Abstract
Objectives
The clinical utility of conventional IV opioids is limited by the occurrence of opioid‐related adverse events. Oliceridine is a novel G protein–biased μ‐opioid receptor agonist designed to provide analgesia with an improved safety and tolerability profile. This phase III, double‐blind, randomized trial (APOLLO‐2 [NCT02820324]) evaluated the efficacy and safety of oliceridine for acute pain following abdominoplasty.
Methods
Patients received a loading dose of either placebo, oliceridine (1.5 mg), or morphine (4 mg), followed by demand doses via patient‐controlled analgesia (0.1, 0.35, or 0.5 mg oliceridine; 1 mg morphine; or placebo) with a 6‐minute lockout interval. The primary endpoint was the proportion of treatment responders over 24 hours for oliceridine regimens compared to placebo. Secondary outcomes included a predefined composite measure of respiratory safety burden (RSB, representing the cumulative duration of respiratory safety events) and the proportion of treatment responders vs. morphine.
Results
A total of 401 patients were treated with study medication. Effective analgesia was observed for all oliceridine regimens, with responder rates of 61.0%, 76.3%, and 70.0% for the 0.1‐, 0.35‐, and 0.5‐mg regimens, respectively, compared with 45.7% for placebo (all P < 0.05) and 78.3% for morphine. Oliceridine 0.35‐ and 0.5‐mg demand dose regimens were equi‐analgesic to morphine using a noninferiority analysis. RSB showed a dose‐dependent increase across oliceridine regimens (mean hours [standard deviation], 0.1 mg: 0.43 [1.56]; 0.35 mg: 1.48 [3.83]; 0.5 mg: 1.59 [4.26]; all comparisons not significant at P > 0.05 vs. placebo: 0.60 [2.82]). The RSB measure for morphine was 1.72 (3.86) (P < 0.05 vs. placebo). Gastrointestinal adverse events increased in a dose‐dependent manner across oliceridine demand dose regimens (0.1 mg: 49.4%; 0.35 mg: 65.8%; 0.5 mg: 78.8%; vs. placebo: 47.0%; and morphine: 79.3%). In comparison to morphine, the proportion of patients experiencing nausea or vomiting was lower with the 2 equi‐analgesic dose regimens of 0.35 and 0.5 mg oliceridine.
Conclusions
Oliceridine is a safe and effective IV analgesic for the relief of moderate to severe acute postoperative pain in patients undergoing abdominoplasty. Since the low‐dose regimen of 0.1 mg oliceridine was superior to placebo but not as effective as the morphine regimen, safety comparisons to morphine are relevant only to the 2 equi‐analgesic dose groups of 0.35 and 0.5 mg, which showed a favorable safety and tolerability profile regarding respiratory and gastrointestinal adverse effects compared to morphine. These findings support that oliceridine may provide a new treatment option for patients with moderate to severe acute pain where an IV opioid is warranted.
Keywords: postoperative, analgesia, patient controlled, clinical trial, abdominoplasty
Introduction
Among the 1.8 million cosmetic surgeries performed in the United States in 2017, abdominoplasty was the fifth most common procedure.1 Post‐procedural pain following abdominoplasty remains a major concern for both surgeons and patients and when adequately managed can lead to earlier mobilization, early discharge, a shorter hospital stay, reduced hospital costs, quicker return to normal activities, and improved patient satisfaction.2, 3, 4 Traditional management of postoperative pain has utilized conventional opioids in the intra/perioperative setting.5 Although these agents provide satisfactory relief of moderate to severe pain, they are also associated with undesirable side effects, including respiratory depression, sedation, nausea, vomiting, constipation, and the risk for dependence.6 When present, these complications can result in negative clinical outcomes, including increased morbidity and mortality, delayed recovery and discharge, and higher overall treatment costs.7, 8, 9, 10, 11 In an attempt to reduce opioid misuse and abuse, clinical guidelines recommend the use of multimodal analgesia involving the adjunctive use of non‐opioid analgesics and reduction in the dose and duration of opioid use.12 While these approaches have been an important clinical advance in care, they have not eliminated the need for IV opioids in all cases.
Morphine and other conventional opioids produce analgesia through their action on centrally located μ‐opioid receptors. Receptor binding leads to a cascade of post‐receptor signaling events, including engagement of G proteins, which are thought to be the principal pathway leading to the analgesic effects of opioids. Similarly, recruitment of post‐receptor β‐arrestin proteins is thought to restrain G protein signaling and to play an important role in the development of opioid‐related adverse events (ORAEs).13, 14, 15, 16, 17, 18, 19 Circumstantial evidence in support of this can be seen from experiments in β‐arrestin knockout mice that display enhanced analgesia and fewer respiratory and gastrointestinal ORAEs under morphine treatment compared to wild‐type mice.17, 20 These findings have prompted an interest in developing μ‐opioid receptor ligands that preferentially engage the G protein pathway with minimal to no recruitment of β‐arrestin signaling.19
Oliceridine (Trevena Inc., Chesterbrook, PA, U.S.A.), an investigational compound, is a novel μ‐opioid receptor ligand that is a full agonist for G protein activation at the μ‐opioid receptor but with markedly reduced β‐arrestin recruitment compared with conventional opioids.18, 21 Preclinical rodent models showed that at equi‐analgesic doses, oliceridine is a potent analgesic, but with less gastrointestinal dysfunction and respiratory suppression than morphine.18 Initial human studies demonstrated similar effects, with oliceridine producing less suppression of hypercarbic‐induced increases in respiratory drive compared to equi‐analgesic and supra‐analgesic doses of morphine in healthy participants.19 Subsequently, single‐center phase II studies in patients undergoing bunionectomy or abdominoplasty showed that as‐needed (PRN) oliceridine regimens provided an analgesic efficacy similar to that of morphine22, 23 but with a clinically notable reduction in the incidence of respiratory and gastrointestinal adverse effects.23 To extend and confirm the phase II study findings, a large multicenter phase III, randomized, controlled APOLLO‐2 trial, which compared IV oliceridine to placebo for the treatment of moderate to severe acute pain following abdominoplasty, was conducted. Morphine (IV) was included in the study design as an active comparator. Based on previous findings from phase II studies, we hypothesized that oliceridine would provide rapid and superior acute postoperative analgesia compared to placebo with a more favorable safety and tolerability profile than morphine. The findings of this study are presented here.
Methods
Study Design
APOLLO‐2 (NCT02820324) was a phase III, multicenter, randomized, double‐blind, placebo‐ and active‐controlled study conducted at 5 sites in the United States among patients recruited for elective abdominoplasty surgery. Each study location was a clinical research facility associated with a surgical unit. Patients were enrolled between May 2016 and January 2017. The study was conducted in compliance with the Declaration of Helsinki and the International Council on Harmonisation Good Clinical Practice Guidelines. The trial protocol was approved by a centralized institutional review board through Advarra®, and all patients provided written informed consent before any study procedures were performed.
Study Participants
Patients were screened for eligibility within 35 days prior to the date of surgery. Eligible patients were 18 to 75 years of age at screening and recruited to undergo an abdominoplasty procedure with no additional collateral procedures. Patients who reported moderate or severe pain within 4 hours after surgery as reported on a categorical scale (none, mild, moderate, or severe) and who rated their pain with a score of ≥5 on a numeric rating scale with a range of 0 = no pain to 10 = worst pain imaginable were eligible for randomization.
Exclusion criteria were body mass index (BMI) > 35 kg/m2 or body weight < 40 kg; women who were pregnant or breastfeeding; history of opioid hypersensitivity; diagnosis of sleep apnea; use of chronic opioid therapy (defined as >15 morphine equivalent units/day for >3 days/week and for >1 month within 1 year of surgery); use of any analgesic medication within 5 half‐lives (or 48 hours if unknown) before surgery; chronic nonsteroidal anti‐inflammatory drug therapy (daily use for >2 weeks within 6 months before surgery with the exception of aspirin ≤ 325 mg daily for cardiovascular prophylaxis if the patient was on a stable regimen for ≥30 days); use of agents that could affect analgesic response (central α‐adrenergic agents, antiepileptics, neuroleptics, antidepressants, antipsychotics) that had not been stably dosed for ≥30 days prior to surgery; use of oral or parenteral corticosteroids within 3 months before surgery; ECG abnormalities (QTcF interval of >450 milliseconds in males and >470 milliseconds in females at screening); or hepatic or renal impairment at screening.
Additional postoperative exclusion criteria included surgical duration of >2.5 hours, evidence of hemodynamic instability or respiratory insufficiency, or surgical/anesthetic complications or protocol violations that could confound interpretation of study data.
All patients were graded with a simplified Apfel risk score24 derived as a value from 1 to 4 based on medical history questions including female sex, nonsmoking status, prior history of motion sickness or postoperative nausea or vomiting, and planned postoperative opioid use.
Concomitant Medications
All patients underwent a semi‐standardized anesthetic protocol, whereby general anesthesia was achieved using fentanyl and propofol, with or without volatile anesthetics or muscle relaxants. Opioids other than fentanyl, intra‐ or postoperative steroids, nonsteroidal anti‐inflammatory drugs, IV acetaminophen, epidural or intrathecal agents, regional or neuraxial anesthesia, long‐acting local anesthetics, or prophylactic antiemetics were prohibited. Analgesia could have been maintained during the immediate postoperative period, if needed, with fentanyl 25‐μg IV boluses. Patients could not initiate the randomized study treatment phase until at least 20 minutes after the last dose of fentanyl.
Prophylactic antiemetics were not permitted perioperatively or during the randomized treatment period. In addition, prophylactic supplemental oxygen was not permitted during the randomized treatment period.
Randomization and Treatment
Patients meeting all eligibility criteria were randomized in equal ratios to double‐blind IV treatment demand dose regimens (placebo, oliceridine 0.1 mg, oliceridine 0.35 mg, oliceridine 0.5 mg, or morphine 1 mg; Table 1). For each regimen, a clinician‐administered IV fixed loading dose (oliceridine 1.5 mg, morphine 4 mg, or volume‐matched placebo) was followed by demand doses administered via a patient‐controlled analgesia (PCA) device and clinician‐administered, blinded supplemental doses. PCA doses were allowed from 10 minutes after the loading dose and limited by a 6‐minute lockout interval. Clinician‐administered IV supplemental doses (oliceridine 0.75 mg and morphine 2 mg) were permitted as often as hourly (PRN). Demand dose regimens for oliceridine were selected based on prior evidence of efficacy in earlier clinical studies.22, 23 Morphine dosing was based on standard clinical guidelines for use.25
Table 1.
Treatment Regimens
| Treatment Regimen | Clinician Administered Loading Dose | Demand Dose Via PCA | Clinician Administered Supplemental Dose |
|---|---|---|---|
| Placebo | Volume‐matched | Volume‐matched | Volume‐matched |
| Oliceridine 0.1 mg regimen | 1.5 mg | 0.1 mg | 0.75 mg |
| Oliceridine 0.35 mg regimen | 1.5 mg | 0.35 mg | 0.75 mg |
| Oliceridine 0.5 mg regimen | 1.5 mg | 0.5 mg | 0.75 mg |
| Morphine 1 mg regimen | 4 mg | 1 mg | 2 mg |
Clinician administered supplemental dosing could start 1 hour after the loading dose and be administered up to hourly, as needed. Further open‐label rescue pain medication (oral etodolac 200 mg every 6 hours) was permitted, as needed. PCA had a 6‐minute lockout.
PCA, patient‐controlled analgesia device.
The dosing limit for all oliceridine treatment groups was 60 mg in the first 12 hours, defined as 3 PCA syringes or 6 clinician‐administered supplemental PRN doses within the first 12 hours. If patients reached their dosing limit, they were discontinued from the study treatment and managed by conventional, clinician‐determined analgesic treatment; however, they continued in the study to provide efficacy and safety assessments per protocol and were included in the final data analysis.
In the event that the study medication, including both demand and supplemental doses, was inadequate, patients could receive protocol‐specified open‐label rescue pain medication (etodolac 200 mg every 6 hours, PRN) if they reported a score ≥ 4 on the pain numeric rating scale (NRS). Patients receiving rescue pain medication continued to be treated with study medication PRN. However, if study medication and rescue pain medication were inadequate, the patient was discontinued from study medication and was managed conventionally with clinician‐determined analgesic treatment.
Primary Endpoint
The primary efficacy endpoint was the proportion of patients who responded to study medication compared to placebo at the end of the randomized 24‐hour treatment period, conforming to the guidance provided by the U.S. Food and Drug Administration on analgesic drug development.26 Response was defined as being present if all of the following criteria were met: (1) at least a 30% improvement in time‐weighted sum of pain intensity difference (SPID) from baseline at 24 hours, (2) no use of protocol‐specified rescue pain medicine, (3) no early discontinuation of study medication for any reason, and (4) did not reach protocol‐specified study medication dosing limit. This composite endpoint was selected with the intention to more clearly depict efficacy that is achieved with only use of the study medication and without detrimental impact on tolerability.
Key Secondary Endpoints
Two key secondary objectives were prespecified in the protocol. The first was the magnitude of respiratory safety burden (RSB) among patients in each treatment regimen. RSB was a novel composite measure reflecting the cumulative duration of clinically meaningful respiratory compromise. RSB was calculated as the mathematical product of the incidence of a defined set of observed respiratory safety events multiplied by the mean expected cumulative duration of these events. The final RSB endpoint was therefore reported in hours. A certified registered nurse anesthetist or anesthesiologist, blinded to study medication allocation, monitored patients during the randomized treatment period according to a protocol‐defined schedule. The clinician intervened when indicated (eg, by administering supplemental oxygen or ordering a dosing interruption) and thereby determined the onset and resolution times of each respiratory safety event. The monitoring clinician combined clinical acumen with bedside observations to identify a respiratory safety event. A respiratory safety event was prespecified among any of the following events reflecting a worsening of respiratory status: changes in respiratory rate, the presence of clinically significant oxygen desaturation, a change in the level of sedation, measured using the Moline‐Roberts Pharmacologic Sedation Scale,27 and changes in the level of end‐tidal carbon dioxide.
The other key secondary endpoint was an efficacy comparison of the proportion of treatment responders in each of the oliceridine regimens to patients receiving morphine. The proportion of treatment responders in each oliceridine regimen compared to those in the morphine regimen used the same responder criteria as was used to calculate the primary endpoint and included both noninferiority and superiority analyses.
Other Secondary Endpoints
Other secondary outcome measures to provide confirmatory evidence of the clinical significance of the primary efficacy endpoint were obtained, including pain intensity over time, the proportion of treatment responders over time, the cumulative response by time point, and the magnitude and time to self‐reported pain relief measured using perceptible and meaningful pain relief with the 2‐stopwatch method.28 In addition, the percentage of patients receiving rescue pain medication over time, and the time to first use of rescue pain medication, were measured to characterize the sufficiency of the analgesic effect. Finally, clinician‐ and patient‐reported satisfaction were also obtained.
Safety and Tolerability Assessment
Overall safety and tolerability of all treatment regimens was assessed using the incidence of treatment‐emergent adverse events (AEs) reported during the treatment period and 7‐day follow‐up period. All AEs were spontaneously reported, and verbatim terms were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 19.0.29 The severity and causal relationship to the study drug for each AE was determined by the treating clinician. Clinical laboratory assessments, vital sign measurements, and an ECG were collected at baseline and following treatment.
Additional secondary safety and tolerability analyses included the proportion of patients reporting gastrointestinal AEs, and the use of rescue antiemetics.
Statistical Analysis
The primary efficacy endpoint (oliceridine vs. placebo) was analyzed using a logistic regression model that contained treatment group as a fixed factor and baseline NRS pain score and study site as covariates. Intermittently missing NRS pain measures were imputed by linear interpolation. In situations where all NRS pain score values were missing after a certain point (due to study discontinuation for example), a model‐based multiple imputation method was applied. Where all NRS pain score values were missing after a certain point (eg, due to study discontinuation), a model‐based multiple imputation method was applied.
The key secondary endpoint of RSB (oliceridine superiority vs. morphine) was analyzed using a zero‐inflated gamma mixture model with treatment group as a factor, and baseline NRS, BMI, and study site as covariates.
The key secondary responder endpoint (oliceridine noninferiority or superiority vs. morphine) was analyzed using 1‐sided linear contrasts on the logarithmic odds scale from a logistic regression model that contained treatment group as a fixed factor and baseline NRS pain score and site as covariates. The noninferiority contrasts were constructed so that any oliceridine treatment regimen shown to have an effect significantly greater than half of the observed morphine effect would be regarded as noninferior to morphine. Oliceridine superiority vs. morphine was then analyzed using the same methods as for the primary comparison of oliceridine vs. placebo.
All other treatment comparisons were 2‐sided, with an unadjusted (nominal) significance level of α = 0.05. Patient demographics and AEs were summarized descriptively.
In order to avoid type I (false positive) errors when testing multiple treatment groups, a Hochberg gatekeeping approach was used to assess the primary and key secondary endpoints.30 The order of analysis was the primary superiority responder analysis of oliceridine vs. placebo, followed by the key secondary RSB analysis of oliceridine vs. morphine, the key secondary noninferiority responder analysis of oliceridine vs. morphine, and finally the key secondary superiority responder analysis of oliceridine vs. morphine. Within each analysis step, a Hochberg adjustment was applied to all P values. The order of the gating was selected based on interest in comparing the respiratory safety profile of oliceridine and morphine, and the significant amount of power expected to be required to achieve noninferiority between active regimens, which were selected based on their potential equi‐analgesia.
Power calculations incorporated the Hochberg gatekeeping multiplicity adjustment and were computed via simulations and assumed responses similar to those observed from the prior phase II clinical trial comparing the efficacy and safety of oliceridine vs. placebo and vs. morphine following abdominoplasty.23 It was expected that identification of noninferiority between oliceridine and morphine regimens designed to be broadly equi‐analgesic would require significant power. Thus, the RSB endpoint was chosen to be the first priority in the gating order.
A sample size of 375 patients (75 per treatment group) was estimated to provide 88% power to demonstrate that at least 2 oliceridine treatment groups would be simultaneously superior to placebo for the primary responder efficacy endpoint, superior to morphine for the key secondary RSB endpoint, and noninferior to morphine for the key secondary responder efficacy endpoint. This planned sample size provided greater than 99% power to demonstrate superiority of all oliceridine groups compared with placebo for the primary endpoint, and 96% power to demonstrate superiority of all oliceridine groups compared to morphine for RSB. The planned sample size also provided 93% power to demonstrate the noninferiority of oliceridine to morphine with respect to the responder efficacy endpoint for at least 2 of the oliceridine groups.
Results
Patient Disposition and Demographic and Clinical Characteristics
During the surgical period, a total of 407 patients were randomized. However, 2 patients did not undergo surgery and 4 patients did not meet the immediate postoperative inclusion criteria. Thus, 401 patients were treated with study medication (placebo: n = 81; oliceridine: 0.1 mg demand dose regimen, n = 77; 0.35 mg demand dose regimen, n = 80; 0.5 mg demand dose regimen, n = 80; morphine: 1 mg demand dose regimen, n = 83). Patient disposition is shown in Figure 1, where 85.5% (n = 348) of patients completed treatment, with all oliceridine regimens combined = 87.6% (n = 212); morphine = 90.4% (n = 75); and 74.4% (n = 61) in the placebo group. Patient demographics were similar across all treatment groups and are shown in Table 2, where most patients were female (n = 398, 99.3%); mean age was 41.4 ± 10.2 years. More than half of all patients reported severe pain at baseline (n = 203, 51.4%), and most had an Apfel risk score of 3 or 4 (95.0%). During the immediate postoperative period and prior to study medication dosing, a total of 399 patients (99.5%) received adjunctive fentanyl 25‐μg IV boluses (placebo n = 81, 100%; oliceridine 0.1‐mg regimen, n = 77, 100%; oliceridine 0.35‐mg regimen, n = 79, 98.8%; oliceridine 0.5‐mg regimen, n = 80, 100%; morphine, n = 82, 98.8%).
Figure 1.
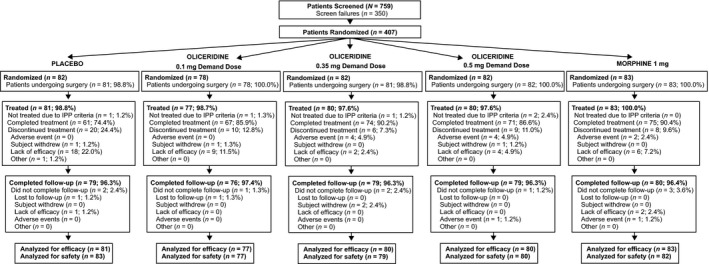
Patient disposition. All percentages are based on the number of patients randomized. Efficacy and safety analyses were conducted on all randomized patients who received ≥1 dose of study medication. Efficacy analyses were based on the randomized treatment assignment, while safety analyses were based on the actual treatment received. This explains the minor discrepancies seen between the number of patients analyzed for efficacy and safety in some treatment regimens. IPP, immediate postoperative period.
Table 2.
Patient Demographics and Baseline Characteristics
| Placebo (n = 81) |
Oliceridine Demand Dose Regimen | Morphine Regimen 1 mg (n = 83) |
|||
|---|---|---|---|---|---|
| 0.1 mg (n = 77) | 0.35 mg (n = 80) | 0.5 mg (n = 80) | |||
| Gender, n (%) | |||||
| Male | 0 | 1 (1.3) | 0 | 0 | 2 (2.4) |
| Female | 81 (100.0) | 76 (98.7) | 80 (100.0) | 80 (100.0) | 81 (97.6) |
| Mean age, years (SD) | 42.2 (10.3) | 41.8 (10.6) | 42.0 (10.0) | 40.4 (10.0) | 40.4 (10.4) |
| Mean BMI, kg/m2 (SD) | 27.0 (3.5) | 28.0 (3.4) | 27.6 (3.0) | 27.0 (3.2) | 26.8 (3.3) |
| BMI ≥ 30 kg/m2, n (%) | 17 (20.9) | 25 (32.5) | 16 (20) | 12 (15) | 12 (14.5) |
| Race, n (%) | |||||
| White | 52 (64.2) | 45 (58.4) | 55 (68.8) | 50 (62.5) | 55 (66.3) |
| Black/African American | 27 (33.3) | 24 (31.2) | 22 (27.5) | 28 (35.0) | 24 (28.9) |
| American Indian/Alaska native | 0 | 1 (1.3) | 0 | 0 | 0 |
| Asian | 1 (1.2) | 3 (3.9) | 2 (2.5) | 1 (1.3) | 2 (2.4) |
| Native Hawaiian/Pacific Islander | 0 | 2 (2.6) | 0 | 0 | 1 (2.2) |
| Other | 1 (1.2) | 2 (2.6) | 1 (1.3) | 1 (1.3) | 1 (1.2) |
| Mean surgery duration, min (SD) | 75.7 (19.4) | 72.9 (16.3) | 77.9 (17.8) | 74.3 (18.4) | 76.7 (18.3) |
| Mean baseline pain score (SD)* | 7.2 (1.4) | 7.4 (1.4) | 7.4 (1.6) | 7.5 (1.6) | 7.3 (1.5) |
| Baseline categorical pain rating, n (%)† | |||||
| Mild | 1 (1.2) | 0 | 0 | 2 (2.6) | 0 |
| Moderate | 41 (50.6) | 35 (45.5) | 36 (45.6) | 34 (44.2) | 43 (53.1) |
| Severe | 39 (48.1) | 42 (54.5) | 43 (54.4) | 41 (53.2) | 38 (46.9) |
| Apfel risk score, n (%)† , ‡ | |||||
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 (1.2) | 3 (3.9) | 4 (5.0) | 7 (8.8) | 5 (6.0) |
| 3 | 67 (82.7) | 57 (74.0) | 60 (75.0) | 66 (82.5) | 67 (80.7) |
| 4 | 13 (16.0) | 17 (22.1) | 16 (20.0) | 7 (8.8) | 11 (13.3) |
*Patients self‐rated pain on a scale from 0 = no pain to 10 = worst pain imaginable.
†Percentages based on the total number of patients who responded; 3 patients were missing baseline pain scores in the oliceridine 0.5 mg regimen, 2 were missing scores in the morphine regimen, and 1 was missing scores in the oliceridine 0.35 mg regimen.
‡The Apfel score assesses a patient's risk for postoperative nausea and vomiting based on known risk factors. The total score ranges from 0 to 4, with higher scores indicating greater risk, and is the sum of positive responses to the following questions: is the patient female; does the patient have a history of postoperative nausea, vomiting, or motion sickness; is the patient a nonsmoker; and does the patient have postoperative opioid use. In this study, all patients were considered as having postoperative opioid use.
BMI, body mass index; SD, standard deviation.
Study Drug Exposure and Rescue Medication Use
With dosing administered as needed, each active treatment group contained a range of drug exposures. Mean (standard deviation [SD]) cumulative exposure to study medication over the 24‐hour treatment period was 9.7 (5.1) mg in the oliceridine 0.1‐mg regimen, 21.2 (12.9) mg in the oliceridine 0.35‐mg regimen, 26.2 (18.2) mg in the oliceridine 0.5‐mg regimen, and 39.7 (27.6) mg in the morphine regimen.
The highest incidence of rescue pain medication utilization during the randomized treatment period was observed among patients in the placebo treatment regimen. All active treatment regimens showed a substantially lower incidence of rescue medication use across the treatment interval. The mid‐ and higher demand dose oliceridine rescue medication use was indistinguishable from morphine, while the lower demand dose of oliceridine showed a level of rescue medication use intermediate between placebo and the other active medication regimens (Figure 2A).
Figure 2.
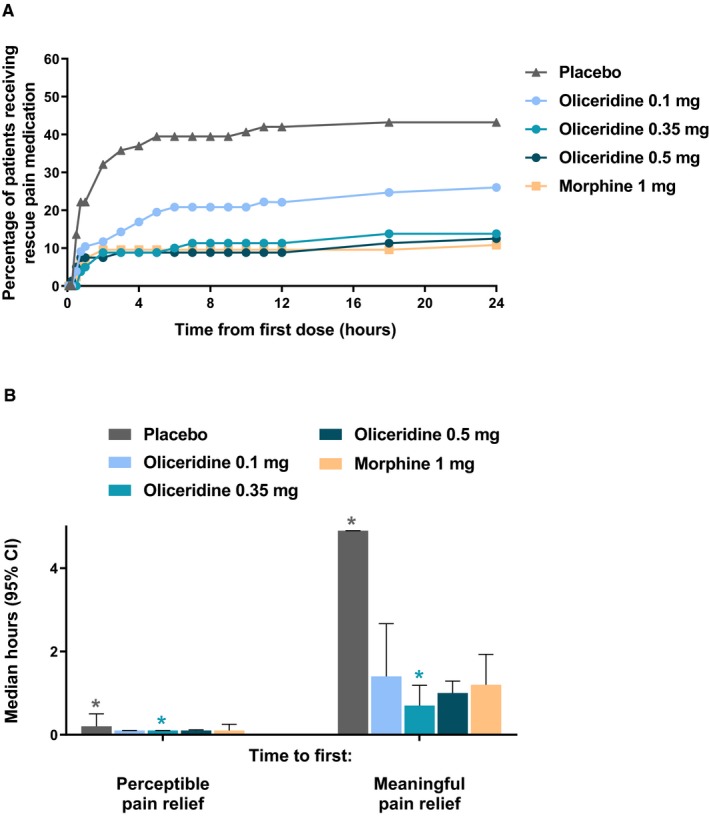
A, Cumulative use of rescue pain medication. B, First perceptible and first meaningful pain relief. In (A), the cumulative percentage of patients using rescue pain medication in each regimen is shown at predefined time points throughout the 24‐hour treatment period. Patients could receive open‐label rescue pain medication (oral etodolac 200 mg every 6 hours PRN) if they reported a score ≥ 4 on the pain NRS. In (B), the median time to first perceptible and first meaningful pain relief is presented, as reported by patients using the two‐stopwatch method. *P < 0.05 compared to morphine. CI, confidence interval; NRS, numeric rating scale; PRN, as needed.
Primary End Point
The proportion of treatment responders at the 24‐hour NRS assessment was statistically significantly higher in all oliceridine assigned patient cohorts (61%, P = 0.029; 76.3%, P < 0.0001; and 70%, P = 0.0004 for oliceridine 0.1, 0.35, and 0.5 mg demand dose regimens, respectively, compared to the placebo regimen (45.7%) with Hochberg adjustment; Figure 3A).
Figure 3.
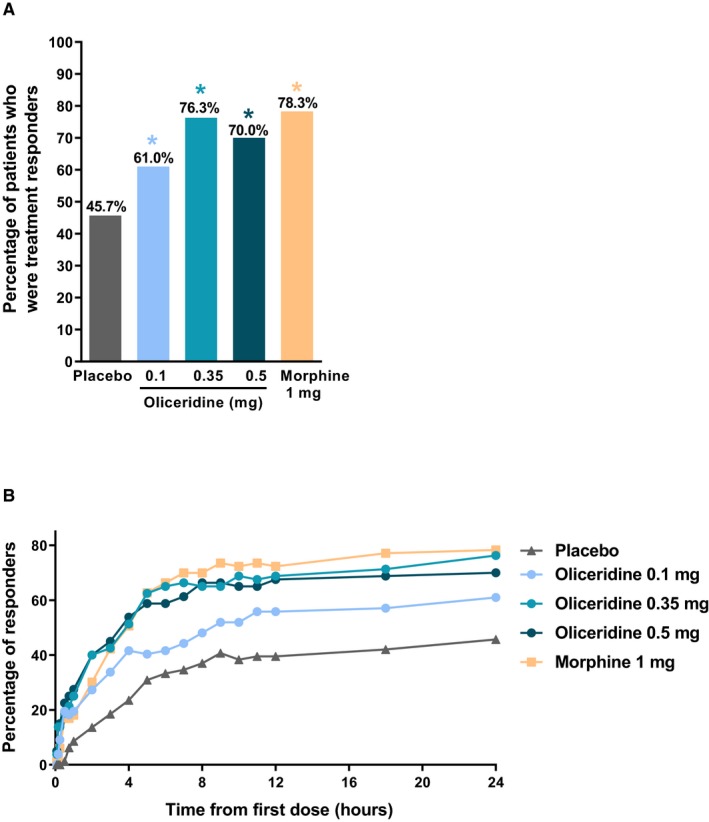
A, Primary treatment response analysis of oliceridine compared with placebo (treatment responders at 24 hours). B, Treatment responders over the full treatment period. This primary endpoint analysis compared the percentage of treatment responders in each oliceridine regimen with the percentage of responders in the placebo regimen at 24 hours post loading dose. Responders were those who reached a ≥30% improvement in time‐weighted sum of pain intensity difference (SPID‐24) from baseline, whilst (1) not received rescue pain medication, (2) not discontinuing study medication early, and (3) without reaching dosing limits. *P < 0.05 vs. placebo (Hochberg adjusted). CI, confidence interval.
Although the primary endpoint analysis compared oliceridine to placebo, for context, the proportion of treatment responders in the morphine group vs. placebo was 78.3% (P < 0.001).
Key Secondary Endpoint (Respiratory Safety Burden)
The key secondary outcome of RSB (defined in the Methods section, denoting a composite, calculated measure of respiratory compromise) showed a dose‐dependent increase across oliceridine regimens (mean hours [SD], 0.1 mg: 0.43 [1.56]; 0.35 mg: 1.48 [3.83]; 0.5 mg: 1.59 [4.26]), with all comparisons not statistically different from placebo (P > 0.05 vs. placebo: 0.60 [2.82]). The RSB outcome for morphine was 1.72 (3.86), which was statistically significantly worse than placebo (P < 0.05 vs. placebo). There was no statistically significant difference in the key secondary outcome of RSB for the higher demand doses of oliceridine 0.35‐mg (P = 0.27 vs. morphine) and 0.5‐mg regimens (P = 0.54 vs. morphine) compared to the morphine‐treated patients.
The component elements that comprised the composite RSB endpoint included the incidence of respiratory safety events and the mean cumulative duration of those events. Both of these components of RSB also showed a dose‐dependent increase across the oliceridine groups (Table 3 and Figure 4). In an exploratory analysis of nonadjusted comparisons, the 2 higher demand dose regimens of oliceridine had numerically lower incidences of respiratory safety events compared to morphine (oliceridine 0.35‐mg regimen: 21.5%; oliceridine 0.5‐mg regimen: 22.5%; morphine: 26.8%; placebo: 6%), although this was not significant (P = 0.20 and P = 0.32 for the 0.35‐mg and 0.5‐mg oliceridine regimens, respectively, vs. morphine). However, the cumulative duration of events was not statistically different for placebo or any oliceridine regimen vs. morphine (P > 0.75 vs. morphine). Among the clinically observed respiratory safety events reported, the proportion of patients receiving supplemental oxygen therapy or experiencing a dosing interruption due to respiratory safety showed a dose‐dependent increase across oliceridine demand dose regimens, all numerically lower than that seen with morphine (Figure 5). The odds ratio (vs. morphine) for the proportion of patients receiving supplemental oxygen therapy was 0.18 (P = 0.0005), 0.54 (P = 0.11), and 0.65 (P = 0.25) with oliceridine 0.1‐mg, 0.35‐mg, and 0.5‐mg regimens, respectively. Likewise, the odds ratio (vs. morphine) for proportion of patients with dosing interruption was 0.15, 0.57, and 0.64 with oliceridine 0.1‐mg (P = 0.0002), 0.35‐mg (P = 0.13), and 0.5‐mg (P = 0.23) regimens, respectively.
Table 3.
Components of the Respiratory Safety Burden and Respiratory Safety Event (RSE) Measures
| Patients, n (%) unless stated | Placebo (n = 83) |
Oliceridine Demand Dose Regimen | Morphine Regimen 1 mg (n = 82) |
||
|---|---|---|---|---|---|
| 0.1 mg (n = 77) | 0.35 mg (n = 79) | 0.5 mg (n = 80) | |||
| Components of the respiratory safety burden | |||||
| Proportion patients with at least 1 RSE | 5 (6.0) | 6 (7.8) | 17 (21.5) | 18 (22.5) | 22 (26.8) |
| Odds ratio vs. placebo | — | 1.2 (P = 0.76) | 3.96 (P = 0.0087) | 4.42 (P = 0.0045) | 6.49 (P = 0.0003) |
| Odds ratio vs. morphine | 0.15 (P = 0.0003) | 0.19 (P = 0.0007) | 0.61 (P = 0.20) | 0.68 (P = 0.32) | — |
| Duration of event, mean hours (SD) | 9.88 (7.0) | 5.51 (1.91) | 6.88 (5.66) | 7.07 (6.56) | 6.40 (5.09) |
| P value* | — | 0.25 | 0.45 | 0.66 | 0.52 |
| P value† | 0.52 | 0.29 | 0.78 | 0.76 | — |
| Respiratory safety event measures | |||||
| Oxygen saturation < 90% | 7 (8.4) | 6 (7.8) | 15 (19) | 16 (20) | 20 (24.4) |
| P value* | — | 0.86 | 0.06 | 0.04 | 0.02 |
| P value† | 0.02 | 0.01 | 0.57 | 0.76 | — |
| Respiratory rate ≤ 8 breaths/min | 1 (1.2) | 0 | 4 (5.1) | 6 (7.5) | 8 (9.8) |
| P value* | — | 0.96 | 0.18 | 0.07 | 0.054 |
| P value† | 0.054 | 0.95 | 0.38 | 0.84 | — |
| Sedation (Moline Roberts scale ≥ 3) | 15 (18.1) | 8 (10.4) | 19 (24.1) | 18 (22.5) | 21 (25.6) |
| P value* | — | 0.17 | 0.35 | 0.49 | 0.25 |
| P value† | 0.25 | 0.02 | 0.83 | 0.65 | — |
*P value comparison vs. placebo.
† P value comparison vs. morphine.
SD, standard deviation.
Figure 4.
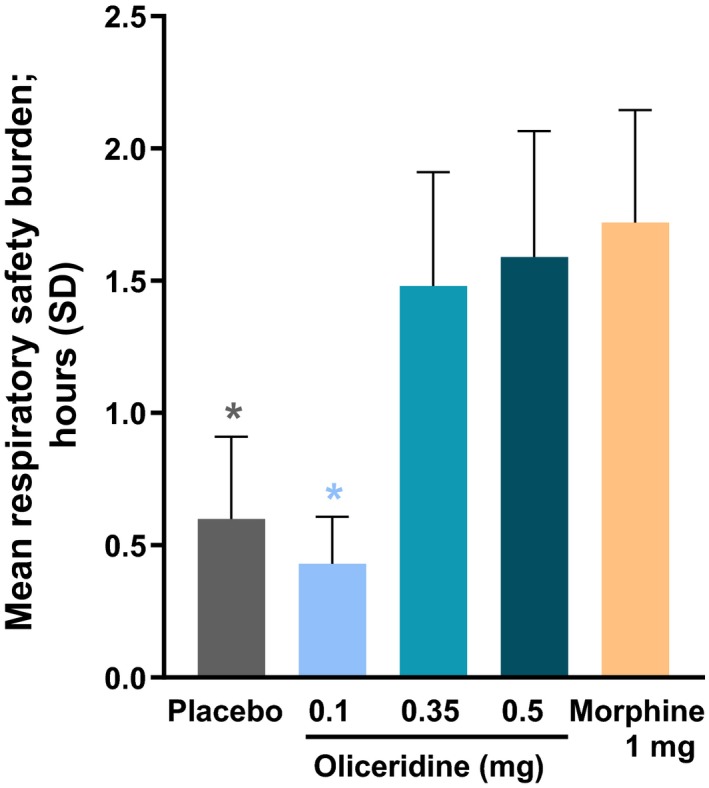
The key prespecified secondary endpoint of cumulative respiratory safety burden. During the randomized treatment period, patients were monitored on a protocol‐defined schedule by either a certified registered nurse anesthetist or an anesthesiologist, blinded to study medication assignment. The monitoring professional intervened when clinically indicated and determined the onset and resolution of each respiratory safety event. Respiratory safety burden was defined as the expected cumulative duration of respiratory safety events in a particular treatment group and was calculated as the mathematical product of the prevalence of respiratory safety events and the mean conditional duration (ie, mean duration in affected patients) of such events (see Table 3). *P < 0.05 compared to morphine (unadjusted). Mean respiratory safety burden from the model‐based estimate was 7, 5, 19, 25, and 32 minutes for the placebo, oliceridine 0.1, 0.35, and 0.5 mg, and morphine groups, respectively. SD, standard deviation.
Figure 5.
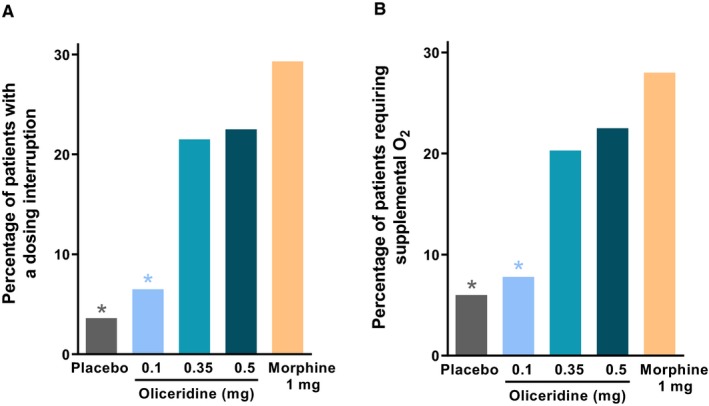
Clinical interventions. A, The proportion of patients in each regimen who experienced any dosing interruption of study medication during the study is presented. Exploratory analyses showed that the odds ratio of an interruption compared to morphine was 0.09 (P < 0.0001) for placebo, 0.15 (P = 0.0002) for oliceridine 0.1 mg, 0.57 (P = 0.13) for oliceridine 0.35 mg, and 0.64 (P = 0.23) for oliceridine 0.5 mg regimens. B, The proportion of patients in each regimen who required supplemental oxygen therapy is presented. Exploratory analyses showed that the odds ratio of an interruption compared to morphine was 0.15 (P = 0.0002) for placebo, 0.18 (P = 0.0005) for oliceridine 0.1 mg, 0.54 (P = 0.11) for oliceridine 0.35 mg, and 0.65 (P = 0.25) for oliceridine 0.5 mg regimens. *P < 0.05 odds ratio vs. morphine.
Other Secondary Efficacy Endpoints
Since the secondary statistical gating assessment for the key secondary endpoint of RSB did not reach statistical significance, no formal noninferiority analyses were conducted between oliceridine and morphine responder rates. However, exploratory analyses (not corrected for multiplicity) indicated that the oliceridine 0.35‐ and 0.5‐mg demand dose regimens were noninferior to morphine, while the 0.1‐mg demand dose regimen did not meet the prespecified noninferiority margin compared to morphine.
The magnitude of pain relief seen with oliceridine 0.35‐ and 0.5‐mg demand dose regimens was comparable to that observed in morphine‐treated patients, including a similar proportion of treatment responders over the full treatment period (Figure 3B). In addition, fewer patients discontinued oliceridine or morphine treatment due to lack of efficacy (see Figure 1) or required rescue analgesia compared to patients allocated to placebo (see Figure 2A).
Using the 2‐stopwatch method, the median time to self‐reported “perceptible pain relief” was shorter (6 minutes) for all oliceridine demand dose regimens, compared with placebo (12 minutes, P ≤ 0.0005). The median time to first perceptible pain relief was also 6 minutes for the morphine regimen. Likewise, median time to first “meaningful pain relief” was also shorter for all oliceridine regimens (1.4, 0.7, and 1.0 hours for the oliceridine 0.1‐, 0.35‐, and 0.5‐mg demand dose regimens, respectively) compared to placebo (4.9 hours; P < 0.05; see Figure 2B). As observed with oliceridine regimens, the median time to first “meaningful pain relief” was shorter for the morphine regimen compared with the placebo (1.2 hours, P = 0.0077). Overall, for both the “perceptible pain relief” as well as “meaningful pain relief,” there was no statistical difference among the active treatment groups. (Pain assessed by SPID scores and NRS scores is shown in Figure S1)
The proportion of early responders over the first hour of treatment was greater in the oliceridine groups and most apparent in the 0.35‐ and 0.5‐mg demand dose regimens at 10 to 15 minutes than for morphine (Figure 6A). During this time, supplemental study medication doses were prohibited, and rescue medication was discouraged. The time to a 1‐point change in NRS score from baseline also appeared to be shorter, particularly for the 0.35‐mg demand dose regimen (Figure 6B). The percentage of clinicians who reported being “mostly or completely satisfied” with study medication results was greater with oliceridine 0.35‐ and 0.5‐mg regimens compared to placebo, and the percentage of patients who reported being “mostly or completely satisfied” with study medication benefits was greater with all oliceridine regimens compared to placebo (Figure S2).
Figure 6.
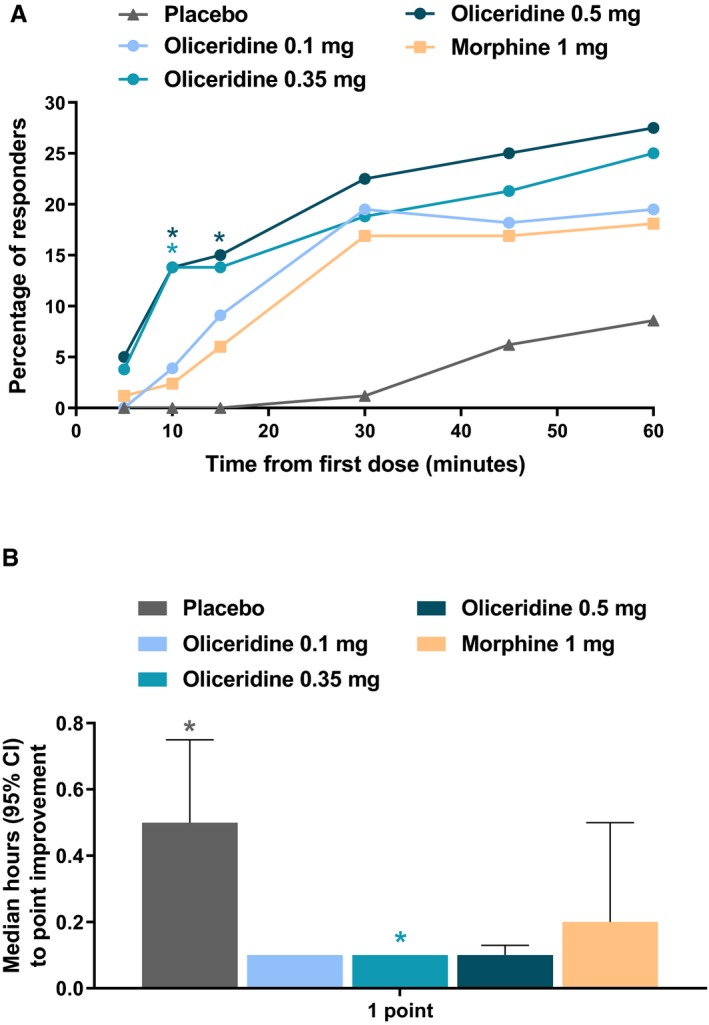
(A) The proportion of responders over the first 60 minutes of treatment and (B) time to 1‐point improvement in NRS score. A, Treatment response during this period can largely be attributed to study drug (loading dose at Time 0 and demand doses starting at 10 minutes) since supplemental study medication doses were prohibited and rescue pain medication was discouraged during this time. *P < 0.05 compared to morphine. B, NRS pain scores were reported at baseline (up to 10 minutes before loading dose [Time 0]); at 5, 10, 15, 30, and 45 minutes; and at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 18, and 24 hours post‐loading dose. *P < 0.05 vs. morphine. CI, confidence interval; NRS, numeric rating scale.
Safety and Tolerability
Overall Safety and Tolerability
No deaths were reported in the trial. Serious adverse events (SAEs) were reported in 5 patients (4 patients in the oliceridine treatment regimens and 1 patient in the morphine treatment regimen). Among the SAEs with oliceridine, post‐procedural hemorrhage, syncope, and lethargy were reported with the 0.5‐mg regimen, and abdominal wall hematoma with the 0.35‐mg regimen. Except for the syncope and lethargy, none of the other SAEs were considered related to oliceridine. Pulmonary embolism and respiratory failure (both in the same patient) were the SAEs reported with the morphine regimen and considered not related to treatment. All SAEs resolved without further sequelae.
Treatment‐emergent AEs are shown in Table 4. The overall proportion of patients experiencing at least 1 AE was lowest with placebo (78.3%) and increased in a dose regimen–dependent manner across the oliceridine 0.1‐, 0.35‐, and 0.5‐mg demand dose regimens (89.6%, 93.7%, and 95%, respectively). The proportion of patients experiencing at least 1 AE with morphine was 97.6%. The majority of the AEs were reported by the treating clinician as mild to moderate in intensity. The incidence of patients experiencing AEs leading to early study medication discontinuation was low: 8 (3.4%) oliceridine‐treated patients (4 each in the 0.35‐mg and 0.5‐mg regimens and 2 [2.4%] with morphine) and none with placebo. The most common AEs were nausea (placebo: 45.8%; oliceridine 0.1 mg: 44.2%; oliceridine 0.35 mg: 62.0%; oliceridine 0.5 mg: 75.0%; morphine: 74.4%); vomiting (placebo: 13.3%; oliceridine 0.1 mg: 23.4%; oliceridine 0.35 mg: 21.5%; oliceridine 0.5 mg: 42.5%; morphine: 53.7%); headache (placebo: 28.9%; oliceridine 0.1 mg: 15.6%; oliceridine 0.35 mg: 29.1%; oliceridine 0.5 mg: 26.3%; morphine: 29.3%); and hypoxia (placebo: 4.8%; oliceridine 0.1 mg: 7.8%; oliceridine 0.35 mg: 20.3%; oliceridine 0.5 mg: 17.5%; morphine: 23.2%).
Table 4.
Summary of Treatment‐Emergent Adverse Events (AE)*
| Placebo (n = 83) |
Oliceridine Demand Dose Regimen | Morphine Regimen1 mg (n = 82) | |||
|---|---|---|---|---|---|
| 0.1 mg (n = 77) | 0.35 mg (n = 79) | 0.5 mg (n = 80) | |||
| Total number of AEs | 208 | 238 | 296 | 300 | 388 |
| Patients with ≥ 1 AE, n (%) | 65 (78.3) | 69 (89.6) | 74 (93.7) | 76 (95.0) | 80 (97.6) |
| Patients with ≥ 1 serious AE, n (%) | 0 | 0 | 1 (1.3) | 3 (3.8) | 1 (1.2) |
| Patients with AE by intensity, n (%) | |||||
| Mild | 29 (34.9) | 22 (28.6) | 21 (26.6) | 21 (26.3) | 18 (22.0) |
| Moderate | 32 (38.6) | 42 (54.5) | 47 (59.5) | 48 (60.0) | 58 (70.7) |
| Severe | 4 (4.8) | 5 (6.5) | 6 (7.6) | 7 (8.8) | 4 (4.9) |
| Patients discontinued due to AE, n (%) | 0 | 0 | 4 (5.1) | 4 (5.0) | 2 (2.4) |
| Most common AE, n (% of patients)† | |||||
| Nausea | 38 (45.8) | 34 (44.2) | 49 (62.0) | 60 (75.0) | 61 (74.4) |
| Vomiting | 11 (13.3) | 18 (23.4) | 17 (21.5) | 34 (42.5) | 44 (53.7) |
| Somnolence or sedation | 8 (9.6) | 7 (9.1) | 11 (13.9) | 11 (13.8) | 25 (30.5) |
| Headache | 24 (28.9) | 12 (15.6) | 23 (29.1) | 21 (26.3) | 24 (29.3) |
| Pruritus or generalized pruritus | 5 (6.0) | 11 (14.3) | 14 (17.7) | 14 (17.5) | 19 (23.2) |
| Hypoxia | 4 (4.8) | 6 (7.8) | 16 (20.3) | 14 (17.5) | 19 (23.2) |
| Dizziness | 9 (10.8) | 11 (14.3) | 7 (8.9) | 7 (8.8) | 13 (15.9) |
| Constipation | 6 (7.2) | 12 (15.6) | 13 (16.5) | 9 (11.3) | 9 (11.0) |
| Back pain | 5 (6.0) | 3 (3.9) | 10 (12.7) | 9 (11.3) | 7 (8.5) |
*Occurring during the treatment or follow‐up period (until 7 days after the last dose of study medication).
†Occurring in ≥10% of patients in any treatment group.
There were no clinically meaningful differences between treatment regimens in vital sign measurements, ECG findings (including any abnormalities in the length of the QRS or QT interval at any predefined assessment points at 1 and 24 hours), hematology, or clinical chemistry parameters, with the exception of elevations from baseline in alanine aminotransferase and aspartate aminotransferase with oliceridine 0.1‐mg (n = 3), 0.35‐mg (n = 3), and 0.5‐mg (n = 1) demand dose regimens and in 2 patients allocated to morphine. All the patients received sevoflurane and propofol anesthesia and at least 1 concomitant medication (acetaminophen/paracetamol and/or ondansetron) that may have contributed to these abnormalities. All elevations were transient and had returned or were returning to normal during follow‐up.
Gastrointestinal Safety/Tolerability
The proportion of patients experiencing gastrointestinal AEs, defined using MedDRA‐coded preferred terms, increased with higher oliceridine demand dose regimens (0.1 mg: 49.4%; 0.35 mg: 65.8%; 0.5 mg: 78.8%) compared to placebo (47.0%). The incidences were generally lower in patients receiving an oliceridine demand dose of 0.35 mg or lower than in those receiving morphine. With the higher dose of 0.5 mg, the incidences were similar to those observed with morphine (see Table 4). Furthermore, numerically lower proportions of oliceridine‐treated patients received rescue antiemetic medications (0.1 mg: 32.5%; 0.35 mg: 55.0%; 0.5 mg: 61.3%), than morphine (65.1%) (Figure 7).
Figure 7.
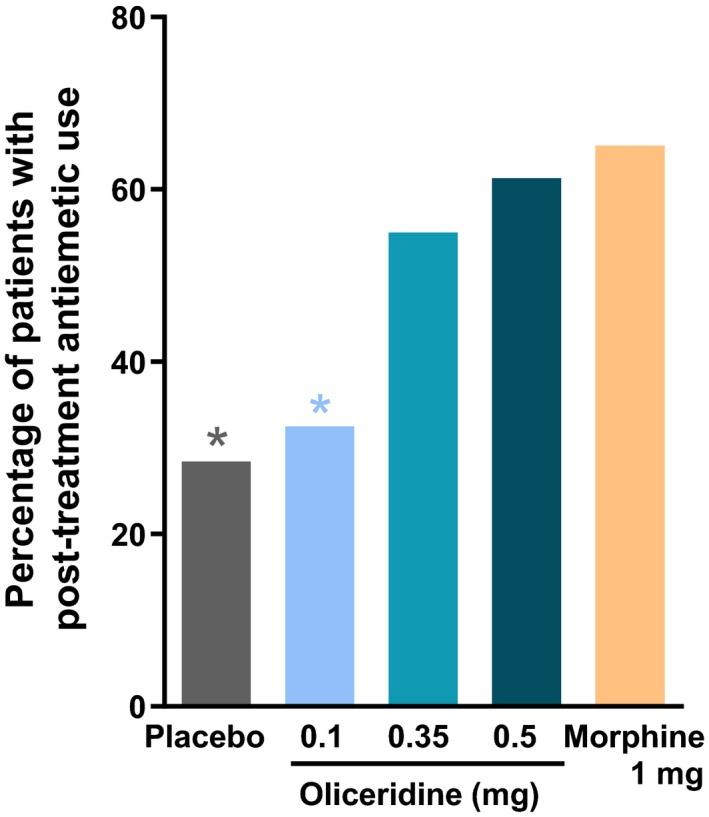
Rescue antiemetic use. This analysis examined post‐treatment rescue antiemetic use. Patients could receive rescue antiemetic medication if they were actively vomiting, or if they requested an antiemetic and reported moderate to severe nausea on a 4‐point scale (none, mild, moderate, severe). Prophylactic antiemetics were not permitted perioperatively or during the randomized treatment period. *P < 0.05 for odds ratio for rescue antiemetic use vs. morphine.
Discussion
In the APOLLO‐2 study, all oliceridine dosing regimens provided statistically superior analgesia to placebo, with a higher proportion of treatment responders in the 0.35‐ and 0.5‐mg demand dose regimens over the 24‐hour treatment period in subjects with moderate to severe pain following abdominoplasty. In addition, across the various secondary efficacy endpoints, including the proportion of treatment responders over time, the magnitude and time to self‐reported pain relief, the percentage of patients receiving rescue pain medication over time, and the time to first use of rescue pain medication, as well as clinician and patient‐reported satisfaction, oliceridine 0.35‐ and 0.5‐mg demand dose regimens were consistently superior to placebo. In comparison to morphine, only the 2 higher dose regimens of 0.35 and 0.5 mg oliceridine were considered equi‐analgesic; thus, safety and tolerability comparisons with morphine are relevant for only these 2 groups. In exploratory analyses using the gatekeeping statistical approach (described previously in the Methods section), oliceridine (0.35‐mg regimen) showed a favorable safety and tolerability profile regarding respiratory and gastrointestinal adverse effects compared to morphine. The safety/tolerability profile of the 0.5‐mg dose regimen, in general, was similar to that of the morphine regimen. Although these exploratory findings merit further investigation, they support the findings reported previously from a single‐center study, in which patients receiving similar demand dose regimens had significantly greater reductions in pain and experienced rapid analgesia compared to placebo, with an acceptable safety/tolerability profile compared to morphine.23
Findings from the APOLLO‐2 study showed a rapid onset of effect with all oliceridine regimens compared to placebo at early time points, particularly at 10 and 15 minutes. The rapid onset of analgesia with oliceridine was also demonstrated by the 2‐stopwatch assessments of perceptible and meaningful pain relief, and the time to a 1‐point change in NRS score (Figures 2B and 6B). The odds of achieving responder status were higher for the 0.35‐ and 0.5‐mg oliceridine treatment regimens compared with placebo at all time intervals through the entire 24‐hour randomized treatment period. Moreover, the proportion of patients using rescue pain medication any time post‐treatment was lower for the 0.1‐, 0.35‐, and 0.5‐mg oliceridine treatment regimens (26.0%, 13.8%, and 12.5%, respectively) compared with the placebo regimen (43.2%), and the time to first use of rescue pain medication was longer for the oliceridine treatment regimens compared with placebo. Taken together, all these observations support a greater magnitude of analgesic effect with all oliceridine dose regimens compared to placebo. It is noteworthy that as observed in a previously conducted phase III study (APOLLO‐1), evaluating oliceridine for the management of moderate to severe acute pain following bunionectomy,31 in an exploratory analysis the higher demand dose regimens of 0.35 mg or 0.5 mg provided an analgesic effect comparable to that of morphine. Furthermore, both APOLLO‐131 and APOLLO‐2 studies demonstrated a comparable level of analgesia between the 0.35‐ and 0.5‐mg dosing regimens.
In this study, the overall reported incidence of AEs ranged from 89.6% to 95.0% in the oliceridine treatment regimens, 97.6% in the morphine group, and 78.3% for placebo. Most AEs in the study were mild to moderate in intensity across all treatment regimens, with no clinically meaningful differences between treatments in vital signs or ECG findings (including the length of the QT interval) at any of the predefined assessment time points. Likewise, the incidence of severe AEs and AEs leading to early study medication discontinuation was also low in the study.
Nausea and vomiting are often cited by patients as key concerns following surgery,9, 32, 33, 34 and opioid‐induced nausea and vomiting are among the most frequent ORAEs. Previous clinical trials have shown evidence of significantly less frequent or less severe nausea and vomiting with oliceridine than with morphine.20, 23, 28 In APOLLO‐2 there were fewer patients treated with oliceridine who experienced nausea and vomiting (in a dose‐dependent manner, with a more notable difference seen with the 0.35‐mg demand dose regimen compared to morphine). In the placebo group, nausea and vomiting were reported in as high as 45.8% and 13.3% of patients, respectively, possibly reflecting a higher baseline Apfel score in the majority of patients.
Opioid‐induced respiratory depression (OIRD) is perhaps the most serious of ORAEs, with potentially fatal consequences.8, 10, 35 Episodes of respiratory depression can increase the length of hospital stay and further increase treatment costs. Particularly serious episodes can progress to cardiopulmonary or respiratory arrest.10 In an earlier phase Ib study, oliceridine was associated with a reduced suppression of respiratory drive at equi‐analgesic doses to morphine using the ventilatory response to hypercapnia experimental model.19 This was further supported by findings from a phase II trial of oliceridine, for acute postsurgical pain following abdominoplasty, in which there was a significantly reduced incidence of respiratory safety events, including reduced respiratory rate, respiratory effort, or hypoxia compared to morphine.23 Although there are no validated or well‐characterized methods to assess OIRD in clinical trials, due to the novel mechanism of action, it was of interest to thoroughly evaluate the potential for improved respiratory safety with oliceridine in the APOLLO‐2 study. The RSB composite endpoint was developed in an attempt to provide a comprehensive overview or “global index” of respiratory safety by incorporating both the incidence of respiratory safety events and their duration.
As per protocol, respiratory function was frequently monitored by experienced and trained medical professionals who were blinded to study medication. The RSB was expressed as the mathematical product of the prevalence of respiratory events and their duration, intentionally multifactorial to help identify clinically relevant indicators of respiratory distress. Of note, the enrolled population in APOLLO‐2 was not enriched for at‐risk patients (such as those with sleep apnea or obesity).24, 35 The reduction in RSB in APOLLO‐2 with oliceridine compared to morphine was consistent with previously reported findings19, 23; however, the differences between treatments using this new composite outcome measure did not reach statistical significance. There may be several contributing factors to this outcome. Overall there was a lower than expected rate of observed respiratory safety events in this trial compared to earlier work.22 Power calculations and sample size estimations for APOLLO‐2 were based on the prevalence of underlying respiratory safety events observed in the phase 2 abdominoplasty trial, which was higher than what was observed in this phase III study.23 Thus, underpowering may partially explain the lack of statistical significance. The more intensive per‐protocol patient monitoring, compared to prior studies, may also have contributed to this lower overall event rate. In an exploratory analysis, although the proportion of patients experiencing a respiratory safety event was numerically lower in all the oliceridine treatment regimens compared to morphine, the cumulative duration of the respiratory safety events was similar or numerically higher with oliceridine compared to morphine treatment regimens, though not statistically significant. Since only the 0.35‐ and 0.5‐mg demand dose regimens are considered equi‐analgesic, comparisons only of these 2 doses were made with morphine, and there was a lack of statistical significance for the numerical differences in favor of oliceridine for the respiratory safety events or RSB. Another aspect of evaluating respiratory safety is the use of clinical interventions such as dosing interruptions and/or the requirement for supplemental oxygen. When looking at individual events in APOLLO‐2, consistent with the observed reduction in respiratory safety events in the olicerdine treatment regimens, the proportion of patients requiring supplemental oxygen or with a dosing interruption was numerically lower with the oliceridine regimens than with morphine, although this was not statistically significant for either of the 0.35‐ and 0.5‐mg oliceridine demand dose regimens compared to morphine. Taking all of the results into consideration, the safety profile of the 0.35‐mg oliceridine demand dose regimen was particularly notable, considering this regimen provided analgesic efficacy similar to both the 0.5‐mg oliceridine regimen and that observed with morphine.
The safety and tolerability findings for oliceridine in APOLLO‐2 were comparable to those in previous oliceridine nonclinical studies and clinical trials of acute postsurgical pain in hard and soft tissues.19, 22, 23, 31 Across different study populations, dosing regimens, and using a variety of measures of respiratory safety endpoints, findings from oliceridine trials to date consistently suggest a potentially improved respiratory safety profile and reduced incidence of nausea and vomiting with oliceridine than with morphine at comparable levels of analgesia. Nevertheless, these safety findings merit further investigation.
From a clinical perspective, abdominoplasty is associated with moderate to severe postoperative acute pain warranting use of IV opioids. However, ORAEs remain a practical concern, particularly in higher risk populations.9, 24, 35 Finding the lowest effective dose of an opioid that provides rapid analgesia, while minimizing AEs, is an important goal in multimodal analgesia regimens.36 Oliceridine is a novel μ‐opioid receptor ligand that is a full agonist for G protein activation at the μ‐opioid receptor but with markedly reduced β‐arrestin recruitment compared with conventional opioids.18, 21 This selective mechanism is predicted to correspond to a lower incidence (but not elimination) of several ORAEs. Preclinical and early clinical evidence, including in a previous phase IIb study of patients with moderate to severe pain following abdominoplasty, has shown that oliceridine is at least as efficacious as morphine but with a lower incidence of gastrointestinal and respiratory AEs at comparable analgesic doses, thus providing a wider therapeutic window.18, 19, 22, 23 The APOLLO‐2 study was designed to support these findings in patients with moderate to severe pain following abdominoplasty, an established soft tissue model of acute postoperative pain. To reflect clinical practice, this study employed PRN, on‐demand dosing delivered via PCA with a range of exposures within each treatment regimen, supporting the premise that analgesic demand and dosing can be variable depending on the clinical situation. The primary efficacy endpoint using a responder analysis included important clinical elements of both efficacy and tolerability, since one should not occur at the expense of the other when trying to achieve optimal patient comfort. In APOLLO‐2, a responder was defined as a patient who experienced a ≥30% reduction in time‐weighted average pain score; did not receive rescue analgesics; did not discontinue study medication; and did not reach predefined dosing limits. Inclusion of not receiving rescue analgesia in the criteria is particularly important, and a clear indicator of analgesic sufficiency from the patients’ perspective, since they are self‐titrating to a level of comfort. This responder assessment complements the efficacy measures provided by a change in NRS score, which only measures intensity or magnitude of analgesic effect. When seeking the lowest necessary dose of opioids, analgesic outcomes that incorporate measures of analgesic sufficiency and tolerability are important considerations in determining overall clinical effectiveness.37
In this study, a standard dose of morphine (the active control) was compared to 3 experimental oliceridine treatment regimens to provide a comparative context for understanding the benefit/risk profile of oliceridine in this post‐surgical setting. The exploratory analysis on responder rates did not show significant differences between the oliceridine higher dose regimens and morphine, and overall data from APOLLO‐2 suggest that IV oliceridine can offer equi‐analgesic efficacy compared to morphine with the 0.35‐ and 0.5‐mg dose regimens. The optimal or lowest effective dose of oliceridine may differ when used as part of multimodal analgesic regimens, or in different clinical situations. Higher doses may be needed in cases of intense pain. In the setting of the APOLLO‐2 trial, the 0.35‐mg oliceridine demand dose regimen appeared to provide a balance of sufficient analgesia and tolerability, with efficacy comparable to morphine but with a reduced incidence of ORAEs. Nevertheless, based on the primary objectives of the study comparing to placebo, all 3 oliceridine demand dose regimens provided superior efficacy. With a need in clinical practice for alternative therapies to conventional opioids, these findings are encouraging.
There are some limitations of the study. The study used a surgical model with a low representation among men, limiting the generalizability of the findings across genders. However, other clinical trials of oliceridine inclusive of both genders have been conducted and will help to address this issue. It is important to note that while oliceridine was studied across a therapeutically active dose range, only a single dosage strength of morphine was included in the study design. While this permits validation of the assay sensitivity of the study design to detect clinically apparent analgesic effects, it limits conclusions that can be drawn regarding the comparative efficacy of oliceridine and morphine. This is most apparent with the 0.1‐mg demand dose, which appears clinically analgesic; however, its effectiveness was slightly lower than what was observed for the 0.35‐, 0.5‐mg, and morphine demand dose regimens. Nevertheless, the data are consistent with prior work and suggest that the development of a biased agonist holds considerable therapeutic promise for improving opiate tolerability. While the novel endpoint for measuring respiratory safety fell short of achieving statistical significance, the findings provide clinically important information and will inform future study design and choice of respiratory endpoints. Indeed, opioid‐induced respiratory disorders are serious AEs that may result in fatal outcomes and need vigilance, especially within the first 24 hours post‐surgery. Thus, further studies to evaluate this endpoint are warranted.
Conclusions
Findings from the APOLLO‐2 trial demonstrate the efficacy and safety of PRN oliceridine for the management of moderate to severe pain following abdominoplasty, an established postsurgical model of acute pain. Efficacy, safety, and tolerability data provide important context for evaluating the benefit/risk profile of IV oliceridine as compared to morphine. The results from APOLLO‐2 combined with those of previous studies further support the finding that oliceridine may be associated with a lower incidence of AEs at dosing regimens associated with comparable analgesia. Clinicians are seeking treatment alternatives for patients with moderate to severe acute pain. These data suggest that oliceridine may provide an important new treatment option for the management of moderate to severe postoperative pain where an IV opioid is warranted.
Sources of Financial Support
This study was sponsored by Trevena Inc. The sponsor had a role in the design and conduct of the study, in the analysis of the data, and in the decision to publish this manuscript.
Conflicts of Interest
Mark Demitrack is a full‐time employees of Trevena Inc. and owns stock in Trevena Inc. David G. Soergel, Franck Skobieranda, Monica Salamea and David A. Burt were full‐time employees, and stockholders, of Trevena Inc. at the time the research was conducted and the manuscript was fully written. Neil Singla is the founder and CEO of Lotus Clinical Research LLC, an analgesic CRO and research site; in this capacity he has served as a consultant and provided clinical trial services for Trevena Inc. Eugene R. Viscusi has served as a consultant to Trevena Inc.
Supporting information
Figure S1. Pain response by (A) Sum of Pain Intensity Difference and (B) numerical rating scale.
Figure S2. (A) Clinician‐ and (B) patient‐reported satisfaction with assigned study medication.
Acknowledgements
The authors wish to acknowledge the contributions of the patients involved in this study. Medical writing support and manuscript preparation with guidance from authors were provided by Kanaka Sridharan, MS R.Ph, Scientific Communications, Trevena Inc., and Jennifer Bodkin, PhD, CMPP, of Engage Scientific Solutions (funded by Trevena Inc.). The authors would also like to thank Linda Wase, MD, Medical and Clinical Affairs, Trevena Inc., for critical review of the manuscript.
References
- 1. Thomazeau J, Rouquette A, Martinez V, et al. Acute pain factors predictive of post‐operative pain and opioid requirement in multimodal analgesia following knee replacement. Eur J Pain. 2016;20:822–832. [DOI] [PubMed] [Google Scholar]
- 2. Fiala T. Tranversus abdominis plane block during abdominoplasty to improve postoperative patient comfort. Aesthet Surg J. 2015;35:72–80. [DOI] [PubMed] [Google Scholar]
- 3. Morales R Jr, Mentz H 3rd, Newall G, Patronella C, Masters O 3rd. Use of abdominal field block injections with liposomal bupivicaine to control postoperative pain after abdominoplasty. Aesthet Surg J. 2013;33:1148–1153. [DOI] [PubMed] [Google Scholar]
- 4. Feng LJ. Painless abdominoplasty: the efficacy of combined intercostal and pararectus blocks in reducing postoperative pain and recovery time. Plast Reconstr Surg. 2010;126:1723–1732. [DOI] [PubMed] [Google Scholar]
- 5. Khansa I, Koogler A, Richards J, Bryant R, Janis JE. Pain management in abdominal wall reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein C. New concepts in opioid analgesia. Expert Opin Investig Drugs. 2018;27:765–775. [DOI] [PubMed] [Google Scholar]
- 7. Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 suppl):S105–S120. [PubMed] [Google Scholar]
- 8. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3:159–180. [DOI] [PubMed] [Google Scholar]
- 9. Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid‐based postsurgical pain control using administrative claims data from a large health system: opioid‐related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33:383–391. [DOI] [PubMed] [Google Scholar]
- 10. Overdyk FJ, Dowling O, Marino J, et al. Association of opioids and sedatives with increased risk of in‐hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11:e0150214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habib AS, Chen Y‐T, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Curr Med Res Opin. 2006;22:1093–1099. [DOI] [PubMed] [Google Scholar]
- 12. Wardhan R, Chelly J. Recent advances in acute pain management: understanding the mechanisms of acute pain, the prescription of opioids, and the role of multimodal pain therapy. F1000Res. 2017;6:2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al‐Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor‐dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. [DOI] [PubMed] [Google Scholar]
- 15. Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist‐directed interactions with specific β‐arrestins determine μ‐opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem. 2011;286:31731–31741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bohn LM, Gainetdinov RR, Lin F‐T, Lefkowitz RJ, Caron MG. μ‐Opioid receptor desensitization by β‐arrestin‐2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. [DOI] [PubMed] [Google Scholar]
- 17. Raehal KM, Walker JK, Bohn LM. Morphine side effects in β‐arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. [DOI] [PubMed] [Google Scholar]
- 18. DeWire SM, Yamashita DS, Rominger DH, et al. A G protein‐biased ligand at the μ‐opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–717. [DOI] [PubMed] [Google Scholar]
- 19. Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the μ‐opioid receptor by TRV130 increases analgesia and reduces on‐target adverse effects versus morphine: a randomized, double‐blind, placebo‐controlled, crossover study in healthy volunteers. Pain. 2014;155:1829–1835. [DOI] [PubMed] [Google Scholar]
- 20. Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin F‐T. Enhanced morphine analgesia in mice lacking β‐arrestin 2. Science. 1999;286:2495–2498. [DOI] [PubMed] [Google Scholar]
- 21. Manglik A, Lin H, Aryal D, et al. Structure‐based discovery of opioid analgesics with reduced side effects. Nat Biotechnol. 2016;537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viscusi ER, Webster L, Kuss M, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ‐opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–272. [DOI] [PubMed] [Google Scholar]
- 23. Singla N, Minkowitz HS, Soergel DG, et al. A randomized, phase 2b study investigating oliceridine (TRV130), a novel μ receptor G protein pathway selective (μ‐GPS) modulator, for management of moderate to severe acute pain following abdominoplasty. J Pain Res. 2017;10:2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Apfel CC, Läärä E, Koivuranta M, Greim C‐A, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross‐validations between two centers. Anesthesiology. 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- 25. Physician Desk Reference . Morphine Sulfate Drug Summary from Physician's Desk Reference Digital Format. https://www.pdr.net/drug-summary/Morphine-Sulfate-Oral-Solution-morphine-sulfate-1228.8394#6 (accessed January 14, 2019)
- 26. U.S. Food and Drug Administration . Guidance for Industry. Analgesic Indications: Developing Drug and Biological Products. U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2014. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm384691.pdf (accessed January 14, 2019)
- 27. Moline B, Roberts M, Houser J. Validity and interrater reliability of the Moline‐Roberts Pharmacologic Sedation Scale. Clin Nurse Spec. 2012;26:140–148. [DOI] [PubMed] [Google Scholar]
- 28. Sunshine A, Mulhern SA, Olson N, Elkind A, Almas M, Sikes C. Comparative sensitivity of stopwatch methodology and conventional pain assessment measures for detecting early response to triptans in migraine: results of a randomized, open‐label pilot study. Clin Ther. 2006;28:1107–1115. [DOI] [PubMed] [Google Scholar]
- 29. Modena V, Bianchi G, Roccatello D. Cost‐effectiveness of biologic treatment for rheumatoid arthritis in clinical practice: an achievable target? Autoimmun Rev. 2013;12:835–838. [DOI] [PubMed] [Google Scholar]
- 30. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 31. Viscusi ER, Skobieranda F, Soergel DG, Cook E, Burt DA, Singla N. APOLLO‐1: a randomized, placebo‐ and active‐controlled Phase 3 study investigating oliceridine (TRV130), a novel μ receptor G protein pathway selective modulator, for management of moderate to severe acute pain following bunionectomy. J Pain Res. 2019;12:927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gan TJ, Epstein R, Menzel B, et al. Physician practice patterns and treatment challenges in acute postoperative pain management. PAINWeek abstract book 2017. Postgrad Med. 2017;129(suppl 1):1–85 (abs. 96).27915490 [Google Scholar]
- 33. Hsia H‐L, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen XT, Pitis P, Liu G, et al. Structure‐activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3‐methoxythiophen‐2‐yl)methyl]({2‐[(9R)‐9‐(pyridin‐2‐yl)‐6‐oxaspiro‐[4.5]decan‐ 9‐yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56:8019–8031. [DOI] [PubMed] [Google Scholar]
- 35. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid‐induced sedation and respiratory depression. Pain Manag Nurs. 2011;12:118–145.e10. [DOI] [PubMed] [Google Scholar]
- 36. Patanwala AE, Keim SM, Erstad BL. Intravenous opioids for severe acute pain in the emergency department. Ann Pharmacother. 2010;44:1800–1809. [DOI] [PubMed] [Google Scholar]
- 37. Levy N, Sturgess J, Mills P. “Pain as the fifth vital sign” and dependence on the “numerical pain scale” is being abandoned in the US: why? Br J Anaesth. 2018;120:435–438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pain response by (A) Sum of Pain Intensity Difference and (B) numerical rating scale.
Figure S2. (A) Clinician‐ and (B) patient‐reported satisfaction with assigned study medication.


