Abstract
Dapagliflozin is associated with greater reductions in HbA1c and weight than saxagliptin in management of type 2 diabetes mellitus (T2DM). The present post hoc analyses compared the durability of these effects over short‐ and long‐term follow‐up in patients with T2DM who were inadequately controlled with metformin (≥1500 mg/day) and who were receiving either dapagliflozin (10 mg/day) or saxagliptin (5 mg/day). Failure of glycaemiccontrol was assessed using the slope of the change in HbA1c from baseline‐over‐time regression line (coefficient of failure [CoF]). CoF was compared directly (dapagliflozin vs saxagliptin) over the short term (NCT01606007, 24 weeks) and indirectly (placebo‐adjusted) over the long term (NCT00528879 and NCT00121667, 102 weeks). A low CoF value indicated greater durability. CoF was lower for dapagliflozin versus saxagliptin over 18–24 weeks (−1.38%/year; 95% CI, −2.41 to −0.35; P = .009) and 20–102 weeks (−0.37%/year; 95% CI, −0.73 to −0.02; P = .04). Fewer dapagliflozin‐treated patients versus saxagliptin‐treated patients required rescue medication or discontinued the study because of failure to achieve glycaemic control at 24 weeks (3.4% vs 9.4%; P = .0191). In patients with T2DM who were inadequately controlled with metformin, dapagliflozin was associated with greater durability of glycaemic control than saxagliptin over 18–24 and 20–102 weeks.
Keywords: coefficient of failure, CoF, dapagliflozin, DPP4 inhibitor, durability, glycaemic control, saxagliptin, SGLT2 inhibitor, T2DM, type 2 diabetes mellitus
1. INTRODUCTION
Although metformin is recommended as a first‐line oral antihyperglycaemic drug, sodium‐glucose cotransporter‐2 (SGLT2) inhibitors and dipeptidyl peptidase‐4 (DPP4) inhibitors offer additional glycaemic control and weight control, with minimal risk of hypoglycaemia.1, 2 Dapagliflozin, an SGLT2 inhibitor, exhibits greater glycaemic efficacy than saxagliptin, a DPP4 inhibitor, with additional reductions in weight and systolic blood pressure.3, 4 Persistence of the benefits of dapagliflozin over saxagliptin may affect the choice of therapy. To date, no head‐to‐head trials have evaluated the durability of control of HbA1c, fasting plasma glucose (FPG), SBP, and body weight with an SGLT2 inhibitor as compared to a DPP4 inhibitor. We reviewed data from three phase 3 randomized trials of dapagliflozin versus saxagliptin in patients with type 2 diabetes mellitus (T2DM) (NCT01606007, NCT00528879, NCT00121667) and compared the durability of these effects over short‐ and long‐term follow‐up using coefficient of failure (CoF) methodology.3, 5, 6
2. METHODS
2.1. Short term: direct statistical comparison of dapagliflozin and saxagliptin
Patients with HbA1c between 8% and 12%, inclusive (64–108 mmol/mol), who were receiving a stable dose of metformin (≥1500 mg/day) were randomized to receive either dapagliflozin (10 mg/day) or saxagliptin (5 mg/day) as add‐on therapy.3 Patients with inadequate glycaemic control from Weeks 6 to 24 were eligible to receive open‐label rescue medication (details of rescue criteria in Table S1). Patients were included in the CoF analysis if there was at least one change in HbA1c from baseline (ΔHbA1c) value at Week 18 and a repeat value between Week 18 and Week 24 available for them in both the dapagliflozin and saxagliptin groups. Patients with uncontrolled hypertension (SBP ≥160 mm Hg and diastolic blood pressure ≥100 mm Hg), FPG of at least 270 mg/dL during the 4‐week lead‐in period, cardiovascular disease within 3 months of screening, congestive heart failure (New York Heart Association [NYHA] functional class IV), estimated glomerular filtration rate less than 60 mL/min/1.73 m2 or serum creatinine of at least 1.5 mg/dL in men and 1.4 mg/dL in women, or significant hepatic disease were excluded. The endpoints assessed included: (a) number of patients with HbA1c above 9% (75 mmol/mol) at Week 24 or who received rescue medication because of failure to achieve pre‐specified glycaemic targets or who discontinued treatment because of lack of glycaemic control, and (b) time to rescue medication usage or study discontinuation because of failure to achieve glycaemic targets up to 24 weeks of treatment. Durability of glycaemic control was assessed as CoF for ΔHbA1c, based on an earlier study.7 CoF was measured using the slope from a random coefficient model of ΔHbA1c over Weeks 18–24, excluding data following rescue medication usage.
2.2. Long term: indirect statistical comparison of dapagliflozin and saxagliptin
Patients receiving either dapagliflozin (10 mg/day) or saxagliptin (5 mg/day) or corresponding placebo plus a stable dose of metformin (≥1500 mg/day) for more than 8 weeks, with HbA1c between 7% and 10%, inclusive (53–86 mmol/mol), C‐peptide greater than 1.0 ng/mL, body mass index of 45 kg/m2 or less for dapagliflozin or 40 kg/m2 or less for saxagliptin, and serum creatinine of 1.5 mg/dL or less in men and 1.4 mg/dL or less in women were assessed. Metformin up‐titration was not permitted in either study. Patients with a lack of glycaemic control from Week 4 to Week 102 were eligible to receive open‐label rescue medication. Rescue criteria were similar in both studies (Table S1). Data concerning patients who received rescue medication prior to Week 20 and those who received it after Week 20 were excluded from CoF analysis. Data proximal to initiation of treatment, prior to Week 20, were also excluded to allow time for glycation of haemoglobin under treatment. There were at least three ΔHbA1c observed values at three different visits available for the patients included in the CoF analysis for the long‐term study of dapagliflozin and saxagliptin (Weeks 20–102). Patients were excluded if they had symptoms of poorly controlled diabetes or a history of diabetic ketoacidosis or hyperosmolar nonketotic coma; if they had used any other antihyperglycaemic medication 8 weeks prior to the study or insulin 1 year prior to the study; if they experienced a cardiovascular event within 6 months of study entry or NYHA stage III/IV congestive heart failure and/or had a known left ventricular ejection fraction of 40% or less; if they underwent chronic or repeated intermittent corticosteroid treatment or treatment with potent systemic cytochrome P450 3A4 inhibitors or inducers; if they had active liver disease and/or clinically significant abnormalities on screening tests of hepatic, renal, endocrine, metabolic or haematologic function; or if they had received assessment of an immunocompromised state. The endpoints assessed were: (a) time to study discontinuation (TTSD) because of lack of glycaemic control or rescue medication usage because of failure to achieve the glycaemic target up to 102 weeks, and (b) durability of glycaemic control between Weeks 20 and 102, with assessed differences in the placebo‐adjusted dapagliflozin and placebo‐adjusted saxagliptin slopes obtained from random coefficient models of ΔHbA1c over Weeks 20 to 102, excluding data following rescue medication usage. Exploratory endpoints included changes in FPG, SBP, and body weight in the 20‐ to 102‐week CoF population.
2.3. Safety
Safety parameters for all three studies3, 5, 6 have been reported previously. Adverse events (AEs), serious AEs, and AEs of special interest, including hypoglycaemia, genital infections, and urinary tract infections for studies NCT00528879 and NCT00121667 are summarised herein.
2.4. Statistical analyses
Patients in both the short‐ and long‐term analyses were categorized into two sets: overall (all randomized patients) and CoF (patients included in the CoF analysis). TTSD because of lack of glycaemic control or rescue medication usage because of failure to achieve pre‐specified glycaemic targets3, 5, 6 was assessed using the Kaplan–Meier method. Indirect statistical comparisons (long‐term analysis) were performed using area‐under‐the‐curve estimates for the unweighted difference in TTSD because of lack of glycaemic control or rescue medication usage during the periods 20–102 weeks and 96–102 weeks. Direct statistical comparison of the CoF was performed for HbA1c between the dapagliflozin and saxagliptin groups (short‐term analysis). Placebo‐adjusted changes from baseline in HbA1c, FPG, SBP, and body weight were assessed using random‐coefficient‐models analysis with terms for baseline value, treatment, and treatment by time, with random subject effects of time and intercept (long‐term analysis). Indirect statistical comparison of the placebo‐adjusted dapagliflozin versus the placebo‐adjusted saxagliptin groups (Weeks 20–102) was performed for the following variables: HbA1c, FPG, SBP, and body weight using Review Manager (RevMan) Version 5.3. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).8
3. RESULTS
Demographic and baseline characteristics of dapagliflozin‐ and saxagliptin‐treated patients were similar in both the short‐ and long‐term analysis populations (Table S2).
Dapagliflozin‐treated patients showed 6.0% (95% CI, 1.0, 11.0; P < .05) fewer discontinuations because of inadequate glycaemic control or instances of rescue medication usage because of failure to achieve glycaemic targets as compared to saxagliptin‐treated patients over 24 weeks. The difference in discontinuations increased to 12.3% (95% CI, 4.5, 20.0; P < .01) when HbA1c greater than 9% (75 mmol/mol) at 24 weeks was added to the above criteria. Dapagliflozin delayed TTSD or rescue medication usage because of failure to achieve pre‐specified glycaemic targets up to Week 24 as compared to saxagliptin (Figure 1A). Importantly, dapagliflozin‐treated patients experienced a significantly greater improvement in HbA1c over Weeks 18–24 as compared to saxagliptin‐treated patients (Figure 1B). Mean (standard deviation) CoF was −0.93% (0.37)/year in dapagliflozin‐treated patients as compared to 0.45% (0.38)/year in saxagliptin‐treated patients (Figure 1B). The difference in CoF estimates was −1.38%/year (0.53) (95% CI, −2.41 to −0.35, P = .009).
Figure 1.
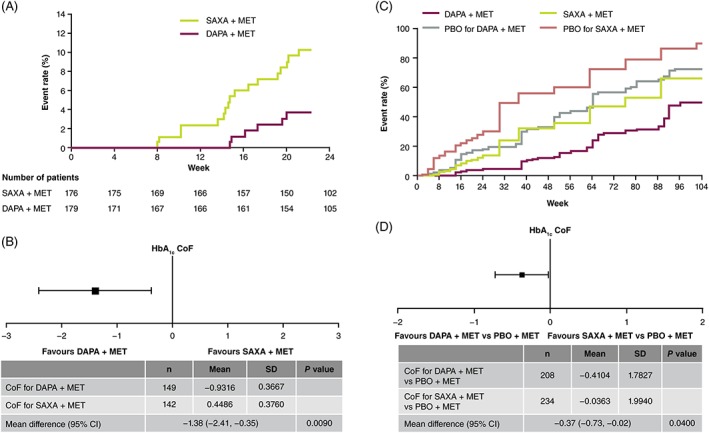
A, Time to study discontinuation because of lack of glycaemic control or requirement for rescue medication through Week 24; B, forest plot showing direct comparison of HbA1c CoF for DAPA vs SAXA (Weeks 18–24); C, time to study discontinuation because of lack of glycaemic control or requirement for rescue medication for DAPA + MET vs SAXA + MET through Week 104; D, forest plot showing placebo‐adjusted indirect comparison of 20‐week to 102‐week HbA1c CoF for DAPA + MET vs SAXA + MET. CoF, coefficient of failure; DAPA, dapagliflozin; MET, metformin; PBO, placebo; SAXA, saxagliptin; SD, standard deviation
Dapagliflozin‐treated patients had 3% fewer discontinuations or rescue medication usage as compared to saxagliptin‐treated patients over 20–102 weeks (Figure 1C). When CoF data analysis was restricted to Weeks 96–102, discontinuation or rescue medication usage was reported 6% more often (unweighted difference) in saxagliptin‐treated patients as compared to dapagliflozin‐treated patients. Dapagliflozin‐treated patients experienced a significantly greater improvement in HbA1c over Weeks 20–102 as compared to patients who received placebo. Mean (standard error) CoF was 0.15% (0.08)/year in dapagliflozin‐treated patients as compared to 0.56% (0.09)/year in patients who received placebo. Mean (standard error) CoF was 0.46% (0.08)/year in saxagliptin‐treated patients as compared to 0.50% (0.10)/year in patients who received placebo. Indirect statistical comparison revealed a significant negative difference in HbA1c in placebo‐adjusted CoF with dapagliflozin versus saxagliptin (mean difference [95% CI], −0.37 [−0.73, −0.02]; P = .04) (Figure 1D). Indirect statistical comparison indicated a relatively greater reduction in HbA1c with dapagliflozin versus saxagliptin of 0.37%/year, representing glycaemic control over Weeks 20–102.
Dapagliflozin‐treated patients exhibited significantly greater maintenance of FPG, SBP, and body weight over Weeks 20–102 compared with saxagliptin‐treated patients (Figure 2A–C). The positive effects of dapagliflozin and saxagliptin on SBP were maintained throughout the study (Figure 2B).
Figure 2.
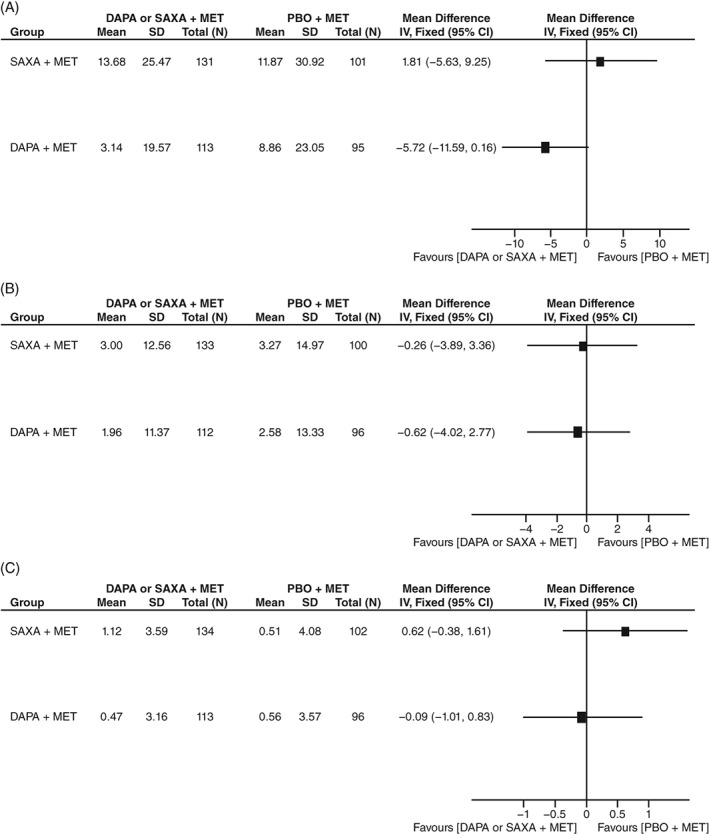
Forest plots showing placebo‐adjusted indirect comparisons of 20‐week to 102‐week CoF for DAPA + MET vs SAXA + MET with respect to A, FPG, B, SBP and C, body weight. CoF, coefficient of failure; DAPA, dapagliflozin; FPG, fasting plasma glucose; IV: instrumental variable; MET, metformin; PBO: placebo; SAXA, saxagliptin; SBP, systolic blood pressure; SD, standard deviation
Concerning safety, both dapagliflozin and saxagliptin were generally well tolerated among patients (Table S3). Incidences of hypoglycaemia were low in both dapagliflozin‐ and saxagliptin‐treated patients, although the rates of genital and urinary tract infections were higher in dapagliflozin‐treated patients than in saxagliptin‐treated patients.
4. DISCUSSION
The durability of the beneficial effects of antihyperglycaemic drugs is important to sustain glycaemic control and counter the chronic progressive course of T2DM. In the present post hoc analysis, the TTSD or requirement for rescue medication was delayed by approximately 6 weeks in dapagliflozin‐treated patients as compared to saxagliptin‐treated patients over 24 weeks. In addition, dapagliflozin‐treated patients showed a greater ability to sustain glycaemic control than saxagliptin‐treated patients over 24 weeks. Although the 18‐week to 24‐week HbA1c CoF for dapagliflozin was significantly lower than that for saxagliptin, longer durability of the glucose‐lowering effect of dapagliflozin as compared to that of saxagliptin was attenuated, although still corroborated, in a Week 20 to Week 102 interval (indirect comparison–based CoF analysis).
Consistent with the β‐cell–independent mechanism of action of dapagliflozin, lower CoF was observed with dapagliflozin than with saxagliptin where efficacy may be limited by reduced β‐cell capacity, especially in patients with higher HbA1c.9, 10, 11 The greater durability of HbA1c control with dapagliflozin versus saxagliptin over 20–102 weeks is consistent with a recent meta‐analysis that showed that SGLT2 inhibitors achieved greater efficacy for glycaemic control than DPP4 inhibitors up to 52 weeks.12 Long‐term dapagliflozin treatment in patients with T2DM has achieved glucose‐lowering efficacy and associated reductions in blood pressure and body weight across a broad spectrum of patients, including those with cardiovascular and renal comorbidities.13 Thus, our findings support the long‐term efficacy and safety profile of dapagliflozin previously reported, as monotherapy or as an adjunct to metformin in patients with inadequately controlled T2DM.
The similar maintenance of FPG, SBP, and body weight from Weeks 20–102 in indirect (CoF) comparison of dapagliflozin and saxagliptin reflects the fact that most of the changes in these parameters occurred in the first 20 weeks from treatment initiation. A recent post hoc analysis4 of the present short‐term trial3 showed that dapagliflozin significantly reduced the FPG, SBP, and body weight as compared to saxagliptin over 24 weeks.
Rates of hypoglycaemia with either dapagliflozin or saxagliptin were low and comparable to the rates observed with placebo; however, in the long‐term analysis, numerically higher rates of both genital and urinary tract infections were noted in dapagliflozin‐treated patients as compared to saxagliptin‐treated patients, which is consistent with dapagliflozin's mechanism of action and safety profile.14
A strength of the CoF method is that it allows for use of all collected data, without need for an arbitrary threshold.15 In addition, the CoF method used reflects both improvement and deterioration of glycaemia.15 Regarding limitations, the short‐term study may not allow sufficient time to adequately assess long‐term durability of the efficacy of dapagliflozin as compared to saxagliptin. Moreover, indirect comparisons, while allowing for assessment of long‐term relative efficacy, are associated with the potential for greater interference of modifying factors such as intercurrent illness and changes in other medications.16 Additionally, the CoF method relies on “regression slopes” and does not allow evaluation of conditions of maintained efficacy unaccompanied by incremental worsening or improvement over time. Alternative methods must be considered in such instances. Finally, in this short‐term study, baseline HbA1c of patients was required to be at least 8.0% (64 mmol/mol) and less than 12.0%, inclusive (108 mmol/mol) and, therefore, our analysis may not apply to patients with baseline HbA1c less than 8.0% (64 mmol/mol).
In conclusion, dapagliflozin offered greater durability of glucose control than saxagliptin, in both short‐term (head‐to‐head comparison) and long‐term (indirect comparison) analyses. Consistent with lower CoF, dapagliflozin‐treated patients had fewer discontinuations or requirements for rescue medication than saxagliptin‐treated patients in both short‐term and long‐term analyses.
ACKKNOWLEDGMENTS
The data were previously presented in abstract form at the American Diabetes Association 78th Scientific Sessions, June 22–26, 2018, Orlando, Florida.
Medical writing assistance was provided by Varunkumar Pandey, PhD, with some editorial assistance from Steven Tresker, both from Cactus Communications.
CONFLICT OF INTEREST
C. J. B. has been involved in several AstraZeneca‐sponsored studies with dapagliflozin and reports remuneration for participation in research, advisory boards, travel, and educational presentations supported by AstraZeneca. C. W., D. R. and G. S. are current employees of AstraZeneca. S. D. P. has served on advisory panels for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Co., GlaxoSmithKline, Merck & Co., Novartis Pharmaceuticals, Novo Nordisk, Sanofi, Laboratoires Servier, and Takeda Pharmaceuticals, and has received research support from AstraZeneca, Boehringer Ingelheim, Merck & Co., and Novartis Pharmaceuticals.
AUTHOR CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, had full access to the data in this study, take complete responsibility for the integrity of the data and accuracy of the data analysis, and give final approval for the version to be published. C. J. B. is the guarantor who takes full responsibility for the work as a whole, including the decision to submit and publish the manuscript. All authors contributed to the review and editing of the manuscript. S. D. P. contributed to the data analysis plan. D. R. contributed to the study design and conception, statistical analysis, and the summary and description of results.
Supporting information
Table S1. Key details of the studies included in the analyses; short‐term (24‐week) study (NCT01606007) direct statistical comparison.
Table S2. Rescue medication criteria.
Table S3. Baseline characteristics and demographics of patients included in the CoF short‐term direct analysis (weeks 18–24).
Table S4. Baseline characteristics and demographics of patients included in the long‐term CoF analysis (weeks 20–102).
Table S5. Summary of safety data reported in the long‐term studies used for the indirect analysis.
Bailey CJ, Del Prato S, Wei C, Reyner D, Saraiva G. Durability of glycaemic control with dapagliflozin, an SGLT2 inhibitor, compared with saxagliptin, a DPP4 inhibitor, in patients with inadequately controlled type 2 diabetes. Diabetes Obes Metab. 2019;21:2564–2569. 10.1111/dom.13841
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13841.
Funding information This study was funded by AstraZeneca Pharmaceuticals LP.
REFERENCES
- 1. Ferrannini E, Berk A, Hantel S, et al. Long‐term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active‐controlled, parallel‐group, randomized, 78‐week open‐label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015‐4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care. 2013;36:2508‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenstock J, Hansen L, Zee P, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376‐383. [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock J, Mathieu C, Chen H, Garcia‐Sanchez R, Saraiva GL. Dapagliflozin versus saxagliptin as add‐on therapy in patients with type 2 diabetes inadequately controlled with metformin. Arch Endocrinol Metab. 2018;62:424‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med. 2013;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeFronzo RA, Hissa MN, Garber AJ, et al; Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Prato S, Foley JE, Kothny W, et al. Study to determine the durability of glycaemic control with early treatment with a vildagliptin‐metformin combination regimen vs. standard‐of‐care metformin monotherapy‐the VERIFY trial: a randomized double‐blind trial. Diabet Med. 2014;31:1178‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Indirect comparisons methods and validity. Summary report. 2009. Haute Autorité de Santé. https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-02/summary_report__indirect_comparisons_methods_and_validity_january_2011_2.pdf. Accessed February 5, 2018.
- 9. Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513‐519. [DOI] [PubMed] [Google Scholar]
- 10. Godinho R, Mega C, Teixeira‐de‐Lemos E, et al. The place of dipeptidyl peptidase‐4 inhibitors in type 2 diabetes therapeutics: a "Me Too" or "the Special One" antidiabetic class? J Diabetes Res. 2015;2015:806979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deacon CF. Dipeptidyl peptidase‐4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13:7‐18. [DOI] [PubMed] [Google Scholar]
- 12. Monami M, Liistro F, Scatena A, Nreu B, Mannucci E. Short and medium‐term efficacy of sodium glucose co‐transporter‐2 (SGLT‐2) inhibitors: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20:1213‐1222. [DOI] [PubMed] [Google Scholar]
- 13. Jabbour S. Durability of response to dapagliflozin: a review of long‐term efficacy and safety. Curr Med Res Opin. 2017;33:1685‐1696. [DOI] [PubMed] [Google Scholar]
- 14.Farxiga (dapagliflozin) [package insert]. 2014. Princeton, NJ: Bristol‐Myers Squibb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s000lbl.pdf.
- 15. Wallace TM, Matthews DR. Coefficient of failure: a methodology for examining longitudinal beta‐cell function in type 2 diabetes. Diabet Med. 2002;19:465‐469. [DOI] [PubMed] [Google Scholar]
- 16. Kim H, Gurrin L, Ademi Z, Liew D. Overview of methods for comparing the efficacies of drugs in the absence of head‐to‐head clinical trial data. Br J Clin Pharmacol. 2014;77:116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Key details of the studies included in the analyses; short‐term (24‐week) study (NCT01606007) direct statistical comparison.
Table S2. Rescue medication criteria.
Table S3. Baseline characteristics and demographics of patients included in the CoF short‐term direct analysis (weeks 18–24).
Table S4. Baseline characteristics and demographics of patients included in the long‐term CoF analysis (weeks 20–102).
Table S5. Summary of safety data reported in the long‐term studies used for the indirect analysis.


