bagga B, Woods CW, Veldman TH, et al. Comparing influenza and RSV viral and disease dynamics in experimentally infected adults predicts clinical effectiveness of RSV antivirals. Antivir Ther. 2013;18(6):785‐791. [DOI] [PubMed] [Google Scholar]
- 41. Garcia‐Mauriño C, Moore‐Clingenpeel M, Thomas J, et al. Viral load dynamics and clinical disease severity in infants with respiratory syncytial virus infection. J Infect Dis. 2018;219:1207‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vergouwe Y, Steyerberg EW, Eijkemans MJC, Habbema JDF. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58:475‐483. [DOI] [PubMed] [Google Scholar]
- 43. Van Smeden M, Moons KGM, de Groot JAH, et al. Sample size for binary logistic prediction models: Beyond events per variable criteria. Stat Methods Med Res. 2018;1:962280218784726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Collins GS, Ogundimu EO, Altman DG. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med. 2016;35(2):214‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information


