Abstract
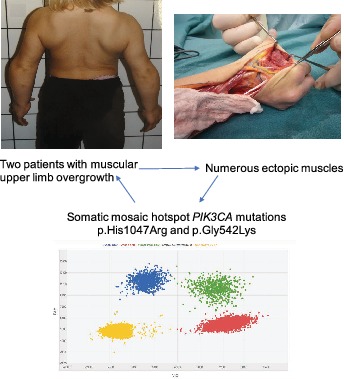
PIK3CA‐related overgrowth spectrum is a group of rare genetic disorders with asymmetric overgrowth caused by somatic mosaic PIK3CA mutations. Here, we report clinical data and molecular findings from two patients with congenital muscular upper limb overgrowth and aberrant anatomy. During debulking surgery, numerous ectopic muscles were found in the upper limbs of the patients. DNA sequencing, followed by digital polymerase chain reaction, was performed on DNA extracted from biopsies from hypertrophic ectopic muscles and identified the somatic mosaic PIK3CA hotspot mutations c.3140A > G, p.(His1047Arg) and c.1624G > A, p.(Glu542Lys) in a male (patient 1) and a female (patient 2) patient, respectively. Patient 1 had four ectopic muscles and unilateral isolated muscular overgrowth while patient 2 had 13 ectopic muscles and bilateral isolated muscular overgrowth of both upper limbs, indicating that her mutation occurred at early pre‐somitic mesoderm state. The finding of PIK3CA mutations in ectopic muscles highlights the importance of PIK3CA in cell fate in early human embryonic development. Moreover, our findings provide evidence that the disease phenotype depends on the timing of PIK3CA mutagenesis during embryogenesis and confirm the diagnostic entity PIK3CA‐related muscular overgrowth with ectopic accessory muscles.
Keywords: accessory, cell fate, ectopic, muscle hypertrophy, muscular hypertrophy, PIK3CA, PROS
1. INTRODUCTION
Activating somatic mutations in the phosphatidylinositol‐4,5‐bisphospate 3‐kinase, catalytic subunit alpha gene (PIK3CA) occur frequently in congenital overgrowth syndromes and in human cancer.1 PIK3CA encodes the p110 α catalytic subunit of phosphoinositide 3‐kinase (PI3K), that phosphorylates phosphatidylinositol to generate phosphatidylinositol 3,4,5‐trisphosphate (PIP3). PIP3 plays a key role in activating signaling cascades including inhibition of apoptosis, activation of protein synthesis, and enhanced cell survival.2
The umbrella term PIK3CA‐related overgrowth spectrum (PROS) is a heterogeneous group of rare genetic disorders with overgrowth caused by somatic mosaic PIK3CA mutations.3 The variable expression of symptoms within PROS is mainly explained by the timing and location of the initiating PIK3CA mutation, but the reason behind the high degree of interindividual phenotypic heterogeneity is unknown.4 Diseases within PROS are classified on the basis on anatomical differences, for example, isolated macrodactyly (OMIM 155500), megalencephaly‐capillary malformation‐polymicrogyria syndrome (OMIM 602501), and congenital lipomatous asymmetric overgrowth of the trunk with lymphatic, capillary, venous, and combined‐type vascular malformations, epidermal nevi, scoliosis/skeletal and spinal anomalies (OMIM 612918).
The terminology “aberrant muscle syndrome” or “accessory muscle syndrome” has been suggested to describe patients with “hypertrophy of the hand and arm because of aberrant muscles with or without hypertrophy of the muscles.”5 It has been proposed that an increased number of neuromuscular junctions and a change in the muscle‐tendon ratio is involved in muscle hypertrophy development.6 The etiology remains still undetermined but it has recently been reported that isolated congenital muscular upper limb overgrowth can be related to somatic mosaic PIK3CA mutations.7, 8
Somatic activating PIK3CA mutations are common in at least 12 different cancer types.1, 9, 10, 11 Previous studies show that the same PIK3CA hotspot mutations found in cancer are found in PROS.12, 13, 14, 15, 16 To date, the only malignancy that has been reported in the 419 known individuals with PROS is Wilms tumor (OMIM 194070), which has been described in 12 individuals (2.86%); six children with a confirmed molecular diagnosis12, 17, 18, 19, 20, 21 and six children where genetic testing has not been performed.22, 23, 24, 25, 26 It has recently been suggested to use monitoring of cell‐free DNA in urine to screen for renal involvement in PROS.27
Although it is critical for normal cell growth and survival, the role of PIK3CA in early human development is poorly characterized. Because not all cells in overgrown tissues in PROS appear to have PIK3CA mutation, it has been suggested that PIK3CA mutation‐positive cells exert growth‐promoting effects on adjacent or distant cells.4 In cells with strong activation of PI3K signaling pathway, PIK3CA mutations may lead to lineage‐specific cell loss during or after differentiation because of mechanisms such as oncogene‐induced senescence.28 Previous studies have highlighted that PI3K signaling is crucial for embryonic development and plays an important role in the control of pluripotency and differentiation.29 Recent studies have also highlighted a prominent role for PI3K signaling during cell differentiation and determination of cell fate in mouse models.30, 31
In this study, we confirm a novel PROS phenotype with isolated muscle overgrowth and ectopic muscles and show evidence that PIK3CA regulates cell fate in early human embryonic development.
2. MATERIAL AND METHODS
2.1. Patients and ethical approvals
We included two patients with local muscular overgrowth of the upper extremity that were seen at the Department of Clinical Genetics, Karolinska University Hospital, Stockholm. The study was performed in accordance with the Declaration of Helsinki, and the local ethical board in Stockholm approved the study. Informed consents were obtained from each participating individual or their legal guardians according to local ethical guidelines and the parents have approved publication of clinical data and pictures.
2.2. Oncomine solid tumor panel
DNA from patient 1 was extracted from paraffin‐embedded tissue containing pathological lesions from dorsal ectopic muscle of the hand. We used the Ion Torrent Oncomine Solid Tumor DNA Panel kit, on the Ion Torrent S5 (Thermo Fisher Scientific, Massachusetts) according to the manufacturer's instructions. The analyzed genes were KRAS, BRAF, EGFR, NRAS and PIK3CA (NM_006218.1).
2.3. Whole genome sequencing
Whole genome sequencing (WGS) was performed on DNA extracted from affected tissue and blood from patient 2 together with blood samples from the healthy parents. Libraries for sequencing on Illumina HiSeq X (Illumina Inc, San Diego, California) were prepared from genomic DNA using the Illumina TruSeq polymerase chain reaction (PCR)‐free kit with a mean insert size of >350 base pairs. This resulted in an average of 798 million mapped unique sequences per sample (range 737‐932 million) with a mean coverage of 38× (range 35‐44×). Reading mapping and somatic variant calling was performed with SpeedSeq framework 0.1.0, using reference genome build GRCh37/hg19.32 All PIK3CA variants were explored with GEMINI33 and visualized in the integrative genomics viewer.34
2.4. Digital polymerase chain reaction
Fifty nanograms of genomic DNA extracted from affected muscle tissue from patients 1 and 2 was amplified with 1X QuantStudio 3D Digital PCR Master mix (Applied Biosystems, California) and commercially available TaqMan assay for PIK3CA p.(His1047Arg) and p.(Glu542Lys) mutation (Applied Biosystems, California), according to the manufacturer's instructions. Extracted DNA was quantified using Qubit fluorometry (Thermo Fisher Scientific, Massachusetts). Fifteen microliters of PCR reaction mixes were loaded into QS3D Digital 20 K V2 chips (Applied Biosystems, California), which were then placed into GeneAmp PCR System 9700 thermocycler (Thermo Fisher Scientific). The chips were incubated at 96°C for 10 minutes, followed by 39 cycles at 54°C for 2 minutes and 98°C for 30 seconds. PCR was completed with a final incubation at 60°C for 2 minutes. After completion of PCR, each chip was scanned using QuantStudio 3D reader (Applied Biosystems). Digital PCR (dPCR) data were analyzed using PoissonPlus algorithm (version 4.4.10) with a confidence interval of 95% and the desired precision of 10% by QuantStudio 3D AnalysisSuite (version 3.1.2‐PRC‐build‐03). Using the two‐dimensional scatter plots, partitions for positive and negative amplification signals were classified for target and reference sequences using 6‐carboxyfluorescein and 2′‐chloro‐7′phenyl‐1,4‐dichloro‐6‐carboxy‐fluorescein channels.
2.5. Pathology
Patient 1: When the patient was 3 years old, macroscopic ectopic muscle tissue was removed from the dorsal part of the left hand. Hematoxylin‐eosin (H‐E) stained frozen sections and formalin fixed paraffin embedded (FFPE) sections stained with H‐E and van Gieson as well as with antibodies against p62 were analyzed.
Patient 2: Biopsies from ectopic dorsal interosseous muscle (I) and ectopic extensor digitorum muscle (II) were taken from the right side when the patient was almost 11 years old. H‐E stained sections from FFPE tissue were available for microscopic evaluation.
3. RESULTS
3.1. Clinical findings
Patient 1: The 4‐year‐old male patient was born as the second child to healthy, non‐consanguineous parents. Pregnancy and delivery were normal and he was born at 40 + 4 weeks of gestational age. His birth length was 49 cm and his weight was 3780 g. Hemihypertrophy of the left arm and hand was noted directly after birth (Figure 1A). Magnetic resonance imaging and ultrasound of the left hand showed thickened muscles between metacarpals I and II and enlarged tendon to extensor pollicis brevis. Also, hypertrophy of extensor pollicis brevis and adductor pollicis longus muscles was noted. X‐ray showed widening of the metacarpals in a fan‐shaped matter. Ulnar deviation of the second to fifth finger was seen. A swan neck like deformity of the second finger was interpreted to be caused by intrinsic tightness. The thumb was abducted and hyperextended in the carpometacarpal joint (Figure 1B). The functional grip of the affected hand adapted well despite the malformations. During growth, a mass on the dorsum of his hand and a subcutaneous string in the volar forearm developed slowly and were removed surgically (Figure 1C). Operative exploration showed at least four different ectopic muscles of the hand and forearm (Table 1).
Figure 1.
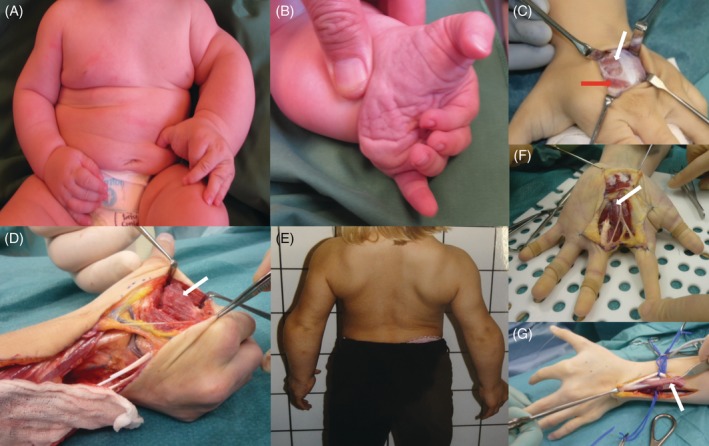
Clinical findings in patients. A, B, Overgrowth of left arm and hand with characteristic ulnar deviation of the index finger and adducted thumb in patient 1. C, White arrow shows atrophic ectopic muscle mass on the dorsum of left hand in patient 1. Red arrow shows normal junctura replaced by ectopic muscle. D, White arrow shows ectopic adductor muscle in patient 2. E, Bilateral overgrowth of shoulders, arms and hands in patient 2. F, White arrow shows ectopic palmar muscles extending proximally into carpal tunnel. G, White arrow shows ectopic extensor muscle dorsal to normal extensor digiti communis tendons and muscle
Table 1.
Summary of molecular findings and phenotype in patients 1 and 2
| Patient | 1 | 2 |
|---|---|---|
| Sex | M | F |
| PIK3CA mutation | c.3140A > G | c.1624G > A |
| Protein alteration | p.(His1047Arg) | p.(Glu542Lys) |
| Affected cells (%) | 36 |
52 (biopsy I) 34 (biopsy II) |
| Tissue analyzed | Ectopic muscle, dorsum of the left hand |
I. Ectopic dorsal interosseous muscle, right side II. Ectopic extensor digitorum muscle, right side |
| Method for detection |
Gene panel dPCR |
Whole genome sequencing (WGS) Digital polymerase chain reaction (dPCR) |
| Description | Unilateral overgrowth of the left arm and forearm muscle, with no signs of edema neither fatty infiltration nor vascular anomaly. Swan neck‐deformity of the index finger and ulnar deviation | Bilateral overgrowth of arms and forearms muscle, with no signs of edema nor fatty infiltration |
|
Ectopic muscles. Localization and characterization. ‐ In hand |
‐ Larger fibrotic muscle mass around the extensor tendons on dorsal side of the hand ‐ Transverse ectopic muscle on the dorsal side of the proximal phalanx in the index finger ‐ Normal junctura between second and third extensor indicis communis tendons was replaced by ectopic muscle |
Right hand: ‐ Fibrotic and hypertrophic muscle above the first dorsal interosseous muscle attached to the ulnar side of first metacarpal ‐ Hypertrophic abductor pollicis brevis ‐ Six accessory longitudinal muscles attached to the proximal phalanx of digiti two (ulnar), three (ulnar and radial), four (ulnar and radial) and five (radial) originating in separate tendons below the carpal tunnel. Attached to fascia in the forearm ‐ Ectopic abductor digiti minimi muscle ‐ Ectopic short flexor digiti minimi muscle ‐ Broad and extended adductor pollicis muscle inserting in fascia above fifth metacarpal ‐ Palmar aponeuroses and carpal‐ligament replaced by ectopic muscle mass to a high extent |
|
Ectopic muscles. localization and characterization. ‐ In forearm |
‐ Ectopic pale fibrotic muscle in volar forearm next to the normal flexor carpi radialis muscle. No wrist or finger movement was noted when traction was applied to the attached tendon |
Bilateral in forearms: ‐ Accessory muscle mass originating deep to extensor digiti communis from middle of forearm to insertion in digit two to five via separate broad tendons. Muscle had separate nerve branches. ‐ Accessory extensor pollicis longus (EPL) muscles with tendons parallel to normal EPL Right forearm: ‐ Accessory hypertrophic muscle above the normal brachioradialis muscle ‐ Accessory abductor pollicis longus muscle |
| Other aberrations |
‐Swan‐neck deformity of the left index finger ‐Intrinsic plus position finger two and three ‐Widening between metacarpals |
‐ Broad junctura ‐ Missing extensor retinaculum ‐ Missing palmar fascia ‐ Intrinsic plus position finger two and three ‐ Abducted thumbs ‐ Widening between metacarpals |
| Vascular anomalies | ‐ | Absent digital volar arteries |
| Pathology | Increased perimysial and endomysial fibrosis, associated with marked fiber size variability, with scattered hypertrophic fibers and many small fibers. Occasional fibers with rimmed vacuoles were found. There were also rounded eosinophilic fibers. | The pathology differed between the two muscles. In muscle I, variation in muscle fiber size and increase of connective tissue was seen while in muscle II, eosinophilic rounded fibers were present in otherwise rather well‐preserved tissue. |
F = female, M = male. The aberrations in patient 2 seemed symmetrical but because of different operations performed on the right and left hand it was not confirmed in all locations.
Patient 2: An 18‐year‐old female with normal physical and cognitive development was born as the second child to healthy, non‐consanguineous parents. Pregnancy and delivery were normal; her birth weight was 3300 g and her length was 49 cm. Bilateral symmetric hypertrophic hands, arms and upper part of her trunk were noted already during the neonatal period. During childhood, she was very strong in her arms and hands and could climb using only her upper limbs (Figure 1E). She was referred for hand surgery because the muscular hypertrophy caused functional limitations with difficulties to grip small objects and problems with the pinch grip. Wide hands and abducted thumbs with difficulty to perform the thumb opposition movement were observed. Rotations osteotomy of the first metacarpal and repeated botulinum toxin injections in the hands' intrinsic muscles were performed to improve the pinch grip. The growth was proportional and no progression of the muscular hypertrophy was noted. The difficulty to move the wrists, fingers rotations and elbows deformity led to several surgical procedures. At least 13 ectopic muscles were discovered during surgery (Table 1, Figure 1D, F, G).
3.2. Genetic findings
Patient 1: In the affected tissue, we identified a mosaic PIK3CA hotspot mutation; c.3140A > G, p.(His1047Arg) in 1154 out of 6524 reads (18%) with the oncomine solid tumor panel. The mutation was confirmed and quantified using digital PCR (dPCR), which showed a mutant allelic frequency of 18%, indicating 36% affected cells in the analyzed sample (Table 1).
Patient 2: WGS identified a mosaic PIK3CA hotspot mutation; c.1624G > A, p.(Glu542Lys) in 10 out of 42 reads (24%) in the affected tissue. The mutation was confirmed and quantified using dPCR, which showed a mutant allelic frequency of 26% in muscle biopsy I and 17% in muscle biopsy II, indicating 52%, respectively, 34% affected cells in the analyzed materials (Figure 2A‐B). The mutation was not detected in blood samples from the patient.
Figure 2.
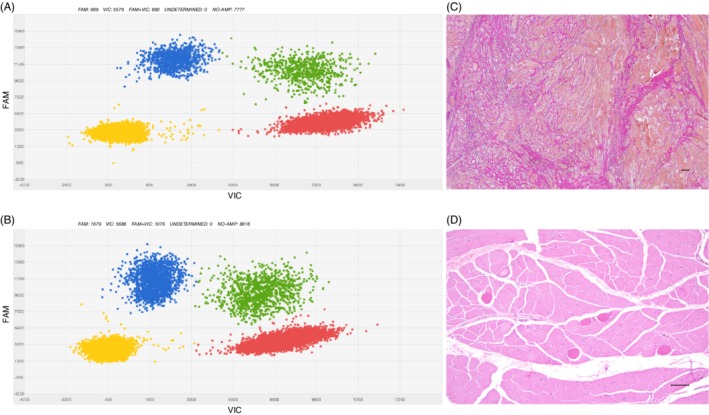
Genetic and pathologic findings in patients. A, B, Representative results from dPCR of DNA from ectopic muscle biopsies. Digital PCR quantifies the load of mosaic mutation, c.1624G > A (p.Glu542Lys), in DNA extracted from ectopic dorsal interosseous muscle and ectopic extensor digitorum muscle from patient 2. Blue cluster (FAM) shows signals from mutant allele, red signals (VIC) from reference allele and green signals from both mutant and reference alleles. Yellow cluster represents the wells where no amplification signal was detected. C, Histopathological findings from affected muscle in patient 1. Severe changes with increase in connective tissue (van Gieson). D, Histopathological findings from affected muscle patient 2. Scattered rounded eosinophilic muscle fibers in otherwise normal looking tissue (H‐E). Bars: 100 μm
3.3. Pathological findings
Patient 1: Cutting of the unfixed tissue showed muscle with fibrotic streaks. Frozen H‐E stained sections and paraffin sections stained with H‐E and van Gieson showed extensive changes. Great variation in fiber size was seen with scattered hypertrophic fibers and many small fibers. Muscle fiber splitting was present. In some fibers, rimmed vacuole‐like structures were found, which showed immunoreactivity for the antibody p62. A few fibers looked necrotic and some fibers seemed to be regenerating. The number of internalized nuclei was increased. Rounded fibers with increased eosinophilia in H‐E staining were present in the tissue. Inflammatory cells were scarce. In some fascicles, the muscle fibers were separated by excessive endomysial connective tissue and the perimysium was also thickened (Figure 2C). Occasional fascicles had a more normal appearance. Some adipose tissue was present in the material.
Patient 2: The pathology was different between the two muscle biopsies I and II (Table 1). In biopsy I, widespread, prominent lesions were found with variation in fiber size and increased number of internalized nuclei. Occasional fibers appeared necrotic and some seemed to be regenerating. A few small aggregates of inflammatory cells were seen. The amount of connective tissue was increased separating the muscle fibers. Some adipose tissue was also found. In regions of more normal appearing muscle, foci of pathologically changed fibers with variation in diameter and an increase of internalized nuclei were noticed together with increased connective tissue. Some rounded eosinophilic fibers could be seen in both muscles with pronounced pathology as well as in otherwise normal tissue. In biopsy II, the pathology consisted mainly of the presence of somewhat rounded eosinophilic muscle fibers. In some fascicles, only one was found, in others they were frequent (Figure 2D). Only occasionally these lesions showed variation in fiber size and an increase in connective tissue.
4. DISCUSSION
In this study, we report two individuals with somatic mosaic PIK3CA hotspot mutations and a similar phenotype with upper limb muscle overgrowth and ectopic muscles. Patient 1 has unilateral engagement with at least four ectopic muscles in his left upper limb, while patient 2 has a bilateral upper limb overgrowth and at least 13 ectopic muscles. Both patients displayed supernumerary muscles in locations in the upper extremity where there normally should not exist muscles. Therefore, the PIK3CA mutated cells seem to have acquired an ability to become muscles when they normally should have differentiated to tendons or fascia. Studies in mouse have shown that activation of mTORC1 signaling in tendons causes impaired collagen fibrillogenesis, disorganized fibers, hypercellularity, and neovascularization35 and it is possible that this is similar to what we describe in our two patients. We speculate that our findings indicate that PIK3CA has a role in pluripotency and cell fate in early human development.
All vertebrate skeletal muscles, apart from superficial neck muscles, derived from the paraxial mesoderm.36 The mesoderm is derived from the primitive streak in the middle of the epiblast plate.37 The exact position in the primitive streak decides cell fate.38 After invagination, the bilateral paraxial mesoderm, caudal to the head, forms somites which eventually develop into different compartments, such as the sclerotome and the syndetome, which in turn give rise to axial bones, tendons and connective tissues, and the dermomyotome containing proliferating progenitors of all skeletal muscles of the body.39, 40 The somatic mosaic PIK3CA mutation in patient 2 must have occurred at an earlier stage than previous cases that have been reported to occur at day 20 to 56.4 Because she has bilateral findings with symptoms involving the trunk and because the somites are located on either side of the midline, the most probable explanation is that the mutation occurred before day 15 post‐fertilization in a cell of the primitive streak in the middle of the embryo just before the process of invagination. During invagination, a group of mutated cells in the primitive streak, predetermined by location to mesoderm, split and migrated to each side of the embryo. In patient 1, who has a unilateral involvement of isolated muscular hypertrophy and supernumerary muscles, mutagenesis must have occurred after day 21 post‐fertilization.
In 2010, Ogino et al described 35 cases with muscular hypertrophy of the hands and/or arms. Similar to our patient 1, 34 out of 35 had unilateral involvement. Similar to our patient 2, one 8‐month‐old male was bilaterally affected. Other common findings were ulnar deviation of the fingers, flexion of the metacarpophalangeal joints, widening of metacarpal spaces, and abducted thumbs. In three operated patients, aberrant accessory muscles were detected but none of these cases were confirmed by molecular analysis. Aberrant accessory muscles have also been reported in other surgically treated patients with upper limb muscle hypertrophy5 and in some cases of carpal tunnel syndrome,41 but no genetic analysis of aberrant muscles have been performed to rule out somatic mosaic PIK3CA mutations. To the best of our knowledge, there are three patients previously described with isolated upper limb muscle overgrowth and with a confirmed PIK3CA mutation.7, 8 In 2014, Castiglioni et al reported the first mosaic PIK3CA mutation in a patient with unilateral isolated muscular hypertrophy of the left hand and arm and aberrant muscles. In 2018, al‐Quattan et al reported two additional patients with mosaic PIK3CA mutation, isolated muscular overgrowth and hypoplasia of the index finger. In addition, one patient with segmental symmetric overgrowth of the hands, arms, shoulders, and neck, and a lipomatous mass on the upper back, shoulder and neck, has been described.42
The somatic mosaic PIK3CA mutations c.3140A > G and c.1624G > A in our patients are both hotspot mutations that have previously been detected in neuroectodermal and mesodermal tissue derivatives in PROS patients. The same hotspot mutations are also common in endodermal tissue derivatives in cancer. The reason behind this tissue specificity is unknown.4 The endoderm is the innermost of the three germ layers that is formed first. We suggest that early mosaic constitutional mutations in the epiblast might be lethal already during gastrulation and that somatic mutations that occur in differentiated cells are tolerated by the cell but give rise to cancer. A possible explanation could be that PIK3CA, depending on cell of origin, switches cell fate in different directions.
Because of the relatively low prevalence, the psychological burden for the families and possible publication bias, it has been debated whether individuals with PROS are at increased risk of Wilms tumor and should be considered for a surveillance program, similar to what is established for Beckwith‐Wiedemann syndrome (OMIM 130650) and hemihyperplasia patients (OMIM 235000).17, 21, 25 There is a possibility that the only patients who are at risk of Wilms tumor are those with a significant burden of renal cells with the activating PIK3CA mutation.27 If so, dPCR analysis of cell‐free DNA in urine seems promising to identify PROS patients at increased risk of Wilms tumor. However, more studies are needed.
In conclusion, this study adds information about timing of PIK3CA mutagenesis during embryogenesis in correlation to phenotype and confirms the diagnostic entity PIK3CA‐related muscular overgrowth with ectopic muscles. However, as our study points out, our understanding of PIK3CA's role during human embryogenesis is still restricted. Further studies of induced pluripotent stem cells, animal models, and cancer cell lines on the role of somatic mosaic activating PIK3CA mutations in cell fate are warranted and could be of great importance for precision medicine in both cancer and PROS.
CONFLICT OF INTEREST
Nothing to declare.
ACKNOWLEDGEMENTS
We thank the families for their participation. This work was supported by grants from the Swedish Childhood Cancer Foundation (A.N., B.T.), the Cancerfonden (A.N.), the Swedish Research Council (A.N.), Berth von Kantzow's foundation (A.N.), Karolinska Institutet (A.N.), the Stockholm County Council (S.F., B.T.), the Hjärnfonden (A.N.) and The Hållsten Research Foundation (A.N.).
Frisk S, Taylan F, Blaszczyk I, et al. Early activating somatic PIK3CA mutations promote ectopic muscle development and upper limb overgrowth. Clin Genet. 2019;96:118–125. 10.1111/cge.13543
Funding information Barncancerfonden; Berth von Kantzows Stiftelse; Bertil Hållstens Forskningsstiftelse; Cancerfonden; Hjärnfonden; Karolinska Institutet; Stockholms Läns Landsting; Vetenskapsrådet; Hållsten Research Foundation; Hjärnfonden; Karolinska Institutet; Swedish Research Council; Cancerfonden
Data Availability Statement: Data for this article is available through the corresponding author upon request.
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/cge.13543/
DATA AVAILABILITY STATEMENT
Data for this article is available through the corresponding author upon request.
REFERENCES
- 1. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. [DOI] [PubMed] [Google Scholar]
- 2. Tatton‐Brown K, Weksberg R. Molecular mechanisms of childhood overgrowth. Am J Med Genet C Semin Med Genet. 2013;163(2):71‐75. [DOI] [PubMed] [Google Scholar]
- 3. Keppler‐Noreuil KM, Rios JJ, Parker VE, et al. PIK3CA‐related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A. 2015;167a(2):287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madsen RR, Vanhaesebroeck B, Semple RK. Cancer‐associated PIK3CA mutations in overgrowth disorders. Trends Mol Med. 2018;24(10):856‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogino T, Satake H, Takahara M, et al. Aberrant muscle syndrome: hypertrophy of the hand and arm due to aberrant muscles with or without hypertrophy of the muscles. Congenit Anom. 2010;50(2):133‐138. [DOI] [PubMed] [Google Scholar]
- 6. Takka S, Doi K, Hattori Y, Kitajima I, Sano K. Proposal of new category for congenital unilateral upper limb muscular hypertrophy. Ann Plast Surg. 2005;54(1):97‐102. [DOI] [PubMed] [Google Scholar]
- 7. al‐Qattan MM, Hadadi A, al‐Thunayan AM, Eldali AA, AlBalwi MA. Upper limb muscle overgrowth with hypoplasia of the index finger: a new over‐growth syndrome caused by the somatic PIK3CA mutation c.3140A>G. BMC Med Genet. 2018;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castiglioni C, Bertini E, Orellana P, et al. Activating PIK3CA somatic mutation in congenital unilateral isolated muscle overgrowth of the upper extremity. Am J Med Genet A. 2014;164a(9):2365‐2369. [DOI] [PubMed] [Google Scholar]
- 9. Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505(7484):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of Phosphatidylinositol‐3‐kinase pathway alterations across 19 784 diverse solid tumors. JAMA Oncol. 2016;2(12):1565‐1573. [DOI] [PubMed] [Google Scholar]
- 12. Kurek KC, Luks VL, Ayturk UM, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet. 2012;90(6):1108‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K‐AKT3‐mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44(8):941‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindhurst MJ, Parker VER, Payne F, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44(8):928‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maclellan RA, Luks VL, Vivero MP, et al. PIK3CA activating mutations in facial infiltrating lipomatosis. Plast Reconstr Surg. 2014;133(1):12e‐19e. [DOI] [PubMed] [Google Scholar]
- 16. Riviere JB, Mirzaa GM, O'Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44(8):934‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gripp KW, Baker L, Kandula V, et al. Nephroblastomatosis or Wilms tumor in a fourth patient with a somatic PIK3CA mutation. Am J Med Genet A. 2016;170(10):2559‐2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hucthagowder V, Shenoy A, Corliss M, et al. Utility of clinical high‐depth next generation sequencing for somatic variant detection in the PIK3CA‐related overgrowth spectrum. Clin Genet. 2017;91(1):79‐85. [DOI] [PubMed] [Google Scholar]
- 19. Kuentz P, St‐Onge J, Duffourd Y, et al. Molecular diagnosis of PIK3CA‐related overgrowth spectrum (PROS) in 162 patients and recommendations for genetic testing. Genet Med. 2017;19(9):989‐997. [DOI] [PubMed] [Google Scholar]
- 20. Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr. 2015;166(4):1048‐1054.e1041‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Postema FAM, Hopman SMJ, Deardorff MA, Merks JHM, Hennekam RC. Correspondence to Gripp et al. nephroblastomatosis or Wilms tumor in a fourth patient with a somatic PIK3CA mutation. Am J Med Genet A. 2017;173(8):2293‐2295. [DOI] [PubMed] [Google Scholar]
- 22. Ehrich JH, Ostertag H, Flatz S, Kamran D. Bilateral Wilms's tumour in Klippel‐Trenaunay syndrome. Arch Dis Child. 1979;54(5):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lapunzina P, Gairi A, Delicado A, et al. Macrocephaly‐cutis marmorata telangiectatica congenita: report of six new patients and a review. Am J Med Genet A. 2004;130a(1):45‐51. [DOI] [PubMed] [Google Scholar]
- 24. Mankad VN, Gray GF, Miller DR. Bilateral nephroblastomatosis and Klippel Trenaunay syndrome. Cancer. 1974;33(5):1462‐1467. [DOI] [PubMed] [Google Scholar]
- 25. Peterman CM, Fevurly RD, Alomari AI, et al. Sonographic screening for Wilms tumor in children with CLOVES syndrome. Pediatr Blood Cancer. 2017;64(12):e26684. [DOI] [PubMed] [Google Scholar]
- 26. Wright DR, Frieden IJ, Orlow SJ, et al. The misnomer "macrocephaly‐cutis marmorata telangiectatica congenita syndrome": report of 12 new cases and support for revising the name to macrocephaly‐capillary malformations. Arch Dermatol. 2009;145(3):287‐293. [DOI] [PubMed] [Google Scholar]
- 27. Biderman Waberski M, Lindhurst M, Keppler‐Noreuil KM, et al. Urine cell‐free DNA is a biomarker for nephroblastomatosis or Wilms tumor in PIK3CA‐related overgrowth spectrum (PROS). Genet Med. 2018;20(9):1077‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JS, Lee C, Bonifant CL, Ressom H, Waldman T. Activation of p53‐dependent growth suppression in human cells by mutations in PTEN or PIK3CA. Mol Cell Biol. 2007;27(2):662‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu JSL, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050‐3060. [DOI] [PubMed] [Google Scholar]
- 30. Van Keymeulen A, Lee MY, Ousset M, et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature. 2015;525(7567):119‐123. [DOI] [PubMed] [Google Scholar]
- 31. Ying Z, Sandoval M, Beronja S. Oncogenic activation of PI3K induces progenitor cell differentiation to suppress epidermal growth. Nat Cell Biol. 2018;20(11):1256‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiang C, Layer RM, Faust GG, et al. SpeedSeq: ultra‐fast personal genome analysis and interpretation. Nat Methods. 2015;12(10):966‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paila U, Chapman BA, Kirchner R, Quinlan AR. GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput Biol. 2013;9(7):e1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson JT, Thorvaldsdottir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim J, Munivez E, Jiang MM, et al. mTORC1 signaling is a critical regulator of postnatal tendon development. Sci Rep. 2017;7(1):17175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pu Q, Patel K, Huang R. The lateral plate mesoderm: a novel source of skeletal muscle. Results Probl Cell Differ. 2015;56:143‐163. [DOI] [PubMed] [Google Scholar]
- 37. Fan H, Sakamoto N, Aoyama H. From the primitive streak to the somitic mesoderm: labeling the early stages of chick embryos using EGFP transfection. Anat Sci Int. 2018;93(4):414‐421. [DOI] [PubMed] [Google Scholar]
- 38. Wymeersch FJ, Huang Y, Blin G, et al. Position‐dependent plasticity of distinct progenitor types in the primitive streak. Elife. 2016;5:e10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deries M, Thorsteinsdottir S. Axial and limb muscle development: dialogue with the neighbourhood. Cell Mol Life Sci. 2016;73(23):4415‐4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pourquie O. The chick embryo: a leading model in somitogenesis studies. Mech Dev. 2004;121(9):1069‐1079. [DOI] [PubMed] [Google Scholar]
- 41. Steele J, Coombs C. Carpal tunnel syndrome in aberrant muscle syndrome: a case report and review of the literature. J Hand Surg Asian Pac Vol. 2018;23(02):290‐293. [DOI] [PubMed] [Google Scholar]
- 42. Rasmussen M, Sunde L, Weigert KP, Bogaard PW, Lildballe DL. Segmental overgrowth syndrome due to an activating PIK3CA mutation identified in affected muscle tissue by exome sequencing. Am J Med Genet A. 2014;164a(5):1318‐1321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this article is available through the corresponding author upon request.


