Abstract
Aim
Albumin–bilirubin (ALBI) grade was investigated to predict prognosis of patients with cirrhosis. It was defined using the ALBI score calculated based on serum total bilirubin and albumin, which represent liver function. The diagnostic accuracy for liver fibrosis staging in patients with chronic hepatitis using the ALBI score has not been investigated well. This study aimed to evaluate the diagnostic abilities of the ALBI score for liver fibrosis staging in chronic hepatitis and cirrhosis in Japanese patients with hepatitis C virus (HCV) infection.
Methods
Japanese patients with HCV infection who underwent liver biopsy examinations were enrolled in a retrospective study. Fibrosis staging and activity grading were assessed using the modified METAVIR score. The ALBI score was calculated according to the following equation: Log10 total bilirubin (μmol/L) × 0.66 + albumin (g/L) × (−0.085).
Results
A total of 382 patients were enrolled in this study. The ALBI score differentiated fibrosis stage 4 from 3 and stage 3 from 2 (P < 0.05). When an ALBI score of −2.125 was adopted as a cut‐off value, the sensitivity and specificity were 73.2% and 87.1%, respectively, with a positive likelihood ratio of 5.67 to differentiate stage 4 from stages 1–3. Kaplan–Meier analysis showed that smaller ALBI scores at baseline correlated with better hepatocellular carcinoma (HCC)‐free and overall survival (P < 0.05).
Conclusions
The ALBI score indicates liver fibrosis staging in Japanese patients with HCV infection. Furthermore, smaller ALBI scores predict better HCC‐free survival and overall survival. The ALBI score has the potential to expand its application from cirrhosis to chronic hepatitis.
Keywords: hepatitis C, chronic; hepatocellular carcinoma; liver cirrhosis; prognosis
Introduction
Liver biopsy examination is the gold standard for determining fibrosis stage and for stratifying risk of hepatocellular carcinoma (HCC), decompensated hepatic failure, and liver‐related death.1 However, the volume of biopsy samples obtained in daily practice could be insufficient for accurate staging of liver fibrosis.2 The diagnostic accuracy of this technique is also decreased by intra‐ and interobserver variability in pathological assessment.3 Serious complications, such as bleeding, are also recognized.4
Therefore, several indices and serum biomarkers have been developed as non‐invasive methods using blood samples to determine liver fibrosis stage. The Fibrosis‐4 (Fib‐4) index and aspartate aminotransferase (AST) to platelet ratio index (APRI) enable liver fibrosis staging using a routine blood examination for AST, alanine aminotransferase (ALT), and platelet count (Plt).5, 6, 7 To date, a non‐invasive strategy of liver fibrosis staging has been proposed to minimize the application of liver biopsy.8 However, the Fib‐4 index and APRI include Plt in their equation. Estimating liver fibrosis stage is difficult in patients with thrombocytopenia caused by diseases other than liver fibrosis, such as idiopathic thrombocytopenia and drug‐induced thrombocytopenia.
Albumin–bilirubin (ALBI) grade was investigated to predict prognosis of patients with cirrhosis with or without HCC.9 The ALBI grade is defined by using the ALBI score calculated based on serum total bilirubin and albumin, which represent liver function in cirrhosis. However, the diagnostic accuracy for liver fibrosis staging in patients with chronic hepatitis using the ALBI score has not been investigated well. The purpose of this study was to evaluate the diagnostic abilities of the ALBI score for liver fibrosis staging in chronic hepatitis and cirrhosis in Japanese patients with hepatitis C virus (HCV) infection.
Methods
Ethics
This study was carried out in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Kagawa University, Faculty of Medicine (Heisei‐30‐151) (Miki, Japan).10 Informed consent was obtained to analyze the clinical data for the current study. For patients who died and had no relatives listed in their clinical records, we provided opt‐out methods for the relatives of the dead participants by publishing a summary of this study on our university website.11, 12
Patients
Japanese patients with HCV infection, who underwent percutaneous liver biopsy examinations in a clinical setting between January 1, 1989 and December 31, 2002, were enrolled. Patients who had hepatocellular carcinoma when the liver biopsy examinations were carried out were excluded.
Clinical data
The following clinical data were extracted from the participants’ medical records: age, gender, Plt, AST, ALT, total bilirubin (T‐Bil), and serum albumin (Alb). Total bilirubin (mg/dL) was converted to T‐Bil (μmol/L) according to the following equation: T‐Bil (mg/dL) × 17.1. The ALBI score was calculated according to its original report: Log10 T‐Bil (μmol/L) × 0.66 + Alb (g/L) × (−0.085).9 The Fib‐4 index was calculated using the following equation: age × AST (U/L) / (Plt [109/L] × √ALT [U/L]).13 The APRI was calculated using the following equation: 100 × (AST [U/L] / upper limit of normal AST values [U/L]) / Plt (109/L).7 In our hospital, 35 U/L was applied as the upper limit of the normal AST values. Hepatitis C virus infection was suspected by positive HCV antibody II or III in sera and confirmed by polymerase chain reaction, combined reverse transcription–polymerase chain reaction, or branched chain DNA probe assay. For interferon (IFN) therapy, non‐pegylated IFNs, including natural IFN‐α and ‐β, and recombinant IFN‐α2a, ‐α2b, and ‐β, were used. Sustained virologic response (SVR) was defined as negative HCV‐RNA in sera 6 months or later after IFN therapy was completed. Patients who failed to achieve SVR were regarded no viral response (NR).
Histopathological analysis
The extent of fibrosis was assessed using the modified METAVIR score as follows: stage 1, portal or central fibrosis; stage 2, some septa; stage 3, many septa; and stage 4, cirrhosis.3 The METAVIR grading system was used to assess hepatic inflammatory activity.14 Staging fibrosis and grading activity were undertaken by an experienced pathologist who specialized in liver pathology.
Statistical analysis
Continuous or discrete variables are presented as the median and interquartile range (IQR) and analyzed using the Mann–Whitney U‐test, Wilcoxon's matched‐pairs signed rank test, or Spearman's rank correlation coefficient. Kaplan–Meier curves were analyzed using the log–rank (Mantel–Cox) test. Statistical analyses mentioned above were carried out using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Area under receiver operating characteristic curves (AUROCs) were statistically compared using DeLong's test.15 DeLong's test was carried out using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R software (The R Foundation, Vienna, Austria).16, 17 P‐values less than 0.05 were considered statistically significant.
Results
Patients’ characteristics
A total of 382 patients comprising 259 men and 123 women were enrolled in this study (Table 1). Based on the liver biopsy examinations, 92 patients had stage 1 fibrosis, 169 had stage 2, 80 had stage 3, and 41 had stage 4. Among them, 125 patients were followed up for 1 year or more. The longest follow‐up period was 28 years for four patients. In our hospital, 85 patients underwent subsequent non‐pegylated IFN therapy. Patients with stage 4 fibrosis were classified using Child–Pugh score. Among them, 11 patients could not be determined by Child–Pugh classification because data on prothrombin time at the initial liver biopsy examinations were not available. Hepatocellular carcinoma was diagnosed in 39 patients: 2 patients were complicated with HCC within 1 year after liver biopsy examinations, and 37 patients later than 1 year. Other clinical data at the time of liver biopsy, including age, albumin, AST, ALT, and T‐Bil, are presented in Table 1.
Table 1.
Baseline characteristics of 382 Japanese patients with Hepatitis C virus infection at the time of liver biopsy
| Fibrosis staging | Total | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| Patient number | 382 | 92 | 169 | 80 | 41 |
| Follow‐up period <1 year | 257 | 67 | 127 | 35 | 28 |
| Follow‐up period >1 year | 125 | 25 | 42 | 45 | 13 |
| Follow‐up period >1 year, years | 14 (8.5–23) | 16 (10–25) | 17.5 (10–25) | 12 (6.5–21.5) | 8 (4–11.5) |
| Age, years | 51 (40–59) | 46 (34–57) | 47 (40–57) | 54 (44–60) | 62 (56–66) |
| Gender, male/female | 259/123 | 64/28 | 127/42 | 53/27 | 15/26 |
| Albumin, g/L | 39 (35–41) | 40 (37–42) | 40 (38–42) | 37 (34–39) | 34 (29–40) |
| AST, U/L | 58 (42–84) | 44 (26–61) | 54 (43–79) | 75 (56–105) | 65 (49–105) |
| ALT, U/L | 83 (51–131) | 60 (37–98) | 88 (59–139) | 110 (76–158) | 55 (35–100) |
| ALP, U/L | 199 (158–251) | 187 (140–227) | 187 (154–226) | 219 (179–286) | 255 (186–336) |
| γGTP, U/L | 48 (25–85) | 36 (19–67) | 47 (25–85) | 66 (40–112) | 44 (22–79) |
| Total bilirubin, μmol/L | 13.7 (10.3–18.8) | 12.0 (10.3–15.4) | 12.0 (10.3–17.1) | 16.3 (12.0–20.5) | 18.8 (13.7–29.1) |
| Alcohol consumption | 111 | 23 | 60 | 21 | 7 |
| Co‐infection with hepatitis B virus | 15 | 6 | 5 | 3 | 1 |
| Complication of diabetes mellitus | 4 | 1 | 1 | 2 | 0 |
| Child–Pugh classification, A/B/C/undetermined | – | – | – | – | 16/12/2/11 |
| Interferon, SVR/NR/not treated/unknown | 31/54/36/261 | 2/10/7/73 | 16/20/5/128 | 12/22/12/34 | 1/2/12/26 |
| HCC prevalence, patient number | 39 | 4 | 13 | 14 | 8 |
Continuous variables are presented as the median and interquartile range.
γGTP, γ‐glutamyltransferase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCC, hepatocellular carcinoma; NR, no viral response; SVR, sustained viral response; –, not applicable.
Among the 382 patients, Plt during the initial liver biopsy examinations was available for 198 patients. Clinical data including Plt for the 198 patients are summarized in Table 2.
Table 2.
Baseline characteristics of Japanese patients with Hepatitis C virus infection, limited to 198 patients with available baseline platelet count
| Fibrosis staging | Total | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| Patient number | 198 | 42 | 71 | 56 | 29 |
| Age, years | 53 (42–60) | 47 (35–59) | 48 (40–58) | 54 (44–60) | 62 (56–65) |
| Gender, male/female | 117/81 | 24/18 | 45/26 | 35/21 | 13/16 |
| Albumin, g/L | 38 (35–41) | 40 (36–42) | 40 (38–42) | 36 (33–39) | 33 (28–37) |
| AST, U/L | 63 (44–96) | 46 (28–71) | 56 (41–83) | 88 (53–105) | 76 (49–105) |
| ALT, U/L | 95 (53–139) | 60 (36–103) | 103 (52–140) | 111 (77–158) | 70 (41–104) |
| Total bilirubin, μmol/L | 13.7 (10.3–20.5) | 12.0 (10.3–15.4) | 12.0 (10.3–17.1) | 18.8 (12.0–22.2) | 18.8 (13.7–29.1) |
| Platelet count, 109/L | 162.5 (122.5–203.3) | 189.5 (162.5–239.0) | 189.0 (150.0–223.0) | 134.5 (110.0–161.0) | 92.0 (67.5–127.0) |
Continuous variables are presented as the median (interquartile range).
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Diagnostic ability of liver fibrosis staging
Differences in median values between two fibrosis stages were analyzed using Mann–Whitney U‐test (Fig. 1). As a result, ALBI score differentiated fibrosis stage 3 from 2 and stage 4 from 3 (P < 0.05). The median value of ALBI score for stage 2 was not significantly different from that for stage 1 (P > 0.05).
Figure 1.
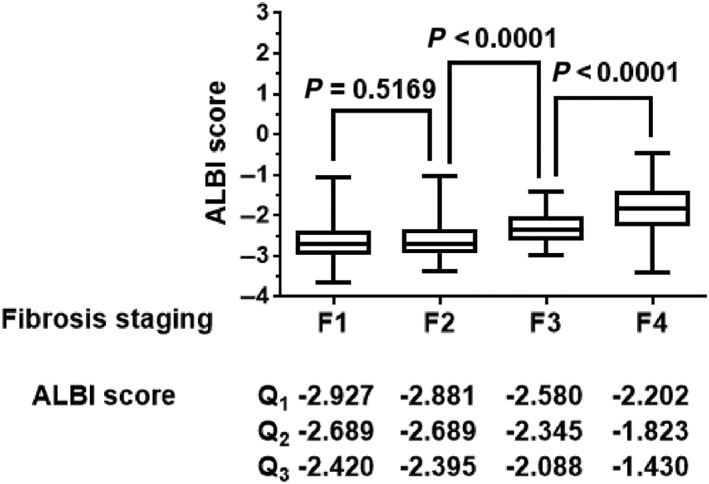
Albumin–bilirubin (ALBI) score in each fibrosis stage among 382 patients with chronic hepatitis C. Difference in the median ALBI scores between two fibrosis stages. The median ALBI score distinguished stage 4 from 3 and stage 3 from 2 (P < 0.05). The median value for stage 2 was not significantly different from that for stage 1 (P > 0.05). Data were analyzed using the Mann–Whitney U‐test. P‐values <0.05 were considered statistically significant.
Receiver operating characteristic analysis was carried out to assess the ability of ALBI score to distinguish advanced fibrosis (F3–4) from non‐advanced fibrosis (F1–2) and cirrhosis (F4) from non‐cirrhotic stages (F1–3). The AUROC to distinguish advanced fibrosis from non‐advanced fibrosis was 0.786 (Fig. 2a). The AUROC to detect cirrhosis differentially from non‐cirrhotic stages was 0.815 (Fig. 2b).
Figure 2.
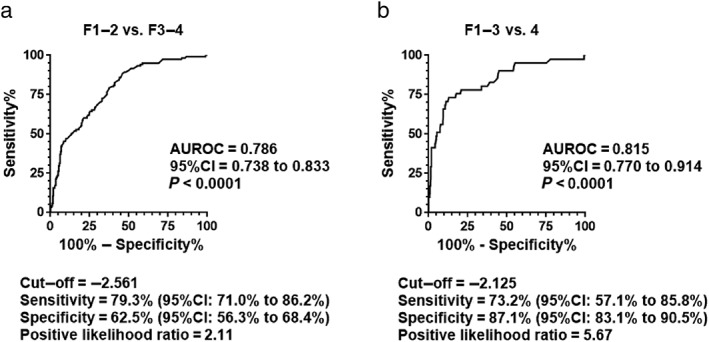
Diagnostic ability of liver fibrosis staging. (a) Receiver operating characteristic (ROC) analysis to assess the ability of albumin–bilirubin (ALBI) score to differentiate advanced liver fibrosis (F3–4) from non‐advanced fibrosis (F1–2) showed an area under the ROC curve (AUROC) of 0.786. (b) The AUROC to diagnose cirrhosis (F4) from non‐cirrhotic stages (F1–3) was 0.815. When an ALBI score of −2.561 was adopted as a cut‐off value, the sensitivity, specificity, and positive likelihood ratio to differentiate advanced fibrosis from non‐advanced fibrosis were 79.3%, 62.5%, and 2.11 (a). When a cut‐off value of −2.125 was used for differential diagnosis of cirrhosis from non‐cirrhotic stages, cirrhosis was detected with a sensitivity of 73.2% and a specificity of 87.1%, with a positive likelihood ratio of 5.67 (b). P‐values <0.05 were considered statistically significant. CI, confidence interval.
When an ALBI score of −2.561 was adopted as a cut‐off value, the sensitivity and specificity were 79.3% and 62.5%, respectively, with a positive likelihood ratio of 2.11 to differentiate advanced fibrosis from non‐advanced fibrosis (Fig. 2a). When a cut‐off value of −2.125 was used for differential diagnosis of cirrhosis from non‐cirrhotic stages, cirrhosis was detected with a sensitivity of 73.2% and a specificity of 87.1%, with a positive likelihood ratio of 5.67 (Fig. 2b).
When patients with stage 4 fibrosis were divided into Child–Pugh A (16 patients), B (12 patients), or C (2 patients), the ALBI score of the Child–Pugh A group was significantly smaller than that of the Child Pugh B or C group (14 patients) (P < 0.05, Fig. 3a). Using the cut‐off value of −2.125, the ROC analysis (AUROC 0.801) proved that the ALBI score distinguished the Child–Pugh A group from the non‐cirrhotic stages (F1–3) with a specificity of 87.1% identical to that of differentiating cirrhosis (F4) from non‐cirrhotic stages (F1–3). The sensitivity and positive likelihood ratio decreased to 56.3% and 4.36, respectively (Fig. 3b).
Figure 3.

Differential diagnosis of Child–Pugh A liver cirrhosis from non‐cirrhotic stages. (a) When patients with stage 4 fibrosis were divided into Child–Pugh A (16 patients), B (12 patients), or C (2 patients), albumin–bilirubin (ALBI) score of the Child–Pugh A group was significantly smaller than that of the Child–Pugh B or C group (14 patients) (P < 0.05). (b) Using the cut‐off value of −2.125, the receiver operating characteristic analysis (area under the ROC curve [AUROC] 0.801) proved that the ALBI score distinguished the Child–Pugh A group from the non‐cirrhotic group (F1–3) with a specificity of 87.1%, identical to that of differentiating cirrhosis (F4) from non‐cirrhotic stages (F1–3). The sensitivity and positive likelihood ratio decreased to 56.3% and 4.36, respectively. P‐values <0.05 were considered statistically significant. CI, confidence interval.
Comparison between ALBI score and conventional fibrosis indices
Diagnostic ability of the ALBI score for liver fibrosis staging was compared to that of conventional fibrosis indices, Fib‐4 index and APRI, based on a cohort of 198 patients (Table 2). Fibrosis‐4 index was able to distinguish stage 3 from 2 and stage 4 from 3, similar to the ALBI score (P < 0.05, Fig. 4a). The APRI was able to differentiate stage 3 from 2 (P < 0.05) but failed to distinguish stage 2 from 1 or stage 4 from 3 (P > 0.05, Fig. 4b).
Figure 4.

Conventional liver fibrosis indices in each fibrosis stage. The diagnostic ability of two conventional fibrosis indices, Fibrosis‐4 index and aspartate aminotransferase (AST) to platelet ratio index (APRI), in differentiating liver fibrosis stage were evaluated based on a cohort of 198 patients, whose baseline platelet counts were available. (a) Fibrosis‐4 index was able to distinguish stage 3 from 2 and stage 4 from 3, similar to albumin–bilirubin ALBI score (P < 0.05). (b) APRI was able to differentiate stage 3 from 2 (P < 0.05) but failed to distinguish stage 2 from 1 or stage 4 from 3 (P > 0.05). P‐values <0.05 were considered statistically significant.
Receiver operating characteristic analysis showed that Fib‐4 index was able to distinguish advanced fibrosis (F3–4) from non‐advanced fibrosis (F1–2) and cirrhosis (F4) from non‐cirrhotic stages (F1–3) with AUROCs of 0.842 and 0.859, respectively (Fig. 5a,b). The AUROCs of APRI were 0.804 in the classification of advanced fibrosis (F3–4) from non‐advanced fibrosis (F1–2) and 0.724 in distinguishing cirrhosis (F4) from non‐cirrhotic stages (F1–3) (Fig. 5c,d).
Figure 5.
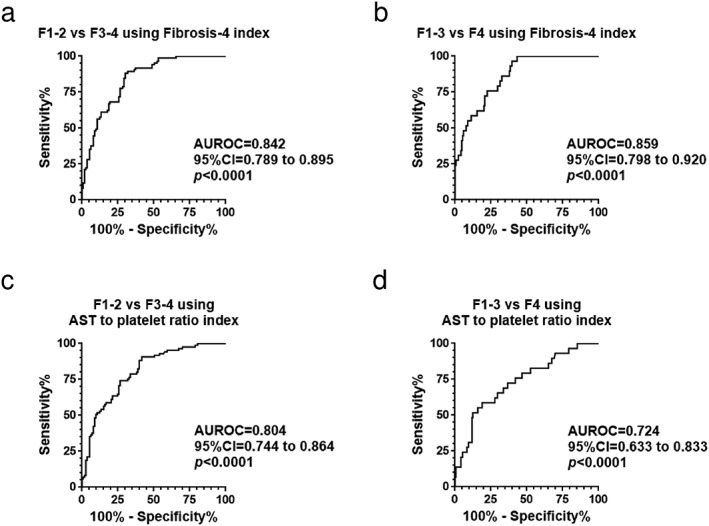
Receiver operating characteristic (ROC) analysis of conventional liver fibrosis indices. (a) ROC analysis indicated that Fibrosis‐4 index was able to distinguish advanced fibrosis (F3–4) from non‐advanced fibrosis (F1–2) (area under the ROC curve [AUROC], 0.842). (b) Cirrhosis (F4) was differentiated from non‐cirrhotic stages (F1–3) using Fibrosis‐4 index (AUROC, 0.859). (c,d) AUROCs of aspartate aminotransferase (AST) to platelet ratio index were 0.804 in the classification of advanced fibrosis (F3–4) from non‐advanced fibrosis (F1–2) (c) and 0.724 in distinguishing cirrhosis (F4) from non‐cirrhotic stages (F1–3) (d).
Limited to 198 patients in Table 2, AUROCs of ALBI scores were calculated and compared to those of Fib‐4 index and APRI using DeLong's test. As presented in Table 3, a significant difference was detected in a single pair, between Fib‐4 index and APRI in distinguishing cirrhosis (F4) from non‐cirrhotic stages (F1–3) with the greater AUROC value for Fib‐4 index than for APRI. The AUROCs of ALBI scores were not different compared to the two conventional indices in differentiation between non‐advanced fibrosis (F1–2) and advanced fibrosis (F3–4) or non‐cirrhotic stages (F1–3) and cirrhosis (F4).
Table 3.
DeLong's test to determine difference of two area under receiver operating characteristic curves (AUROCs)
| P‐value | ALBI score vs. Fibrosis‐4 index | ALBI score vs. APRI | Fibrosis‐4 index vs. APRI |
|---|---|---|---|
| F1–2 vs. F3–4 | 0.4640 | 0.7230 | 0.0767 |
| F1–3 vs. F4 | 0.3680 | 0.1640 | 0.0003 * (Fibrosis‐4 index > APRI) |
AUROC of Fibrosis‐4 index is significantly greater than that of APRI in comparison between non‐cirrhotic status (F1–3) and cirrhosis (F4). P‐values <0.05 were considered statistically significant.
APRI, aspartate aminotransferase to platelet ratio index.
Influence of hepatitis activity on ALBI score
Values of serum fibrosis biomarkers have been reported to fluctuate depending on not only fibrosis stage but also activity grade of hepatitis.18 To assess the variability of ALBI score and the other two indices to histopathological activity grade in liver biopsy specimens, 56 patients with fibrosis stage 3 were divided into 15 patients of activity grade 1, 29 patients of grade 2, and 12 patients of grade 3 based on the modified METAVIR score.
The ALBI scores for the grade 2 group were significantly different from those for the grade 1 group using the Mann–Whitney U‐test (P < 0.05, Fig. 6). The data indicated that the values of the ALBI score were variables of activity grade. This result suggests that fibrosis staging using ALBI scores has the potential to overestimate or underestimate it.
Figure 6.
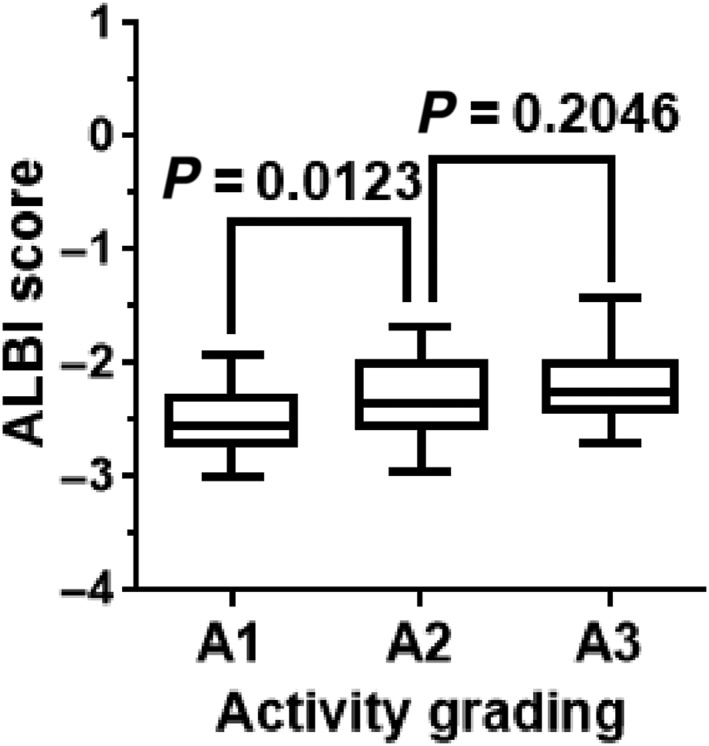
Influence of hepatitis activity on albumin–bilirubin (ALBI) score. In 80 patients with fibrosis stage 3, the median ALBI score of the activity grade 2 group was significantly different from that of the grade 1 group (P < 0.05). The median ALBI score for the hepatitis grade 3 group was not statistically significant from that of the grade 2 group. The value of ALBI score was a variable of activity grade, suggesting that fibrosis staging using the ALBI score has the potential to overestimate or underestimate it. Data were analyzed using the Mann–Whitney U‐test. P‐values <0.05 were considered statistically significant.
Change of ALBI score after IFN therapy
In the current cohort, 85 patients underwent non‐pegylated IFN therapy after the liver biopsy examinations. The viral response was determined when the median 6.5 years (IQR, 2.5–10.5 years) passed after the completion of IFN therapy. As a result, 31 patients achieved SVR and 54 patients failed.
The median value of ALBI score presented the smaller value after SVR was confirmed compared with baseline, but it was not statistically significant using Wilcoxon's matched‐pairs signed rank test (P > 0.05, Fig. 7a). Among the patients who failed to achieve SVR, ALBI score significantly increased after NR was confirmed compared with baseline (P < 0.05, Fig. 7b). The ALBI scores increased following progression of fibrosis and activity.
Figure 7.

Change of albumin–bilirubin (ALBI) score after interferon (IFN) therapy. In the current cohort, 85 patients underwent subsequent non‐pegylated IFN therapy after liver biopsy examinations. As a result, 31 patients achieved sustained virologic response (SVR) and 54 patients failed (no viral response, NR). The viral response was determined the median 6.5 years (interquartile range, 2.5–10.5 years) after the completion of IFN therapy. (a) Median value of the ALBI score presented the smaller value after SVR was confirmed compared with baseline, but it was not statistically significant using Wilcoxon's matched‐pairs signed rank test (P > 0.05). (b) Among the patients who failed to achieve SVR, ALBI score significantly increased after NR was confirmed compared with baseline (P < 0.05). ALBI score increased following progression of fibrosis and activity in the NR group. Data were analyzed using Wilcoxon's matched‐pairs signed rank test. P‐values <0.05 were considered statistically significant.
Prevalence of HCC and overall survival
In the current cohort, 39 patients were diagnosed with HCC during follow‐up. Limiting to 125 patients who were observed 1 year or more, 37 patients were diagnosed with HCC during the observation periods. The median period between the time of liver biopsy and HCC diagnosis was 9.0 years (IQR, 6.0–17.0 years). In 88 patients, those observations were censored with no incidence of HCC; ALBI scores at censoring were not significantly greater than that at baseline liver biopsy examinations (P > 0.05, Fig. 8a). In contrast, ALBI scores at the time of HCC diagnosis were significantly greater compared with baseline at the time of liver biopsy examinations (baseline) in 37 patients with HCC incidence (P < 0.05, Fig. 8b). Smaller ALBI scores at baseline inversely correlated with the length of time between liver biopsy and HCC diagnosis (r = −0.4017; 95% confidence interval [CI], –0.6480 to −0.0795, P < 0.05; Fig. 8c).
Figure 8.
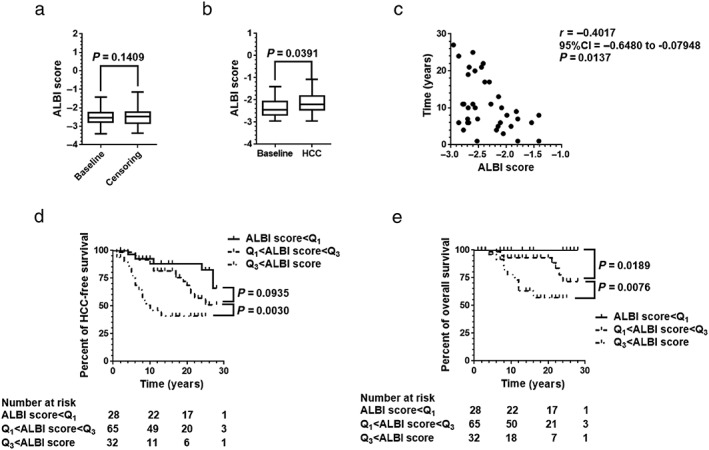
Hepatocellular carcinoma (HCC) prevalence and overall survival. In the current cohort, 39 patients were diagnosed with HCC during follow‐up. Limiting to 125 patients who were observed 1 year or more, 37 patients were diagnosed with HCC in their observation periods. (a) In 88 patients, those observations were censored with no incidence of HCC, albumin–bilirubin (ALBI) score at censoring was not significantly greater than that at baseline liver biopsy examinations (P > 0.05). (b) In contrast, ALBI score at the time of HCC diagnosis was significantly greater compared with baseline at the time of liver biopsy examinations (baseline) in 37 patients with HCC (P < 0.05). (c) Smaller ALBI scores at baseline inversely correlated with the length of time between liver biopsy and HCC diagnosis (r = −0.4017; 95% confidence interval [CI], –0.6480 to −0.0795; P < 0.05). (d) Using 25th percentile value (Q1; ALBI score = −2.773) and 75th percentile value (Q3; ALBI score = −2.215) at baseline as cut‐off values, 125 patients were divided into three groups: smaller ALBI score < Q1 group, greater ALBI score > Q3 group, and middle ALBI score group between Q1 and Q3. Kaplan–Meier analysis revealed that HCC‐free survival was significantly poor in the greater ALBI score group compared with the middle ALBI score group (P < 0.05). (e) For overall survival, smaller ALBI scores correlated with better survival among the three groups (P < 0.05). Data were analyzed using the log–rank (Mantel–Cox) test. P‐values <0.05 were considered statistically significant.
Using the 25th percentile value (Q1; ALBI score = −2.773) and 75th percentile value (Q3; ALBI score = −2.215) at baseline as cut‐off values, 125 patients were divided into three groups: the smaller ALBI score < Q1 group, the greater ALBI score > Q3 group, and the middle ALBI score group between Q1 and Q3. Kaplan–Meier analysis revealed that HCC‐free survival was significantly poorer in the greater ALBI score group compared with the middle ALBI score group (P < 0.05, Fig. 8d). For overall survival, smaller ALBI scores correlated with better survival among the three groups (P < 0.05, Fig. 8e).
Differences of HCC prevalence and overall survival depending on ALBI scores were also evaluated under stratification of the cohort by baseline liver fibrosis stage. The 125 patients were divided into two smaller cohorts: 67 patients with non‐advanced fibrosis (F1–2) and 58 patients with advanced fibrosis (F3–4). Using the quantile values above (Q1 = −2.773 and Q3 = −2.215), 25 patients with smaller ALBI scores resulted in longer HCC‐free survival (Fig. 9a) and overall survival (Fig. 9b) than 34 patients in the middle ALBI score group in the non‐advanced fibrosis stage (F1–2) cohort (P < 0.05). Hepatocellular carcinoma‐free survival of eight patients in the greater ALBI score group did not significantly differ from those of other two groups (P > 0.05, Fig. 9a). Overall survival curve for the greater ALBI score group overlapped with that of the smaller ALBI score group, yielding no significant difference with other groups either (P > 0.05, Fig. 9b).
Figure 9.
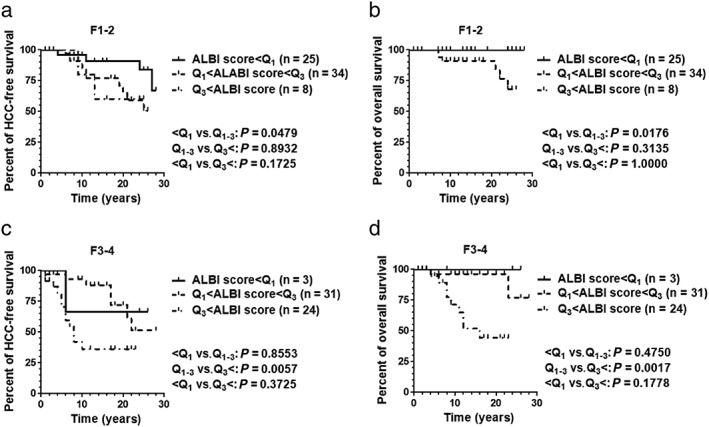
Prevalence of hepatocellular carcinoma (HCC) and overall survival stratified by baseline liver fibrosis staging. Differences in HCC prevalence and overall survival depending on albumin–bilirubin (ALBI) score were also evaluated under stratification by baseline liver fibrosis stage. The 125 patients were divided into two smaller cohorts: 67 patients with non‐advanced fibrosis (F1–2) and 58 patients with advanced fibrosis (F3–4). (a,b) Using the quantile values Q1 = −2.773 and Q3 = −2.215, 25 patients in the smaller ALBI score group experienced longer HCC‐free survival (a) and overall survival (b) than the 34 patients in the middle ALBI score group in the non‐advanced fibrosis stage (F1–2) cohort (P < 0.05). HCC‐free survival of eight patients in the greater ALBI score group did not significantly differ from those of other two groups (P > 0.05) (a). Overall survival curve for the greater ALBI score group was overlapped with that of the smaller ALBI score group, yielding no significant difference with other groups either (P > 0.05) (b). (c,d)In the advanced fibrosis stage (F3–4) cohort, 31 patients from the middle ALBI score group finished the observation with longer HCC‐free survival (c) and overall survival (d) compared to the 24 patients in the greater ALBI score group (P < 0.05). The smaller ALBI score group consisted of three patients and its HCC‐free curve and overall survival curve were not significantly different from those of other groups (c,d). Data were analyzed using the log–rank (Mantel–Cox) test. P‐values <0.05 were considered statistically significant.
In the advanced fibrosis stage (F3–4) cohort, 31 patients in the middle ALBI score group finished the observation with longer HCC‐free survival (Fig. 9c) and overall survival (Fig. 9d) than the 24 patients in the greater ALBI score group (P < 0.05). The smaller ALBI score group consisted of three patients and its HCC‐free curve and overall survival curve were not significantly different from those of other groups (P > 0.05, Fig. 9c,d).
Discussion
The current study obtained the following results. First, the ALBI score is able to diagnose fibrosis stage, particularly advanced liver fibrosis and cirrhosis. Second, smaller ALBI scores correlate with better HCC‐free survival and overall survival. Finally, ALBI score has the potential to expand its application from cirrhosis to chronic hepatitis.
Several serum biomarkers to evaluate liver fibrosis stage are able to differentiate advanced fibrosis from non‐advanced fibrosis.19 The diagnostic accuracy of blood tests is evaluated using AUROC. An AUROC of 0.9–1.0 is classified as excellent, 0.8–0.9 as good, and 0.7–0.8 as fair.20, 21 Values of serum biomarkers fluctuate depending not only on fibrosis progression but also hepatitis activity.18, 22
The ALBI score has a moderate diagnostic ability to distinguish cirrhosis from non‐cirrhotic stages when the AUROC is between 0.8 and 0.9, which might be comparable to conventional indirect biomarkers, Fib‐4 index and APRI.19 The AUROC of the ALBI score to diagnose advanced fibrosis is between 0.7 and 0.8, which might be smaller than those of the Fib‐4 index and APRI as reported in the past studies.6, 23 However, our data indicate that the ALBI score shares comparable accuracy to diagnose advanced fibrosis and cirrhosis with current indirect fibrosis biomarkers.
The ALBI score was originally established to classify patients with cirrhosis according to their prognosis.9 The preoperative ALBI score has a long‐term impact on liver function after curative therapy for HCC.24 Accumulating evidence indicated that the ALBI score predicted prognosis of patients with HCC who underwent oral chemotherapy using sorafenib, radioembolization, and drug‐eluting embolic chemoembolization.25, 26, 27 The ALBI score also correlated with recurrence of HCC after curative treatments, surgical resection, or ablative therapy.28 A risk of post‐hepatectomy liver failure and long‐term survival is stratified using the simple score.29 An ALBI score of <−2.600 indicates better prognosis, classified as grade 1, −2.600< ALBI score <−1.390 indicates moderate prognosis, classified as grade 2, and −1.390< ALBI score indicates worse prognosis, classified as grade 3. Application of the ALBI score is limited in patients with cirrhosis similar to Child–Pugh score. Accuracy of the ALBI score might surpass that of the Child–Pugh score in predicting the occurrence of severe post‐hepatectomy complications. 30 Combined with the tumor–node–metastasis classification, prognosis prediction for recurrence‐free survival using the ALBI score improved its accuracy in a large cohort of post‐hepatectomy patients.31
In contrast to past studies, our cohort includes 89.3% (341 of 382) of patients with chronic hepatitis and 10.7% (41 of 382) of patients with cirrhosis. In the current study, ALBI score increased when non‐pegylated IFN therapy failed. It also increased when HCC was identified compared with that when the liver biopsy examination was carried out at baseline. Furthermore, smaller ALBI scores at baseline inversely correlated with the period of time between liver biopsy and HCC diagnosis. Our data showed that ALBI scores in patients with chronic hepatitis, including smaller proportions of patients with cirrhosis, stratified the HCC‐free survival and overall survival.
Stratification of patients’ prognosis using the ALBI score in our study could depend on the development of more effective antiviral therapy than non‐pegylated IFN. Patients with smaller ALBI scores should have longer follow‐up periods until HCC develops and more chances to undergo next‐generation antiviral therapies, such as pegylated IFN therapy, combination therapy with pegylated IFN and telaprevir or simeprevir, and then IFN‐free oral treatments.32, 33, 34, 35
Our data suggest that the ALBI score has the potential to expand its application from cirrhosis to chronic hepatitis. For example, a patient with an ALBI score >−2.125 is diagnosed with cirrhosis with 87.1% of specificity according to our data. The patient is classified into the moderate or poor prognosis group of cirrhosis based on the original study of the score.9
The strength of the ALBI score is that it does not include Plt. The diagnostic accuracy of the ALBI score for liver fibrosis staging is preserved in thrombocytopenic patients caused by diseases not related to liver fibrosis, such as idiopathic thrombocytopenia, drug‐induced thrombocytopenia, and Helicobacter pylori infection. Additionally, Plt increases in iron deficiency anemia. A weakness of the ALBI score is that it depends on serum albumin in its equation. Viral hepatitis currently complicates with albuminuria and decreases in serum albumin caused by glomerulonephritis. Diabetic nephropathy with albuminuria and oligoalbuminemia could be observed more frequently in daily practice.
The limitations of the current study are as follows. First, the diagnostic accuracy of the ALBI score for advanced liver fibrosis was not compared with that of shear wave elastography or magnetic resonance elastography.36, 37 Second, two direct serum biomarkers, enhanced liver fibrosis score and Wisteria floribunda agglutinin‐positive Mac‐2 binding protein, were not included in the assessment.38, 39 Finally, the HCV genotype was not determined in most patients.
In conclusion, the ALBI score indicates liver fibrosis staging in Japanese patients with HCV infection. The diagnostic accuracy of the ALBI score to differentiate cirrhosis from non‐cirrhotic stages is comparable to those of the Fib‐4 index and APRI. Furthermore, smaller ALBI scores predict better HCC‐free survival and overall survival. The clinical application of the ALBI score has the potential to expand its application from cirrhosis to chronic hepatitis.
Fujita, K. , Oura, K. , Yoneyama, H. , Shi, T. , Takuma, K. , Nakahara, M. , Tadokoro, T. , Nomura, T. , Morishita, A. , Tsutsui, K. , Himoto, T. , and Masaki, T. (2019) Albumin–bilirubin score indicates liver fibrosis staging and prognosis in patients with chronic hepatitis C. Hepatol Res, 49: 731–742. 10.1111/hepr.13333.
Conflict of interest: The authors have no conflict of interest.
Financial support: None declared.
References
- 1. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344: 495–500. [DOI] [PubMed] [Google Scholar]
- 2. Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 2003; 39: 239–244. [DOI] [PubMed] [Google Scholar]
- 3. The French METAVIR Cooperative Study Group . Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994; 20: 15–20. [PubMed] [Google Scholar]
- 4. Seeff LB, Everson GT, Morgan TR et al Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT‐C trial. Clin Gastroenterol Hepatol 2010; 8: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guha IN, Parkes J, Roderick P et al Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008; 47: 455–460. [DOI] [PubMed] [Google Scholar]
- 6. Vallet‐Pichard A, Mallet V, Nalpas B et al FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and FibroTest. Hepatology 2007; 46: 32–36. [DOI] [PubMed] [Google Scholar]
- 7. Wai CT, Greenson JK, Fontana RJ et al A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38: 518–526. [DOI] [PubMed] [Google Scholar]
- 8. Tapper EB, Lok ASF. Use of liver imaging and biopsy in clinical practice. N Engl J Med 2017; 377: 2296–2297. [DOI] [PubMed] [Google Scholar]
- 9. Johnson PJ, Berhane S, Kagebayashi C et al Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach – the ALBI grade. J Clin Oncol 2015; 33: 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 11. Vellinga A, Cormican M, Hanahoe B, Bennett K, Murphy AW. Opt‐out as an acceptable method of obtaining consent in medical research: a short report. BMC Med Res Methodol 2011; 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montoy JC, Dow WH, Kaplan BC. Patient choice in opt‐in, active choice, and opt‐out HIV screening: randomized clinical trial. BMJ 2016; 532: h6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterling RK, Lissen E, Clumeck N et al Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 14. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996; 24: 289–293. [DOI] [PubMed] [Google Scholar]
- 15. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 16. Hens N, Aerts M, Molenberghs G. Model selection for incomplete and design‐based samples. Stat Med 2006; 25: 2502–2520. [DOI] [PubMed] [Google Scholar]
- 17. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujita K, Kuroda N, Morishita A et al Fibrosis staging using direct serum biomarkers is influenced by hepatitis activity grading in hepatitis C virus infection. J Clin Med 2018; 7: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 2013; 158: 807–820. [DOI] [PubMed] [Google Scholar]
- 20. Altman DG, Bland JM. Statistics notes: diagnostic tests 3: receiver operating characteristic plots. BMJ 1994; 309: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zweig MH, Campbell G. Receiver‐operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39: 561–577. [PubMed] [Google Scholar]
- 22. Fagan KJ, Pretorius CJ, Horsfall LU et al ELF score ≥9.8 indicates advanced hepatic fibrosis and is influenced by age, steatosis and histological activity. Liver Int 2015; 35: 1673–1681. [DOI] [PubMed] [Google Scholar]
- 23. Lin ZH, Xin YN, Dong QJ et al Performance of the aspartate aminotransferase‐to‐platelet ratio index for the staging of hepatitis C‐related fibrosis: an updated meta‐analysis. Hepatology 2011; 53: 726–736. [DOI] [PubMed] [Google Scholar]
- 24. Toyoda H, Lai PB, O'Beirne J et al Long‐term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016; 114: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogasawara S, Chiba T, Ooka Y et al Liver function assessment according to the albumin‐bilirubin (ALBI) grade in sorafenib‐treated patients with advanced hepatocellular carcinoma. Invest New Drugs 2015; 33: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 26. Gui B, Weiner AA, Nosher J et al Assessment of the albumin‐bilirubin (ALBI) grade as a prognostic indicator for hepatocellular carcinoma patients treated with radioembolization. Am J Clin Oncol 2018; 41: 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carling U, Røsok B, Line PD, Dorenberg EJ. ALBI and P‐ALBI grade in Child‐Pugh A patients treated with drug eluting embolic chemoembolization for hepatocellular carcinoma. Acta Radiol 2018; 11: 284185118799519. [DOI] [PubMed] [Google Scholar]
- 28. Matsushima H, Takami Y, Ryu T et al Prognosis of hepatocellular carcinoma patients who achieved long‐term recurrence‐free survival after curative therapy: impact of the ALBI grade. J Gastrointest Surg 2018; 22: 1230–1238. [DOI] [PubMed] [Google Scholar]
- 29. Zhang ZQ, Xiong L, Zhou JJ et al Ability of the ALBI grade to predict posthepatectomy liver failure and long‐term survival after liver resection for different BCLC stages of HCC. World J Surg Oncol 2018; 16: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li MX, Zhao H, Bi XY et al Prognostic value of the albumin‐bilirubin grade in patients with hepatocellular carcinoma: validation in a Chinese cohort. Hepatol Res 2017; 47: 731–741. [DOI] [PubMed] [Google Scholar]
- 31. Harimoto N, Yoshizumi T, Sakata K et al Prognostic significance of combined albumin‐bilirubin and tumor‐node‐metastasis staging system in patients who underwent hepatic resection for hepatocellular carcinoma. Hepatol Res 2017; 47: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 32. Fried MW, Shiffman ML, Reddy KR et al Peginterferon α‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–982. [DOI] [PubMed] [Google Scholar]
- 33. McHutchison JG, Everson GT, Gordon SC et al Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360: 1827–1838. [DOI] [PubMed] [Google Scholar]
- 34. Jacobson IM, Dore GJ, Foster GR et al Simeprevir with pegylated interferon α2a plus ribavirin in treatment‐naive patients with chronic hepatitis C virus genotype 1 infection (QUEST‐1): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet 2014; 384: 403–413. [DOI] [PubMed] [Google Scholar]
- 35. Baumert TF, Berg T, Lim JK, Nelson DR. Status of direct‐acting antiviral therapy for HCV infection and remaining challenges. Gastroenterology 2018; 156: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barr RG, Zhang Z. Shear‐wave elastography of the breast: value of a quality measure and comparison with strain elastography. Radiology 2015; 275: 45–53. [DOI] [PubMed] [Google Scholar]
- 37. Loomba R, Wolfson T, Ang B et al Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014; 60: 1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuno A, Ikehara Y, Tanaka Y et al A serum “sweet‐doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013; 3: 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nobili V, Parkes J, Bottazzo G et al Performance of ELF serum markers in predicting fibrosis stage in pediatric non‐alcoholic fatty liver disease. Gastroenterology 2009; 136: 160–167. [DOI] [PubMed] [Google Scholar]


