Abstract
Purpose
We aimed to determine myopia control efficacy with novel contact lenses (CL) that (1) reduced both central and peripheral defocus, and (2) provided extended depth of focus with better global retinal image quality for points on, and anterior to, the retina and degraded for points posterior to the retina.
Methods
Children (n = 508, 8–13 years) with cycloplegic spherical equivalent (SE) −0.75 to −3.50D were enrolled in a prospective, double blind trial and randomised to one of five groups: (1) single vision, silicone hydrogel (SH) CL; (2) two groups wearing SH CL that imposed myopic defocus across peripheral and central retina (test CL I and II; +1.00D centrally and +2.50 and +1.50 for CL I and II at 3 mm semi‐chord respectively); and (3) two groups wearing extended depth of focus (EDOF) hydrogel CL incorporating higher order aberrations to modulate retinal image quality (test CL III and IV; extended depth of focus of up to +1.75D and +2.50D respectively). Cycloplegic autorefraction and axial length (AL) measurements were conducted at six monthly intervals. Compliance to lens wear was assessed with a diary and collected at each visit. Additionally, subjective responses to various aspects of lens wear were assessed. The trial commenced in February 2014 and was terminated in January 2017 due to site closure. Myopia progression over time between groups was compared using linear mixed models and where needed post hoc analysis with Bonferroni corrections conducted.
Results
Myopia progressed with control CL −1.12 ± 0.51D/0.58 ± 0.27 mm for SE/AL at 24 months. In comparison, all test CL had reduced progression with SE/AL ranging from −0.78D to −0.87D/0.41–0.46 mm at 24 months (AL: p < 0.05 for all test CL; SE p < 0.05 for test CL III and IV) and represented a reduction in axial length elongation of about 22% to 32% and reduction in spherical equivalent of 24% to 32%. With test CL, a greater slowing ranging from 26% to 43% was observed in compliant wearers (≥6 days per week; Control CL: −0.64D/0.30 mm and −1.14D/0.58 mm vs test CL: −0.42D to −0.47D/0.12–0.18 mm and −0.70 to −0.81D/0.19–0.25 mm at 12 and 24 months respectively).
Conclusions
Contact lenses that either imposed myopic defocus at the retina or modulated retinal image quality resulted in a slower progression of myopia with greater efficacy seen in compliant wearers. Importantly, there was no difference in the myopia control provided by either of these strategies.
Keywords: central and peripheral plus contact lenses, extended depth of focus contact lenses, myopia, progression
Introduction
Myopia is a common yet insidious condition, with each dioptre increasing the burden associated with the condition. Particularly, high myopia (≤−5.00D) increases the risk of vision impairment due to complications such as myopic macular degeneration, chorio‐retinal atrophy and glaucoma.1, 2, 3, 4, 5, 6, 7, 8, 9 Given the substantially high prevalence of myopia in many East Asian countries and rising prevalence elsewhere, its impact on eye health and the associated economic burden is significant.10
Although the biological processes that result in myopia are not well understood, there is significant evidence for the role of visual feedback in emmetropization and development of refractive errors. Notably, experiments in animal models found optical defocus to predictably alter eye growth, with myopic defocus resulting in slower growth and hyperopic defocus resulting in increased growth.11, 12, 13 Furthermore, over the years, hypotheses formulated to explain onset and progression of myopia have implicated hyperopic defocus, either on‐axis or off‐axis, as the impetus for increasing axial length of the eye and optical strategies aimed at reducing on‐axis hyperopic defocus such as under‐correction, bifocal spectacles, multifocal spectacles and bifocal contact lenses were assessed in clinical trials.14, 15, 16, 17, 18, 19, 20 Generally, these were effective (excepting under‐correction that was either not different or resulted in worse progression compared to comparative group), but the level of control (i.e. slowing of eye growth) varied between strategies and was not considered to be sufficient to translate to clinical practice. More recent studies in animal models found the peripheral retina to play an active role in emmetropization and refractive error development, and peripheral or off‐axis hyperopic defocus was considered to be instrumental in axial elongation.21 A number of innovative lens designs aimed at reducing off‐axis defocus and inducing myopic defocus were assessed in animal and human clinical trials and shown to reduce the axial elongation of the eye.22, 23, 24, 25, 26, 27, 28, 29
Although the evidence indicates that myopia progression can be slowed, there is a need to determine if efficacy can be further improved. We developed novel contact lenses based on two design principles and assessed them for their efficacy in slowing progression of myopia. The first design principle aimed to reduce hyperopic defocus and induce myopic defocus across a large portion of the retina. The second design principle used extended depth of focus (EDOF) contact lenses that were designed to result in a global retinal image quality (i.e., across both the central and peripheral retina) that was improved for points on, and anterior to, the retina and degraded for points posterior to the retina to prevent axial elongation.30, 31 Two lens designs based on each of the principles were developed and assessed in clinical trials involving myopic children in China. The clinical trial was scheduled for 2 years in duration, but the trial was terminated early due to closure of the clinic. We present results for the participants who attended the 2‐year visit.
Materials and methods
Study population
A total of 508 Chinese children with myopia, aged 7–13 years were enrolled and randomised into a prospective, parallel‐arm, randomised, double‐masked clinical trial. The study was conducted at the Zhongshan Ophthalmic Center, Sun Yet Sen University, Guangzhou, China from February 2014 to January 2017. Enrolled children were of Chinese ethnicity, were myopic in both eyes with cycloplegic spherical equivalent refractive error ranging from −0.75 to −3.50D and astigmatism no more than 0.75D, were willing to comply with the wearing and trial schedule, had ocular health findings considered to be normal and did not preclude contact lens wear, and had vision correctable to at least 6/9.5. Children with any pre‐existing ocular or systemic conditions that precluded lens fitting and safe wear of lenses, those that underwent corneal refractive surgery, those with keratoconus, allergies to topical drops, mydriatics and anaesthetics, or systemic/syndromic conditions associated with myopia such as Marfan syndrome, those that underwent atropine treatment, or other forms of myopia control such as progressive addition spectacles or orthokeratology were excluded from the trial. The study was approved by the Institutional Ethics Committee of Zhongshan Ophthalmic Center and adhered to the Declaration of Helsinki for experimentation on human subjects. Informed consent was provided by parents and/or legal carers of the participant. The study was registered with the Chinese Clinical Trial Registry (ChiCTR‐TRC‐14004227.) and study procedures were conducted in accordance with the Good Clinical Practice Guidelines (ICH‐GCP guidelines for clinical trials ICH 135/95) The study commenced in February 2014 and was terminated in January 2017 due to closure of the clinic facility.
Study design
Participants attended a baseline visit, and were randomised to one of four treatment groups or a single vision contact lens control group. Randomisation scheme for the study was generated using randomly permuted blocks of 25 participants per block, wherein each participant was randomly assigned to one of the five parallel treatment arms in a ratio of 1:1:1:1:1. The randomisation plan was generated by the statistician using http://www.randomization.com and applied via a computer based data management system. The study visits took place at the Brien Holden Vision Institute clinical trial facility located at Zhongshan Ophthalmic Centre, Guangzhou.
Investigational lenses
Lenses were dispensed for use on a daily disposable wear schedule. The control contact lens was clariti® 1 day somofilcon A (http://www.coopervision.com) silicone hydrogel lens material. The optic zones of test lenses I and II somofilcon A (http://www.coopervision.com) incorporated relative plus power in the periphery (commencing at 1.5 mm chord diameter) in a stepped manner with a maximum relative positive power of +2.50D at 3.0 mm with test lens I and +1.50D with test lens II. In addition, test lenses I and II also incorporated a stepped, relative positive power centrally (up to 1.0 mm semi‐chord) of up to +1.00D. Test lenses III and IV were hydrogel Aquamax contact lenses (http://www.pegavision.com) that incorporated and manipulated selective higher order aberrations to achieve a through focus global (both central and peripheral) retinal image quality that was optimised for points at and anterior to retina and degraded for points posterior to retina. The refractive power profile of the lens across the optic zone was non‐monotonic and aperiodic (i.e., there were no discrete power zones and the power varied above and below the normal mean power) across the optic zone.31 Test III and IV were configured to offer extended depth of focus of up to +1.75D and +1.25D, respectively (Figure 1).
Figure 1.
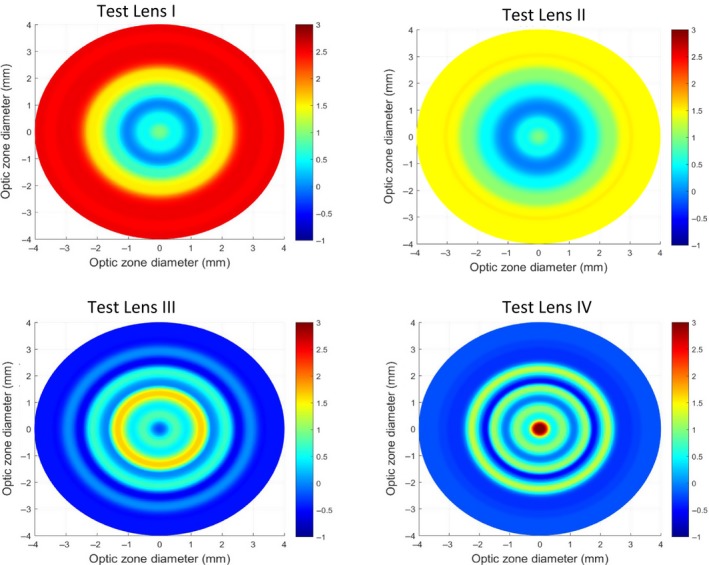
2‐D plot depicting the power profile across the optical zone of the test lenses. x‐ and y‐ axes represent the optical zone diameter and the colours represent the power.
Procedures
Following a baseline examination that included patient history, visual acuity, cycloplegic auto refraction and axial length measures, participants were randomised and fit into one of the five contact lens types. A fit and vision assessment with contact lens was conducted and participants were trained in lens insertion and removal. If children needed further training, a second visit was scheduled and if not, children were dispensed with lenses and advised to return for a 1 month follow up visit. Thereafter, visits were scheduled at three monthly intervals. At each visit, vision and lens fit were assessed. High and low contrast visual acuity was assessed with LogMAR charts. Visual acuity was measured by asking the participant to commence reading letters from the 6/9.5 line. If they could not read the line, they were asked to start at the line they could comfortably read. Errors were cumulative and the end point reached when three or more errors were made. Acuity was read as the smallest line read less the errors. Cycloplegic auto refraction and axial length were measured at six monthly intervals by masked observers using the Shin‐Nippon SRW 500 (http://www.shin-nippon.jp) and the Lenstar 900 (http://www.mylenstar.com). An average of five measurements were considered for autorefraction and three measurements (considered acceptable on the Lenstar software) for axial length. Participants were cyclopleged with 1% tropicamide (two drops, 5 min apart) preceded by topical anaesthetic 0.4% oxybuprocaine hydrochloride. Thirty minutes after instillation of drops, pupils were checked for dilation and nonresponsiveness to light before measurements were conducted. In the event that high contrast visual acuity had fallen by one line or more in comparison to previous or baseline visit, or if the difference in subjective refraction was ≥±0.50D, then the contact lens power was appropriately adjusted.
At each visit, children were asked to fill in a questionnaire on subjective assessment of vision and comfort related to lens wear as well as compliance to lens wear since their last visit.
Sample size
Approximately 58 subjects were needed to complete 1 year to demonstrate a 35% difference in myopia progression of 0.26 ± 0.45D between the test and control groups at the 5% level of significance with 90% power using a two‐tailed distribution and an intra‐ocular correlation of 0.8. The 2‐year progression was estimated at −1.10D based on a meta–analysis of children wearing single vision spectacle lenses.32 Thus, approximately 45 subjects were required in each study group to complete 2 years to demonstrate a statistically significant difference in myopia progression of 35% between the test and control groups at the 5% level of significance with 90% power, using a 2‐tailed distribution and assuming an intra‐ocular correlation of 0.8. Thereafter, based on data from previous studies, wherein the drop out ranged from 11% to 46% with contact lenses in children,24, 28, 33, 34 the minimum sample size was estimated as 97 per treatment arm based on a drop‐ out rate adjustment of 40%.
Statistical analysis
Data from all 508 participants were included in the analysis dataset. Demographic variables between study groups were compared using chi‐square tests and analysis of variance. Change in spherical equivalent (sphere + cylinder/2) and axial length were the primary outcome variables and were recorded on an interval scale. Myopia progression, defined as the change in spherical equivalent or axial length from baseline, was computed for each eye and summarised as grouped mean ± standard deviation at each visit. The effect of lens types on the progression of spherical equivalent and axial length was analysed at each visit using linear mixed model with subject random intercepts. This model accounted for the correlation between eyes. To obtain unbiased treatment effects, the model was adjusted for confounding factors such as age, gender, parental myopia and baseline refractive error. Interactions of confounding factors with lens types were tested and if significant, effect of lens type was estimated within the subgroups of the interacting factor. Compliance was recorded at each visit as average number of days of wear per week and included in the model as a covariate. Compliance was also categorised as lens wear ≥6 days per week or ≤5 days per week. Based on the model, estimated mean progression with 95% confidence limits were reported for each lens type. Additionally, a model that included all the six monthly visits was used to determine the rate of change in progression over time. Here, time (follow‐up months) was factored as a covariate and the model tested for the interaction of lens type with time. A significant interaction of lens type with time would indicate that the rate of change in progression was dependent on lens type. Estimated mean progression and 95% confidence limits were reported based on the model. Progression was also converted to a binary outcome based on a cut off value of −0.75D. The time to the outcome (i.e. −0.75D or more progression) and survival probabilities over 24 months were compared between lens types using Cox proportional hazard model. Visual acuity measured in LogMAR were analysed at each study visit and compared between lens types using linear mixed model. Subjective ratings of vision and comfort related to lens wear were recorded on a scale of 1–10 (1 = poor and 10 = excellent) were binned and summarised as percentages and compared between lens types using chi‐square tests if frequency assumptions were valid. All post hoc multiple comparisons between lens types were adjusted using Bonferroni correction. Level of significance was set at 5%. SPSS Statistics v21 (https:www.ibm.com/spss/modeler) and Stata v10 (http://www.stata.com) were used for statistical analysis.
Results
Table 1 presents the baseline demographic and ocular data for the 508 participants randomised to one of five groups in the study. No significant difference existed between the groups for baseline age, gender, parental myopia, spherical equivalent and axial length. The progression of children through the trial is presented in Figure 2. A large number of children discontinued soon after lens dispensing (129/508, 25.4%) and prior to the 1 month visit. The main reasons for dropping out were: discomfort with lens wear (26/129, 20.2%); safety concern with contact lenses (25/129, 19.4%); no interest in contact lens wear (25/129, 19.4%); handling (15/129 or 11.6%); time conflicts and issues with attending follow up (10/129 or 7.8%); and other reasons such as red eye, rhinitis, preferred orthokeratology and unable to attend due to relocation. One participant complained of poor vision. Of the remaining 379 participants that continued to wear lenses, 89 participants (23.5%) were discontinued/lost to follow up over 2 years and a further 56 children were terminated due to site closure in January 2017. The main reasons for discontinuation were discomfort (19/89, 21%); time conflicts (12/89, 14%); lost to follow (10/89, 11%); handling (8/89, 9%); increased myopia progression (9/89, 10%); vision problems with lenses (2/89, 2%); and prefer to switch to orthokeratology (3/89, 3%).
Table 1.
Biometric data of participants enrolled in the trial
| Control (n = 102) | Test lens I (n = 103) | Test lens II (n = 101) | Test lens III (n = 98) | Test lens IV (n = 104) | p‐value | |
|---|---|---|---|---|---|---|
| Age (years) | 10.5 ± 1.3 | 10.4 ± 1.3 | 10.4 ± 1.3 | 10.4 ± 1.3 | 10.3 ± 1.3 | 0.76 |
| Female % | 43 | 49 | 52 | 57 | 56 | 0.29 |
| Parental Myopia % (None: One: Two) | 26:36:38 | 23:43:34 | 33:39:28 | 26:46:28 | 22:50:28 | 0.45 |
| S.E. (D) | −2.29 ± 0.75 | −2.38 ± 0.82 | −2.39 ± 0.79 | −2.41 ± 0.82 | −2.44 ± 0.73 | 0.70 |
| Axial length (mm) | 24.7 ± 0.8 | 24.7 ± 0.8 | 24.5 ± 0.7 | 24.5 ± 0.7 | 24.6 ± 0.8 | 0.44 |
Figure 2.
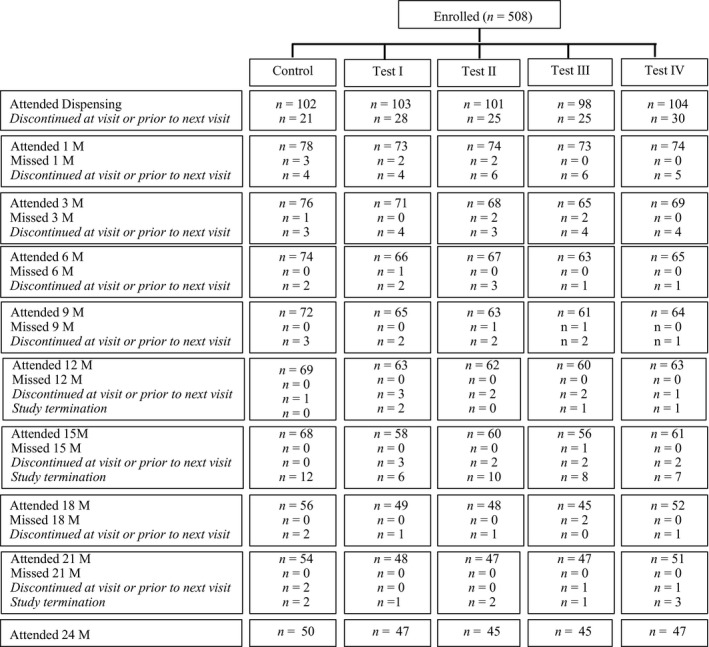
Flow chart of the participants through the parallel, five group randomised trial detailing the number of participants that attended and discontinued at each visit.
Change in spherical equivalent over time
The raw means were presented in Table 2, and Table 3 presents the means adjusted for confounders, namely, age, gender, parental myopia, compliance and baseline refractive error. Table 3 presents the adjusted means (estimated for a baseline age of 10.4 years, spherical equivalent refractive error of −2.40D and compliance of 5.8–6.2 days per week). Less progression was observed in test groups compared to control group at all visits (p = 0.002, 0.004, 0.034 and 0.016 for 6, 12, 18 and 24 months, respectively). At 12 months, estimated progression in controls was −0.66D (95% CI −0.58D to −0.74D) compared to −0.46D to −0.52D in the test groups. The dioptric difference between control and test lens wearing eyes was 0.20–0.14D (31–21%) and significant for lens types I, III and IV (p = 0.006, 0.004 and 0.014). At 24 months, progression in controls was −1.15D (95% CI: −0.99 to −1.30D) as compared to test groups that progressed from −0.78D to −0.87D [dioptric difference of 0.37D to 0.27D (32–24%)]. The difference was significant for lens types III and IV (p = 0.005 and 0.030 for III and IV respectively). There was no difference in progression between the test groups at any of the visits (p > 0.05).
Table 2.
Raw, unadjusted observed means for each of the lens types at the scheduled visits
| BL‐VISIT | Control | Test I | Test II | Test III | Test IV |
|---|---|---|---|---|---|
| Change in spherical equivalent (D) | |||||
| 6 months | −0.39 ± 0.25 | −0.25 ± 0.29 | −0.27 ± 0.31 | −0.25 ± 0.33 | −0.27 ± 0.24 |
| 12 months | −0.66 ± 0.33 | −0.50 ± 0.35 | −0.53 ± 0.43 | −0.47 ± 0.39 | −0.50 ± 0.34 |
| 18 months | −0.88 ± 0.40 | −0.71 ± 0.53 | −0.70 ± 0.48 | −0.62 ± 0.57 | −0.68 ± 0.48 |
| 24 months | −1.12 ± 0.51 | −0.92 ± 0.56 | −0.87 ± 0.56 | −0.81 ± 0.65 | −0.89 ± 0.56 |
| Change in axial length (mm) | |||||
| 6 months | 0.19 ± 0.09 | 0.11 ± 0.11 | 0.12 ± 0.11 | 0.12 ± 0.10 | 0.11 ± 0.09 |
| 12 months | 0.33 ± 0.14 | 0.21 ± 0.17 | 0.24 ± 0.17 | 0.22 ± 0.16 | 0.22 ± 0.14 |
| 18 months | 0.47 ± 0.21 | 0.34 ± 0.25 | 0.35 ± 0.24 | 0.34 ± 0.24 | 0.34 ± 0.20 |
| 24 months | 0.58 ± 0.27 | 0.44 ± 0.29 | 0.45 ± 0.29 | 0.45 ± 0.28 | 0.44 ± 0.25 |
Table 3.
Adjusted means with 95% CI (lower; upper)
| Visit‐Baseline | Control | Test I | Test II | Test III | Test IV |
|---|---|---|---|---|---|
| Change in spherical equivalent (D) | |||||
| 6 months | −0.40 (−0.34; −0.46) | −0.23 (−0.17; −0.30) | −0.27 (−0.20; −0.33) | −0.25 (−0.19; −0.32) | −0.26 (−0.20; −0.33) |
| 12 months | −0.66 (−0.58; −0.74) | −0.46 (−0.38; −0.55) | −0.52 (−0.44; −0.61) | −0.46 (−0.37; −0.55) | −0.49 (−0.40; −0.57) |
| 18 months | −0.88 (−0.76; −1.00) | −0.68 (−0.55; −0.81) | −0.70 (−0.57; −0.83) | −0.60 (−0.46; −0.74) | −0.66 (−0.53; −0.79) |
| 24 months | −1.15 (−0.99; −1.30) | −0.87 (−0.71; −1.03) | −0.88 (−0.72; −1.03) | −0.78 (−0.62; −0.94) | −0.85 (−0.69; −1.00) |
| Change in axial length (mm) | |||||
| 6 months | 0.19 (0.17; 0.21) | 0.10 (0.07; 0.12) | 0.12 (0.09; 0.14) | 0.12 (0.10; 0.14) | 0.11 (0.09; 0.13) |
| 12 months | 0.33 (0.30; 0.36) | 0.19 (0.15; 0.22) | 0.23 (0.20; 0.27) | 0.22 (0.19; 0.26) | 0.22 (0.18; 0.25) |
| 18 months | 0.47 (0.42; 0.52) | 0.32 (0.27; 0.38) | 0.35 (0.29; 0.40) | 0.33 (0.27; 0.39) | 0.34 (0.28; 0.39) |
| 24 months | 0.60 (0.53; 0.66) | 0.41 (0.34; 0.48) | 0.46 (0.39; 0.53) | 0.45 (0.38; 0.52) | 0.43 (0.36; 0.50) |
Further analysis showed a significant lens by time interaction, with the controls showing a faster rate of change of spherical equivalent compared to test groups (Figure 3 a). However, the rate of change between the control and the test groups was significant for the first 6 months only (p = 0.005, 0.029, 0.010 and 0.015 for test lenses I to IV compared to control lens respectively) and not for the remaining consecutive periods, indicating that a greater treatment effect occurred in the first 6 months of the trial.
Figure 3.

Estimated rate of progression (change in spherical equivalent and axial length from baseline).
Change in axial length over time
Tables 2 and 3 present the unadjusted and adjusted axial length at each of the visits. Considering the adjusted means, significantly less axial elongation was observed with test groups compared to control groups at all visits (p < 0.001 at 6 and 12 months; p = 0.003, 0.002, 0.008 and 0.001 at 18 months and p = 0.001, 0.030, 0.012 and 0.004 at 24 months for lens types I to IV, respectively). The estimated mean axial length progression in the control group was 0.33 and 0.60 mm at 12 and 24 months respectively. In comparison, progression in test groups ranged from 0.19 to 0.23 mm (30–43% less elongation) at 12 months and 0.41–0.46 mm (22–32%) at 24 months. There was no difference in progression between the test lenses at any visit (p > 0.05). Additionally, there was a significant lens by time interaction with controls showing a faster rate of change compared to test groups (Figure 3 b). As seen with change in spherical equivalent, the rate of change between the control and test groups was significant for the first 6 months only (p < 0.001 for test lenses I to IV) and not for the remaining time in study, thus indicating a greater treatment effect in the first 6 months of commencing lens wear.
Effect of compliance
Figure 4 presents the results at 12 and 24 months. When lens wear compliance was ≤5 days per week, progression between test (combined) and control groups was similar at both 12 and 24 months. For lens wear compliance ≥6 days per week, greater efficacy, i.e. significantly less progression was observed with test groups compared to control groups (p < 0.001).
Figure 4.
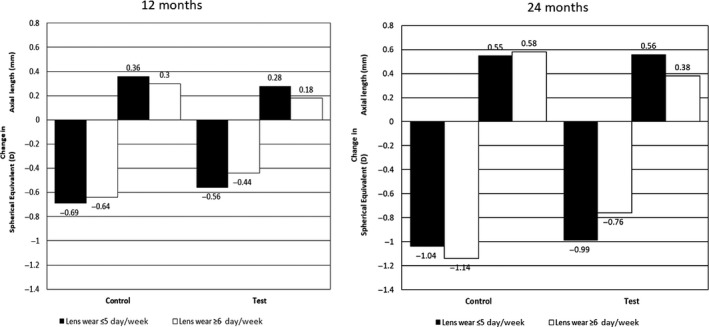
Change in spherical equivalent and axial length from baseline at 12 and 24 months in compliant (≥6 days per week) and non‐ compliant wearers (≤5 days per week).
In the compliant group, at 12 months, change in spherical equivalent was −0.64D for control group and −0.44 for test groups. The dioptric difference was 0.20D (31%). The change in axial length was 0.30 mm for control group and was 0.18 with the test lenses (40%).
At 24 months, in the compliant wearers, the control group progressed on average by −1.14D and the test groups progressed by −0.76D. The difference in estimated means between test and control lenses was 0.38D (dioptric difference of 33%). The change in axial length was 0.58 mm for the control group and 0.383 mms for test groups. The difference in estimated means between test and control lenses was 0.20 mm (difference of 34%).
Survival estimates for progression ≥ 0.75D
Figure 5 provides the survival estimates for progression of ≥0.75D with the test (all combined) compared to control lenses. At each time point, eyes wearing control lenses had a lesser chance of survival compared to eyes wearing test lenses (p ≤ 0.005). There were no significant differences in survival between the test groups (p > 0.1). At 24 months, test lenses improved survival by 36% compared to control lenses.
Figure 5.
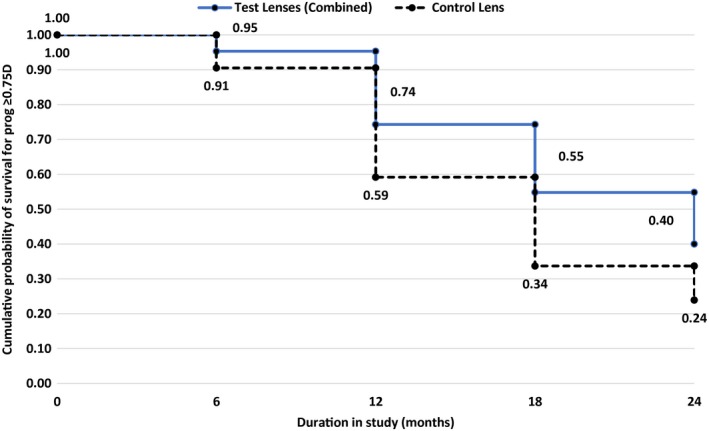
Probability of surviving progression of −0.75D or more during the 24 month period.
Effect of discontinuation
Table 4 details the rate of progression from baseline for individuals that discontinued/lost to follow‐up compared to those that continued in the study. Greater progression was observed in children that discontinued/were lost to follow up compared to those that continued in the study (p < 0.001).
Table 4.
Spherical equivalent progression from baseline for discontinued/lost to follow‐up vs eyes that continued
| Visit | Test lenses (Combined) | Control lens | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Discontinued/Lost to follow‐up | Completed study* | Discontinued/Lost to follow up | Completed study* | ||||||
| N (Eyes) | Mean ± S.D. | N (Eyes) | Mean ± S.D. | N (Eyes) | Mean ± S.D. | N (Eyes) | Mean ± S.D. | ||
| Spherical equivalent progression (D) | 6 M | 68 | −0.33 ± 0.26 | 436 | −0.25 ± 0.30 | 20 | −0.49 ± 0.28 | 128 | −0.38 ± 0.24 |
| 12 M | 42 | −0.58 ± 0.36 | 444 | −0.50 ± 0.38 | 10 | −0.83 ± 0.39 | 128 | −0.65 ± 0.33 | |
| 18 M | 6 | −1.10 ± 0.26 | 372 | −0.67 ± 0.51 | 8 | −1.03 ± 0.43 | 102 | −0.87 ± 0.40 | |
| Axial length progression (mm) | 6 M | 68 | 0.16 ± 0.10 | 438 | 0.10 ± 0.10 | 20 | 0.25 ± 0.10 | 127 | 0.18 ± 0.08 |
| 12 M | 42 | 0.30 ± 0.14 | 446 | 0.22 ± 0.16 | 10 | 0.42 ± 0.16 | 127 | 0.32 ± 0.14 | |
| 18 M | 6 | 0.46 ± 0.15 | 374 | 0.34 ± 0.23 | 8 | 0.52 ± 0.21 | 102 | 0.46 ± 0.21 | |
*Includes data of those that completed study and also those that were terminated.
Visual performance
At each visit, distance visual acuity (monocular and binocular high contrast and monocular low contrast) were assessed with new lenses. However, at baseline and six monthly intervals, vision performance was conducted with dilated pupils. Therefore, monocular high and low contrast visual acuity at the 1 and 3 month visit(s) was considered for the analysis (Figure 6). High contrast and low contrast visual acuity (VA) was maximum with control lens and the difference between test and control group was significant except for Test II for high contrast VA (p < 0.001). Compared to control lens, reduction in high contrast VA ranged from one letter (test lens II) to four to five letters (test lens III). With low contrast VA, the drop with test lenses ranged from one to two lines.
Figure 6.
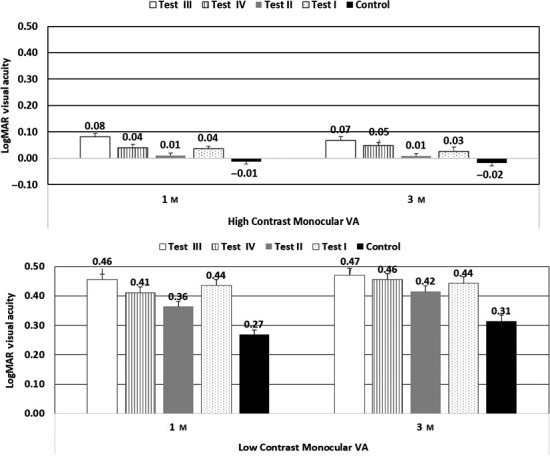
Monocular high and low contrast visual acuity with test and control lenses at 1 and 3 month visits.
Subjective responses
Subjective responses related to vision and comfort to lens wear were determined using a questionnaire (ranked 1–10 where 1 = very poor and 10 = excellent). Subjective responses to distance, intermediate and near vision was generally high (commonly rating of 9 and above, Figure 7) There was no difference in the ratings between the lens types for subjective vision (p > 0.05). With overall comfort, there was a difference between the groups at both 12 and 24 months (p = 0.006 and 0.002) with a greater no of eyes wearing test lens I reporting <9 rating with comfort.
Figure 7.
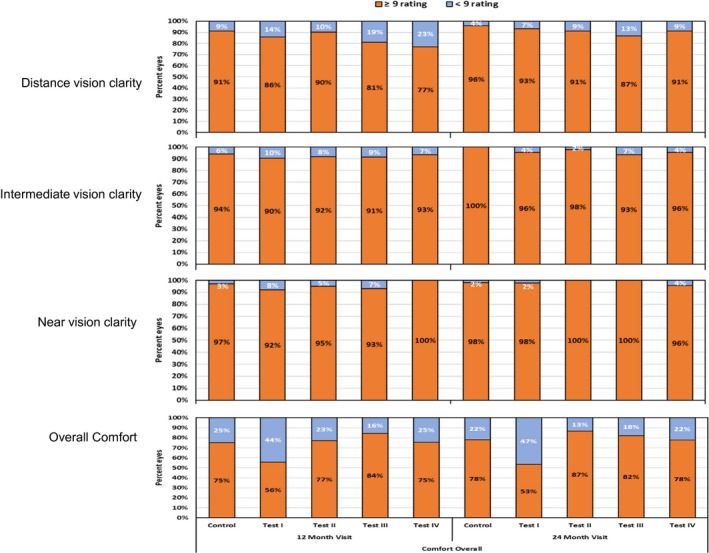
Per cent eyes with subjective ratings of ≥9 and <9 for aspects related to vision and comfort (scale of 1–10 where 1 = poor and 10 = excellent).
Discussion
A significant slowing of myopia was observed with test contact lenses and results from this trial adds to the growing evidence that demonstrates that it is feasible to slow the progression of myopia.16, 20, 22, 23, 24, 27, 28, 29, 35, 36
Previous trials have supported the use of multifocal contact lenses (centre‐distance type) to slow the progression of myopia and it was hypothesised that these lenses reduced either off‐axis or on‐axis hyperopic defocus.24, 27, 28, 33 In the present study, test lenses I and II were directed to reducing peripheral hyperopic defocus as well as inducing myopic defocus across a large portion of the retina, and at 2 years, both lenses slowed myopia by 24% and axial length elongation by 32% and 24%, respectively. Significantly, efficacy was greater in compliant wearers (≥6D week−1) where there was 35% and 39% reduction in myopia and 43% and 37% reduction in axial length elongation for test lenses I and II, respectively. Test lenses I and II differed in the relative peripheral plus at the periphery (Test lens I had +2.50D and Test lens II: +1.50D). Figure 8 presents the refractive error profile of the eye for both the unaided state as well as with the lenses, and it is observed that the test lenses induced myopic defocus (greater with test lens I). However, it is worth noting that the lens design of test lenses I and II (central plus power) and the lens centration on eye may have likely influenced the refractive error measurements taken through these lenses.
Figure 8.
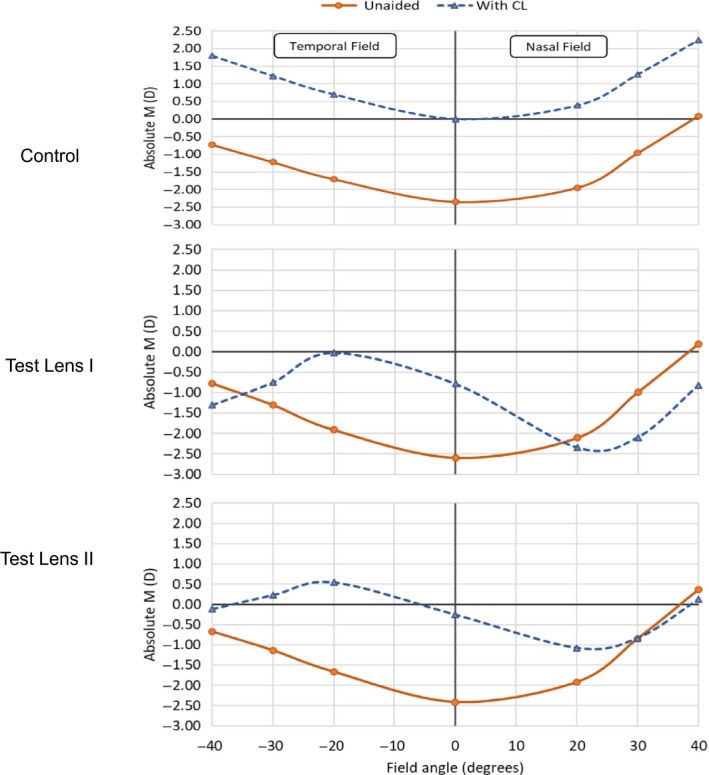
Absolute refractive error profile (measured at fovea and peripheral angles up to 30 degrees horizontally) unaided and with control lens, test lens I and test lens II.
Test lenses III and IV were directed to providing a global retinal image quality that was optimised for points at and anterior to retina and deliberately degraded for points posterior to the retina. It was hypothesised that the poor image quality posterior to the retina prevents axial elongation. The power profile of these novel lenses varied across the entire optical zone with no discrete zones of constant power. At 2 years, test lenses III and IV slowed myopia by 32% and 26% and reduced axial length elongation by 25% and 27%, respectively. In compliant wearers (≥6 days per week), efficacy with test lens III was similar at 29% and 26% slowing of myopia and axial length elongation and improved with test lens IV to 31% and 32% for slowing of myopia and axial length elongation. Test lenses III and IV differ in the higher order aberration combinations that resulted in different magnitudes of extended depth‐of‐focus ranges offered (test III: up to +1.75D; test IV: up to +1.25D) but the efficacy was not significantly different between these lenses or all of the four groups.
Comparing these results to previously published data for 2 years of lens wear, rate of slowing of myopia progression with a centre distance multifocal was 50% and axial elongation was 29%.28 In another trial, where the lens had a clear centre with concentric rings, the lens slowed myopia by 25% but improved to 50% when compliance was considered.22 Similarly, the results of the current trial suggest that compliance is an important factor to consider for efficacy.
Given that the lens designs were unique across the four test groups, the lack of difference in efficacy between the various test lenses is puzzling. However, the results are based on grouped means and the current study design where individuals were randomised to a particular group makes it difficult to explore if certain individuals or eyes derived a treatment benefit compared to others.
A greater efficacy was observed in the first 6 month period in the trial. A reduction in efficacy over time was previously reported.15, 24, 37 Drop‐outs related to fast progression may be one of the factors for the reduced efficacy with time. Individuals that dropped out from the trial had faster progression compared to those continuing and resulting in the mean progression skewed to a lower progression rate. An individual's (or their carer's) motivation to participate and continue in a trial is mainly linked to efficacy, with many likely to discontinue if and when they do not perceive the benefit of participation in the trial. Indeed, few of the participants that discontinued cited rapid progression or indicated a preference for Orthokeratology, which in many Asian countries is considered superior to other myopia control options. A bias towards survivors with lower rates of progression may fail to appropriately discriminate between treatments. Another reason for diminishing efficacy with time may be related to adherence and compliance to lens wear. Unlike trials where there is symptomatic relief with the intervention (for example, asthma medication), there is no incentive for children to wear contact lenses at all times especially if they have low amounts of myopia or if they have access to spectacles. In addition, other lifestyle related reasons such as limited time in the morning for insertion were reported by few. Such reasons may lead to reduced compliance in lens wear over time.
With respect to visual performance, decrement of visual performance with test lenses was more with low contrast testing conditions. Interestingly, a greater number of participants wearing test lens I reported <9 rating for comfort. As the lens material (somofilcon A) was similar across test lens I, II and control and the area of relative central positive zone was similar across test lens I and II, it is possible that this difference may be attributable to vision.
The trial suffers from a number of limitations. An intent‐to‐treat analysis could not be undertaken due to the large number of lost to follow‐ups and discontinuations soon after enrolment and the early termination of the study. The high number of discontinuations that were observed soon after dispensing was anticipated and could have been limited by fitting children with contact lenses for a trial period prior to enrolment. However, this approach was not adopted as there were issues related to additional visits and additionally fitting children with single vision contact lenses and randomising them soon after into a myopia control lens may have resulted in subjective preferences and bias with treatment.
Due to the nature of the current trial where an individual was randomised to one of the four test groups or control and the progression presented as grouped means, it is not clear if a particular individual benefitted from the treatment. It is likely that there may be many other factors – both lens and patient related that may influence efficacy such as for example the pupil size, decentration, refractive error profile at both centre and periphery and fit of the lens and far more research is needed to understand if efficacy can be optimised for each individual myopic eye.
Overall, the data from the current trial shows that test lenses slow myopia progression and axial elongation compared to control single vision lenses and support data from a number of human and animal trials that demonstrated that it is feasible to slow eye growth.22, 23, 24, 25, 27, 28, 35, 38 The percent reduction observed in this trial is somewhat similar to the reduction rates reported in previous contact lens trials conducted in this region with similar ethnic populations22, 24 but less than those reported with predominantly other ethnicities.28, 35 It is well known that myopic individuals of Asian ethnicity progress faster and it is not understood if this played a role in our study.32
In summary, we have demonstrated that in children with myopia, significant reductions in progression of myopia can be obtained with the use of novel contact lens aimed at reducing peripheral and central defocus, and those aimed at optimising the retinal image quality for points on, and in front of, the retina resulted in slower eye growth compared to use of conventional, single vision contact lenses.
Disclosures
Brien Holden Vision Institute has commercial interests in myopia control products. PS: P; RC: P; TJ: None; XC: None; RW: None; DT: None; PX: None; WL: None; ES: P; KE: P.
Acknowledgements
The authors acknowledge grant support from the Brien Holden Vision Institute. Some of the contact lenses used in the study were supplied by Sauflon Pharmaceuticals, United Kingdom. NB: Sauflon was acquired by CooperVision in August 2014.
Sankaridurg P, Bakaraju RC, Naduvilath T, Chen X, Weng R, Tilia D, Xu P, Li W, Conrad F, Smith EL III & Ehrmann K. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt 2019; 39: 294–307. 10.1111/opo.12621
Author contributions: All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis and writing or revision of the manuscript.
The copyright line for this article was changed on 27 June 2019 after original online publication.
References
- 1. Tideman JW, Snabel MC, Tedja MS et al Association of zxial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmol 2016; 134: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 2. Mitchell P, Hourihan F, Sandbach J & Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology 1999; 106: 2010–2015. [DOI] [PubMed] [Google Scholar]
- 3. Kuzin AA, Varma R, Reddy HS, Torres M & Azen SP. Ocular biometry and open‐angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology 2010; 117: 1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu L, Wang Y, Wang S, Wang Y & Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology 2007; 114: 216–220. [DOI] [PubMed] [Google Scholar]
- 5. Jonas JB, Weber P, Nagaoka N & Ohno‐Matsui K. Glaucoma in high myopia and parapapillary delta zone. PLoS One 2017; 12: e0175120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu HH, Xu L, Wang YX, Wang S, You QS & Jonas JB. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology 2010; 117: 1763–1768. [DOI] [PubMed] [Google Scholar]
- 7. Hsu WM, Cheng CY, Liu JH, Tsai SY & Chou P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology 2004; 111: 62–69. [DOI] [PubMed] [Google Scholar]
- 8. Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H & Kitazawa Y. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology 2006; 113: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 9. Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB & Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology 2004; 111: 53–61. [DOI] [PubMed] [Google Scholar]
- 10. Holden BA, Fricke TR, Wilson DA et al Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 11. Schaeffel F, Glasser A & Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res 1988; 28: 639–657. [DOI] [PubMed] [Google Scholar]
- 12. Smith EL 3rd, Hung LF & Harwerth RS. Effects of optically induced blur on the refractive status of young monkeys. Vision Res 1994; 34: 293–301. [DOI] [PubMed] [Google Scholar]
- 13. Diether S & Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res 1997; 37: 659–668. [DOI] [PubMed] [Google Scholar]
- 14. Chung K, Mohidin N & O'Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res 2002; 42: 2555–2559. [DOI] [PubMed] [Google Scholar]
- 15. Gwiazda J, Hyman L, Hussein M et al A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci 2003; 44: 1492–1500. [DOI] [PubMed] [Google Scholar]
- 16. Edwards MH, Li RW, Lam CS, Lew JK & Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci 2002; 43: 2852–2858. [PubMed] [Google Scholar]
- 17. Aller TA & Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clin Exp Optom 2008; 91: 394–399. [DOI] [PubMed] [Google Scholar]
- 18. Yang Z, Lan W, Ge J et al The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic Physiol Opt 2009; 29: 41–48. [DOI] [PubMed] [Google Scholar]
- 19. Fulk GW, Cyert LA & Parker DE. A randomized clinical trial of bifocal glasses for myopic children with esophoria: results after 54 months. Optometry 2002; 73: 470–476. [PubMed] [Google Scholar]
- 20. Cheng D, Woo GC, Drobe B & Schmid KL. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three‐year results of a randomized clinical trial. JAMA Ophthalmol 2014; 132: 258–264. [DOI] [PubMed] [Google Scholar]
- 21. Smith EL 3rd, Hung LF & Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res 2009; 49: 2386–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam CS, Tang WC, Tse DY, Tang YY & To CH. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2‐year randomised clinical trial. Br J Ophthalmol 2014; 98: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sankaridurg P, Donovan L, Varnas S et al Spectacle lenses designed to reduce progression of myopia: 12‐month results. Optom Vis Sci 2010; 87: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sankaridurg P, Holden B, Smith E 3rd et al Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one‐year results. Invest Opthalmol Vis Sci 2011; 52: 9362–9367. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y & Wildsoet C. The effect of two‐zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci 2011; 52: 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benavente‐Perez A, Nour A & Troilo D. Axial eye growth and refractive error development can be modified by exposing the peripheral retina to relative myopic or hyperopic defocus. Invest Ophthalmol Vis Sci 2014; 55: 6765–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anstice NS & Phillips JR. Effect of dual‐focus soft contact lens wear on axial myopia progression in children. Ophthalmology 2011; 118: 1152–1161. [DOI] [PubMed] [Google Scholar]
- 28. Walline JJ, Greiner KL, McVey ME & Jones‐Jordan LA. Multifocal contact lens myopia control. Optom Vis Sci 2013; 90: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 29. Ruiz‐Pomeda A, Perez‐Sanchez B, Valls I, Prieto‐Garrido FL, Gutierrez‐Ortega R & Villa‐Collar C. MiSight Assessment Study Spain (MASS). A 2‐year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 2018; 256: 1011–1021. [DOI] [PubMed] [Google Scholar]
- 30. Tilia D, Bakaraju RC, Chung J et al Short‐term visual performance of novel extended depth‐of‐focus contact lenses. Optom Vis Sci 2016; 93: 435–444. [DOI] [PubMed] [Google Scholar]
- 31. Bakaraju RC, Ehrmann K & Ho A. Extended depth of focus contact lenses vs. two commercial multifocals: Part 1. Optical performance evaluation via computed through‐focus retinal image quality metrics. J Optom 2018; 11: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL 3rd & Holden BA. Myopia progression rates in urban children wearing single‐vision spectacles. Optom Vis Sci 2012; 89: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng X, Xu J, Chehab K, Exford J & Brennan N. Soft Contact lenses with positive spherical aberration for myopia control. Optom Vis Sci 2016; 93: 353–366. [DOI] [PubMed] [Google Scholar]
- 34. Paune J, Morales H, Armengol J, Quevedo L, Faria‐Ribeiro M & Gonzalez‐Meijome JM. Myopia control with a novel peripheral gradient soft lens and orthokeratology: a 2‐year clinical trial. Biomed Res Int 2015; 2015: 507572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Back A, Chamberlain P, Logan N et al Clinical evaluation of a dual-focus myopia control 1 day soft contact lens - 2-year results. Optom Vis Sci 2016; 93: E-abstract 160035. [Google Scholar]
- 36. Cho P & Cheung SW. Retardation of myopia in orthokeratology (ROMIO) study: a 2‐year randomized clinical trial. Invest Ophthalmol Vis Sci 2012; 53: 7077–7085. [DOI] [PubMed] [Google Scholar]
- 37. Hiraoka T, Kakita T, Okamoto F, Takahashi H & Oshika T. Long‐term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5‐year follow‐up study. Invest Ophthalmol Vis Sci 2012; 53: 3913–3919. [DOI] [PubMed] [Google Scholar]
- 38. Benavente‐Perez A, Nour A & Troilo D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Invest Ophthalmol Vis Sci 2012; 53: 6479–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]


