Abstract
Objective
Lip treatment products often incorporate oils and waxes in their formulations, and a desired outcome of their use is to prevent lip dryness and roughness as well as help to repair this condition. The objective of this study was to combine confocal Raman spectroscopy with skin capacitance (corneometry) and transepidermal water loss (closed chamber Aquaflux system) measurements, in the evaluation of the degree of moisturization and lip skin penetration of a fruit wax (Rhus vernicula peel cera) and natural oil‐based (Cocos nucifera fruit oil and Olea europea oil) lip care product, following a single application.
Methods
The study was conducted on a total of 15 healthy female volunteers. Instrumental measurements were performed before and 30 min, 2 h and 6 h after a single application of the product.
Results
Lip skin barrier function as well as lip hydration were significantly improved and penetration of olive oil was maintained for at least 6 h post product application. The deposition of the three component lipids (berry fruit wax, coconut oil and olive oil) into the stratum corneum after a single application of the lip care product was maintained and data significant for 2–6 h post product application. Lipid deposition was regarded as a positive long‐lasting skin care (depot‐) effect combined with a profound hydrating effect for about 6 h.
Conclusion
The tri‐method approach taken in this study is deemed relevant and valid for measuring lip hydration offering a complimentary assessment of the barrier function of lip skin and interactive effects of cosmetic ingredients.
Keywords: chapped lips, in vivo confocal Raman spectroscopy, lip care, lip penetration, plant lipid deposition
The lip water content, deposition of plant lipids after single application of a lip care product was assessed using Confocal Raman Spectroscopy, Corneometry, and transepidermal water‐loss measruement. That tri‐method approach was deemed relevant and valid for measuring lip hydration offering a complimentary assessment of the barrier function of lip skin and interactive effects of cosmetic ingredients.

Résumé
Objectifs
Les formulations des produits de soins des lèvres contiennent souvent des huiles et des cires. En outre, la prévention, voire la réparation de la sécheresse et de la rugosité des lèvres font partie des résultats attendus de l'utilisation de ces produits. Cette étude avait pour objectif d'associer une spectroscopie confocale Raman à des mesures de la capacitance de la peau (cornéométrie) et de la perte d'eau transépidermique (système à chambre fermée Aquaflux), dans l’évaluation du niveau d'hydratation et de pénétration cutanées des lèvres d'une cire à base de fruits (cire d’écorce de Vernis du Japon) et d'un produit de soins des lèvres à base d'huiles naturelles (huile de coco et huile d'olive), après une seule application.
Méthodes
Au total, l’étude a été menée auprès de 15 volontaires en bonne santé de sexe féminin. Des mesures instrumentales ont été réalisées avant, puis 30 minutes, 2 heures et 6 heures après une seule application du produit.
Résultats
Une amélioration significative de la fonction barrière et de l'hydratation de la peau des lèvres a été observée, et la pénétration cutanée de l'huile d'olive est demeurée stable pendant au moins 6 heures après l'application du produit. Le dépôt des trois lipides entrant dans sa composition (la cire de baies, l'huile de coco et l'huile d'olive) dans la couche cornée s'est prolongé pendant 2 à 6 heures après une seule application du produit de soins des lèvres, présentant ainsi un intérêt significatif pour le recueil de données. Les résultats concernant le dépôt lipidique ont décrit un effet positif et durable dans le soin de la peau associé à une hydratation intense pendant environ 6 heures.
Conclusion
L'approche à trois méthodes adoptée dans le cadre de cette étude pour mesurer l'hydratation des lèvres est jugée pertinente et valable, car elle offre une évaluation complémentaire de la fonction barrière de la peau des lèvres et des effets interactifs des ingrédients entrant dans la composition des cosmétiques.
Introduction
The lips (labia oris) or vermillion borders form an extension from the mucosal membrane to the outer skin and are the only part of the face where the oral mucosa is permanently exposed to the environment 1. Their prominent presence on the face makes them vulnerable to a variety of diseases and a target for both cosmetic and pharmaceutical treatments. Lips are highly susceptible to environmental exposure, such as wind, sun, smoking and temperature extremes. Environmental damage as well as certain treatments can cause the lips to become dry, chapped and less bright in colour 2. The main cause of dryness and chapping is considered as a result of low stratum corneum (SC) moisture capacity and ineffective barrier function 3, 4. It has been reported that incomplete corneocyte formation of the lip surface is responsible for poor barrier function and water‐holding capacity 5 with the upper lip being more hydrated than the lower. However, it appears that there is no correlation between lip capacitance and clinical scores of lip dryness 6.
A thin layer of SC covers the lips and their red coloration is believed to result from a combination of decreased density of keratin and translucency of the tissue, thus allowing the underlying capillaries to be observed 7, 8. In general, variations in the SC and thus, the barrier function, can be linked to many intrinsic factors, as for example ethnical background, gender, age and diseases, but also to extrinsic factors such as the environmental humidity, temperature, UV or especially for facial and lip skin SC the individual usage of facial cleansing products. These, and other factors, can influence or even disturb the barrier function of the lip skin leading to a higher transepidermal water loss (TEWL) and thus, result in rough and dry lips which often can be observed, for example in the winter season or perennially in elderly lip skin 9, 10, 11. Age‐related changes to the lips and perioral skin show that wrinkle number and visibility are linearly related to age, becoming more visible during the fourth and fifth decades. Furthermore, histological analysis of the upper lip reveals that elastic and collagen fibres in the cutis undergo a degeneration process during the ageing process with resulting thinning of the cutis 9 – the intercommissural distance increases with age, whereas lip height decreases.
A number of studies have used corneometry and TEWL as methods for examining the hydration and dryness of lips 10, 11. The relationship between lip roughness and ceramide profiles has been reported, suggesting that not only the level of total ceramides but also the specific ceramide species and their carbon numbers affect the maintenance of SC function in the lips 10. Furthermore, although xerosis represents a physiological response of the SC to environmental threats, the influence of the environmental dew point is not fully understood in terms of its relationship with the water‐holding capacity of the lips and their environment 12.
In vivo confocal Raman spectroscopy (CRS) is a sound investigative and widely accepted method 13, 14, 15, often employed to study the composition of the epidermal barrier in a space‐resolved manner 16. As a sensitive method, in an automated non‐invasive manner, it enables provision of biochemical information about the state of skin tissue, while maintaining the capability of delivering this information real‐time. By employing this method, a semi‐quantitative analysis of skin barrier components can be performed, for example SC lipids, natural moisturizing factors. CRS has emerged for high spatial and temporal resolution evaluation of SC barrier function and hydration 17, 18, 19, 20. The combination of a confocal signal acquisition with inelastic (Raman) photon scattering permits the direct determination of the SC molecular composition and distribution.
The signal, coming from a small and spatially defined volume of tissue, can be defined as an ‘optical sectioning’ of the skin. Raman measurements in the so called fingerprint region with wave numbers of 400–1800 allow the quantification of externally applied product ingredients to detect their rate of penetration 13.
Conventional methods such as the well‐established capacitance method measure the degree of hydration, which includes the widely used corneometry 21, 22. Decreasing the amount of water loss from the skin is an important parameter in efficacy of moisturizers and TEWL is well‐recognized as the main indicator of skin barrier function 22, 23. While the open‐chamber method of measurement has established itself as the main method for TEWL measurement, it has a number of limitations, especially disturbance by ambient air movements. This important limitation can be overcome by closing the measurement chamber 24. Closed condenser‐chamber instruments bring a new dimension to TEWL measurements, providing an all‐important greater sensitivity and repeatability. Especially for measurements on lips a closed chamber device is required to avoid the effect of breathing that disturbs measurements. Combining closed chamber TEWL measurements on the lips with the use of a dental tongue fixation device, to avoid lip licking, enables reproducible results with a low scatter.
Within this context, the scope and objective of this study was to utilize CRS accompanied by corneometry and TEWL in the evaluation of the degree of moisturization and lip skin penetration of a fruit wax and natural oil‐based lip care product. Lip treatment products often incorporate oils and waxes in their formulations, and a desired outcome of their use is to prevent skin barrier dryness and roughness as well as help to repair this condition. Many products are mineral oil and/or petroleum‐based formulations, yet to the consumer such products do not always meet their preferred needs, with many consumers choosing more natural and traditional formulations. Native cultures in tropical countries applied plant oils for centuries; the oil of the coconut (Cocos nucifera L.) was used to soften, moisturize, protect and improve dry skin 25. Studies have shown that coconut oil has comparable effects as mineral oil on skin hydration and skin surface lipid levels 26 or revealed an even better improvement of the TEWL and skin capacitance, for example in paediatric care 27. Furthermore, coconut oil is known to positively affect skin barrier repair, wound recovery and skin ageing, as well as possessing anti‐bacterial, anti‐inflammatory and antioxidant effects 27, 28, 29, 30, 31, 32, 33. In general, plant oils contain up to 30 different fatty acids in higher concentrations, which have shown diverse effects on human skin. Olive oil (Olivea europaea) contains about 60–70% oleic acid which is regarded as a penetration enhancer for active ingredients. Oleic acid fluidizes the barrier by displacing a number of the saturated acids 34. Additionally, many studies have demonstrated the positive effects of olive oil on wound recovery, as well as anti‐inflammatory and anti‐oxidative effects 28, 35, 36, 37, 38, 39, 40, 41.
In order to gain a further understanding of the effects of plant oils on lip skin, we utilized a CRS led tri‐method approach for clinically evaluating and characterizing SC hydration, water loss and ingredient penetration of lip skin treated with a formula rich in berry fruit wax (Rhus verniciflua peel cera), coconut oil (Cocos nucifera oil) and olive oil (Olea europaea fruit oil).
Materials and methods
Test materials
The test product under investigation was a proprietary cosmetic product (Kneipp GmbH, Würzburg, Germany), with a formula rich in berry fruit wax (Rhus verniciflua peel cera), coconut oil (Cocos nucifera oil) and olive oil (Olea europaea fruit oil). These three lipids are the ‘active emollients’ of the formulation.
Volunteers
A total of 15 healthy female volunteers were recruited onto the study aged 18–65 years with a median age of 44.7 ± 14.5 years (mean ± standard deviation). In a prior pilot study, conducted on six volunteers, the feasibility of methods was checked and the main study completed with further nine subjects.
Study inclusion criteria
Inclusion onto the study required volunteers willing to actively participate into the study and conform with all volunteer requirements. The study was open to female participants aged 18–65 years with a BMI of <30 and Fitzpatrick skin types I‐III with uniform skin colour and absence of erythema or dark pigmentation within the test area.
Study exclusion criteria
Potential volunteers excluded from study participation were: Pregnant or lactating females; drug addicts and alcoholics; AIDS, HIV‐positive or infectious hepatitis; active skin disease at the test area; documented allergies to cosmetic products and/or ingredients; illnesses that might require regular systemic medication; moles, tattoos, scars, irritated skin, etc. in the test area; any topical medication at the test area or systemic therapy with immunosuppressive drugs (e.g. corticosteroids) and/or antihistamines (e.g. anti‐allergics) within the last 7 days prior to study commencement; regular use of tanning beds.
All inclusion and exclusion criteria were checked by a questionnaire during the screening phase and during the study.
Instructions prior to study commencement
Screened and approved volunteers came to the study site and were provided with the following instructions. The subjects were instructed not to apply any decorative cosmetics (e.g. make‐up, powder, tinted day cream and other cover products) on the face on the day of study commencement; apply any detergents (e.g. face washing) in the test area within the last 12 h prior to the start of the study; use any lip conditioning products (e.g. lipstick, lip‐gloss, lip balms, lip pencils, chap stick) 2 days prior to the study; expose the test area to the sun, UV‐therapy and/or artificial tanning within the last 6 weeks prior to study commencement.
Safety criteria and adverse event reporting
Assessment of adverse events (AEs) was recorded. All adverse events occurring during the study (excluding those parameters being scored as part of the protocol) were documented in the study records. Details recorded include the nature of the adverse event, onset date/time, duration, severity, outcome and relationship with test product. Any adverse events requiring medical attention were referred to the appropriate proDERM medical personnel.
Measurements
Study Schedule The study was broken down according to the schedule summarized in Table 1. The study was conducted according to the study protocol and principles of GCP.
Table 1.
Study schedule
| Day 1 | ||||
|---|---|---|---|---|
| T0 Baseline | T1 30 min after product application | T2 2 h after product application | T3 6 h after product application | |
| Informed consent | X | |||
| In‐/exclusion criteria | X | |||
| Acclimatization | X | X | X | X |
| Application of test materials | Xa | |||
| Visual evaluation | X | X | X | X |
|
Instrumental measurements: Aquaflux Corneometer Raman |
X | X | X | X |
First application under control of a trained technician.
Climatic conditions
Instrumental measurements took place in an air‐conditioned room at a temperature of 21 ± 1°C and at 50 ± 5% relative humidity. Beforehand, subjects remained climatized for at least 30 min. To avoid undesired ‘moisturization’ of the lower lip during acclimatization and measurements, a dental plastic device was fixed to the subject's tongue.
Test procedure
Volunteers came to the study site and provided informed written consent. On all subjects Raman measurements, Aquaflux and corneometer measurements were performed post acclimatization. Three strokes of the lip care stick were applied on the upper as well as on the lower lip by a study technician.
The visual evaluation and instrumental measurements were repeated 30 min, 2 h and 6 h after application.
Raman measurement(s)
Approximately eight Raman spectra from the centre of the lower lip (see Fig. 2) were obtained by focusing low power laser light in the skin and by measuring the Raman scattered light from the laser focus with a confocal Raman Spectrometer Model 3510 Skin Analyzer (River Diagnostics, Rotterdam, Netherlands). A small amount of the scattered light is found at wavelengths higher than the incident laser light. This part of the scattered light provides information about the molecular composition of the skin.
Figure 2.
Measurement areas of the lower lip.
Water profile: The concentration profiles were calculated from Raman spectra (high wave number range from 2600 to 3800 cm−1) that were taken at different depths. Profiles were defined by water content at skin depths of 0, 2, 4, 6, up to 24 μm, measured from the surface of the lip skin, in steps of 2 μm (exposure time of 1 s per step). Approximately eight profiles per test area and assessment time were taken. The following parameters were assessed from obtained water profiles: water content at a depth of 0 μm, water content within SC and stratum disjunctum (SD) (water content in total and water content in three equally divided parts); water gradient within the SC and SD; thickness of SC and SD together (Fig. 1).
Figure 1.
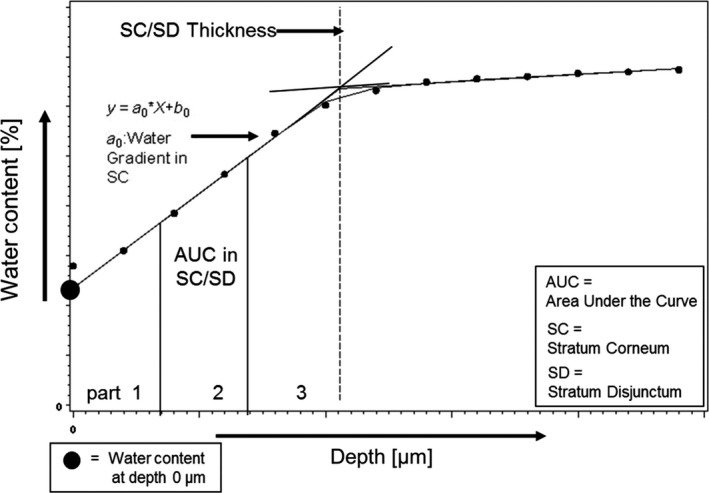
Scheme for Raman water curve with the assessed parameters.
Penetration profiles by Raman spectrometer: The penetration rate of three test product components: berry fruit wax (Rhus verniciflua peel cera), coconut oil (Cocos nucifera oil) and olive oil (Olea europaea fruit oil) was measured. The penetration was calculated from Raman spectra (wave number range from 400 to 1800 cm−1) that were taken at different depths. Variances could occur according to differences in skin depth and measurement conditions. Profiles are defined at skin depths of 0, 5 and 10 μm measured from the surface of the skin (approximately 8 profiles, exposure time of 10 s per step).
Transepidermal water loss
Transepidermal water loss is a non‐invasive method to measure the barrier function of the skin and is regarded as a sensitive parameter to quantify skin barrier damage and re‐epithelialisation. TEWL was measured with a closed chamber system using an Aquaflux AF200 (Biox Systems Ltd., London, UK). Water evaporation from the skin was measured by placing the cylindric chamber on the skin. When in contact with the test area, the chamber was closed and the air within was protected against disturbances from ambient air movements. The humidity difference between skin and condenser causes water vapour to migrate from source to sink by passive diffusion, leading to a linear distribution of humidity parallel to the axis of the chamber under steady conditions. The water vapour flux was calculated from measurements of this humidity gradient and Fick's first law of diffusion. One measurement per test area and assessment time was performed (Fig. 2).
Stratum corneum hydration
The measurement of SC hydration was performed by the electrical capacitance method with a Corneometer CM 825 (Courage & Khazaka, Cologne, Germany). The measuring principle is based on changes in the capacitance of the measuring head, functioning as a condensator. Between the conductors consisting of gold, an electrical field is built. By these means, the di‐electricity of the upper skin layer was measured. As the di‐electricity varies as a function of the skin's state of hydration, the SC hydration can thus be measured and five measurements per test area and assessment time were performed (Fig. 2).
Visual evaluation
At each assessment time an expert grader evaluated objective skin parameters in the lip test area (dryness, scaling, cracking/fissures), according to the following scale and scores documented: 0 = none; 0.5 = very slight; 1 = slight; 2 = moderate and 3 = strong.
Data managements and statistics
Valid subjects were defined as enrolled subjects who had finished the study without any major deviations from the protocol, and who had not withdrawn their consent. No replacement of missing data was performed, and demographic variables (age, gender) were given for the analysis population. Data were summarized using frequency distributions (number and percentage) for categorical/ordinal variables and mean, standard deviation and range for continuous variables. No unblinding or de‐randomization was applicable in this study.
Data listing
The assessment time before product application was defined as baseline.
Raman water profiles and Raman penetration factors (berry wax, coconut oil and olive oil): Per parameter and assessment time, the mean over all measurements was taken for further data analysis.
Transepidermal water loss: Per assessment time, the single value was used as raw data for further analysis. Skin hydration: After removal of the minimum and maximum value, the mean of the remaining measurements was used as raw data per assessment time for further analysis. Objective dermatological evaluation: Per assessment time, the single score was used as raw data for further analysis. All raw data of all valid subjects as well as differences to baseline were listed.
Statistical data analysis
Visual analysis of data revealed that pooling of the six volunteers of the pilot study with the nine volunteers of the main study was appropriate. The analysis of this study was therefore performed on the whole panel (n = 15). A significance level of 0.05 (alpha) was chosen for statistical analysis. Due to the explorative character of the study, no adjustment for multiplicity was calculated.
For skin hydration, TEWL and each water parameter of Raman spectroscopy (SC and SD thickness, water gradient within SC and SD, AUC and three AUC parts within SC and SD, and water content at depth 0 μm) as well as each penetration factor (berry wax, coconut oil and olive oil), comparisons of assessment times were performed using paired t‐test on raw data. The computation of the statistical data was carried out with commercially available statistics software (SAS Version 9.4 for Windows, SAS Institute, NC, USA).
Results
There were no subject exclusions or drop outs and all subjects completed the study. No adverse reactions were reported.
Raman water profile measurements
The water content on the skin surface (depth 0 μm) and within the uppermost part of the SC (part 1) was lower after 30 min and 2 h after product application compared to before product application (Baseline). In addition, the water content measured in the middle part of the SC (part 2) was lower 30 min after product application than at all other assessment times. In the lowest part of the SC (part 3), the water content measured remained relatively constant at all time points (Fig. 3 and Table 2). Due to the decrease in the water content within the uppermost layers and the unchanged water content in the innermost part of the SC, the water gradient within the total SC was steeper after 30 min and 2 h compared to baseline (Fig. 4). After 6 h, water content and water gradient had almost returned to baseline values. Overall, the thickness of the SC of the lips was measured at 22–23 ± 4 μm. No relevant change was observed in this study (Table 3).
Figure 3.
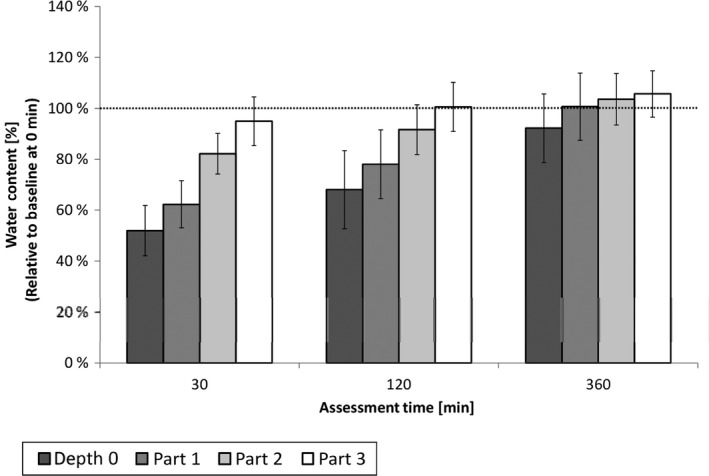
Water Content in the SC at different depth and points of time relative to baseline before product application (= 100%), means and 95% confidence intervals (n = 15). At skin surface (depth 0 μm) and AUC within SC+SD: part 1 = upper SC, part 2 = middle SC, part 3 = deep SC as illustrated in Fig. 2.
Table 2.
Water content – at skin surface (depth 0 μm) and AUC within SC + SD (upper, middle and lower part) – mean values and standard deviations for calculated values, differences to baseline and results for the comparison of times on calculated values by paired t‐test (n = 15)
| Depth | Time | Mean values (standard deviations) | Comparison of times | |||
|---|---|---|---|---|---|---|
| Calculated values | Differences to baseline | Versus baseline | Versus 30 min | Versus 2 h | ||
| Skin Surface (0 μm) | Baseline | 34.28 (6.38) | – | – | – | – |
| 30 min | 17.47 (6.21) | −16.80 (7.66) | <0.001a | – | – | |
| 2 h | 22.50 (8.09) | −11.78 (10.38) | <0.001a | 0.005a | – | |
| 6 h | 30.79 (6.36) | −3.49 (8.37) | 0.129n.s. | <0.001a | <0.001a | |
| Upper SC (part 1) | Baseline | 271.69 (47.00) | – | – | – | – |
| 30 min | 167.80 (48.92) | −103.89 (55.91) | <0.001a | – | – | |
| 2 h | 211.60 (71.02) | −60.10 (67.86) | 0.004a | 0.004a | – | |
| 6 h | 268.00 (57.71) | −3.70 (73.01) | 0.847n.s. | <0.001a | <0.001a | |
| Middle SC (part 2) | Baseline | 348.42 (56.78) | – | – | – | – |
| 30 min | 282.54 (50.85) | −65.88 (61.85) | 0.001a | – | – | |
| 2 h | 317.50 (73.99) | −30.92 (68.58) | 0.103n.s. | 0.041a | – | |
| 6 h | 356.71 (66.74) | 8.29 (68.67) | 0.647n.s. | <0.001a | 0.013a | |
| Lower SC (part 3) | Baseline | 423.52 (73.42) | – | – | – | – |
| 30 min | 394.87 (63.70) | −28.65 (79.17) | 0.183n.s. | – | – | |
| 2 h | 421.19 (85.89) | −2.33 (80.51) | 0.912n.s. | 0.208n.s. | – | |
| 6 h | 443.77 (84.50) | 20.25 (70.48) | 0.285n.s. | 0.013a | 0.243n.s. | |
n.s.: not significant.
Significant, P ≤ 0.05.
Figure 4.
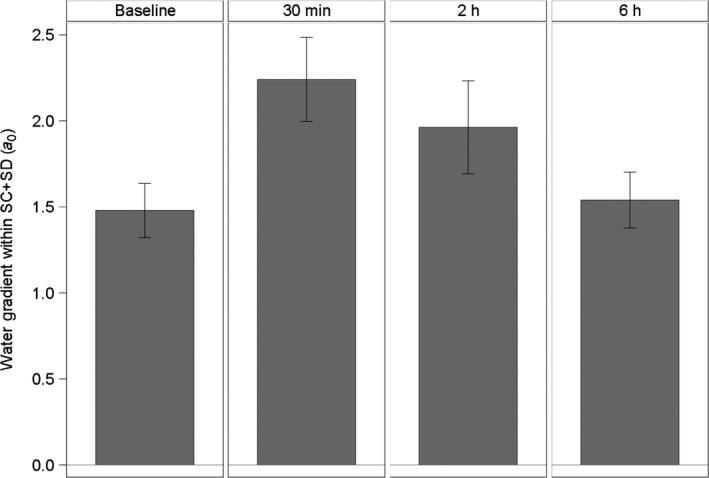
Water Gradients within the SC and SD at different points of time, means and 95% confidence intervals (n = 15). Measurements were taken at baseline, 30 min, 2 h and 6 h after application.
Table 3.
Stratum corneum (SC) thickness [μm] – mean values and standard deviations: (differences to baseline) and results for the comparison of times by paired t‐test (n = 15)
| Time | Mean values (standard deviations) | P‐values | |||
|---|---|---|---|---|---|
| SC thickness [μm] | Differences to baseline [μm] | Versus baseline | Versus 30 min | Versus 2 h | |
| Baseline | 21.70 (4.11) | – | – | – | – |
| 30 min | 21.72 (3.70) | 0.01 (4.58) | 0.990n.S. | – | – |
| 2 h | 22.43 (4.21) | 0.72 (4.38) | 0.532n.s. | 0.502n.s. | – |
| 6 h | 22.93 (4.50) | 1.22 (3.33) | 0.177n.s. | 0.242n.s. | 0.624n.s. |
n.s.: not significant.
significant, P ≤ 0.05.
Lip skin penetration rate
Figure 5 and Table 4 show the mean values and the 95% CV of the calculated penetration rate of the test product's main components. Following test product application, berry fruit wax was detected on the skin surface and down to a depth of 5 μm for a period of at least 2 h. At the skin surface, berry fruit wax was detected for at least 6 h post application. A significant higher berry fruit wax content was found in depths of 5–10 μm for a period of at least 2 h post application compared to baseline.
Figure 5.
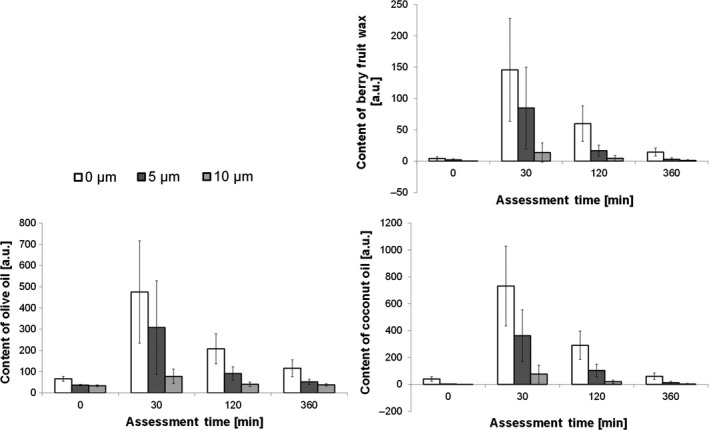
Penetration of berry fruit wax, coconut oil and olive oil in arbitrary units, means and 95% confidence intervals (n = 15). Measurements were taken at depths of 0, 5 and 10 μm in SC over a time of 6 h.
Table 4.
Mean values and standard deviations for area under the curve (AUC) on depth 5–10 μm and results for the comparison to baseline by paired t‐test (n = 15)
| Parameter | Time | AUC mean values (standard deviations) | Comparison to baseline P‐values |
|---|---|---|---|
| Berry fruit wax | Baseline | 6.19 (8.35) | – |
| 30 min | 246.66 (356.46) | 0.020a | |
| 2 h | 53.74 (51.20) | 0.003a | |
| 6 h | 9.86 (17.42) | 0.408n.s. | |
| Coconut oil | Baseline | 9.95 (7.69) | – |
| 30 min | 1099.18 (1150.22) | 0.003a | |
| 2 h | 310.62 (261.85) | <0.001a | |
| 6 h | 39.54 (61.97) | 0.086n.s. | |
| Olive oil | Baseline | 176.37 (33.38) | – |
| 30 min | 964.38 (1126.78) | 0.017a | |
| 2 h | 329.58 (182.51) | 0.004a | |
| 6 h | 224.05 (76.42) | 0.028a |
n.s.: not significant.
Significant, P ≤ 0.05.
Under the same conditions, coconut oil was detected from the skin surface down to a depth of 10 μm for a period of at least 2 h. Six hours post application, the level of coconut oil was comparable to baseline at all measured skin depths. A significant higher coconut oil content was found in depths of 5–10 μm for at least 2 h post application compared to baseline.
Olive oil was detected on the skin surface and to a depth of 5 μm which was maintained for at least 6 h. 30 min post product application, olive oil was also found to a skin depth of 10 μm. A significant higher olive oil content was found in depths of 5–10 μm for at least 6 h post product application when compared with baseline.
Transepidermal water loss
As shown in Fig. 6 and Table 5, TEWL (Aquaflux) was significantly reduced and maintained for at least 2 h post product application (berry fruit wax, coconut oil and olive oil). At 6 h post application, transepidermal water loss had returned to a comparable level to baseline.
Figure 6.
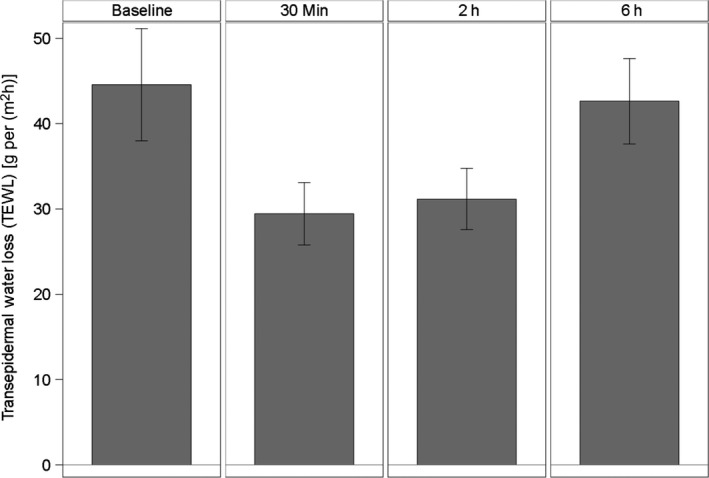
Transepidermal Water Loss (TEWL) by Aquaflux [g/(m2h)], means and 95% confidence intervals (n = 15).
Table 5.
Transepidermal water loss (TEWL) by aquaflux [g/(m²h)] – mean values and standard deviations, differences to baseline and results for the comparison of times on raw data by paired t‐test (n = 15)
| Time | Mean values (standard deviations) | Comparison of times P‐values | |||
|---|---|---|---|---|---|
| Raw data | Differences to baseline | Versus baseline | Versus 30 min | Versus 2 h | |
| Baseline | 44.56 (11.87) | – | – | – | – |
| 30 min | 29.43 (6.59) | −15.12 (12.89) | <0.001a | – | – |
| 2 h | 31.16 (6.48) | −13.40 (9.98) | <0.001a | 0.324n.s. | – |
| 6 h | 42.62 (9.02) | −1.93 (10.35) | 0.481n.s. | <0.001a | <0.001a |
n.s.: not significant.
Significant, P ≤ 0.05.
Skin hydration
Skin hydration measured as skin capacitance by a Corneometer was significantly increased and maintained for at least 2 h post product application (Fig. 7 and Table 6). As with TEWL, skin hydration returned to near baseline levels 6 h post product application.
Figure 7.
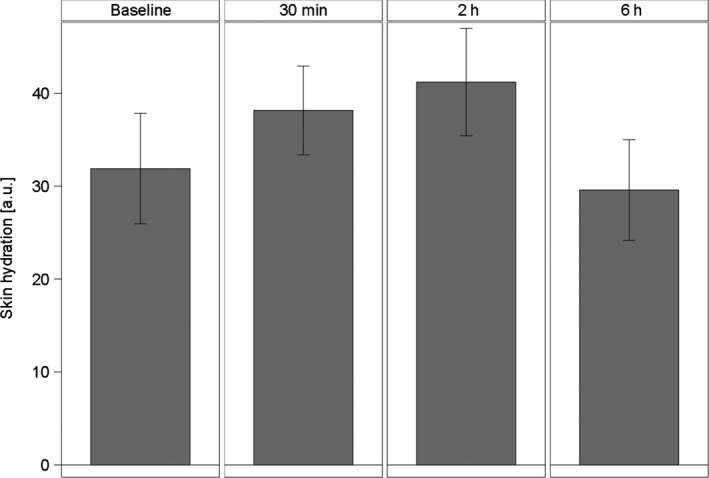
Skin hydration by measuring skin capacitance with corneometer [a.u.], means and 95% confidence intervals (n = 15).
Table 6.
Skin hydration by measuring skin capacitance with corneometer [a.u.] – mean values and standard deviations, differences to baseline and results for the comparison of times on raw data by paired t‐test (n = 15)
| Time | Mean values (standard deviations) | Comparison of times P‐values | |||
|---|---|---|---|---|---|
| Raw data | Differences to baseline | Versus baseline | Versus 30 min | Versus 2 h | |
| Baseline | 31.89 (10.73) | – | – | – | – |
| 30 min | 38.16 (8.63) | 6.27 (7.48) | 0.006a | – | – |
| 2 h | 41.21 (10.44) | 9.32 (6.71) | <0.001a | 0.166n.s. | – |
| 6 h | 29.59 (9.78) | −2.31 (7.85) | 0.274n.s. | <0.001a | <0.001a |
n.s.: not significant.
Significant, P ≤ 0.05.
Visual evaluation
Visual evaluation of dryness, scaling and cracking/fissures by a trained grader showed a definite improvement of all parameters for at least 2 h post application of the test product (Table 7). Six hours post product application, visible dryness and scaling were still slightly lower and cracking/fissures were comparable to the level evaluated at baseline. This may also be because of wearing off of the lip product which only had one application.
Table 7.
Visual dermatological assessment of lip skin – for dryness, scaling and cracking/fissures (n = 15)
| Code A | Counts of scores for dermatological evaluation | Mean values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| None (0) | Very slight (0.5) | Slight (1) | Moderate (2) | Strong (3) | Counts = 0 | Counts >0 | |||
| Dryness | Baseline | 1 | 7 | 7 | 0 | 0 | 1 | 14 | 0.7 |
| 30 min | 15 | 0 | 0 | 0 | 0 | 15 | 0 | 0.0 | |
| 2 h | 11 | 4 | 0 | 0 | 0 | 11 | 4 | 0.1 | |
| 6 h | 3 | 7 | 5 | 0 | 0 | 3 | 12 | 0.6 | |
| Scaling | Baseline | 4 | 7 | 3 | 1 | 0 | 4 | 11 | 0.6 |
| 30 min | 13 | 1 | 1 | 0 | 0 | 13 | 2 | 0.1 | |
| 2 h | 12 | 2 | 1 | 0 | 0 | 12 | 3 | 0.1 | |
| 6 h | 9 | 3 | 2 | 1 | 0 | 9 | 6 | 0.4 | |
| Cracking/fissures | Baseline | 10 | 3 | 2 | 0 | 0 | 10 | 5 | 0.2 |
| 30 min | 13 | 2 | 0 | 0 | 0 | 13 | 2 | 0.1 | |
| 2 h | 12 | 3 | 0 | 0 | 0 | 12 | 3 | 0.1 | |
| 6 h | 11 | 3 | 1 | 0 | 0 | 11 | 4 | 0.2 | |
Discussion
The purpose and objective of this study was to utilize CRS accompanied by corneometry and TEWL in the evaluation of the degree of moisturization and lip skin penetration of a fruit wax and natural oil‐based lip care product. For reproducible measurements, the tongue was fixed with a dental device to prevent the involuntary reflex of lip moisturization. Due to visible observation, most of the product had penetrated within 30 min after application. However, partial removal of remaining product residue by the measurement procedures was tolerated in our study, as the amount of penetrating actives was the main interest and not the low amounts of lip surface residue
Published studies have demonstrated the combination of TEWL (open‐chamber) and corneometry 10, 11 as tools for characterizing barrier function of lip skin, however, they do not provide the detailed information of the changes in hydration of SC that can be achieved with Raman spectroscopy. Skin barrier function, at the SC level, is normally evaluated using well‐established, non‐invasive biophysical methods such as TEWL and capacitance. Yet, these methods do not measure the skin's structure or molecular composition. In many cases, these facts will hamper the detection of causes of skin barrier alterations. Moreover, confocal Raman spectroscopy (CRS) can evaluate the structural and molecular composition of the skin and is thus a versatile technique in skin research.
The results of the water profile measured with Raman spectroscopy indicated a replacement of water molecules within the upper parts of the SC by ingredients of the test product. This displacement effect demonstrated the penetration of ingredients into the SD. Six hours post product application, this effect was no longer detectable except for olive oil that was still measurable at a low level. This deposition of test product ingredients is regarded as positive skin (depot‐)effect for about 6 h that contributes to the improvement of lip skin barrier and of visible skin dryness. The three main test product ingredients berry fruit wax, coconut oil and olive oil penetrated the SC down to a depth of 10 μm. That means all three components were mainly found in the SD.
The lip skin barrier protective semi‐occlusive effect by the test product wax and lipids was clearly shown. An improvement of lip skin barrier because of the deposition of lipids (shown by a decrease in TEWL), of skin hydration (corneometry) and of visible dryness parameters was recorded for at least 2 h but has almost faded 6 h post product application. Raman measurements also showed the deposition of the lipids into the outer SC, the SD. Data provided from Aquaflux and Raman measurements showed the presence of the test products ingredients at the lip skin surface and in the SD. Whereas the semi‐occlusive effect was expected in this type of study and for this product category, the hydrating effect observed by Corneometer measurements in combination with wax and lipid deposition could be regarded as profound. Initial observations might be considered contradictory in that the Corneometer measurements clearly increased after treatment, whereas water content as measured with CRS decreased. We assume that two effects contribute to this effect. First, on dry lips Corneometer contact with the lip surface is poor. The air between dry lip flakes influences the capacitance results significantly, since air has a very low capacitance. Clinical (visual) assessment of lip dryness is mainly an assessment of the flakiness. Therefore, the Corneometer correlates in an excellent manner with the clinical grading of lip dryness, but not with the true water content of the SC. The second reason is that moisturization of lips with lipids (emollients) can only function through the deposition of lipids on and in the SC. This means that water in the outer layers is replaced by the lipids and the water content in the SC is thus reduced. Therefore it is not the water content but the water gradient in SC that is the parameter that positively correlates with clinical skin dryness, as well as Corneometer measurements 42.
Any variances observed in levels of TEWL and corneometry are likely related to incomplete corneocyte formation of the lip surface which is responsible for poor barrier function and water‐holding capacity 5. Open chamber systems for measuring transepidermal water loss (TEWL) have limitations related to ambient and body‐induced airflows near the probe, probe size, measurement sites and angles and measurement range. The closed chamber system for TEWL measurement is accurate without significant blocking of normal evaporation through the skin. Furthermore, using a closed chamber system, the disturbance related to external or body‐induced air flows on the measurement, such as the lips, can be avoided. In order to achieve a complete dataset and a high level of statistical significance it is important to ensure, that by incorporating a pre‐pilot study, a statistically and confidently precise number of subjects are enrolled.
Conclusions
The lip care product under evaluation in this study comprising berry fruit wax (Rhus verniciflua peel cera), coconut oil (Cocos nucifera oil) and olive oil (Olea europaea fruit oil) markedly improved the skin barrier, skin hydration as well as penetrating the surface of lip skin. The deposition of these three main test product lipids into the SC after a single application of a formulated lip care product was maintained and under the conditions of normal wear, this may be regarded as a long‐lasting effect for this product type (lipstick). Lipid deposition was regarded as a positive long‐lasting skin care effect of at least 6 h combined with a profound hydrating effect.
CRS remains an interesting and useful technique which not only follow the penetration of cosmetic constituents into lip skin and their effect on water gradient, but also to evaluate the extent and longevity of this delivery. The tri‐method approach taken in this study is deemed relevant and valid for measuring skin lip hydration, offering a complimentary assessment of the barrier functioning of lip skin and interactive effects of cosmetic ingredients.
Acknowledgements
The authors thank Dr. Theresa Callaghan, Callaghan Consulting International, Hamburg, Germany for the support in the preparation of this manuscript. The study was initiated and sponsored by Kneipp GmbH, Würzburg, Germany. In addition, the test products were provided by Kneipp GmbH.
References
- 1. Zugerman, C. The lips: anatomy and differential diagnosis. Cutis 38, 116–120 (1986). [PubMed] [Google Scholar]
- 2. Caisey, L. , Gubanova, E. , Camus, C. , Lapatina, N. , Smetnik, V. and Lévêque, J. Influence of age and hormone replacement therapy on the functional properties of the lips. Skin Res. Technol. 14, 220–225 (2008). [DOI] [PubMed] [Google Scholar]
- 3. Tamura, E. , Ishikawa, J. , Naoe, A. and Yamamoto, T. The roughness of lip skin is related to the ceramide profile in the stratum corneum. Int. J. Cosmet. Sci. 38, 615–621 (2016). [DOI] [PubMed] [Google Scholar]
- 4. Tamura, E. , Ishikawa, J. , Sugata, K. et al Age‐related differences in the functional properties of lips compared with skin. Skin Res. Technol. 24, 472–478 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Kobayashi, H. and Tagami, H. Distinct locational differences observable in biophysical functions of the facial skin: with special emphasis on the poor functional properties of the stratum corneum of the perioral region. Int. J. Cosmet. Sci. 26, 91–101 (2004). [DOI] [PubMed] [Google Scholar]
- 6. Lévêque, J. and Goubanova, E. Influence of age on the lips and perioral skin. Dermatology 208, 307–313 (2004). [DOI] [PubMed] [Google Scholar]
- 7. Kar, M. , Muluk, N. , Bafaqeeh, S. and Cingi, C. Is it possible to define the ideal lips? Acta Otorhinolaryngol. Ital. 38, 67–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barrett, A. , Morgan, M. , Nwaeze, G. et al The differentiation profile of the epithelium of the human lip. Arch. Oral Biol. 50, 431–438 (2005). [DOI] [PubMed] [Google Scholar]
- 9. Penna, V. , Stark, G. , Eisenhardt, S. et al The aging lip: a comparative histological analysis of age‐related changes in the upper lip complex. Plast. Reconstr. Surg. 124, 624–628 (2009). [DOI] [PubMed] [Google Scholar]
- 10. Ishikawa, J. , Shimotoyodome, Y. , Ito, S. et al Variations in the ceramide profile in different seasons and regions of the body contribute to stratum corneum functions. Arch. Dermatol. Res. 305, 151–162 (2013). [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi, H. and Tagami, H. Functional properties of the surface of the vermilion border of the lips are distinct from those of the facial skin. Br. J. Dermatol. 150, 563–567 (2004). [DOI] [PubMed] [Google Scholar]
- 12. Devillers, C. , Piérard, G. , Quatresooz, P. and Piérard, S. Environmental dew point and skin and lip weathering. J. Eur. Acad. Dermatol. Venereol. 24, 513–517 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Caspers, P. , Lucassen, G. , Carter, E. et al In vivo confocal Raman micro‐spectroscopy of the skin: noninvasive determination of molecular concentration profiles. J. Invest. Dermatol. 116, 434–442 (2001). [DOI] [PubMed] [Google Scholar]
- 14. Caspers, P. , Lucassen, G. , Bruining, H. et al Automated depth‐scanning confocal Raman micro‐spectrometer for rapid in vivo determination of water concentration profiles in human skin. J. Raman Spectrosc. 31, 813–818 (2000). [Google Scholar]
- 15. Chrit, L. , Hadjur, C. , Morel, S. et al In vivo chemical investigation of human skin using a confocal Raman fiber optic microprobe. J. Biomed. Opt. 10, 44007 (2005). [DOI] [PubMed] [Google Scholar]
- 16. Pudney, P. , Bonnist, E. , Caspers, P. et al A new in vivo Raman probe for enhanced applicability to the body. Appl. Spectrosc. 66, 882–891 (2012). [DOI] [PubMed] [Google Scholar]
- 17. Broding, H. , Pol, A. , Sterke, J. et al In vivo monitoring of epidermal absorption of hazardous substances by confocal Raman micro‐spectroscopy. J. Ger. Soc. Dermatol. 9, 618–626 (2011). [DOI] [PubMed] [Google Scholar]
- 18. Petry, T. , Bury, D. , Fautz, R. et al Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicol. Lett. 280, 70–78 (2017). [DOI] [PubMed] [Google Scholar]
- 19. Choe, C. , Lademann, J. and Darvin, M. Gaussian‐function‐based deconvolution method to determine the penetration ability of petrolatum oil into in vivo human skin using confocal Raman microscopy. Laser Phys. 24, 105601 (2014). [Google Scholar]
- 20. Choe, C. , Lademann, J. and Darvin, M. Analysis of human and porcine skin in vivo/ex vivo for penetration of selected oils by confocal Raman microscopy. Skin Pharmacol. Physiol. 28, 318–330 (2015). [DOI] [PubMed] [Google Scholar]
- 21. Patzelt, A. , Lademann, J. , Richter, H. et al In vivo investigations on the penetration of various oils and their influence on the skin barrier. Skin Res. Technol. 18, 364–369 (2012). [DOI] [PubMed] [Google Scholar]
- 22. Stamatas, G. , de Sterke, J. , Hauser, M. et al Lipid uptake and skin occlusion following topical application of oils on adult and infant skin. J. Dermatol. Sci. 50, 135–142 (2008). [DOI] [PubMed] [Google Scholar]
- 23. Ghadially, R. , Halkier‐Sorensen, L. and Elias, P. Effects of petrolatum on stratum corneum structure and function. J. Am. Acad. Dermatol. 26, 387–396 (1992). [DOI] [PubMed] [Google Scholar]
- 24. Tagami, H. , Kobayashi, H. and Kikuchi, K. A portable device using a closed chamber system for measuring transepidermal water loss: comparison with the conventional method. Skin Res. Technol. 8, 7–12 (2002). [PubMed] [Google Scholar]
- 25. de Tavera, T. Plantas medicinales de Filipinas: Medicinal Plants of the Philippines. Ayala Foundation Inc., Makati: (2000). [Google Scholar]
- 26. Agero, A. and Verallo‐Rowell, V. A randomized double‐blind controlled trial comparing extra virgin coconut oil with mineral oil as a moisturizer for mild to moderate xerosis. Dermatitis 15, 109–116 (2004). [DOI] [PubMed] [Google Scholar]
- 27. Padilla Evangelista, M.‐T. , Abad‐Casintahan, F. and Lopez‐Villafuerte, L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, doubleblind, clinical trial. Int. J. Dermatol. 53, 100–108 (2014). [DOI] [PubMed] [Google Scholar]
- 28. Korać, R. and Khambholja, K. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 5, 164–173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nevin, K. and Rajamohan, T. Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacol. Physiol. 23, 290–297 (2010). [DOI] [PubMed] [Google Scholar]
- 30. Kim, S. , Jang, J. , Kim, J. , Lee, Y. , Lee, D.W. , Song, S.Y. , Lee, J.H. Enhanced barrier functions and anti‐inflammatory effect of cultured coconut extract on human skin. Food Chem. Toxicol. 106, 367–375 (2017). [DOI] [PubMed] [Google Scholar]
- 31. Preuss, H. , Echard, B. , Enig, M. , Brook, I. and Elliott, T. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram‐positive and gram‐negative bacteria. Mol. Cell. Biochem. 272, 29–34 (2005). [DOI] [PubMed] [Google Scholar]
- 32. Oyi, A. , Onaolapo, J. and Obi, R. Formulation and antimicrobial studies of coconut (Cocos nucifera Linne). Oil. Res. J. Appl. Sci. Eng. Technol. 2, 133–137 (2010). [Google Scholar]
- 33. Esquenazi, D. , Wigg, M. , Miranda, M. et al Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn (Palmae) husk fiber extract. Res. Microbiol. 153, 647–652 (2002). [DOI] [PubMed] [Google Scholar]
- 34. Lautenschläger, H. Vegetable oils. Kosmetik Int. 1, 16–18 (2009). [Google Scholar]
- 35. Nasopoulou, C. , Karantonis, H. , Detopoulou, M. , Demopoulos, C. and Zabetakis, I. Exploiting the anti‐inflammatory properties of olive (Olea europaea) in the sustainable production of functional food and neutraceuticals. Phytochem. Rev. 13, 445–458 (2014). [Google Scholar]
- 36. Donato‐Trancoso, A. , Monte‐Alto‐Costa, A. and Romana‐Souza, B. Olive oil‐induced reduction of oxidative damage and inflammation promotes wound healing of pressure ulcers in mice. J. Dermatol. Sci. 83, 60–69 (2016). [DOI] [PubMed] [Google Scholar]
- 37. Zahmatkesh, M. , Manesh, M. and Babashahabi, R. Effect of Olea ointment and Acetate Mafenide on burn wounds – a randomized clinical trial. Iran. J. Nurs. Midwifery Res. 20, 599–603 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Budiyanto, A. , Ahmed, N. , Wu, A. et al Protective effect of topically applied olive oil against photocarcinogenesis following UVB exposure of mice. Carcinogenesis 21, 2085–2090 (2000). [DOI] [PubMed] [Google Scholar]
- 39. Danby, S. , Al Enezi, T. , Sultan, A. , Lavender, T. , Chittock, J. , Brown, K. , Cork, M.J. Effect of olive and sunflower seed oil on the adult skin barrier: implications for neonatal skin care. Pediatr. Dermatol. 30, 42–50 (2013). [DOI] [PubMed] [Google Scholar]
- 40. Cooke, A. , Cork, M. , Victor, S. , Campbell, M. , Danby, S. , Chittock, J. , Lavender, T. Olive oil, sunflower oil or no oil for baby dry skin or massage: a pilot, assessor‐blinded, randomized controlled trial (the oil in Baby SkincaRE [OBSeRvE] study). Acta Derm. Venereol. 96, 323–330 (2016). [DOI] [PubMed] [Google Scholar]
- 41. Norlen, L. Is oil a balsam for baby skin? Acta Derm. Venereol. 96, 291 (2016). [DOI] [PubMed] [Google Scholar]
- 42. Bielfeldt, S. , Schoder, V. , Ely, U. , Van Der Pol, A. , De Sterke, J. , Wilhelm, K.P. Assessment of human stratum corneum thickness and its barrier properties by in‐vivo confocal Raman spectroscopy. Int. J. Cosmet. Sci. 31, 479–480 (2009). [Google Scholar]


