Abstract
Blood coagulation factor Va serves an indispensable role in hemostasis as cofactor for the serine protease factor Xa. In the presence of an anionic phospholipid membrane and calcium ions, factors Va and Xa assemble into the prothrombinase complex. Following formation of the ternary complex with the macromolecular zymogen substrate prothrombin, the latter is rapidly converted into thrombin, the key regulatory enzyme of coagulation. Over the years, multiple binding sites have been identified in factor Va that play a role in the interaction of the cofactor with factor Xa, prothrombin, or the anionic phospholipid membrane surface. In this review, an overview of the currently available information on these interactive sites in factor Va is provided, and data from biochemical approaches and 3D structural protein complex models are discussed. The structural models have been generated in recent years and provide novel insights into the molecular requirements for assembly of both the prothrombinase and the ternary prothrombinase‐prothrombin complexes. Integrated knowledge of functionally important regions in factor Va will allow for a better understanding of factor Va cofactor activity.
Keywords: binding sites, coagulation factor V, coagulation factor Xa, prothrombin activation, prothrombinase complex
1. INTRODUCTION
Factor Va (FVa) is a blood coagulation protein that is central to the formation of a blood clot following vascular injury. It is generated from its precursor or procofactor factor V (FV) by limited proteolysis and removal of the inhibitory B domain1, 2; for a review on FV activation see Camire and Bos.3 Once FV is activated, it is able to function as a cofactor to the serine protease factor Xa (FXa) in the so‐called prothrombinase complex that is responsible for converting prothrombin to the key regulatory enzyme thrombin. Factor Va assembly with FXa into the prothrombinase complex occurs exclusively in the presence of Ca2+ and on a phosphatidylserine‐exposing lipid membrane provided by activated platelets or endothelial cells. Although FXa is capable of activating prothrombin in the absence of its cofactor, assembly into the prothrombinase complex increases the conversion rate by five orders of magnitude,4 illustrating the essential role of FVa in coagulation. In this review, we will focus on the structural requirements of FVa to assemble into the prothrombinase complex and engage prothrombin, thereby forming the ternary complex. For this purpose, data from in vitro studies and 3D structural protein complex models are discussed. The structural models have been generated in recent years and provide novel insights into the interactive sites in FVa that are involved in the assembly of the prothrombinase and the ternary complexes. Earlier reviews have focused on the general functionality of FV under normal conditions and in disease states; see Kalafatis and Mann5 and Nicolaes and Dahlbäck.6
2. THE FACTOR V PROTEIN STRUCTURE
Factor V circulates as a 330‐kDa procofactor and comprises an A1‐A2‐B‐A3‐C1‐C2 domain structure. The A domains of FV are ~30% identical to the copper‐binding plasma protein ceruloplasmin, while the FV C domains share ~20% sequence homology with the discoidin I‐like domain of phospholipid‐binding lectins in slime mold.7, 8 The large B domain of FV is poorly conserved among species.3 This domain comprises several tandem repeats, yet their role remains largely unknown.7, 8 The inhibitory effect of the B domain is maintained by a basic and a complementary acidic region, creating an autoinhibitory conformation that is considered to obscure FXa‐interactive sites.9, 10 Proteolytic activation of FV is facilitated by FXa and meizothrombin in the initiation phase of coagulation and subsequently by thrombin in the propagation phase.11, 12, 13, 14 Sequential cleavage at the B domain sites Arg709, Arg1018, and Arg1545 results in dissociation of the B domain and generation of the non‐covalently associated heavy (A1‐A2) and light chains (A3‐C1‐C2) that form FVa.2, 15, 16
Downregulation of the cofactor function of FVa is regulated by activated protein C (APC) and its cofactor protein S.17, 18 Activated protein C cleaves FVa at Arg306, Arg506, and Arg679 in the A2 domain.19, 20 The cleavage at Arg506 is kinetically preferred, causing a rapid loss of FVa cofactor activity, while the relatively slow cleavage at Arg306 is required for full FVa inactivation,19, 21 the latter resulting from dissociation of the complete A2 domain.22
The FV molecule contains a copper‐ion and calcium‐ion binding site, located between the A1 and A3 domains. Although the exact mechanism remains unclear, both ions likely stabilize the interaction between the FVa heavy and light chains and are crucial for the FVa cofactor activity.23, 24, 25, 26, 27, 28 Furthermore, FV undergoes multiple posttranslational modifications including glycosylation, sulfation, and phosphorylation5 (for a review on posttranslational modifications in blood coagulation proteins see Hansson and Stenflo29). The 3D FV protein structure is further mediated by seven intradomain disulfide bridges in the A and C domains.30, 31
3. THE FACTOR Va‐FACTOR Xa INTERACTION
To gain insight into the molecular requirements of protein‐protein interactions, the generation of crystal structures of proteins in combination with mutagenesis approaches has greatly contributed to unravel these characteristics. While crystal structures are available for both FVa and FXa, they are lacking for the human prothrombinase complex. As a result, the 3D structure of the human prothrombinase complex has been modeled on the basis of (partial) crystal structures and guided by biochemical studies. The first 3D model of prothrombinase dates from 2006 and was based on the crystal structures of APC‐inactivated bovine FVa and active site‐inhibited human FXa.32 Protein docking simulations revealed five representative prothrombinase structures, of which one proved consistent with most of the reported FVa‐FXa binding data. A second prothrombinase model reported in 2008 by Lee et al. was generated from Molecular Dynamics simulation data.33 Subsequently, the same group constructed the first ternary complex in 2011. By making use of the X‐ray crystal structure of human prethrombin‐1, the human prothrombin 3D structure was modeled and docked onto the prothrombinase complex.34 In 2013, a crystal structure of a prothrombinase‐like complex from the venom of the Australian eastern common brown snake became available.35 The components of this prothrombinase‐like complex, known as pseutarin C, associate with high affinity in the absence of a negatively charged lipid surface.36 As pseutarin C shares ~40% to 60% sequence identity with human FVa‐FXa,35 this crystal structure provides a reliable blueprint for molecular modeling of human prothrombinase. Consequently, the latter was generated by Huntington and coworkers.37 More recently, a full human ternary complex was reported,38 thereby providing important new insights in the structural organization of the prothrombinase complex and prothrombin binding. In the following sections, we will review the molecular details of the FVa‐FXa interactions, guided by the crystal structure of pseutarin C, structural homology models, and biochemical studies.
3.1. The A2 domain Arg306 region in factor Va
The FVa A2 domain has been suggested to play a critical role in the interaction of FVa with FXa, as several distinct FXa binding sites have been identified of which an overview can be found in Table S1. One of these sites is a region in close proximity of the APC cleavage site Arg306 (Figure 1). The first evidence for involvement of this region came from studies in which peptides of FV A2 domain sequences were assessed for their capacity to inhibit prothrombinase activity. In a screen of overlapping 15‐residue peptides covering FVa region Thr271‐Lys345, the peptide encompassing region Ile311‐Phe325 was found to inhibit prothrombin activation significantly in the presence of FVa, while full activity was maintained in the absence of FVa.39 These observations led Kojima et al. to conclude that this peptide competes for FXa binding. Supporting this finding, Kalafatis and Mann obtained evidence indicating that the FVa clotting activity was inhibited by a 42‐amino‐acid peptide comprising residues Asn307‐Arg348.40 Further studies making use of both FV peptides and recombinant FV variants with targeted amino acid substitutions suggested amino acids Glu323, Tyr324, Glu330, Val331, Asp334, and Tyr335 as a FXa‐binding cluster.41, 42, 43
Figure 1.
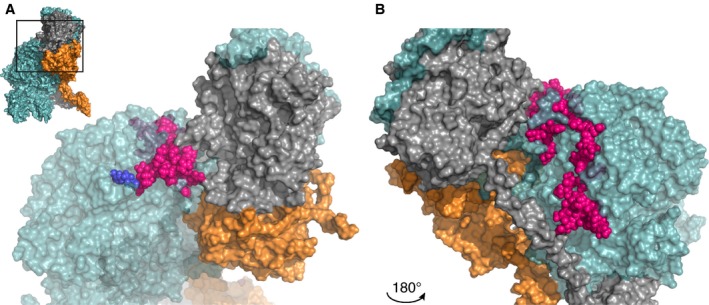
Interface between the factor Va A2 domain Arg306 region and factor Xa or prothrombin. A zoomed‐in region of the human ternary model by Shim et al. is shown38; the insert in panel A outlines the specific region depicted. Panel B displays the same region after a 180° rotation around the Y‐axis. The region (Asn307‐Lys386, specified in Tables S1 and S3) surrounding the Arg306 site (indicated in blue) is in close proximity to prothrombin, suggesting that this specific region is a binding site for prothrombin rather than for FXa. FVa amino acids implicated as contact residues are highlighted in pink; FVa (teal), FXa (orange), and prothrombin (gray) are indicated. See Movie S1 for a 3D overview. Abbreviations: FVa, factor Va; FXa, factor Xa
Interestingly, the crystal structure of pseutarin C reveals that these residues are located at a relatively far distance from FXa (>20 Å). In pseutarin C, only Met318, homologous to human Arg317, was found to interact with FXa,35 and similar observations were made for recent homology models of human prothrombinase37, 38 (Figure 1; Table S1). It therefore seems unlikely that the A2 domain Arg306 region in FVa is directly contributing to FXa binding, but rather seems to be involved in the interaction with prothrombin (see also Section 4). This would be in agreement with the findings by Norstrøm and colleagues, who reported that the Arg306 cleavage site is not protected by FXa,44 suggesting that APC and FXa do not share the Arg306 region in FVa as binding site.
3.2. The A2 domain Arg506 region in factor Va
In contrast to the Arg306 region in the FVa A2 domain, the Arg506 cleavage site is known to be protected from APC‐dependent proteolysis by FXa,17, 44 indicating that this region harbors a competitive binding site for FXa and APC. Several other studies have provided further evidence for a FXa‐binding site in the Arg506 region of the FVa A2 domain (Figure 2; Table S1). For instance, a Gly493‐Arg506 FV peptide was found to inhibit prothrombin activation.45 In addition, a FVa variant in which residues Lys499‐Arg505 were swapped for the homologous FVIIIa residues Tyr555‐Gln561 revealed a significantly reduced FXa affinity, while Arg506 cleavage by APC was unaffected.46 Further evidence was provided by site‐directed glycosylation studies, demonstrating that introduction of N‐glycosylation sites at FVa residues Glu467 and Ala511 attenuated FXa binding, while the affinity for prothrombin remained unaffected.47 Still, these results could potentially be caused by steric hindrance rather than disrupting direct interactions between FVa and FXa. A direct assessment approach in which several charged residues were mutated to neutral amino acids revealed that 4 of the 15 FVa variants exhibited a markedly reduced FXa binding and cofactor activity, thereby identifying an A2 domain binding cluster of Arg501, Arg510, Ala511, Asp513, Asp577, and Asp578.48 Structural analysis confirms that these residues are all located on the A2 domain surface in close proximity to FXa (<4 Å) (Figure 2). Furthermore, these residues and their homologs in pseutarin C have been implicated as FXa contact residues.35, 37, 38
Figure 2.
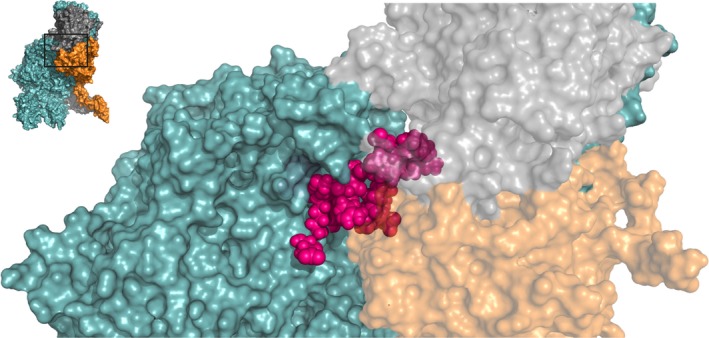
The factor Va A2 domain Arg506 region functions as a binding site for both factor Xa and prothrombin. A zoomed‐in region of the human ternary model by Shim et al. is shown38; the insert outlines the specific region depicted. A cluster of amino acids between Glu467‐Ile514, specified in Tables S1 and S3, forms a binding site for factor Xa, while residues Leu503, Arg505, and Arg506 interact with both FXa and prothrombin. FVa amino acids implicated as contact residues are indicated in pink; FVa (teal), FXa (orange), and prothrombin (gray) are indicated. See Movie S2 for a 3D overview. Abbreviations: FVa, factor Va; FXa, factor Xa
3.3. The A2 domain C‐terminus (A2T) in factor Va
During the activation of FV, the B domain is cleaved and dissociates, resulting in a non‐covalently linked FVa heavy and light chain.3 The resulting A2 domain C‐terminus (A2T) forms a long negatively charged tail that has been implicated as a FXa‐interactive site (Figure 3; Table S1). Initial evidence came from a study in which FVa was treated with a protease from the venom of the snake Naja naja oxiana, which resulted in the cleavage of a 27‐amino‐acid peptide (Asp683‐Arg709) from the FVa A2T.49 The resulting cofactor, FVaNO, displayed a markedly reduced affinity for both FXa and prothrombin, while the catalytic efficiency of prothrombinase‐assembled FVaNO was comparable to that of FVa. This finding indicates that the A2T plays a role in the assembly of the ternary complex rather than affecting its cofactor activity.
Figure 3.
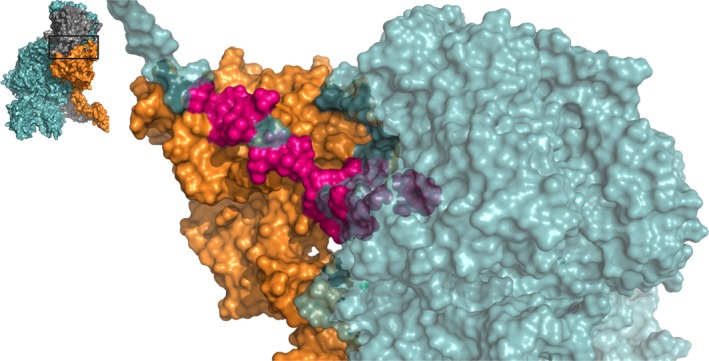
The factor Xa binding region in the A2 domain C‐terminus of factor Va. A zoomed‐in region of the human ternary model by Shim et al. is shown38; the insert outlines the specific region depicted. Several residues in the A2‐domain C‐terminus, spanning from Cys575 to Pro670, as specified in Table S1, are involved in the assembly of the prothrombinase complex by interacting with the heparin‐binding site of FXa. FVa amino acids implicated as contact residues are indicated in pink; FVa (teal) and FXa (orange) are indicated; prothrombin has been removed for clarity. See Movie S3 for a 3D overview. Abbreviations: FVa, factor Va; FXa, factor Xa
Although no crystal structure is available and only structural models exist of the complete A2T, part of the structural interactions (up to Glu683) were revealed by the pseutarin C structure.35 In this crystal structure, the A2T residues Asn656, Tyr657, and Asp671‐Glu683 interact with the highly basic heparin‐binding site of FXa. Interestingly, similar interactions have been reported for human FXa in studies in which potential FVa‐binding residues were identified using inhibitory peptides or a mutagenesis approach.50, 51 The A2T may therefore function as a FXa recognition site and is as such important to prothrombinase complex assembly. However, the exact contribution of the A2T region to the interaction with FXa remains to be determined.
3.4. The factor Va A3 domain
The FVa light chain has been implicated in prothrombinase assembly through the FVa A3 domain‐FXa EGF domain interaction (Figure 4; Table S2). Most data on the FVa residues involved in this interaction have been derived from structural models, and few biochemical studies have provided evidence for the involvement of the A3 domain in FXa binding. A monoclonal antibody recognizing the FVa region Asp1537‐Lys1752 was found to reduce FXa binding and prothrombin conversion dramatically.52 Further data uncovered a possible role for the region surrounding His1683, as introduction of a glycan at this position fully abrogated FXa binding and greatly decreased thrombin formation.48 Furthermore, alanine substitution of residues Arg1551, Glu1650, Trp1665, and His1683 in FVa resulted in a minor reduction in FXa affinity.48 The aforementioned residues have also been identified as FXa contact residues in several prothrombinase structures.37, 38 Within the pseutarin C FV sequence Thr789‐Gln922 (equivalent to human FV Ser1546‐His1683), seven residues were found to be directly involved in FXa binding, while 16 other amino acids were considered as contact residues.35 Similar observations were made for the human homology models, revealing tight connections between the FXa EGF domains and the A3 domain of FVa.37, 38 Together, these studies highlight a role for the FVa A3 domain in prothrombinase complex assembly. These interactions might be important for the initial docking of the complex, allowing for subsequent productive interactions between the FVa A2 domain and FXa. As the structure of pseutarin C comprises a truncated FXa molecule that lacks the EGF1 domain,35 structural data on the interaction with the EGF1 domain are currently provided by prothrombinase models only.
Figure 4.
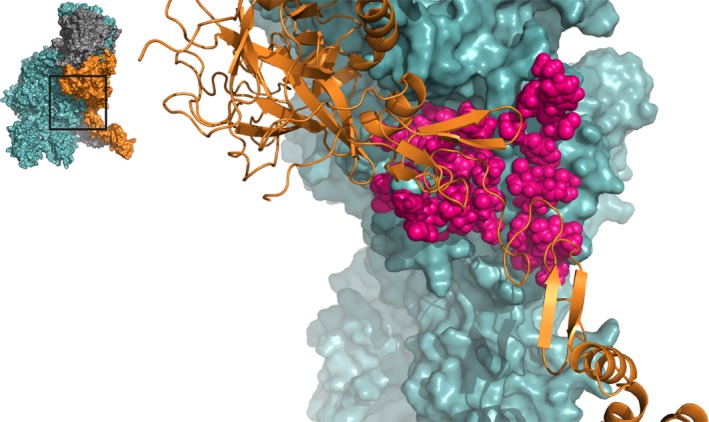
The factor Va A3 domain interacts with the serine protease and EGF domains of factor Xa. A zoomed‐in region of the human ternary model by Shim et al. is shown38; the insert outlines the specific region depicted. All highlighted residues (pink spheres) in FVa, spanning from Asn1547 to Lys1725 (specified in Table S2), are located close to FXa and may be directly involved in the formation of the prothrombinase complex. FVa (teal) and FXa (orange cartoon) are indicated; prothrombin has been removed for clarity. See Movie S4 for a 3D overview. Abbreviations: FVa, factor Va; FXa, factor Xa
4. THE FACTOR Va–PROTHROMBIN INTERACTION
Upon assembly of the prothrombinase complex, the ternary complex is formed following docking of the macromolecular substrate prothrombin. Efficient docking of prothrombin is crucial for its conversion to thrombin, which requires limited proteolysis by FXa at cleavage sites at Arg271 and Arg32053; for a review on prothrombin activation see Krishnaswamy.54 Both molecular models that have been generated for the human ternary complex provide a template for prothrombin binding.34, 38 In addition, several experimental studies have provided evidence for prothrombin binding sites on the FVa heavy and light chains. In the next sections, we will describe the interactive sites in FVa for prothrombin that have been identified on the basis of biochemical studies and the structural models of the ternary complex.
4.1. The A2 domain Arg306 and Arg506 region in factor Va
As described in previous sections, the regions surrounding positions 306 and 506 in the FVa A2 domain have been implicated in FXa binding on the basis of results from both structural models and in vitro studies. In most in vitro studies, a reduction in FVa cofactor activity was linked to an impaired interaction of FVa with either FXa or prothrombin. However, an exact determination of the affected binding partner would require analysis of direct protein‐protein interactions. As a consequence, the regions that have been implicated in FXa binding could function as prothrombin‐binding sites, and vice versa. In support of this, several prothrombin binding residues that are in close proximity to the Arg306 region (Arg313, Arg316, and Arg317) or Arg506 region (Arg503, Arg505, and Arg506) have been identified following assessment of the ternary structural models (Figures 1 and 2; Table S3).34, 38 Interestingly, while FXa has been found to compete for APC‐dependent cleavage at Arg506 only,44 prothrombin is capable of protecting both Arg306 and Arg506 from cleavage by APC.55 This suggests that the Arg506 region in FVa interacts with both FXa and prothrombin, while the FVa Arg306 region acts solely as a prothrombin binding site. Considering these observations, we conclude that those FXa‐binding sites in the FVa Arg306 region that have been identified on the basis of prothrombinase and/or clotting activity analyses might in fact be responsible for the interaction with prothrombin.
4.2. The A2T in factor Va
Apart from its role in the interaction with FXa, the FVa A2T may also serve an important role in prothrombin binding (Figure 5; Table S3). For instance, FVa cleaved at Arg679 by a venom protease found in Naja nigricollis nigricollis exhibited a 60% to 80% reduced clotting activity.56 The study further revealed that a synthetic peptide comprising residues Asp697‐Arg709 was found to inhibit prothrombinase activity competitively by interfering with prothrombin binding, while the FVa‐FXa binding remained unaffected. Similarly, the Asp697‐Arg709 peptide was found to interact directly with thrombin‐agarose, suggestive of a role in prothrombin rather than FXa binding. Next, a FV peptide encompassing region Asp695‐Gln699 (DYDYQ) was observed to interfere with the incorporation of prothrombin and impaired prothrombin conversion.57 In FVa region Asp695‐Gln698, both tyrosines (Tyr696, Tyr698) are sulfated and have been suggested to facilitate the initial binding of prothrombin, thereby securing the position of prothrombin and its cleavage sites Arg271 and Arg320 close to the FXa active site.38 This notion was further supported by alanine substitution of residues Asp695‐Gln698, which resulted in a surprising ~20% increase in k cat for the activation of prothrombin, while, in contrast, an accumulation of meizothrombin and subsequent delay in prothrombin activation were observed.58 Similar results were obtained for FVa variants in which residues Asp659‐Asp663 were either mutated or deleted,59 or in which residues Asn700‐Arg701 were replaced for the bovine homologs Asp‐Glu.60 These results indicate that the A2T plays a prominent role in both the incorporation and catalysis of prothrombin.
Figure 5.
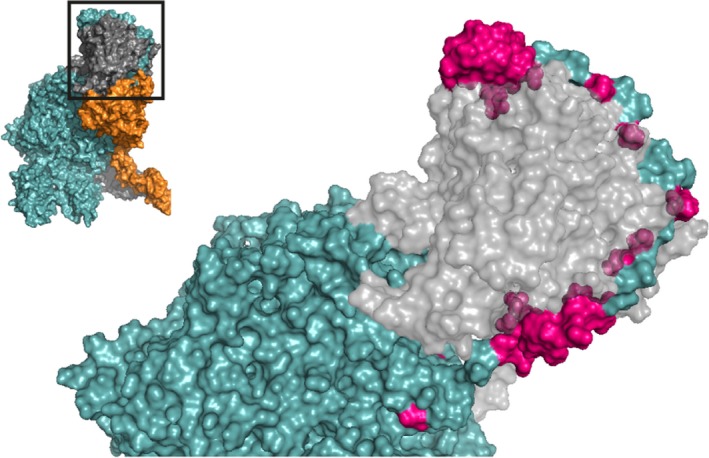
The factor Va A2 domain C‐terminus is predicted to wrap around the serine protease domain of prothrombin. A zoomed‐in region of the human ternary model by Shim et al. is shown38; the insert outlines the specific region depicted. Asp628‐Arg709 (highlighted in pink, specified in Table S3) within the A2 domain C‐terminus has been implicated to function as a prothrombin binding site. This region forms an extended arm that likely facilitates the docking of prothrombin onto the prothrombinase complex. FVa (teal) and prothrombin (gray) are indicated; FXa has been removed for clarity. See Movie S5 for a 3D overview. Abbreviations: FVa, factor Va; FXa, factor Xa
Despite the fact that multiple studies have shown the involvement of the A2T in prothrombin binding and conversion, contradictory studies have also been reported. For example, a 13‐residue C‐terminal peptide of the FV A2 domain (residues Asp697‐Arg709) was not able to inhibit the FVa clotting activity.40 Furthermore, recombinant truncation of the FVa heavy chain between Thr679 and Arg709 did not affect the FVa clotting activity, prothrombin conversion kinetics, or FXa binding.61 Conversely, truncation at Pro658 resulted in a markedly reduced clotting activity and FXa binding, although this variant showed normal prothrombinase kinetics when fully saturated with FXa and anionic membranes.61 Although prothrombin time‐based clotting assays may not be sufficiently sensitive to detect small functional differences in FVa cofactor activity, the observation that truncation of the FVa A2 domain C‐terminus failed to affect the interaction with FXa and/or prothrombin might suggest that this region contributes minimally to the assembly of the ternary complex.
The findings described here have thus far not been supported by mechanistic insights derived from crystal structures, as the FVa A2T has not been fully resolved in the available X‐ray structures of the FV A2 domain. Currently, only an X‐ray structure of active site‐inhibited thrombin bound to FVa residues Glu666‐Glu672 has been reported,62 revealing that these FVa residues interact with exosite I in thrombin. Interestingly, in addition to the reported X‐ray structure, the authors modeled the complete A2T (Ile657‐Arg709) as a peptide wrapping around thrombin, thereby covering a large surface of productive interactions. Similarly modeled interactions of the FVa A2T were obtained from the ternary model created by Shim et al.38 In this model, the A2T first interacts with the heparin‐binding site of FXa and subsequently binds prothrombin, thereby acting as a long arm grasping prothrombin and assisting in its assembly into the ternary complex (Figure 3). Collectively, these structural studies indicate a dual role for the A2T, being involved in both FXa and prothrombin binding. On the basis of the available data, we suggest that the N‐terminal segment of the A2T may interact with FXa, thereby contributing to prothrombinase formation. Subsequently, the remaining C‐terminal part of the A2T region might interact with prothrombin to facilitate efficient substrate docking onto the prothrombinase complex. However, until high‐resolution X‐ray structures of the ternary complex become available, the exact molecular interactions facilitating the interaction of the FVa A2T with both FXa and prothrombin remain undefined.
4.3. The A3 and C1‐C2 domains in factor Va
Currently, direct evidence supporting binding of prothrombin and the FVa light chain is absent. Yet, interactions between the Kringle domains of prothrombin and FVa have been reported. Characterization of prothrombin mutants in which either the Kringle 1 or Kringle 2 domain was deleted suggested that the Kringle 2, but not the Kringle 1 domain, interacts with FVa.63 In contrast, in a later study both Kringle domains of prothrombin were found to contribute to FVa binding.64 It is generally acknowledged that the Kringle 1 domain is located close to the membrane layer as it is separated via a small linker from the Gla domain,65 the latter facilitating phospholipid binding. Given the structural orientation of prothrombin and the Kringle 1 domain, the Kringle 1 domain could have the potential to interact with the FVa A3 domain and/or with the lipid‐binding C domains. Such interactions were observed in the two ternary models, revealing significant interactions between the Kringle 1 and Gla domains of prothrombin and the A3 and C1 domains of FVa.34, 38 Thus far, support for this has come from one in vitro study in which a peptide comprising the sequence of prothrombin's Gla domain (residues 1‐46) was demonstrated to inhibit prothrombinase activity and to interact directly with FVa.66 While the binding site in FVa is likely located in the lipid‐binding light chain, the contribution of the FVa A3‐C1‐C2 domains to prothrombin assembly remains largely unclear. Collectively, the current models and sparse in vitro data suggest that interactive regions are located in the A3 and C1 domains (Figure 6; Tables S4 and S5). Nevertheless, future studies focused on providing solid evidence for the contribution of the FVa A3‐C1 domains to prothrombin binding are required.
Figure 6.
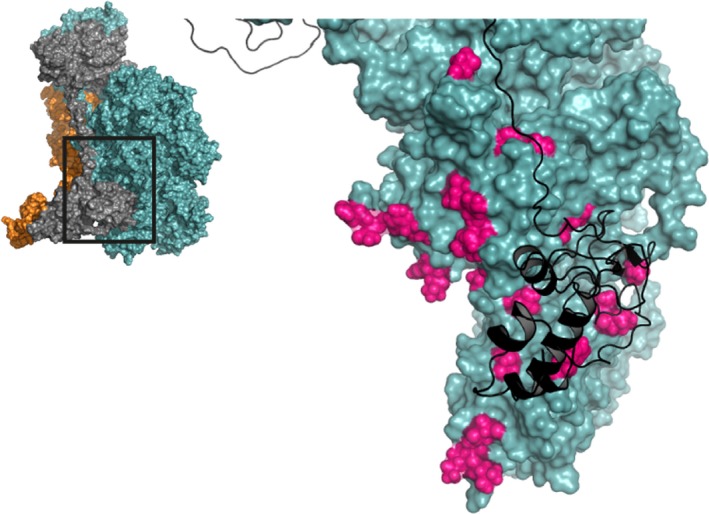
The interaction of the factor Va A3 and C1 domains with prothrombin. A zoomed‐in region of the human ternary model by Shim et al. is shown38; the insert outlines the specific region depicted. Several prothrombin‐interactive regions have been suggested for the FVa A3 and C1 domains (highlighted in pink, specified in Tables S4 and S5) and are proposed to interact with the Kringle 2 and Gla domains of prothrombin. FVa (teal) and prothrombin (black cartoon) are indicated; FXa has been removed for clarity. See Movie S6 for a 3D overview. Abbreviations: FVa, factor Va; FXa, factor Xa
5. THE FACTOR Va–ANIONIC MEMBRANE INTERACTION
A negatively charged membrane surface comprising PS is essential for the formation of the ternary prothrombinase‐prothrombin complex. This anionic phospholipid layer provides a binding surface for the ternary complex assembly and is mainly provided by the membranes of activated platelets and endothelial cells. The interaction of FVa with the PS‐rich membrane is facilitated by its C1 and C2 domains. These domains contain several protruding spikes that are orientated toward the lipid layer. Hydrophobic and positively charged residues are located at the apex of these spikes and have been implicated as the lipid‐binding sites.67, 68 Here, we will review the studies providing evidence for these membrane binding sites and describe our current understanding of the key residues facilitating such interactions.
5.1. The C1 domain of factor Va
Interaction of FVa with the negatively charged membrane surface is, together with contributions of the C2 domain, mediated by the immersion of hydrophobic C1 domain spikes into the phospholipid bilayer (Figure 7; Table S6). The FVa C1 domain was initially considered to facilitate phospholipid binding because of the observation that FVa had a higher membrane affinity relative to the isolated FVa C2 domain.69 Using alanine‐scanning mutagenesis, several membrane binding residues were identified in the C1 domain.70 Of 20 generated FVa variants, alanine substitution of the residue pairs Lys1954 and His1955, Tyr1956 and Leu1957, and Arg2023 and Arg2027 revealed an impaired binding to immobilized PS. Further characterization showed significantly reduced prothrombinase activity of the Tyr1956 and Leu1957, and Arg2023 and Arg2027 substituted variants, although this was only observed at limiting phospholipid concentrations. Conversely, the Lys1954 and His1955 mutated FVa variant displayed a similar prothrombinase activity compared to wild‐type FVa,70 suggesting that these residues might be involved in FVa‐lipid binding but are not essential for prothrombinase assembly. Later studies demonstrated that the binding site for a soluble form of PS (C6PS) was lost upon alanine substitution of the hydrophobic residues Tyr1956 and Leu1957.71, 72 Binding of FVa to soluble PS has been shown to increase the affinity of FVa for FXa.73, 74 Interestingly, substitution of Tyr1956 and Leu1957 resulted in an approximately 200‐fold decreased affinity for FXa in the presence of soluble C6PS,71 and a ~4‐fold reduced affinity was observed on PS membranes.70 Still, at saturating concentrations of FVa, FXa, and PS membranes, the maximal rate of prothrombin activation was indistinguishable from wild‐type FVa. Therefore, mutating residues Tyr1956 and Leu1957 seems to affect the assembly of FVa and prothrombinase on lipid membranes rather than affecting the activity while assembled. This might suggest that binding of the C1 domain to the lipid surface induces conformational changes in FVa, resulting in an enhanced affinity for FXa. It has been proposed that these changes may induce rearrangements in the A2 domain, the main binding region for FXa.71, 75 Alternatively, the conformational changes may also result in the presentation of an FXa binding site on the FVa C1 and/or C2 domain, although no interactions between the C domains and FXa have been identified in recent structural models.37, 38
Figure 7.
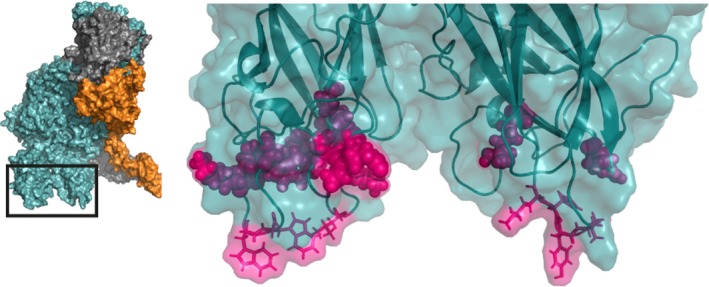
The factor Va C1 and C2 domains facilitate interaction with the anionic lipid membrane surface. A zoomed‐in region of the human ternary model by Shim et al. is shown38; the insert outlines the specific region depicted. The hydrophobic protruding spikes of the C1 and C2 domains (shown in stick configuration) insert into the anionic lipid membrane layer. Additional interactions are provided by the groove between the C domains (residues spanning Lys1954‐Arg2027 and Lys2060‐Arg2187, specified at Tables S6 and S7). FVa amino acids implicated as contact residues are indicated in pink, FVa is indicated in teal. See Movie S7 for a 3D overview. Abbreviations: FVa, factor Va; FXa, factor Xa
5.2. The C2 domain of factor Va
The C2 domain plays a pivotal role in the binding of FVa to the lipid membrane layer, which involves both hydrophobic and electrostatic interactions (Figure 7; Table S7). The first studies demonstrating C2 domain binding to the lipid surface revealed an impaired PS binding upon either deletion of the C2 domain or use of specific inhibitory antibodies directed against the C2 domain.76, 77, 78 Subsequently, chimeric FV variants generated by exchange of exon‐size sequences for the corresponding FVIII regions revealed that the N‐terminal region of the C2 domain (residues Gly2037‐Lys2087) was involved in PS binding.79 In 1998, the first models of the FV C1 and C2 domains, based on the crystal structure of the homologous D1 domain of galactose oxidase, were reported.80 A cluster of hydrophobic/aromatic residues (Lys2060, Lys2061, Trp2063, and Trp2064) was identified in a solvent‐exposed loop in the C2 domain, suggesting a role in membrane binding.80 This suggestion was strengthened by the crystal structure of the C2 domain of FV, which revealed three protruding spikes containing hydrophobic and polar residues, with Trp2063, Trp2064 (spike 1), and Leu2116 (spike 3) suggested as particular candidates for membrane immersion.81 Subsequent alanine‐scanning revealed a critical role for Trp2063 and Trp2064 in PS binding.72, 82, 83, 84, 85, 86 Mutating both residues resulted in a markedly reduced membrane binding affinity in addition to an attenuated prothrombinase activity. However, the attenuated activity was only observed in conditions with limiting PS concentrations (<10%) and was restored in the presence of 25% PS membranes with saturating concentrations of FVa and FXa. Therefore, these results indicate that Trp2063 and Trp2064 are important for PS‐membrane binding, but do not affect the intrinsic activity of FVa. The fact that FVa variants comprising mutations at key lipid‐binding residues fail to show abrogated prothrombinase activity on membranes containing >20% PS may suggest the involvement of other regions in FVa‐membrane binding. However, given that physiological concentrations of PS are considered to be in the range of 5% to 10%,87, 88 the contribution of individual binding sites should be assessed employing relevant compositions of negatively charged phospholipids and direct membrane‐binding assessments.
Two FV isoforms, FV1 and FV2, that exist in plasma display different FVa cofactor activities and slightly different molecular weights as a result of heterogeneity in the light chain caused by partial glycosylation at Asn2181.89, 90, 91 The Asn2181‐glycosylated FVa1 displays an impaired lipid binding and FVa cofactor activity compared to the non‐glycosylated form FVa2. Yet, the reduced prothrombin conversion could only be detected in the presence of limiting PS concentrations; once assembled, FVa1 and FVa2 exhibit comparable catalytic efficiencies.89 These results indicate that although Asn2181 does not directly contribute to membrane binding, glycosylation of Asn2181 interferes with the binding of FVa to PS membranes.
Interestingly, the crystal structure of the FVa C2 domain revealed two distinct conformations referred to as an “open form” and “closed form” that correspond to a membrane binding state and a circulating state, respectively.81 In the open form, the three protruding spikes create a deep groove lined by hydrophobic and polar residues that are ideally arranged to interact with the PS head groups. The hydrophobic residues located at the apex of the spikes could thereby facilitate the initial binding and immersion into the membrane layer. This allows for the formation of additional interactions between the interior of the groove (residues Trp2064, Trp2068, Met2120, and Leu2116) and the PS head groups. An additional PS‐binding site might be localized between the second and third spikes consisting of residues Gln2078, Gly2079, Asn2082, Lys2114, and Arg2187.81 This basic region would be more accessible for acidic phospholipids, explaining the preference of FVa for acidic membranes.92 In the closed form, the first and third spikes are slightly tilted and as a result the interior of the groove is covered by the side chains of Trp2063, Trp2064, and Leu2116, leading to a markedly reduced hydrophobic exposed surface.
5.3. The C1 and C2 domains of factor Va
A mechanistic model describing the FVa C1‐C2‐lipid interaction has been proposed.69, 71 The C2 domain, and perhaps also the C1 domain, circulate in a closed state preventing phospholipid binding and prothrombinase assembly. The basic and polar residues surrounding the binding spikes facilitate the approach to the negatively charged PS membrane. The subsequent PS membrane binding triggers the open state of the C domains, resulting in membrane insertion of hydrophobic residues Tyr1956 and Leu1957 (C1 domain), Trp2063 and Trp2064 (C2 domain), and subsequent interactions between the lipid‐binding groove and PS head groups. Initial membrane binding may be facilitated by the C2 domain,71 and subsequent anchoring of FVa to the phospholipid layer allows the C1 membrane to interact, establishing FVa membrane binding. This proposed mechanism is in agreement with molecular dynamics simulations studies on the homologous FVIII C domains, in which the FVIII C2 domain is the first to interact with the membrane layer, followed by membrane immersion of the C1 spikes.93
6. SUMMARY
In this review, we have evaluated the regions in FVa that are responsible for the interaction with FXa, prothrombin, and the negatively charged lipid membrane. As FVa plays a central role in hemostasis, the binding sites in FVa are essential functional regions that mediate full prothrombinase activity. Important interactive regions for FXa are mainly located within the A2 domain of FVa. The two main FXa‐binding sites within this domain are the A2T and the region surrounding Arg506. Although the Arg306‐surrounding region has previously been implicated as a FXa‐binding site, the pseutarin C structure and homology models of the ternary prothrombinase‐prothrombin complex reveal that this region is likely involved in prothrombin binding rather than FXa binding. Instead, the Arg506 and A2T regions were found to play a dual role, as they contribute to both FXa and prothrombin binding. While the exact contribution of the A2T remains to be elucidated, current observations suggest a role in the initial binding of prothrombin and its positioning close to the FXa active site to facilitate efficient proteolytic conversion to thrombin. Last, we summarized the interaction of FVa with the lipid membrane layer. Membrane binding is facilitated by both C domains, in which the hydrophobic residues of several protruding spikes insert into the membrane layer. These membrane interactions may result in conformational changes within the C domains that have been suggested to induce structural rearrangements that allow for assembly of the ternary complex.
Collectively, this review has provided an overview of the essential binding sites in FVa for incorporation into the prothrombinase and functional ternary complexes. Unfortunately, X‐ray structures of the complete prothrombinase or ternary complexes are lacking, limiting our knowledge to available structural models and biochemical data. Nevertheless, in the last decades considerable knowledge has been gained, as a crystal structure of the prothrombinase‐like pseutarin C complex has been generated and several structural models of the prothrombinase and ternary complexes have become available. While some issues concerning the molecular details and mechanisms of FVa binding remain, the current overview will provide a framework for future research and novel approaches to uncover details on binding sites and functional regions in FVa.
CONFLICT OF INTEREST
P. H. Reitsma owns equity in VarmX B.V. The remaining authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
M. Schreuder drafted the manuscript; P. H. Reitsma reviewed the manuscript; M. H. A. Bos revised the manuscript.
Supporting information
ACKNOWLEDGMENTS
This work was financially supported by the Bayer Hemophilia Awards Program (Special Project Award) and Landsteiner Foundation for Blood Transfusion (LSBR, grant no. 1451). The funding agencies had no role in the preparation, review, or approval of the manuscript.
Schreuder M, Reitsma PH, Bos MHA. Blood coagulation factor Va's key interactive residues and regions for prothrombinase assembly and prothrombin binding. J Thromb Haemost. 2019;17:1229–1239. 10.1111/jth.14487
Manuscript handled by: Rodney Camire
Final decision: Rodney Camire, 7 May 2019
REFERENCES
- 1. Esmon CT, Owen WG, Duiguid DL, Jackson CM. The action of thrombin on blood clotting factor V: conversion of factor V to a prothrombin‐binding protein. Biochim Biophys Acta. 1973;310:289–94. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki K, Dahlback B, Stenflo J. Thrombin‐catalyzed activation of human coagulation factor V. J Biol Chem. 1982;257:6556–64. [PubMed] [Google Scholar]
- 3. Camire RM, Bos MH. The molecular basis of factor V and VIII procofactor activation. J Thromb Haemost. 2009; 7: 1951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface‐dependent reactions of the vitamin K‐dependent enzyme complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 5. Kalafatis M, Mann KG. Factor V: Dr. Jeckyll and Mr. Hyde. Blood. 2003;101:20–30. [DOI] [PubMed] [Google Scholar]
- 6. Nicolaes GA, Dahlback B. Factor V and thrombotic disease: description of a janus‐faced protein. Arterioscler Thromb Vasc Biol. 2002;22:530–8. [DOI] [PubMed] [Google Scholar]
- 7. Kane WH, Davie EW. Cloning of a cDNA coding for human factor V, a blood coagulation factor homologous to factor VIII and ceruloplasmin. Proc Natl Acad Sci USA. 1986;83:6800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jenny RJ, Pittman DD, Toole JJ, Kriz RW, Aldape RA, Hewick RM, et al. Complete cDNA and derived amino acid sequence of human factor V. Proc Natl Acad Sci USA. 1987;84:4846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bos MH, Camire RM. A bipartite autoinhibitory region within the B‐domain suppresses function in factor V. J Biol Chem. 2012; 287: 26342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bunce MW, Bos MH, Krishnaswamy S, Camire RM. Restoring the procofactor state of factor Va‐like variants by complementation with B‐domain peptides. J Biol Chem. 2013; 288: 30151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foster WB, Nesheim ME, Mann KG. The factor Xa‐catalyzed activation of factor V. J Biol Chem. 1983;258:13970–7. [PubMed] [Google Scholar]
- 12. Monkovic DD, Tracy PB. Activation of human factor V by factor Xa and thrombin. Biochemistry. 1990;29:1118–28. [DOI] [PubMed] [Google Scholar]
- 13. Schuijt TJ, Bakhtiari K, Daffre S, Deponte K, Wielders SJ, Marquart JA, et al. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation. 2013; 128: 254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tans G, Nicolaes GA, Thomassen MC, Hemker HC, van Zonneveld AJ, Pannekoek H, et al. Activation of human factor V by meizothrombin. J Biol Chem. 1994;269:15969–72. [PubMed] [Google Scholar]
- 15. Nesheim ME, Mann KG. Thrombin‐catalyzed activation of single chain bovine factor V. J Biol Chem. 1979;254:1326–34. [PubMed] [Google Scholar]
- 16. Dahlback B. Human coagluation factor V purification and thrombin‐catalyzed activation. J Clin Invest. 1980; 66: 583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosing J, Hoekema L, Nicolaes GA, Thomassen MC, Hemker HC, Varadi K, et al. Effects of protein S and factor Xa on peptide bond cleavages during inactivation of factor Va and factor VaR506Q by activated protein C. J Biol Chem. 1995;270:27852–8. [DOI] [PubMed] [Google Scholar]
- 18. Dahlback B, Villoutreix BO. The anticoagulant protein C pathway. FEBS Lett. 2005; 579: 3310–6. [DOI] [PubMed] [Google Scholar]
- 19. Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994;269:31869–80. [PubMed] [Google Scholar]
- 20. Egan JO, Kalafatis M, Mann KG. The effect of Arg306–>Ala and Arg506–>Gln substitutions in the inactivation of recombinant human factor Va by activated protein C and protein S. Protein Sci. 1997; 6: 2016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalafatis M, Mann KG. Role of the membrane in the inactivation of factor Va by activated protein C. J Biol Chem. 1993;268:27246–57. [PubMed] [Google Scholar]
- 22. Mann KG, Hockin MF, Begin KJ, Kalafatis M. Activated protein C cleavage of factor Va leads to dissociation of the A2 domain. J Biol Chem. 1997;272:20678–83. [DOI] [PubMed] [Google Scholar]
- 23. Hibbard LS, Mann KG. The calcium‐binding properties of bovine factor V. J Biol Chem. 1980;255:638–45. [PubMed] [Google Scholar]
- 24. Guinto ER, Esmon CT. Formation of a calcium‐binding site on bovine activated factor V following recombination of the isolated subunits. J Biol Chem. 1982;257:10038–43. [PubMed] [Google Scholar]
- 25. Mann KG, Lawler CM, Vehar GA, Church WR. Coagulation Factor V contains copper ion. J Biol Chem. 1984;259:12949–51. [PubMed] [Google Scholar]
- 26. Song J, Talbot K, Hewitt J, MacGillivray RT, Pryzdial EL. Differential contributions of Glu96, Asp102 and Asp111 to coagulation factor V/Va metal ion binding and subunit stability. Biochem J. 2009; 422: 257–64. [DOI] [PubMed] [Google Scholar]
- 27. Sorensen KW, Nicolaes GA, Villoutreix BO, Yamazaki T, Tans G, Rosing J, et al. Functional properties of recombinant factor V mutated in a potential calcium‐binding site. Biochemistry. 2004; 43: 5803–10. [DOI] [PubMed] [Google Scholar]
- 28. Adams TE, Hockin MF, Mann KG, Everse SJ. The crystal structure of activated protein C‐inactivated bovine factor Va: implications for cofactor function. Proc Natl Acad Sci USA. 2004; 101: 8918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansson K, Stenflo J. Post‐translational modifications in proteins involved in blood coagulation. J Thromb Haemost. 2005; 3: 2633–48. [DOI] [PubMed] [Google Scholar]
- 30. Xue J, Kalafatis M, Mann KG. Determination of the disulfide bridges in factor Va light chain. Biochemistry. 1993;32:5917–23. [DOI] [PubMed] [Google Scholar]
- 31. Xue J, Kalafatis M, Silveira JR, Kung C, Mann KG. Determination of the disulfide bridges in factor Va heavy chain. Biochemistry. 1994;33:13109–16. [DOI] [PubMed] [Google Scholar]
- 32. Autin L, Steen M, Dahlback B, Villoutreix BO. Proposed structural models of the prothrombinase (FXa‐FVa) complex. Proteins. 2006; 63: 440–50. [DOI] [PubMed] [Google Scholar]
- 33. Lee CJ, Lin P, Chandrasekaran V, Duke RE, Everse SJ, Perera L, et al. Proposed structural models of human factor Va and prothrombinase. J Thromb Haemost. 2008; 6: 83–9. [DOI] [PubMed] [Google Scholar]
- 34. Lee CJ, Wu S, Pedersen LG. A proposed ternary complex model of prothrombinase with prothrombin: protein‐protein docking and molecular dynamics simulations. J Thromb Haemost. 2011; 9: 2123–6. [DOI] [PubMed] [Google Scholar]
- 35. Lechtenberg BC, Murray‐Rust TA, Johnson DJ, Adams TE, Krishnaswamy S, Camire RM, et al. Crystal structure of the prothrombinase complex from the venom of Pseudonaja textilis. Blood. 2013; 122: 2777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bos MH, Boltz M, St Pierre L, Masci PP, de Jersey J, Lavin MF, et al. Venom factor V from the common brown snake escapes hemostatic regulation through procoagulant adaptations. Blood. 2009; 114: 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pomowski A, Ustok FI, Huntington JA. Homology model of human prothrombinase based on the crystal structure of Pseutarin C. Biol Chem. 2014; 395: 1233–41. [DOI] [PubMed] [Google Scholar]
- 38. Shim JY, Lee CJ, Wu S, Pedersen LG. A model for the unique role of factor Va A2 domain extension in the human ternary thrombin‐generating complex. Biophys Chem. 2015; 199: 46–50. [DOI] [PubMed] [Google Scholar]
- 39. Kojima Y, Heeb MJ, Gale AJ, Hackeng TM, Griffin JH. Binding site for blood coagulation factor Xa involving residues 311‐325 in factor Va. J Biol Chem. 1998;273:14900–5. [DOI] [PubMed] [Google Scholar]
- 40. Kalafatis M, Mann KG. The role of the membrane in the inactivation of factor va by plasmin. Amino acid region 307‐348 of factor V plays a critical role in factor Va cofactor function. J Biol Chem. 2001; 276: 18614–23. [DOI] [PubMed] [Google Scholar]
- 41. Kalafatis M, Beck DO. Identification of a binding site for blood coagulation factor Xa on the heavy chain of factor Va. Amino acid residues 323‐331 of factor V represent an interactive site for activated factor X. Biochemistry. 2002;41:12715–28. [DOI] [PubMed] [Google Scholar]
- 42. Singh LS, Bukys MA, Beck DO, Kalafatis M. Amino acids Glu323, Tyr324, Glu330, and Val331 of factor Va heavy chain are essential for expression of cofactor activity. J Biol Chem. 2003; 278: 28335–45. [DOI] [PubMed] [Google Scholar]
- 43. Barhoover MA, Orban T, Beck DO, Bukys MA, Kalafatis M. Contribution of amino acid region 334‐335 from factor Va heavy chain to the catalytic efficiency of prothrombinase. Biochemistry. 2008; 47: 6840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Norstrom EA, Tran S, Steen M, Dahlback B. Effects of factor Xa and protein S on the individual activated protein C‐mediated cleavages of coagulation factor Va. J Biol Chem. 2006; 281: 31486–94. [DOI] [PubMed] [Google Scholar]
- 45. Heeb MJ, Kojima Y, Hackeng TM, Griffin JH. Binding sites for blood coagulation factor Xa and protein S involving residues 493‐506 in factor Va. Protein Sci. 1996; 5: 1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gale AJ, Yegneswaran S, Xu X, Pellequer JL, Griffin JH. Characterization of a factor Xa binding site on factor Va near the Arg‐506 activated protein C cleavage site. J Biol Chem. 2007; 282: 21848–55. [DOI] [PubMed] [Google Scholar]
- 47. Steen M, Villoutreix BO, Norstrom EA, Yamazaki T, Dahlback B. Defining the factor Xa‐binding site on factor Va by site‐directed glycosylation. J Biol Chem. 2002; 277: 50022–9. [DOI] [PubMed] [Google Scholar]
- 48. Steen M, Tran S, Autin L, Villoutreix BO, Tholander AL, Dahlback B. Mapping of the factor Xa binding site on factor Va by site‐directed mutagenesis. J Biol Chem. 2008; 283: 20805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bakker HM, Tans G, Thomassen MC, Yukelson LY, Ebberink R, Hemker HC, et al. Functional properties of human factor Va lacking the Asp683‐Arg709 domain of the heavy chain. J Biol Chem. 1994;269:20662–7. [PubMed] [Google Scholar]
- 50. Rezaie AR. Identification of basic residues in the heparin‐binding exosite of factor Xa critical for heparin and factor Va binding. J Biol Chem. 2000;275:3320–7. [DOI] [PubMed] [Google Scholar]
- 51. Chattopadhyay A, James HL, Fair DS. Molecular recognition sites on factor Xa which participate in the prothrombinase complex. J Biol Chem. 1992;267:12323–9. [PubMed] [Google Scholar]
- 52. Kalafatis M, Xue J, Lawler CM, Mann KG. Contribution of the heavy and light chains of factor Va to the interaction with factor Xa. Biochemistry. 1994;33:6538–45. [DOI] [PubMed] [Google Scholar]
- 53. Boskovic DS, Krishnaswamy S. Exosite binding tethers the macromolecular substrate to the prothrombinase complex and directs cleavage at two spatially distinct sites. J Biol Chem. 2000; 275: 38561–70. [DOI] [PubMed] [Google Scholar]
- 54. Krishnaswamy S. Exosite‐driven substrate specificity and function in coagulation. J Thromb Haemost. 2005; 3: 54–67. [DOI] [PubMed] [Google Scholar]
- 55. Tran S, Norstrom E, Dahlback B. Effects of prothrombin on the individual activated protein C‐mediated cleavages of coagulation factor Va. J Biol Chem. 2008; 283: 6648–55. [DOI] [PubMed] [Google Scholar]
- 56. Kalafatis M, Beck DO, Mann KG. Structural requirements for expression of factor Va activity. J Biol Chem. 2003; 278: 33550–61. [DOI] [PubMed] [Google Scholar]
- 57. Beck DO, Bukys MA, Singh LS, Szabo KA, Kalafatis M. The contribution of amino acid region ASP695‐TYR698 of factor V to procofactor activation and factor Va function. J Biol Chem. 2004; 279: 3084–95. [DOI] [PubMed] [Google Scholar]
- 58. Hirbawi J, Bukys MA, Barhoover MA, Erdogan E, Kalafatis M. Role of the acidic hirudin‐like COOH‐terminal amino acid region of factor Va heavy chain in the enhanced function of prothrombinase. Biochemistry. 2008; 47: 7963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hirbawi J, Vaughn JL, Bukys MA, Vos HL, Kalafatis M. Contribution of amino acid region 659‐663 of Factor Va heavy chain to the activity of factor Xa within prothrombinase. Biochemistry. 2010; 49: 8520–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hirbawi J, Kalafatis M. Spellbinding effects of the acidic COOH‐terminus of factor Va heavy chain on prothrombinase activity and function. ACS Omega. 2017; 2: 5529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Toso R, Camire RM. Role of Hirudin‐like factor Va heavy chain sequences in prothrombinase function. J Biol Chem. 2006; 281: 8773–9. [DOI] [PubMed] [Google Scholar]
- 62. Corral‐Rodriguez MA, Bock PE, Hernandez‐Carvajal E, Gutierrez‐Gallego R, Fuentes‐Prior P. Structural basis of thrombin‐mediated factor V activation: the Glu666‐Glu672 sequence is critical for processing at the heavy chain‐B domain junction. Blood. 2011; 117: 7164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kotkow KJ, Deitcher SR, Furie B, Furie BC. The second kringle domain of prothrombin promotes factor Va‐mediated prothrombin activation by prothrombinase. J Biol Chem. 1995;270:4551–7. [DOI] [PubMed] [Google Scholar]
- 64. Deguchi H, Takeya H, Gabazza EC, Nishioka J, Suzuki K. Prothrombin kringle 1 domain interacts with factor Va during the assembly of prothrombinase complex. Biochem J. 1997;321(Pt 3):729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang M, Rigby AC, Morelli X, Grant MA, Huang G, Furie B, et al. Structural basis of membrane binding by Gla domains of vitamin K‐dependent proteins. Nat Struct Biol. 2003; 10: 751–6. [DOI] [PubMed] [Google Scholar]
- 66. Blostein MD, Rigby AC, Jacobs M, Furie B, Furie BC. The Gla domain of human prothrombin has a binding site for factor Va. J Biol Chem. 2000; 275: 38120–6. [DOI] [PubMed] [Google Scholar]
- 67. Krishnaswamy S, Mann KG. The binding of factor Va to phospholipid vesicles. J Biol Chem. 1988;263:5714–23. [PubMed] [Google Scholar]
- 68. Kalafatis M, Rand MD, Mann KG. Factor Va‐membrane interaction is mediated by two regions located on the light chain of the cofactor. Biochemistry. 1994;33:486–93. [DOI] [PubMed] [Google Scholar]
- 69. Srivastava A, Quinn‐Allen MA, Kim SW, Kane WH, Lentz BR. Soluble phosphatidylserine binds to a single identified site in the C2 domain of human factor Va. Biochemistry. 2001;40:8246–55. [DOI] [PubMed] [Google Scholar]
- 70. Saleh M, Peng W, Quinn‐Allen MA, Macedo‐Ribeiro S, Fuentes‐Prior P, Bode W, et al. The factor V C1 domain is involved in membrane binding: identification of functionally important amino acid residues within the C1 domain of factor V using alanine scanning mutagenesis. Thromb Haemost. 2004; 91: 16–27. [DOI] [PubMed] [Google Scholar]
- 71. Majumder R, Quinn‐Allen MA, Kane WH, Lentz BR. A phosphatidylserine binding site in factor Va C1 domain regulates both assembly and activity of the prothrombinase complex. Blood. 2008; 112: 2795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Peng W, Quinn‐Allen MA, Kane WH. Mutation of hydrophobic residues in the factor Va C1 and C2 domains blocks membrane‐dependent prothrombin activation. J Thromb Haemost. 2005; 3: 351–4. [DOI] [PubMed] [Google Scholar]
- 73. Majumder R, Weinreb G, Zhai X, Lentz BR. Soluble phosphatidylserine triggers assembly in solution of a prothrombin‐activating complex in the absence of a membrane surface. J Biol Chem. 2002; 277: 29765–73. [DOI] [PubMed] [Google Scholar]
- 74. Zhai X, Srivastava A, Drummond DC, Daleke D, Lentz BR. Phosphatidylserine binding alters the conformation and specifically enhances the cofactor activity of bovine factor Va. Biochemistry. 2002;41:5675–84. [DOI] [PubMed] [Google Scholar]
- 75. Qureshi SH, Yang L, Manithody C, Rezaie AR. Membrane‐dependent interaction of factor Xa and prothrombin with factor Va in the prothrombinase complex. Biochemistry. 2009; 48: 5034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ortel TL, Devore‐Carter D, Quinn‐Allen M, Kane WH. Deletion analysis of recombinant human factor V. Evidence for a phosphatidylserine binding site in the second C‐type domain. J Biol Chem. 1992;267:4189–98. [PubMed] [Google Scholar]
- 77. Ortel TL, Quinn‐Allen MA, Charles LA, Devore‐Carter D, Kane WH. Characterization of an acquired inhibitor to coagulation factor V. Antibody binding to the second C‐type domain of factor V inhibits the binding of factor V to phosphatidylserine and neutralizes procoagulant activity. J Clin Invest. 1992; 90: 2340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ortel TL, Moore KD, Quinn‐Allen MA, Okamura T, Sinclair AJ, Lazarchick J, et al. Inhibitory anti‐factor V antibodies bind to the factor V C2 domain and are associated with hemorrhagic manifestations. Blood. 1998;91:4188–96. [PubMed] [Google Scholar]
- 79. Ortel TL, Quinn‐Allen MA, Keller FG, Peterson JA, Larocca D, Kane WH. Localization of functionally important epitopes within the second C‐type domain of coagulation factor V using recombinant chimeras. J Biol Chem. 1994;269:15898–905. [PubMed] [Google Scholar]
- 80. Villoutreix BO, Bucher P, Hofmann K, Baumgartner S, Dahlback B. Molecular models for the two discoidin domains of human blood coagulation factor V. J Mol Model. 1998;4:8. [Google Scholar]
- 81. Macedo‐Ribeiro S, Bode W, Huber R, Quinn‐Allen MA, Kim SW, Ortel TL, et al. Crystal structures of the membrane‐binding C2 domain of human coagulation factor V. Nature. 1999; 402: 434–9. [DOI] [PubMed] [Google Scholar]
- 82. Nicolaes GA, Villoutreix BO, Dahlback B. Mutations in a potential phospholipid binding loop in the C2 domain of factor V affecting the assembly of the prothrombinase complex. Blood Coagul Fibrinolysis. 2000;11:89–100. [PubMed] [Google Scholar]
- 83. Kim SW, Quinn‐Allen MA, Camp JT, Macedo‐Ribeiro S, Fuentes‐Prior P, Bode W, et al. Identification of functionally important amino acid residues within the C2‐domain of human factor V using alanine‐scanning mutagenesis. Biochemistry. 2000;39:1951–8. [DOI] [PubMed] [Google Scholar]
- 84. Peng W, Quinn‐Allen MA, Kim SW, Alexander KA, Kane WH. Trp2063 and Trp2064 in the factor Va C2 domain are required for high‐affinity binding to phospholipid membranes but not for assembly of the prothrombinase complex. Biochemistry. 2004; 43: 4385–93. [DOI] [PubMed] [Google Scholar]
- 85. Majumder R, Quinn‐Allen MA, Kane WH, Lentz BR. The phosphatidylserine binding site of the factor Va C2 domain accounts for membrane binding but does not contribute to the assembly or activity of a human factor Xa‐factor Va complex. Biochemistry. 2005; 44: 711–8. [DOI] [PubMed] [Google Scholar]
- 86. Gilbert GE, Novakovic VA, Kaufman RJ, Miao H, Pipe SW. Conservative mutations in the C2 domains of factor VIII and factor V alter phospholipid binding and cofactor activity. Blood. 2012; 120: 1923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bevers EM, Comfurius P, Zwaal RF. Changes in membrane phospholipid distribution during platelet activation. Biochim Biophys Acta. 1983;736:57–66. [DOI] [PubMed] [Google Scholar]
- 88. Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–93. [PubMed] [Google Scholar]
- 89. Rosing J, Bakker HM, Thomassen MC, Hemker HC, Tans G. Characterization of two forms of human factor Va with different cofactor activities. J Biol Chem. 1993;268:21130–6. [PubMed] [Google Scholar]
- 90. Kim SW, Ortel TL, Quinn‐Allen MA, Yoo L, Worfolk L, Zhai X, et al. Partial glycosylation at asparagine‐2181 of the second C‐type domain of human factor V modulates assembly of the prothrombinase complex. Biochemistry. 1999; 38: 11448–54. [DOI] [PubMed] [Google Scholar]
- 91. Nicolaes GA, Villoutreix BO, Dahlback B. Partial glycosylation of Asn2181 in human factor V as a cause of molecular and functional heterogeneity. Modulation of glycosylation efficiency by mutagenesis of the consensus sequence for N‐linked glycosylation. Biochemistry. 1999;38:13584–91. [DOI] [PubMed] [Google Scholar]
- 92. Bloom JW, Nesheim ME, Mann KG. Phospholipid‐binding properties of bovine factor V and factor Va. Biochemistry. 1979;18:4419–25. [DOI] [PubMed] [Google Scholar]
- 93. Du J, Wichapong K, Hackeng KM, Nicolaes GA. Molecular simulation studies of human coagulation factor VIII C domain‐mediated membrane binding. Thromb Haemost. 2015; 113: 373–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


