Abstract
Body weight and fat are major performance variables in many sports. Extreme weight reduction can lead to severe medical problems. Accurate body composition measurements are fundamental for both medical and performance optimization. Relative body weight in terms of mass index (MI1 = 0.53 M/(hs)), and in terms of body mass index (BMI = M/h 2) were determined (h:stature, s:sitting height, M:body mass). Subcutaneous adipose tissue (SAT) was measured using a recently standardized ultrasound (US) method. US thickness sums from eight body sites were measured in 26 female and 35 male judokas of various weight classes. Comparisons of US and skinfold results indicate that the latter can be severely misleading in competitive judokas. Mean MI1 of females was 22.8 kg m−2 (BMI:22.9 kg m−2), males: 26.7 kg m−2 (BMI:26.5 kg m−2), but individual differences MI1‐BMI were larger than 0.5 kg m−2 in 13 and larger than 1.0 kg m−2 in three cases. Medians of SAT thickness sums D I were three‐times higher in females (66.1 mm) than in males (21.8 mm), and the fat patterning differed significantly. Females had 8.6% (median) fibrous structures embedded in SAT, and males 20.2%. Both MI and BMI were not correlated with SAT. Mean pre‐competition weight loss was 4.3% (ie, 3.0 kg), and maximum was 9.2% (7.4 kg), indicating that modifications of weigh‐in procedures are urgently needed. DI‐values mirror the athletes' potential to reduce ballast fat instead of short‐term weight reduction by dehydration; however, weight loss and SAT measured some weeks before the competitions were not correlated. Further, US measurements and medical longitudinal observations are required for discussing the large individual variations and possible fat minimum demands.
Keywords: adipose tissue, body composition, body mass index, sex differences, ultrasound imaging
1. INTRODUCTION
Body weight and body composition are major performance variables in many sports. Among the weight‐sensitive sports are gravitational sports in which the body mass in the gravitational field of the earth limits performance, esthetic sports in which higher scores are expected when the body's shape matches a perceived ideal, and weight class sports in which unhealthy body mass reductions can be observed because of possible advantages when competing in a lower weight category.1, 2, 3, 4, 5, 6 Deliberately induced mass reductions may result in severe medical problems that can also lead to death.7, 8 In 2009, the IOC Medical Commission set up a working group on Body Composition, Health and Performance in Sports to analyze contemporary practices and to counteract these dangerous developments.1, 4, 9 Body composition disturbances in sports are closely related to eating habits and eating disorders. The severe medical consequences are in the focus of many publications in sports medicine.4, 7, 10 A review and position statement on behalf of the Research Working Group on Body Composition, Health and Performance, under the auspices of the IOC Medical Commission has been presented recently.4 However, this is a discussion paper based on current insights and experiences, but not a final consensus statement. Changes of competition rules can contribute to a better health status of athletes. In the gravitational sport of ski jumping, where lighter athletes have the advantage of flying further, regulations that limit ski length for athletes who are too light (“BMI rule” of the International Ski Federation, FIS) improved the severe low weight problem in this sport.11, 12, 13
This research focuses on athletes' weight and body composition in the weight class sport of Judo.
1.1. Measurement of relative body weight
To be allowed to compete in their chosen weight class category, almost all athletes (except for the heavy weight class) reduce their weight before the “weigh‐in” by several kg. Loss of water and thus severe dehydration is a major part of the weight reduction practices. Athletes try to compensate for this, at least partially, in the time between the “weigh‐in” and their first fight.14, 15, 16 In Judo, the weight is measured the day before the tournament, usually in the evening.
The body mass index (BMI) is widely used to measure relative body mass (M): BMI = M/h 2 with respect to height (h); however, the BMI does not consider the individual body dimensions as stated by the World Health Organization (WHO): "Problems arise, however, in adults who's shape differs from the norm, particularly those whose legs are shorter or longer than might be expected for their height".17 Therefore, an improved measure for relative weight had been developed1, 3, 18: the mass index (MI) which is a modified BMI that considers the person's sitting height (s): , with C being the Cormic index C = s/h, s is the sitting height and is a value in the middle of the Cormic index continuum representing “mean sitting height”; k is a weighting exponent for the correction term Persons who have the same body height but lower sitting height (and thus longer legs (l): h = s + al, a considers the individual's proportions) have lower body volume, and therefore, a lower body weight can be expected to be appropriate for them. For athletes with lower sitting height, the relative body weight in terms of MI is higher than their BMI. The MI and the BMI are identic for k = 0. The other extreme would result for k = 2: MI2 = 0.532 M/s 2. The MI2 is related to sitting height only; this measure ignores the contribution of the legs for assessing relative body mass. For a relative measure that considers both s and h, it is appropriate to choose k between these two extremes. For k = 1, we get: MI1 = 0.53 M/(hs). The MI1 is used in this study as an improved measure for relative body mass, but—as the BMI—it cannot distinguish between muscle and fat mass.
1.2. Body composition assessment in sports
Most athletes and their coaches, in particular those in weight‐sensitive sports,1 are interested in their body fat amount. Surprisingly, only a few of them are aware of the principle differences whether fat is measured on the molecular or on the anatomic level.1 The fat amount determined on the molecular level (eg, by multi‐component models including measurements of body density, body water, and bone mineral content1, 19) should be higher when compared to the fat detected on the anatomic level (eg, by medical imaging techniques like US or MRI) because anatomic approaches necessarily miss the dissolved fat and all fat containing structures that can only be detected on the histological or molecular level (eg, in the nervous system, or stored in the liver or in the blood).
But this is only one reason for the wide‐spread confusion when it comes to discussing body fat in athletes. Other reasons are the measurement discrepancies of widely used methods because of their inherent methodical limitations. Currently used body composition assessment methods in sports9 have recently been analyzed by the Working Group on Body Composition, Health and Performance, under the auspices of the Medical Commission of the International Olympic Committee.1 A detailed discussion of best practice (and limitations) in physique assessment in sports can be found in Patria A. et al.20 In top level sports where athletes aim for extremes of their body composition, it is essential to be aware of possible measurement errors: small changes of body composition can have a major effect on performance.
Measuring body fat is not a simple task. Many assumptions on which some measurement techniques rely on are not valid for athletes. Surprising and sometimes obviously wrong data can result: in elite athletes, minus 12% fat were measured using densitometry, and negative fat amounts were measured on the torso using dual‐energy X‐ray absorptiometry (DXA).1 Santos et al15 analyzed the accuracy of DXA in elite judokas and found that DXA overestimated fat mass changes at the lower end and underestimated at the upper end when compared to the 4‐component reference method. These authors concluded: "DXA may not be accurate for detecting small physiological changes in athletes that need to achieve a target body weight prior to a competition".15 Severe errors of DXA can result because using two X‐ray photon energies enables only to distinguish between two sorts of tissue (soft tissue and bone) but not between three (muscle, fat, and bone). Therefore, the evaluation softwares of the companies have to be based on assumptions that are often not fulfilled, particularly not in athletes with their physique that deviates in many cases substantially from the "reference person". The shortcomings of DXA have repeatedly been analyzed and discussed in the literature.1, 15, 21, 22, 23
The use of bio‐impedance (BIA) for body fat measurement in sports has to be questioned because of unrealistic model assumptions that result in low accuracy which is not appropriate for analyzing body composition of athletes on a sufficiently fine scale, and also because of the reliability problems associated with this method.24 The widely used skinfold method9, 25, 26 for measuring subcutaneous adipose tissue (SAT) thickness was shown to deviate substantially from thickness measurements based on high‐resolution ultrasound (US) images.2
1.3. Ultrasound measurements of subcutaneous fat
During the last years, an ultrasound method for measuring subcutaneous adipose tissue patterning with high accuracy and reliability has been developed2, 27 and standardized in cooperation with the IOC Medical Commission Research Group on Body Composition, Health and Performance.28 This method can be applied to groups ranging from extreme leanness to obesity class III.29 With this novel US technique, thicknesses of SAT layers can be measured without compression and with high accuracy that is only limited by the furrowed SAT tissue borders1, 2, 28: the measurement accuracy2, 28, 29 is about 0.1‐0.2 mm (when using a 9‐18 MHz probe). High reliability is obtained by the standardized measurement and evaluation protocol.2, 28, 29, 30 US does not use ionizing radiation and can therefore be applied to children or pregnant women, too. The method uses eight sites to represent fat depots on the trunk, on the arms, and on the legs.28, 29 For defining the US measurement sites, the authors started out from the well‐known skinfold (SF) sites of the International Society for the Advancement of Kinanthropometry (ISAK),31 but several of these sites are not optimal for US measurements and had to be replaced by new ones.2, 27, 28, 29 All distances are relative to body height and marking of the eight sites takes only a few minutes.27 Further advantages of the US method are that SAT thickness layers can accurately be measured across a wide range of thicknesses,28, 29 fibrous tissues embedded in the SAT can be quantified accurately,2, 27, 28, 29 and many semi‐automatically performed thickness measurements from one image result in a small standard error of the mean (SEM) for a given site. US measurement and image evaluation can be done within about 15 minutes, and the method is easily applicable in the field. However, handling of the US system and the evaluation of the images need some practice.28, 29, 30
Here, a group of 61 elite judokas, among them several World Championships and Olympic medalists were investigated with this recently standardized US method. Additionally, ISAK SFs for subcutaneous fat assessment,9, 31 and BMI and MI1 as measures for relative body mass1, 3, 11, 18 were used for testing the following hypotheses:
The sums of SAT thicknesses D (medians) measured by the standardized US method are larger in female than in male judokas.
SAT thicknesses measured by US at the individual sites (medians) are not larger at all sites in female judokas than in male judokas.
Relative body weight (measured in terms of MI1 and BMI) correlates positively with D.
There is a significant correlation between short‐term weight losses for a competition and D measured some weeks before the competition.
There is a significant correlation between short‐term weight losses for an upcoming competition and SAT thicknesses measured some weeks before the competition at the abdomen sites.
Percentages of fibrous structures embedded in the SAT are the same in both sexes.
A higher skinfold sum of an athlete compared with another athlete is associated with a higher US sum D of SAT thicknesses.
Thickness of SAT measured by US in individuals at a given site (d) can be estimated by SF measurements at this site (X) according to d = qX, with q being a constant factor, independent of the site and of the person measured.
2. MATERIALS AND METHODS
All listed Appreviations may be found in the Supplementary Material.
2.1. Participants
Sixtyone elite judokas participated in this study, of whom 50 had competed at an international level (14 medalists of Olympic Games, World Championships, Masters, Grand Slam, and Grands Prix during 2015‐2017), 10 athletes participated in the Olympic Games in Rio de Janeiro 2016, and two won Olympic medals there. Females and males are analyzed separately because of their deviating physiological conditions and body composition necessities.4, 7 The study was approved by the Ethics Committee of the Medical University of Graz (20‐295ex08/09). All participants (or parents of participants younger than 18 years) were informed about the study contents and aims, and signed a written consent form before the examination was started. 51 athletes were measured within 3 days, before the upcoming competition phase (during an international training camp in Mittersill, Austria, January 7‐9, 2016), ten were measured in the following weeks. The most important first competitions for the investigated group of athletes took place on January 30‐31 (European Open) and on February 6‐7 (Grand Slam, Paris). Participants were grouped according to the Judo weight category (WC) borders for females (f) and males (m): (1) Extra‐lightweight (f:48 kg; m:60 kg), (2) Half‐lightweight (f:52 kg; m:66 kg), (3) Lightweight (f:57 kg; m:73 kg), (4) Half‐middleweight (f:63 kg; m:81 kg), (5) Middleweight (f:70 kg;m:90 kg), (6) Half‐heavyweight (f:78 kg; m:100 kg), and (7) Heavyweight (f: + 78 kg; m: + 100 kg).
Further group definitions: G: All participants, Gf: All females, Gm: All males.
Gf,A: Female adults; ≥18 years, excluded heavyweight category + 78 kg,
Gm,A: Male adults; ≥ 18 years, excluded heavyweight category + 100 kg,
Gf,HW: Female heavyweight category + 78 kg and for youth + 70 kg,
Gm,HW: Male heavyweight category + 100 kg,
Gf,Y: Female youth <18 years, excluded heavyweight category + 70 kg,
Gm,Y: Male youth <18 years, excluded heavyweight category + 100 kg,
Gf,A,1‐5: Female adults groups 1‐5 (heavyweight,7, and half‐heavyweight,6, not included),
Gm,A,1‐5: Male adults groups 1‐5 (heavyweight,7, and half‐heavyweight,6, not included),
Gf,A,6‐7: Female adults groups 6‐7 (heavyweight,7, and half‐heavyweight,6),
Gm,A,6‐7: Male adults groups 6‐7 (heavyweight,7, and half‐heavyweight,6).
2.2. Anthropometry
Anthropometric measurements include the following: body mass (M) measured on a calibrated electronic scale (Seca Modell 799) in underwear or tight sport clothes, without shoes; body height (h), sitting height (s), leg length (l) were measured to the nearest 0.1 cm with an anthropometer (GPM 100). A metallic tape (CESCORF) was used for measuring waist circumference. Measurements were performed according to the ISAK protocol.31 BMI = M/h2 29 and the mass index MI1 = 0.53M/(hs) were calculated. Anthropometric variables were measured immediately before US images were captured. Weight reduction was determined by subtracting the maximum weight of the athlete's weight category from the weight measured during this study (50 of the 61 judokas competed at international events after the study).
2.3. Ultrasound site marking
All eight standardized sites (on the right side of the body) were marked according to the standardized US protocol28: the sites upper abdomen (UA), lower abdomen (LA), lateral thigh (LT) were marked in standing position, the erector spinae (ES) in sitting position, distal triceps (DT), and brachioradialis (BR) were marked with the forearm supported, front thigh (FT) and medial calf (MC) with the right foot on a supporting box. US site selection is discussed in detail elsewhere.28, 29
2.4. Skinfold site marking
Skinfolds were measured with a Harpenden skinfold caliper (Baty International; spring pressure of 10 g/mm2) at each of the eight ISAK sites: triceps, subscapular, biceps, iliac crest, supraspinale, abdominal, front thigh, and medial calf.31
2.5. Observers
Two experienced observers performed the measurements. They had been trained according to the ISAK protocol for skinfolds and by the International Association of Sciences in Medicine and Sports (IASMS) for the US technique.
2.6. Ultrasound imaging of SAT
The image procedure for US measurements was made with the participants lying in a supine, prone, or rotated position.28 Conventionally B‐mode (brightness mode) US imaging systems use a speed of sound c = 1540 m s‐1 for calculating distances of reflectors in “soft” tissues according to the pulse‐echo method. The image evaluation software used here considers the speed of sound in SAT accordingly (c = 1450 ms‐1).28 Compression is avoided by using a thick layer of US gel (about 5 mm) between the probe and the skin. Two conventional US systems operated with linear probes (Phillips CX50, linear probe L12‐3, and GE Logic e with probe 18L) were used. US measurements were performed in accordance with the method published recently.28
2.7. Semi‐automatic thickness measurement
The US Tissue‐Scientific software (version 3.3, rotosport.at) enables measuring series of thickness values in US images semi‐automatically. Sound speed was set to 1450 m s‐1 for distance determination in SAT.32 Visual control is necessary because anatomic structures (eg, Camper's fascia in the abdomen region2) may be complex in some cases. Within the appropriate region of interest (ROI) chosen by the observer, the software detects the contour of the adipose tissue layer, and measures typically about hundred thicknesses therein. The ROI was usually chosen symmetrically around the middle line which corresponds to the middle beam of the US probe. The evaluation software started out from the blue seeds (compare to Figure 1B). The SAT detected within the region of interest is colored in red. The software displays both the mean SAT thickness in a given ROI with the fibrous structures included (d I) or excluded (d E). The sums of the thicknesses from the eight sites (D I and D E, respectively) are also computed.
Figure 1.
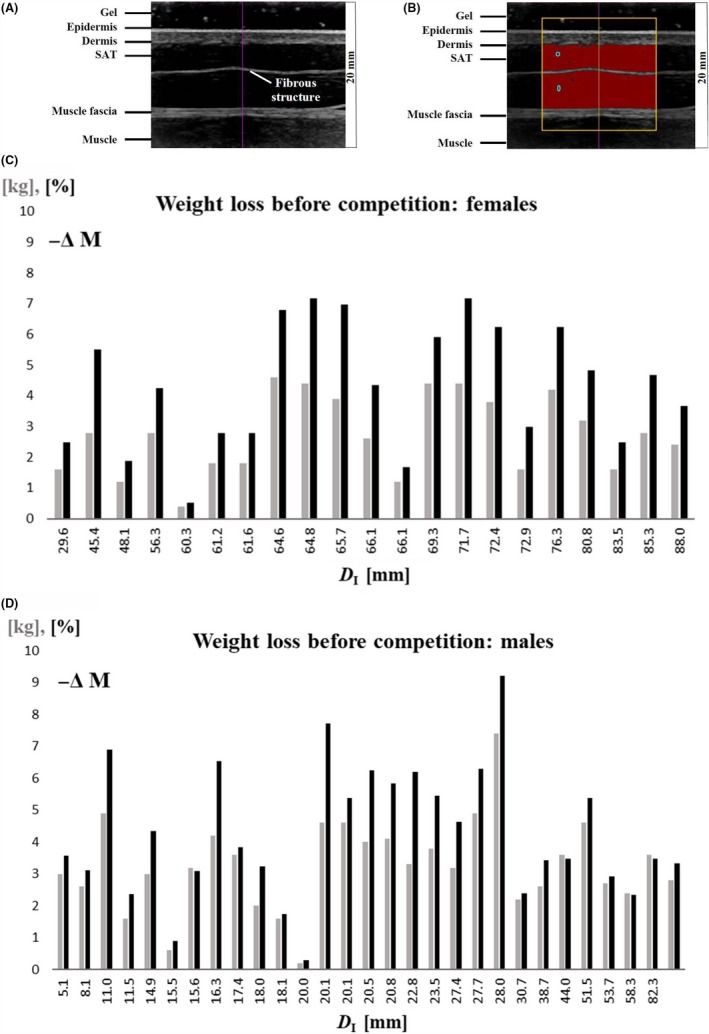
A, Typical example of an ultrasound image (US) of subcutaneous adipose tissue (SAT). Site: front thigh (FT). Tissue structures of relevance for US image evaluation are marked. B, The semi‐automatic SAT detection algorithm starts out from the blue zones. The yellow frame represents the chosen region of interest. The evaluation algorithm measured automatically 235 SAT thicknesses of the SAT layer along 235 vertical lines in this image. Mean d I = 8.10 (± 0.11) mm, d E = 7.61 (± 0.12) mm, and the difference d F = 0.49 mm (represents the mean thickness of the embedded fibrous structure). Image depth was 20 mm. C, Weight loss in all females (except for heavy weight category) before competition, in kg (grey) and in percent of body weight (black). Participants are ordered according to their sums of SAT thicknesses D I. Fig. D, as C, but for males.
2.8. Statistics
Participants' data (Table 1) are given in means ± standard deviation (mean ± sd). Shapiro‐Wilk test revealed that not all datasets were normally distributed, therefore medians and box plots for brief descriptions of data distributions were used. Spearman's or Pearson rank correlation coefficients (ρ) were used for data of Figure 2A‐D. Mann‐Whitneytest was applied to compare female and male values in given datasets (Figures 4 and 5). SPSS (IBM SPSS Statistics V.24) was used.
Table 1.
Anthropometric data of all groups
| N | G 61 | Gf 26 | Gm 35 | Gf,A 16 | Gm,A 26 | Gf,y 8 | Gm,Y 6 | Gf,HW 2 | Gm,HW 3 |
|---|---|---|---|---|---|---|---|---|---|
| a (y) | 21.4 (5.5) | 19.9 (4.2) | 22.5 (6.1) | 21.6 (3.7) | 24.2 (5.8) | 16.1 (1.0) | 15.5 (1.0) | 21.5 (7.8) | 21.7 (4.2) |
| h (m) | 1.730 (0.08) | 1.678 (0.06) | 1.769 (0.08) | 1.681 (0.06) | 1.763 (0.07) | 1.655 (0.05) | 1.739 (0.08) | 1.745 (0.05) | 1.879 (0.06) |
| s (m) | 0.920 (0.04) | 0.895 (0.03) | 0.939 (0.04) | 0.898 (0.03) | 0.936 (0.03) | 0.878 (0.02) | 0.922 (0.04) | 0.935 (0.00) | 0.998 (0.04) |
| l (m) | 0.966 (0.06) | 0.936 (0.04) | 0.990 (0.05) | 0.934 (0.04) | 0.982 (0.05) | 0.928 (0.03) | 0.999 (0.06) | 0.987 (0.04) | 1.039 (0.04) |
| M (kg) | 75.6 (18.3) | 64.7 (7.7) | 83.7 (19.8) | 65.3 (6.3) | 82.1 (12.7) | 59.6 (4.9) | 67.1 (13.8) | 80.3 (1.3) | 130.6 (3.5) |
| BMI (kg m−2) | 25.0 (4.0) | 22.9 (1.9) | 26.5 (4.5) | 23.1 (1.6) | 26.3 (2.7) | 21.8 (1.4) | 22.0 (2.5) | 26.4 (1.1) | 37.1 (2.4) |
| MI1 (kg m−2) | 25.0 (4.0) | 22.8 (1.9) | 26.7 (4.3) | 22.9 (1.8) | 26.2 (2.8) | 21.7 (1.5) | 22.8 (2.3) | 26.1 (0.3) | 37.0 (2.3) |
| w/h (1) | 0.44 (0.05) | 0.42 (0.02) | 0.46 (0.05) | 0.42 (0.02) | 0.46 (0.03) | 0.41 (0.02) | 0.41 (0.02) | 0.43 (0.04) | 0.56 (0.05) |
| C = s/h (1) | 0.53 (0.01) | 0.53 (0.01) | 0.53 (0.01) | 0.53 (0.01) | 0.53 (0.01) | 0.53 (0.00) | 0.53 (0.01) | 0.54 (0.02) | 0.53 (0.00) |
| D I,MEDIAN (mm) | 49.8 | 66.1 | 21.8 | 65.4 | 20.8 | 65.9 | 19.0 | 115.4 | 111.5 |
| D E,MEDIAN (mm) | 44.2 | 60.0 | 17.0 | 59.1 | 16.2 | 59.4 | 15.5 | 109.4 | 106.4 |
| PMEDIAN (%) | 12.9 | 8.6 | 20.2 | 8.8 | 21.7 | 8.8 | 19.3 | 5.1 | 5.5 |
Shown are means (±SD) of: age (a), body height (h), sitting height (s), leg length (l) body mass (M), body mass index (BMI = M/h 2), mass index (MI1 = 0.53M/(hs)), waist‐to‐height ratio (w/h) and the group medians of D I and D E. P is the percentage of fibrous structures P = 100F/D I = 100(D I‐D E/D I) of the group median.
D I: sums of subcutaneous adipose tissue (SAT) thicknesses when the fibrous structures are included.
D E: sums of SAT thicknesses when the fibrous structures are excluded.
F: sum of fibrous structures.
Abbreviations: a: age (years), h: body height (m), s: sitting height (m), l: leg length (m), M: body mass (kg), BMI: body mass index (kg m−2), MI1: mass index (kg m−2), w/h: waist‐to‐height ratio (1), C: Cormic index (1).
Figure 2.
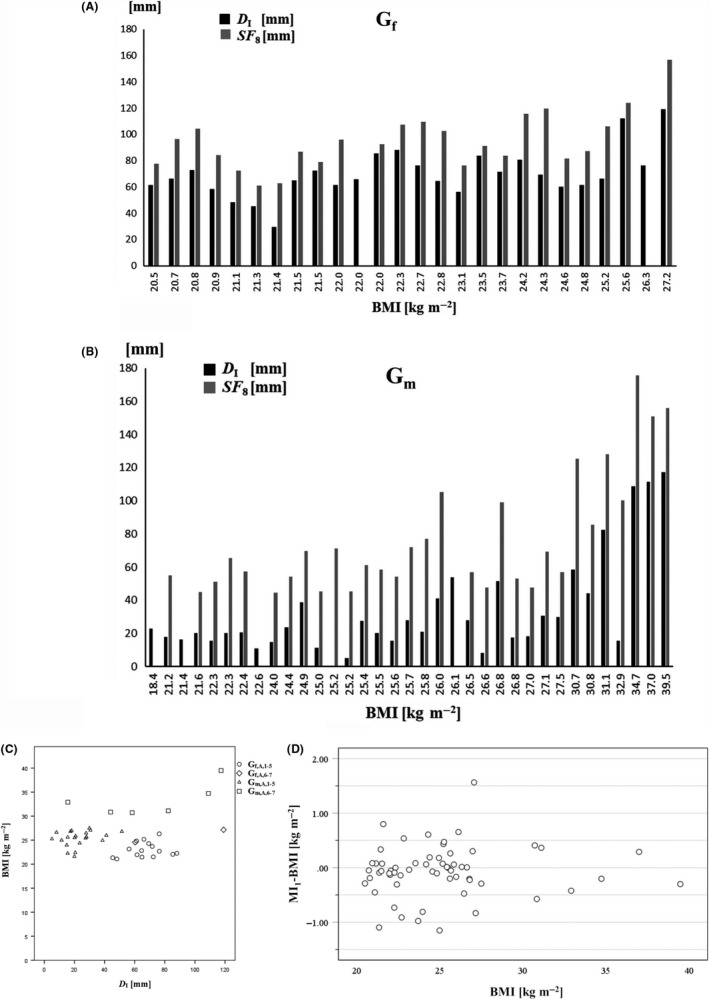
Subcutaneous adipose tissue (SAT) measurement with US and skinfold (SF) technique. Participants are ordered according to their body mass index (BMI). A, black columns represent the D I (sum of SAT thicknesses of the eight standardized US sites, with fibrous structures included); grey columns represent SF 8 (sum of the eight ISAK skinfold thicknesses) in all females (Gf). B, as in (A), but for all men (Gm). C, Relationship between the subcutaneous adipose tissue (SAT) and BMI. The sum of US measurements of SAT thicknesses D I (with fibrous structures included) is compared with the BMI = M/h2. For both female (Gf,A,1‐5) and male (Gm,A,1‐5) adult judo athletes together (ie,: half‐heavy weight and heavy weight not included), there was a weak negative correlation (ρ = −.379, P < .01) between BMI and D I. Gf,A,1‐5: Female adults groups 1‐5 (heavy weight,7 and half‐heavy weight,6, not included). Gf,A,6‐7: Female adults groups 6‐7 (heavy weight,7 and half‐heavy weight,6). Gm,A,1‐5: Male adults groups 1 to 5 (heavy weight,7 and half‐heavy weight, 6, not included). Gm,A,6‐7: Male adults groups 6‐7 (heavy weight,7 and half‐heavy weight,6). D, Differences between mass Index MI1 and body mass index BMI. Number of athletes: N = 60. MI1 = 0.53 M/(hs), and BMI = M/h2; M is the body mass in kg, h the stature and s the sitting height, both in m
3. RESULTS
Table 1 shows participants' anthropometric data and also the sums of SAT thicknesses measured at the eight standardized sites for all groups. In D I, the embedded fibrous structures are included in the thickness measurement, whereas in D E they are excluded. For each group, the median percentage of fibrous structures (P) that are embedded in the SAT is also shown.
A typical US image from the site front thigh (FT) is shown in Figure 1A. The black band on top corresponds to the thick gel layer that avoids tissue compression. The black region between the skin and the muscle fascia represents the SAT layer (1A). A fibrous structure is embedded in this case.
Body weights measured during the research study were compared with the maximum weight of the athletes' individual weight category limits (Figure 1C,D). In the training phase when measurements took place, most judokas (except for the five heavy weight athletes and another six) had body masses above their weight category limits: the mean (N = 50) was 3.0 kg ± 1.4 kg (ie, 4.3% ± 2.0% of body mass), the highest values were 7.4 kg (9.2%), two cases with 4.9 kg (6.9% and 6.3%), and four cases with 4.6 kg (7.7%, 6.8%, 5.4%, and 5.4%). Mean weight loss of females (N = 21) was 2.7 kg ± 1.3 kg (4.4% ± 2.0%), and of males (N = 29) 3.3 kg ± 1.4 kg (4.3% ± 2.0%). There was no significant correlation between weight losses and sums of SAT thicknesses (females: ρ = .26, P = .25; males: ρ = .19, P = .34), and there was also no significant correlation between weight losses and SAT thicknesses measured at the UA and LA site (UA: females: ρ = −.08, P = .73; males: ρ = −.003, P = .99); LA: females: ρ = .24, P = .30; males: ρ = .01, P = .97). The mean weight losses in athletes measured in the same training period (January 7‐9, 2016) (N = 40) was: 3.0 kg ± 1.3 kg (ie, 4.2% ± 1.9% of body mass), and there was also no significant correlation between weight losses and SAT thicknesses (females: ρ = .21, P = .42; males: ρ = .34, P = .12) in both sexes.
Figure 2A,B compare SAT thicknesses measured by US to skinfold (SF) measurements. Athletes are ordered according to their BMI. The pattern of the columns indicates that BMI and SAT are not correlated. This also holds true in many cases when SFs are compared to the US measurements of SAT (D I). The ratios D I,MEDIAN/SF 8,MEDIAN were 0.61 for females (Nf = 24), and 0.40 for males (Nm = 30). However, several cases differed substantially from the medians of the ratios: for example, the male participant with BMI = 21.6 kg m−2 had a ratio of 20.0 mm(D I)/44.9 mm(SF) = .45 and the male participant with BMI = 25.2 kg m−2 had a ratio of 5.1 mm/45.4 mm = 0.11 indicating that the same skinfold sum (45 mm) did not at all correspond to the same SAT thickness sum measured by the standardized US technique.28, 29 SAT thickness sums ranged from 5.1 mm (corresponding to a mean of 0.64 mm) to 119.0 mm (14.88 mm) and SF sums from 44.6 mm (5.58 mm) to 175.8 mm (21.98 mm) (Figure 2). The FT and the MC site were the same for US and SF measurements. At FT, the ratios d/X ranged from 0.03 to 0.62 (median: 0.38; N = 55), and at MC from 0.03 to 0.64 (median: 0.37; N = 60). There was no constant factor relating the SAT thicknesses measured by US and by SFs, indicating the large influence of individual skin thickness and SAT compressibility on the SF measurement results.
Figure 2C shows that there was even a negative correlation (Spearman's rank correlation coefficient ρ was − .379, P < .01) between BMI and the accurately measured sum of SAT thicknesses (D I) when females and males were analyzed together (half‐heavy weight and heavy weight not included). This indicates that BMI is not a useful measure of fatness, at least in athletes. Analysis of females and males separately did not result in a significant correlation.
Figure 2D shows the differences between the measures MI1 and BMI for relative body weight. 13 of the athletes showed differences larger than 0.5 kg m−2, and three differences were larger than 1.0 kg m−2.
Figure 3A,B show the enormous SAT differences of two male judokas (chosen as examples) measured by SFs compared with the highly accurate US technique,28, 29 although these two athletes had the same SF sums. In the US images, one can see the SAT layers immediately (Figure 3B): the much higher SAT thicknesses of the judoka J1 with the BMI of only 21.6 kg m−2 when compared to J2 with the BMI of 25.2 kg m−2 is obvious (Figure 3A,B). Other examples where SF results stand in contrast to the thicknesses measured with the standardized B‐mode US technique can be found in Figure 2.
Figure 3.
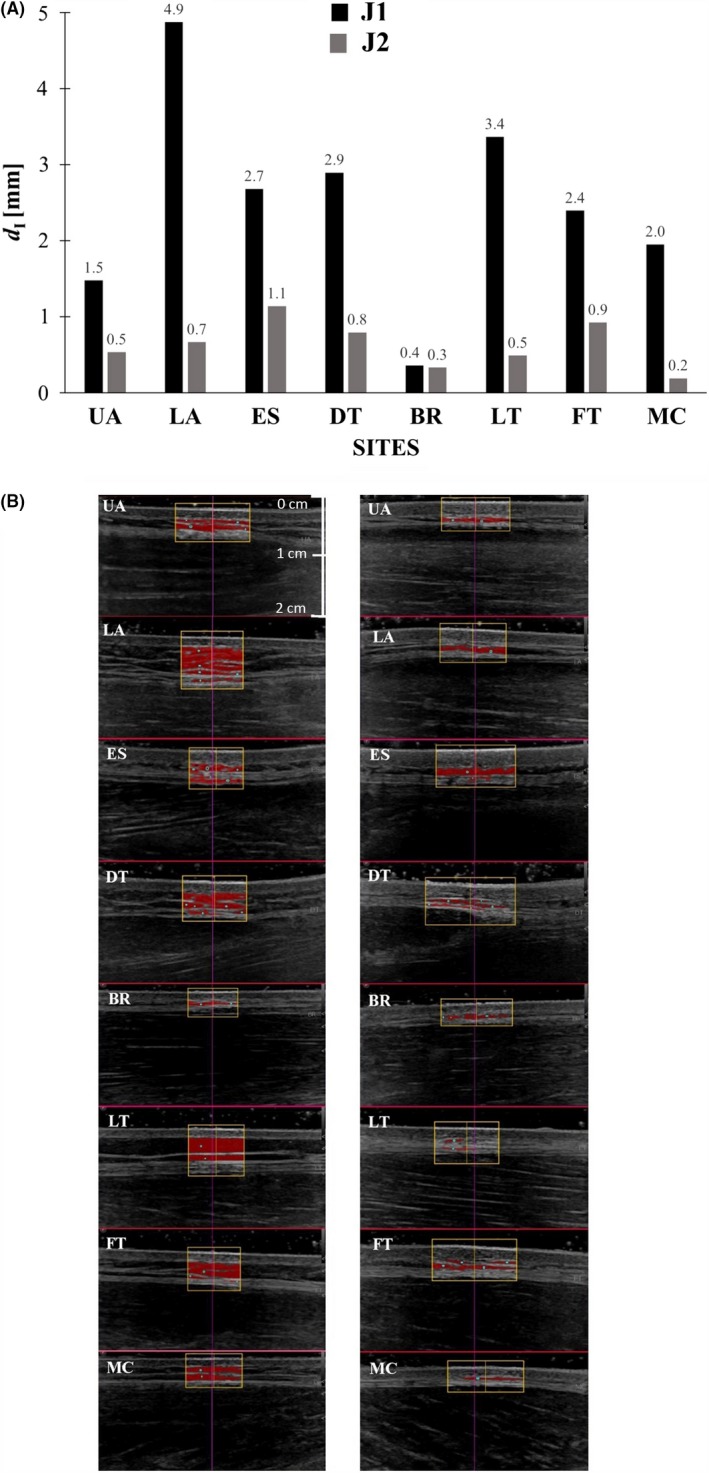
Comparison of subcutaneous fat profiles. Two elite male judokas, J1 (black columns; weight category (WC) 66 kg; body mass (M) 66.2 kg; body height (h) 1.751 m; BMI 21.6 kg m−2) and J2 (grey columns; WC 81 kg; M 84.0 kg; h 1.824 m; BMI 25.2 kg m−2) with almost the same sum of eight ISAK skinfolds (J1: 44.9 mm; J2: 45.4 mm) were compared. Their sums of SAT thicknesses measured by US differed enormously (J1: 20 mm, and J2: 5.1 mm). A, The thickness values of J1 and J2 at the individual sites measured by US (d I) include the fibrous structures. B, Shows the according series of evaluated US images (left side: J1, right side: J2). The yellow frame indicates the region of interest (ROI); in these two exemplarily selected participants, the numbers of measurements (semi‐automatic thickness measurements) contributing to the representative mean at a given site ranged from 132 to 264
At all eight standardized sites, there was a significant difference in SAT thicknesses between females and males (Figure 4). Ratios of median SAT thicknesses of all adult females and males were: UA (4.9 mm/2.2 mm = 2.2), LA (12.1/5.1 = 2.4), ES (5.9/3.7 = 1.6), DT (6.0/2.3 = 2.6), BR (2.1/0.5 = 4.2), LT (22.5/4.2 = 5.4), FT (8.2/2.5 = 3.3), and MC (4.9/1.4 = 3.5). The highest ratio (5.4) was found at LT.
Figure 4.
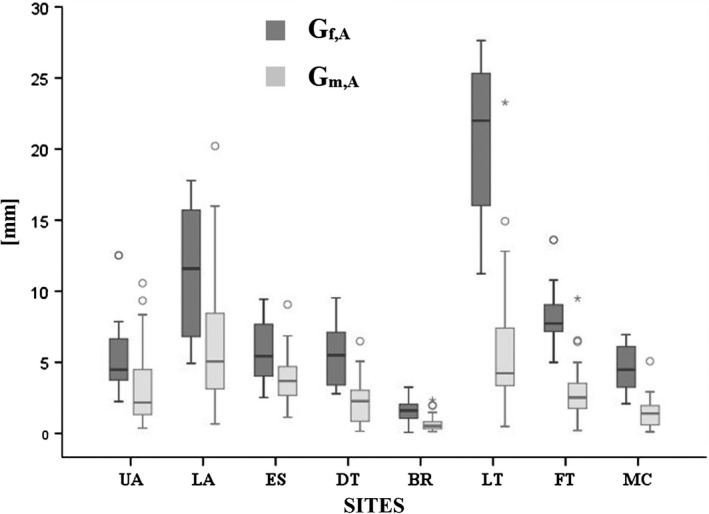
Thickness patterning in female and male judo athletes. Box plots of SAT thicknesses (d I) at the eight standardized sites in adult females (Gf,A) and males (Gm,A): upper abdomen (UA), lower abdomen (LA), erector spinae (ES), distal triceps (DT), brachioradialis (BR), lateral thigh (LT), front thigh (FT), medial calf (MC)
Figure 5A,B show the percentages P of fibrous structures embedded in the SAT (ordered according to D I) for females and males. P = 100F/D I; F: thickness sum of fibrous structures measured at the eight US sites (Table 2). The median of the 16 adult females (Figure 5A) was 8.8%, and that of the 25 adult males was 21.7% (Figure 5B). In other words: the median percentage of embedded fibrous structures was about 2.5 times higher in the male group; this further increases the difference in subcutaneous fat between females and males.
Figure 5.
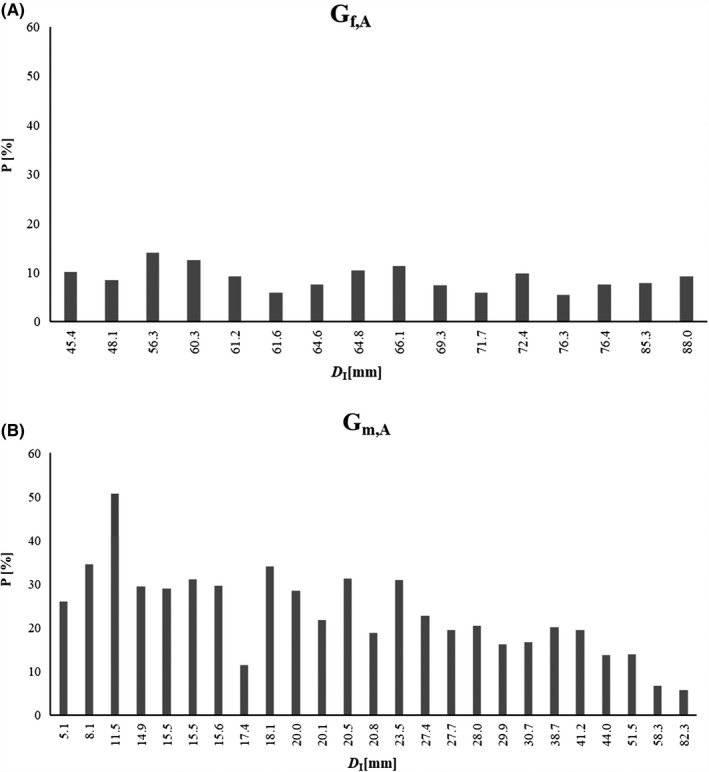
Percentage P of fibrous structures (F) embedded in the SAT. Columns represent: P = 100F/D I = 100(D I‐D E)/D I. D I: sums of subcutaneous adipose tissue (SAT) thicknesses when the fibrous structures are included. D E: sums of SAT thicknesses when the fibrous structures are excluded. (F): sum of fibrous structures. A, adult females: median value of the 16 females was 8.8%, (mean: 8.9 ± 2.4%). B, adult males: median value of the 25 males was: 21.7%, (mean: 23.3 ± 10.0%)
Table 2.
Embedded fibrous structures in adult female (Gf,A) and male (Gm,A) judokas. D I: subcutaneous adipose tissue (SAT) thickness sums of the standardized eight sites with the fibrous structures included in the SAT; F: thickness sums of fibrous structures embedded in the SAT. D I,MEDIAN was 65.4 mm in Gf,A, and 20.8 mm in Gm,A. Median of F was 5.7 mm in Gf,A and 5.1 mm in Gm,A. Participants are ordered according to their D I. Values in mm. The percentages [%] of fibrous structures (F) embedded in the SAT: P = 100F/D I = 100(D I‐D E)/D I are also listed
| Gf,A | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 4.6 | 4.1 | 7.9 | 7.5 | 5.6 | 3.7 | 4.9 | 6.7 | 7.5 | 5.1 | 4.3 | 7.1 | 4.2 | 5.8 | 6.8 | 8.2 | |||||||||
| D I | 45.4 | 48.1 | 56.3 | 60.3 | 61.2 | 61.6 | 64.6 | 64.8 | 66.1 | 69.3 | 71.7 | 72.4 | 76.3 | 76.4 | 85.3 | 88.0 | |||||||||
| P | 10.2 | 8.4 | 14.1 | 12.5 | 9.2 | 5.9 | 7.6 | 10.4 | 11.3 | 7.3 | 6.0 | 9.8 | 5.5 | 7.6 | 7.9 | 9.3 | |||||||||
| Gm,A | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 1.3 | 2.8 | 5.8 | 4.4 | 4.5 | 4.8 | 4.6 | 2.0 | 6.2 | 5.7 | 4.4 | 6.4 | 3.9 | 7.2 | 6.2 | 5.4 | 5.7 | 4.8 | 5.1 | 7.8 | 8.0 | 6.0 | 7.2 | 3.9 | 4.6 |
| D I | 5.1 | 8.1 | 11.5 | 14.9 | 15.5 | 15.5 | 15.6 | 17.4 | 18.1 | 20.0 | 20.1 | 20.5 | 20.8 | 23.5 | 27.4 | 27.7 | 28.0 | 29.9 | 30.7 | 38.7 | 41.2 | 44.0 | 51.5 | 58.3 | 82.3 |
| P | 25.9 | 34.5 | 50.8 | 29.4 | 28.9 | 31.0 | 29.6 | 11.4 | 34.1 | 28.4 | 21.7 | 31.3 | 18.8 | 30.8 | 22.7 | 19.4 | 20.4 | 16.1 | 16.7 | 20.1 | 19.5 | 13.6 | 13.9 | 6.7 | 5.6 |
4. DISCUSSION
4.1. Body weight and rapid weight loss before competitions in elite judokas
As in all weight category sports, body weight, and body composition play a major role in judo and reducing substantial amounts of weight within short time (before the weight‐in) is a usual part of the competition (Figure 1C). Rapid weight loss practices are common 14, 16, 33: among them are extreme dehydration procedures (eg, working out in the sauna in neoprene cloths), extreme reductions of fluid and food in‐take, use of laxatives and diuretics. Mass reduction of 3 kg (4.3%) was the mean in the study presented here, 19 athletes had to reduce their weight by more than 5%, and the highest value found in this group was 9.2%. Similar weight reduction percentages were also reported in other weight class sports. In a meta study, Franchini et al16 reported prevalence's of rapid weight loss practices in judo (89%, Brazil; 70%‐80% in USA), karate (71%, Brazil), taekwondo (63%, Brazil), jujitsu (57%, Brazil), wrestling (62%, Iran; 62% and 70% in another USA high school study, 89% USA college), Olympic boxing (100%, Brazil), and others. Franchini et al 16 also reported several intentional rapid weight loss practices that ended up lethally (causa mortis were heart attacks, hyperthermia, and dehydration). In a specific study in competitive judokas, Artioli et al 33 found that "most athletes cut their weight up to five times a year to compete, but a significant percentage reduced their weight up to 10 times a year or more." The most influential persons in leading athletes to reduce their weight were the coaches, but not physicians or dietitians who could provide the best advice for weight management. Reale et al 14 reviewed "acceptable" dietary strategies for Olympic combat sports and discussed how better health and performance outcome may be achieved. However, rapid body mass reduction can be seen as a prohibited method according to the World Anti‐Doping Agency (WADA) criteria; Artioli et al 34, 35 discuss solid arguments why it is "time to ban rapid weight loss from combat sports". Strong data and health considerations indicate that weigh‐in procedures should urgently be modified to counteract these unhealthy tendencies in judo, and also in other weight category sports. This could be obtained by modifying the weigh‐in regulations and is up to the international combat sport federations. Their medical commissions should take responsibility for the health of their athletes. This was successful in other weight‐sensitive sports, for example, in ski jumping, where competition rules have been changed by the International Ski Federation (FIS) to improve the health situation.11, 13, 36
4.2. Measures for relative body weight applied in female and male judokas
The body mass index is a measure of relative body weight, but not of body fatness,1 although it had originally been intended to measure fat this way. This holds particularly true in highly trained athletes: in the cases of the groups Gf,A (all adult females) and Gm,A (all adult males), there were no correlations between SAT thickness (sum of eight sites) and BMI values, and for both groups evaluated together, there was even a negative correlation.
Also as a measure for relative body weight ("ponderosity"), the BMI has severe shortcomings that have been pointed out by the expert committee of the WHO.17 Therefore, we recommend to use the improved measure "mass index (MI)" for assessing relative body weight: MI1 = 0.53 M/(hs) 3, 11, 18, 28 because this modified BMI takes the individual sitting height s into account (and thus, implicitly, also the leg length l). Mean MI1 of females was 22.8 kg m−2 (BMI:22.9 kg m−2), and of males: 26.7 kg m−2 (BMI:26.5 kg m−2), but individual differences MI1‐BMI were larger than 0.5 kg m−2 in 13, and larger than 1.0 kg m−2 in three cases (Figure 2D).
4.3. Subcutaneous fat patterning in elite female and male judokas
When compared to thickness measurements using high‐resolution US imaging,28, 29 severe errors of skinfold measurements come to light because compressibility of fat and thickness of the skin varies substantially from site to site and among individuals.2 Large deviations were also found in the group of elite judokas investigated here. For example, two competing athletes who had almost the same SF sums at eight sites (44.9 mm and 45.4 mm) differed by a factor of four when their SAT thicknesses were measured with the standardized US method (the sums of eight sites D I were 20.0 mm and 5.1 mm, respectively). The sums of SAT thicknesses were much higher in females (median D I was 66.1 mm) than in males (21.8 mm), although relative body weight was much lower in females (mean BMI was 22.9 kg m−2) than in males (26.5 kg m−2). In the female medalists included in this study (at Olympic Games, World Championships, Masters, Grand Slam, and Grand Prix; 2015‐2017), SAT thickness sums were also much higher (median D I was 61.6 mm; N = 9) than in male medalists (20.1 mm; N = 5). In rowing,37 substantially higher SAT values were also measured in females (65.5 mm; 22.0 kg m−2) when compared to males (27.6 mm; 23.8 kg m−2). This holds also true for several other sports. Not only the sum of SAT thicknesses (Figure 2A‐C) was higher in females, also the mean thickness at each individual site was higher (Figure 4), with the highest difference found at the site lateral thigh (LT). In addition, males showed a higher percentage of fibrous structures embedded in the SAT (Figure 5) which again enlarges the difference in the subcutaneous fat amount between the sexes. Females seem to "need" a much higher amount of subcutaneous fat, although individual differences were large (Figure 2A,C): in the adult female group (Gf,A excluded heavy weight category), the highest sum of SAT thicknesses D I was 88.0mm, and the lowest was 45.4mm. According to a preliminary assessment schedule,38 no female was in the category "extremely low", and one was in the category “very low”; among males, there were four and nine, respectively. The fat values can be used as an important indicator for finding the appropriate weight division for an athlete: athletes who are further away from the extremely low‐fat edges have some potential to reduce their weight in the long run without losing muscle and organ mass, instead of applying unhealthy rapid weight reduction practices before a competition. The US method provides an accurate approach for assessing subcutaneous fat, which forms by far the major part of total body fat.39 The results found here show that medical questions concerning lower fat limits need to be treated separately for females and males.
5. CONCLUSIONS
The median of sums of subcutaneous fat layers (D) in the elite female judokas was about three‐times higher than in the elite male judokas. This implies the hypothesis that females, although when in an intensive phase of training, “need” a substantially larger amount of body fat compared to males and that health questions associated with body composition necessitate separate approaches for females and males.
At all eight (standardized) body sites, medians of SAT thicknesses in the female adult judokas were significantly higher. The highest ratio between females and males was found at the LT site. Although fat patterning differs between female and male judokas, the increased subcutaneous fat mass in female athletes is distributed over all sites.
In both the female and the male group, there were no significant correlations between relative body weight and D. These results show that both MI1 and BMI are measures of ponderosity, but not capable to assess body composition in athletes.
There was no significant correlation between weight loss before a competition and D, indicating that there is an (unused) potential for long‐term weight reduction based on SAT reduction for those athletes who have larger SAT depots.
At the upper and lower abdomen sites, there were also no significant correlations between short‐term weight loss for an upcoming competition and SAT thicknesses measured some weeks before the competition.
Percentages of fibrous structures embedded in the SAT differed significantly between female and male judokas: the median was about 2.5 times higher in the male group. This further increases the difference in subcutaneous fat between females and males.
Listing the athletes according to their skinfold (SF) sums does not result in the same order when listing them according to their US sums of SAT thicknesses. SAT thickness sums (from eight sites) ranged from 5.1 mm to 119.0 mm, and SF sums (from eight sites) from 44.6 mm to 175.8 mm.
When comparing SF and US measurements at the same sites (FT and MC), large deviations were found and the median deviations were significantly higher at FT compared with MC. This can be explained because SFs measure two layers of skin and two layers of fat in an undefined compressed state, and both compressibility and skin thickness differ from site to site and from person to person.
5.1. Perspectives
From the medical point of view, not much is currently known about acceptable lower limits of the individual's fat amount,4 and the large variation found, particularly in female judokas, indicates that this important question needs to be discussed individually. Results clearly show that the enormous differences between sexes have to be considered. The accurate and reliable data obtainable with US will contribute to a solid basis for analyses of the long‐term health development associated with low body fat.4, 40 For this purpose, US SAT data of both sexes and from various sports are needed. Athletes of weight class sports who have a higher body fat amount than others could use this potential to avoid or reduce unhealthy short‐term weight reduction before a competition. There is also large potential for reducing the severe medical problems associated with the common rapid weight loss practices by modifying the weigh‐in procedures; this is up to the International Federations of the weight class sports.
Supporting information
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Austrian Judo Federation and in particular Markus Moser for generously supporting this research. We thank Hans Holdhaus und Gregor Bialowas (IMSB‐Austria) for initializing this project, and all athletes for their participation. Results of the study are presented clearly, honestly and without fabrication, falsification, or inappropriate data manipulation.
Sengeis M, Müller W, Störchle P, Führhapter‐Rieger A. Body weight and subcutaneous fat patterning in elite judokas. Scand J Med Sci Sports. 2019;29:1774–1788. 10.1111/sms.13508
REFERENCES
- 1. Ackland TR, Lohman TG, Sundgot‐Borgen J, et al. Current status of body composition assessment in sport: review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the I.O.C. Medical Commission. Sports Med. 2012;42(3):227‐249. [DOI] [PubMed] [Google Scholar]
- 2. Müller W, Horn M, Fürhapter‐Rieger A, et al. Body composition in sport: a comparison of a novel ultrasound imaging technique to measure subcutaneous fat tissue compared with skinfold measurement. Br J Sports Med. 2013;47(16):1028‐1035. [DOI] [PubMed] [Google Scholar]
- 3. Müller W. Towards research‐based approaches for solving body composition problems in sports: ski jumping as a heuristic example. Br J Sports Med. 2009;43(13):1013‐1019. [DOI] [PubMed] [Google Scholar]
- 4. Sundgot‐Borgen J, Meyer NL, Lohman TG, et al. How to minimise the health risks to athletes who compete in weight‐sensitive sports review and position statement on behalf of the ad hoc research working group on body composition, health and performance, under the auspices of the ioc medical commission. Br J Sports Med. 2013;47(16):1012‐1022. [DOI] [PubMed] [Google Scholar]
- 5. Quatromoni PA. A tale of two runners: a case report of athletes' experiences with eating disorders in college. J Acad Nutr Diet. 2017;117(1):21‐31. [DOI] [PubMed] [Google Scholar]
- 6. Slater G, O'Connor H, Kerr A. Optimising physique for sports performance In: Hume PA, Kerr DA, Ackland T, eds. Best practice protocols for physique assessment in sport, 1st edn Singapore: Springer; 2018:27‐36. [Google Scholar]
- 7. Nattiv A, Loucks AB, Manore MM, et al. American college of sports medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867‐1882. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC) . Hyperthermia and dehydration‐related deaths associated with intentional rapid weight loss in three collegiate wrestlers–North Carolina, Wisconsin, and Michigan, November‐December 1997. MMWR Morb Mortal Wkly Rep. 1998;47(6):105‐108. [PubMed] [Google Scholar]
- 9. Meyer NL, Sundgot‐Borgen J, Lohman TG, et al. Body composition for health and performance: a survey of body composition assessment practice carried out by the ad hoc research working group on body composition, health and performance under the auspices of the IOC medical commission. Br J Sports Med. 2013;47(16):1044‐1053. [DOI] [PubMed] [Google Scholar]
- 10. Mountjoy M, Sundgot‐Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad‐relative energy deficiency in sport (RED‐S). Br J Sports Med. 2014;48(7):491‐497. [DOI] [PubMed] [Google Scholar]
- 11. Müller W, Gröschl W, Müller R, Sudi K. Underweight in ski jumping: the solution of the problem. Int J Sports Med. 2006;27(11):926‐934. [DOI] [PubMed] [Google Scholar]
- 12. Schmölzer B, Müller W. The importance of being light: aerodynamic forces and weight in ski jumping. J Biomech. 2002;35(8):1059‐1069. [DOI] [PubMed] [Google Scholar]
- 13. Schmölzer B, Müller W. Individual flight styles in ski jumping: results obtained during olympic games competitions. J Biomech. 2005;38(5):1055‐1065. [DOI] [PubMed] [Google Scholar]
- 14. Reale R, Slater G, Burke LM. Individualised dietary strategies for olympic combat sports: acute weight loss, recovery and competition nutrition. Eur J Sport Sci. 2017;17(6):727‐740. [DOI] [PubMed] [Google Scholar]
- 15. Santos DA, Silva AM, Matias CN, Fields DA, Heymsfield SB, Sardinha LB. Accuracy of DXA in estimating body composition changes in elite athletes using a four compartment model as the reference method. Nutr Metab (Lond). 2010;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franchini E, Brito CJ, Artioli GG. Weight loss in combat sports: physiological, psychological and performance effects. J Int Soc Sports Nutr. 2012;9(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health . Organization (WHO). Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1‐452. [PubMed] [Google Scholar]
- 18. Müller W. Determinants of ski‐jump performance and implications for health, safety and fairness. Sports Med. 2009;39(2):85‐106. [DOI] [PubMed] [Google Scholar]
- 19. Wang Z, Sardinha LB, Lukasky HC, et al. Body composition models and components In: Heymsfield SB, Lohman TG, Wang ZM, Going SB, eds. Human body composition, 2nd edn Champaign, IL: United States of America Human Kinetics; 2005:163‐239. [Google Scholar]
- 20. Hume P, Kerr D, Ackland TR. Best practice protocols for physique assessment in sport , 1st: edn Singapore: Springer; 2018. [Google Scholar]
- 21. Kerr A, Slater GJ, Byrne N, Nana A. Reliability of 2 different positioning protocols for dual‐energy x‐ray absorptiometry measurement of body composition in healthy adults. J Clin Densitom. 2016;19(3):282‐289. [DOI] [PubMed] [Google Scholar]
- 22. Nana A, Slater GJ, Hopkins WG, Burke LM. Effects of daily activities on dual‐energy X‐ray absorptiometry measurements of body composition in active people. Med Sci Sports Exerc. 2012;44(1):180‐189. [DOI] [PubMed] [Google Scholar]
- 23. Stewart AD, Hannan WJ. Prediction of fat and fat‐free mass in male athletes using dual X‐ray absorptiometry as the reference method. J Sports Sci. 2000;18(4):263‐274. [DOI] [PubMed] [Google Scholar]
- 24. Kerr DA, Hume PA. Non‐imaging method: Bioelectrical impedance analysis In: Hume PA, Kerr DA, Ackland T, eds. Best practice protocols for physique assessment in sport. Singapore: Springer; 2018:88‐99. [Google Scholar]
- 25. Hume PA, Sheerin K, Hans de Ridder J. Non‐Imaging Method: Surface anthropometry In: Hume PA, Kerr A, Ackland T. eds. Best practice protocols for physique assessment in sport. Vol 1. Singapore: Springer Verlag; 2018:64‐69. [Google Scholar]
- 26. Stewart AD, Sutton L. Body composition in sport, exercise and health, 1st edn Abingdon, UK: Routledge; 2012. [Google Scholar]
- 27. Müller W, Horn M, Fürhapter‐Rieger A, et al. Body composition in sport: interobserver reliability of a novel ultrasound measure of subcutaneous fat tissue. Br J Sports Med. 2013;47(16):1036‐1043. [DOI] [PubMed] [Google Scholar]
- 28. Müller W, Lohman TG, Stewart AD, et al. Subcutaneous fat patterning in athletes: selection of appropriate sites and standardisation of a novel ultrasound measurement technique: ad hoc working group on body composition, health and performance, under the auspices of the IOC medical commission. Br J Sports Med. 2016;50(1):45‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Störchle P, Müller W, Sengeis M, et al. Standardized ultrasound measurement of subcutaneous fat patterning: high reliability and accuracy in groups ranging from lean to obese. Ultrasound Med Biol. 2017;43(2):427‐438. [DOI] [PubMed] [Google Scholar]
- 30. Müller W, Müller T, Fürhapter‐Rieger A, Ahammer H. Relative body weight and standardised ultrasound measurement of subcutaneous fat in athletes: an international multicentre reliability study. 2019. Manuscript in preparation. [DOI] [PMC free article] [PubMed]
- 31. Stewart AD, Marfell‐Jones M, Olds T, DeRidder J. International standards for anthropometric assessment. Lower Hutt, New Zealand: International Society for the Advancement of Kinanthropometry; 2011. [Google Scholar]
- 32. Herman IP. Physics of the human body , 2nd: edn New York, NY: Springer International Publishing; 2016. [Google Scholar]
- 33. Artioli GG, Gualano B, Franchini E, et al. Prevalence, magnitude, and methods of rapid weight loss among judo competitors. Med Sci Sports Exerc. 2010;42(3):436‐442. [DOI] [PubMed] [Google Scholar]
- 34. Artioli GG, Saunders B, Iglesias RT, Franchini E. It is time to ban rapid weight loss from combat sports. Sports Med. 2016;46(11):1579‐1584. [DOI] [PubMed] [Google Scholar]
- 35. Artioli GG, Saunders B, Iglesias RT, Franchini E. Authors' reply to Davis: "it is time to ban rapid weight loss from combat sports". Sports Med. 2017;47(8):1677‐1681. [DOI] [PubMed] [Google Scholar]
- 36. Müller W, Platzer D, Schmölzer B. Dynamics of human flight on skis: improvements in safety and fairness in ski jumping. J Biomech. 1996;29(8):1061‐1068. [DOI] [PubMed] [Google Scholar]
- 37. Kelso A, Trajer E, Machus K, Treff G, Müller W, Steinacker JM. Assessment of subcutaneous adipose tissue using ultrasound in highly trained junior rowers. Eur J Sport Sci. 2017;17(5):576‐585. [DOI] [PubMed] [Google Scholar]
- 38. Ackland T, Müller W. Imaging method: ultrasound In: Hume PA, Kerr A, Ackland T, eds. Best practice protocols for physique assessment in sport, vol. 1. Singapore: Springer; 2018:131‐141. [Google Scholar]
- 39. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 40. Lackner S, Mörkl S, Müller W, et al. Novel approaches for the assessment of relative body weight and body fat in diagnosis and treatment of anorexia nervosa: A cross‐sectional study. Clin Nutr. 2019. 10.1016/j.clnu.2018.12.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


