Abstract
Dry skin is one of the most important concerns of consumers worldwide. Despite huge efforts over several decades, the personal care industry still does not offer a perfect solution to satisfy the unmet needs of consumers for moisturising treatments in different ethnic groups. The paucity of data for the underlying cellular and biochemical problems in, and the effects of moisturisers on photodamaged facial skin may partly explain this. Mainly, single point measurements are used to understand the effects of products on skin physiology even on surrogate skin sites such as the non‐photodamaged volar forearm. Some groups have developed discontinuous facial maps of skin biophysical properties, however, in 2014 a continuous facial analysis of bio‐instrumental evaluations was developed using a heat map approach. These maps enabled a continuous visualization of features that not only revealed an unexpected complexity of facial skin but also indicated that use of surrogate skin sites for facial skin is inappropriate. We have demonstrated that remarkable gradients of skin hydration, TEWL, skin surface pH and sebum exist within short distances across the face and the gradients are distinctive among different ethnic groups. In addition, these studies have demonstrated that darkly‐pigmented individuals do not necessarily have a better skin barrier function than their less‐pigmented counterparts and that Caucasians have a lower facial skin surface pH compared with more pigmented subjects. Overall, there are no correlations between capacitance, TEWL and skin surface pH including individual topology angle values. Novel 3D camera approaches have also been used to facilitate a more precise assignment of measurement sites and visualisation. The 3D facial colour mappings illustrated precisely the local moisturising effects of a moisturising cream. There were subtle ethnic differences in efficacy that may be related to underlying skin biochemistry and/or ethnic differences in product application. A placebo‐controlled study using conductance measurements in Chinese subjects is also reported. Finally, a new whole face statistical approach has been taken to prove differences in skin parameters but also of moisturiser treatment that adds further to our understanding of the ethnic differences in skin physiology and product application. This paper reviews the background of the development and application of this methodology.
Keywords: claim substantiation, skin barrier, skin physiology, facial colour mapping, bio‐instrumental evaluation, ethnic groups
The skin mapping technology enables the transformation of bio‐instrumental data to continuous 2D and 3D visualisation of facial skin features. This approach reveals the complexity of facial skin and the differences of differently pigmented skin types.
Résumé
L'assèchement de la peau est l'un des problèmes les plus importants chez les consommateurs à travers le monde. En dépit des efforts fournis dans les dernières décennies, l’industrie du soin ne propose pas encore une solution parfaite qui répond aux attentes des consommateurs de différentes ethnies pour des traitements hydratants. Le manque de données concernant les problèmes de mécanisme cellulaire et biochimique, ainsi que les effets des soins hydratants sur la peau du visage photo‐endommagée peuvent en partie expliquer cela. En général, une mesure ponctuelle est réalisée pour comprendre les effets des produits sur la physiologie de la peau sur des sites de substitution tels que l’avant‐bras non photo‐endommagé. Certains groupes ont développé des cartographies du visages discontinues des propriétés biophysiques de la peau, mais ce n’est qu’en 2014 qu’une analyse continue du visage de l’évaluation bio‐instrumentale a été proposée en utilisant une approche par cartographie de chaleur. Ces cartographies permettent une visualisation continue des caractéristiques qui ne révèlent pas seulement une complexité inattendue de la peau du visage mais indique également que l’utilisation de sites de substitution est inappropriée. Nous avons démontré que certains gradients liés à l’hydratation de la peau, à la PIE, au pH à la surface de la peau et au sébum sont présents sur de faibles distances à travers le visage et que ces gradients sont différents selon les groupes ethniques. De plus, ces études ont démontré que les individus ayant une pigmentation de peau importante n’ont pas nécessairement une meilleure fonction de barrière cutanée que leurs homologues ayant une peau moins pigmentée et que les Caucasiens ont une plus faible surface de pH sur le bas du visage en comparaison avec des sujets ayant plus de pigmentation. Globalement, en incluant les aspects typologiques individuels, il n’y a pas de corrélation entre la capacitance, la PIE et le pH à la surface de la peau. Une nouvelle approche par caméra 3D à également été utilisée pour faciliter l’attribution et la visualisation plus précise de la mesure par site. Les cartographies du visage 3D en couleur illustrent précisément les effets hydratants localisés d’une crème hydratante. Il y avait des différences ethniques subtiles dans l’efficacité qui peuvent être liées au mécanisme de la biochimie cutanée et/ou dans l’application des produits des différentes ethnies. Une étude contrôlée par placebo utilisant une mesure de conductance chez les sujets d’origine chinoise est également communiquée. Enfin, une nouvelle approche statistique sur le visage complet a été adoptée afin de prouver les différences dans les paramètres de la peau mais aussi dans le traitement hydratant, ce qui nous permet de mieux comprendre les différences ethniques dans la physiologie de la peau et l'application des produits. Cette publication retrace les éléments de développement ainsi que l’application des méthodologies.
Introduction
Our aim is to understand in more depth the complexity of facial skin and also the need for different products for differently pigmented skin types 1. Although controversial data exist, it has been reported that the barrier of darkly pigmented skin has some advantages compared with that of lightly‐pigmented skin 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14. However, most of these studies have been conducted on volar forearms and hands but not on the face. Moreover, visual dryness is more apparent in pigmented skin 3, 10.
Facial skin is one of the most environmentally‐challenged areas of the body, naturally being affected by humidity, pollution and sunlight, all of which contribute to the signs of accelerated aging on facial skin but also to perturbations to stratum corneum (SC) biochemistry and physiology 15. Paradoxically, the SC of the face is particularly delicate. Compared to most other body sites on the face, there are fewer cell layers and smaller corneocytes, of which some contain fragile corneocyte envelopes 16, 17. On the forearm there are approximately 15–20 cell layers; facial SC has only approximately 7–10 cell layers with the eye region having a SC as thin as 6 µm 18, 19. Compared to other body sites, facial skin shows elevated transepidermal water loss (TEWL, a measure of skin barrier function), and increased SC cohesion together with lower levels of SC ceramides and natural moisturising factors and higher serine, aspartic and matrix metalloprotease protease activity resulting in premature corneodesmosomal degradation and thinning of the SC 1, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. Relating to the immaturity of the cells, reduced loricrin levels together with lowered transglutaminase and 12R‐lipoxygenase activities have been reported in photodamaged facial skin 31, 32, 33. A summary of this biochemistry is described in Fig. 1, which highlights key pathways in desquamation and SC maturation. However, changes in this biochemistry have only been measured on single specific facial sites rather than for the face globally.
Figure 1.
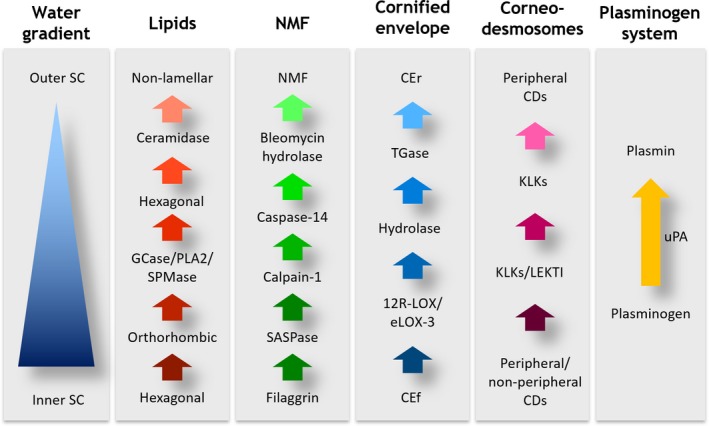
Key pathways in desquamation and stratum corneum maturation.
Recently a multiethnic facial skin study including Albino African subjects living in Pretoria examining skin barrier function, hydration, skin surface pH and SC integrity together with cohesion was reported 34. Differences in barrier function especially in the Albino African subjects compared with the Black African and Caucasian subjects were observed. Not only did the Albino subjects have a better barrier integrity, with little differences between the Caucasian and Black African subjects, barrier recovery was faster on their cheeks. SC cohesion was also greater. Equally when comparing the Caucasian and Black African subjects there was no correlation among values of pH, individual topology angle (ITA°) values, TEWL, barrier integrity and barrier recovery but there was a weak correlation between pH and SC cohesion. Nevertheless, ITA° values were correlated with skin hydration. This data appeared to be in conflict with the other data showing that the more pigmented the skin, the better the barrier of the skin 7, 12, 13. Nevertheless, these measurements were only on a defined location of the face. However, in an exploratory trial using these subjects large differences in skin hydration and barrier function in different regions of the face were shown. Rather than these results being inconsistent differences in facial skin physiology must have been the reason for the observed site variations. As a result, it was concluded that single point measures do not adequately describe the variability in hydration, TEWL or skin surface pH of the face. Thus, our aim was to create continuous facial maps of these measures, test the effects of moisturisers and develop a novel whole face visualisation approach to their statistical comparisons.
Facial skin mapping procedures have already been undertaken by other research groups, however, the mapping was discontinuous between the measurement areas 35, 36, 37, 38.
Our vision was to measure skin hydration, TEWL and skin surface pH on 30 pre‐defined facial sites of subjects (half face) of different ethnicities (Fig. 2) (Table 1), then to interpolate the areas between the test sites, transcribe the data into colours and finally to superimpose the colours onto digital facial images of the subjects for distinct visualisation and eventually using a whole face statistical measure 39. Throughout all our studies the bio‐instrumental procedures were conducted following published guidelines 40, 41, 42. TEWL (Aquaflux) was used as a measure of skin barrier function. Capacitance (Corneometer) and conductance (Skicon) were used for skin hydration measurements with the latter measuring skin hydration more superficially in the SC. Skin surface pH (Skin‐pH‐Meter) and sebum (Sebumeter) measurements were also taken.
Figure 2.
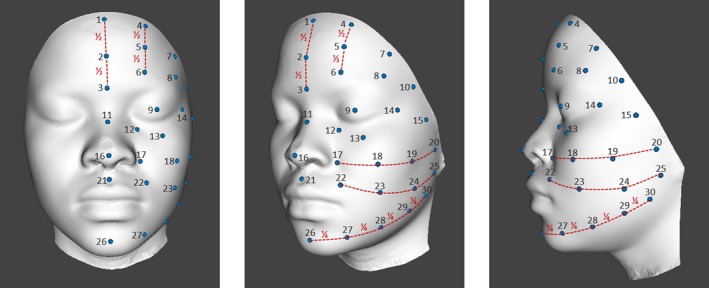
Mapping layout, anterior, oblique and lateral images of 30 pre‐defined facial measurement points.
Table 1.
Mapping layout, description of the 30 pre‐defined facial measurement points
| Site # | Description of site | Localization of site |
|---|---|---|
| 01 | Forehead, central, upper | Central brow top, close to hairline |
| 02 | Forehead, central, middle | Central brow mid, exactly between 01 and 03 |
| 03 | Forehead, central, lower | Central brow lower, between eyebrows |
| 04 | Forehead, middle left, upper | Mid brow top, close to hairline, exactly between 01 and 07 |
| 05 | Forehead, middle left, middle | Mid brow mid, exactly between 04 and 06 |
| 06 | Forehead, middle left, lower | Mid brow lower, exactly between 03 and 08 |
| 07 | Forehead, left, middle | Outer brow mid; close to hairline |
| 08 | Forehead, left, lower | Temple brow |
| 09 | Eyelid | Eyelid |
| 10 | Forehead, outer, level with eyebrow | Temple, outer edge of brow |
| 11 | Nose, bridge | Nose, bridge |
| 12 | Under eye, inner corner | 1.5 cm below medial canthus, slightly outward bended, parallel to nose |
| 13 | Under eye, middle | 2 cm below pupil, slightly below the central infra orbital margin |
| 14 | Outer eye canthus | 1.5 cm horizontally from outer lateral eye canthus |
| 15 | Cheek, lateral | Outer cheekbone ± 4 cm below site 10 |
| 16 | Nose, apex | End/top of nose |
| 17 | Nasolabial sulcus, top | 0.5 cm left of nostril |
| 18 | Cheek, middle, oblique | In slightly curved line with 17, 19 and 20 |
| 19 | Cheek, middle, oblique/lateral | In slightly curved line with 17, 18 and 20 |
| 20 | Cheek, middle, lateral | In slightly curved line with 17, 18 and 19 |
| 21 | Philtrum | Middle of upper up in cleft |
| 22 | Nasolabial sulcus, midpoint | Midpoint of nasolabial fold |
| 23 | Cheek, lower, oblique | In slightly curved line with 22, 24 and 25 |
| 24 | Cheek, lower, oblique/lateral | In slightly curved line with 22, 23 and 25 |
| 25 | Cheek, lower, lateral | In slightly curved line with 22, 23 and 24 |
| 26 | Chin, central | Middle of chin |
| 27 | Jaw, anterior/oblique | Exactly between 26 and 28 |
| 28 | Jaw, oblique | Exactly between 26 and 30 |
| 29 | Jaw, oblique/lateral | Exactly between 28 and 30 |
| 30 | Jaw, lateral | Slightly above mandibular angle |
Egawa et al. developed an alternative approach for facial hydration mapping based on near infrared (NIR) absorption of –OH at 1920 nm 43, 44. Characteristic facial points were set as references to create similar number of meshes for the subjects. The average signal intensity of each mesh was calculated and then averaged for all subjects. The numerical data were used for analysis of the water distribution in facial skin. Each mesh was then colored using the intensity values. A drawback of this methodology is the covering of water of the living epidermis and partially of the dermis, leading to results that are not directly comparable to the methodology discussed in this review.
In the colour mapping approach discussed here each of the pre‐defined facial sites was precisely positioned on the images, and XY coordinates for 2D reconstruction and XYZ coordinates for 3D reconstruction were recorded. A model was computed to link the bio‐instrumental data to the corresponding facial positions. Between each measuring site, physiological values were interpolated using a thin plate spline transform to obtain for each pixel of the facial image a value. These values were then transformed into colours and continuous colour maps were created representing a continuous distribution of the bio‐instrumental measures. The choice was made to represent good skin condition in deep blue and impaired skin condition in deep red. Limit skin conditions were set to white colour, for capacitance initially at 40 AU, later at 45 AU, conductance at 135 µS, skin surface pH at 5.9, TEWL initially at 16 g m−2 h−1 later at 23.5 g m−2 h−1 and sebum level at 100 mg cm−2. These changes were made as we progressed with our understanding of the complexity of facial skin physiology but were originally related to skin dryness levels. Between these landmarks, colours were linearly interpolated in the CIELAB space. Finally, skin pixels on the images were segmented and, depending on their position, biophysical data were attached to them. The corresponding colour was superimposed to the original value with a transparency level 45. The colour mapping images were generated from the mean values of each ethnic group.
Part 1: Observational study: exploratory inter‐ethnic 2D approach using capacitance and TEWL measurements
For this exploratory observational study, we enrolled sixteen young females (21.8 ± 1.1 years old) without visual signs of photoaging, all living in Pretoria 39. There were four subjects each of Black Africans (phototype V‐VI), Caucasians (phototype II‐III), Chinese (phototype II‐III) and Indians (phototype III‐IV). The study took place end of May to mid of June 2014 in the South African winter season.
Stratum corneum capacitance was measured using a Corneometer CM825 (Courage & Khazake electronic, Cologne, Germany) and basal TEWL using an Aquaflux AF200 (Biox Systems, London, UK) on 30 predefined sites on the left‐hand side of the face (Fig. 2 and Table 1). Three digital images were taken with the Visia‐CR imaging system (Canfield Scientific Inc., Fairfield, NJ, USA), one each from anterior, oblique and lateral view. To reduce any possible inter‐individual variation of the measurements, a template was used to ensure the same facial site was measured on each occasion. The facial colour maps for capacitance and TEWL were created as described above. The mean data of each ethnicity were applied to the face of a selected subject of each ethnic group.
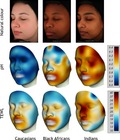
Although a certain level of heterogeneity was expected the complexity of facial hydration and barrier properties was surprising as shown in the images of Fig. 3. Remarkable skin hydration and TEWL gradients were observed. On some areas of the face, subtle differences were found, particularly in the Chinese subjects, but in others, there were steep particular gradients within short distances. The gradients are distinctive in the different ethnic groups.
Figure 3.
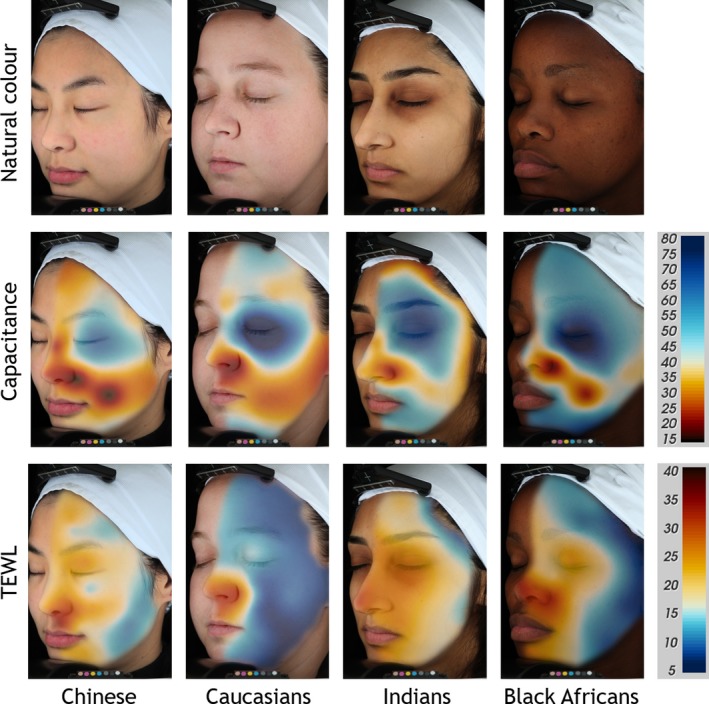
Continuous facial colour mappings of transepidermal water loss (TEWL) and capacitance, displayed on one selected subject per ethnicity. From left: Chinese, Caucasians, Indians, Black Africans. Top row: standard VISIA‐CR portraits in cross‐polarised light mode, natural skin colour, middle row shows capacitance and bottom row TEWL mappings. Colour code for Corneometer values (15–80 AU) and TEWL values (5–40 g m−2 h−1) shown on the colour scales on the right side (blue = good skin condition and red = impaired skin condition). Limit skin condition is set to white: 40 AU for capacitance and 16 g m−2 h−1 for TEWL, mean values of each group.
When considering the overall ethnicity and overall skin hydration values, Corneometer readings were greatest for Black Africans > Indians > Caucasians > Chinese (Fig. 3, Table 2). In all groups the nasolabial region and the cheek showed the lowest and the eye region the highest capacitance values. When examining the 30 individual sites, the findings were more complex. However, as shown in other studies it seems that darkly pigmented skin is more hydrated 46, 47.
Table 2.
Mean TEWL and capacitance data of the 30 facial test sites of four ethnic groups
| P‐value of comparison | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chinese | Caucasians | Indians | Black Africans | Ch vs. Ca | Ch vs. In | Ch vs. Bl | Ca vs. In | Ca vs. Bl | In vs. Bl | |
| TEWL | 18.6 ± 3.5 | 12.4 ± 2.4 | 20.4 ± 2.6 | 16.7 ± 1.6 | <0.001 | n.s. | n.s. | <0.001 | <0.01 | n.s. |
| Capacitance | 41.5 ± 3.1 | 46.8 ± 1.2 | 51.0 ± 2.7 | 55.0 ± 1.3 | n.s. | <0.01 | <0.001 | n.s. | <0.01 | n.s. |
Results represent mean ± SEM.
Ch, Chinese; Ca, Caucasians; In, Indians; BA, Black Africans; n.s., not significant.
For TEWL a similar level of complexity was demonstrated. Considering overall SC barrier properties, TEWL values (g m−2 h−1) were greatest for Indians > Chinese > Black Africans > Caucasians, with the Chinese group showing the most complexity (Fig. 3, Table 2). The lowest values were found on the middle and lower cheek and jaw regions, and the highest values around the eyes, the nasolabial fold and the philtrum. However, the results are more clearly integrated by the continuous mapping images.
The lack of concordance between skin capacitance and TEWL is clearly apparent. Big differences could be demonstrated on particular facial sites. Clearly darkly pigmented skin was more hydrated, but the opposite was observed for skin barrier properties. Depending on the facial location, darkly pigmented skin does not have a superior barrier function. Indeed, compared with Caucasian subjects the Black African subjects had a numerically or significantly higher TEWL which is diametrically‐opposed to the forearm and hand data from the group of Elias 7, 12, 13.
Part 2: Proof of concept moisturisation study: inter‐ethnic 3D approach using capacitance, TEWL and skin surface pH measurements
In this proof of concept study, we wanted to understand better how a moisturising cream acts on the different facial sites among different ethnic groups. We chose a 3D camera system and created mean faces for each ethnic group enabling a more precise transfer of the mean data 48, 49, 50.
Thirty‐two healthy female subjects (22.5 ± 1.7 years old) from three different skin ethnicities (Black Africans, ITA° −49.9 ± 2.7; Indians, ITA° −21.5 ± 8.6; and Caucasians, ITA° 29.2 ± 2.3) living in Pretoria, South Africa, with normal skin condition and no signs of photodamage were enrolled. The study took place from beginning of June to end of July 2015.
The study was composed of a 3‐day conditioning phase and a longitudinal 28‐day application phase. For the conditioning phase, the subjects did not apply any dermatological or cosmetic products. In the application phase a moisturising cream containing saccharide isomerate, niacinamide and glycerin (Table 3) was applied twice daily, once in the morning and once in the afternoon, under normal conditions of use. The evaluations on day 28 were taken at least 12 h after the last application of the moisturising cream.
Table 3.
INCI list of the moisturiser cream
| Aqua, Diisopropyl Sebacate, C12‐15 Alkyl Benzoate, Niacinamide, Glycerin, Glyceryl Myristate, Saccharide Isomerate, Butyl Methoxydibenzoylmethane, Polysilicone‐15, Octocrylene, Potassium Cetyl Phosphate, Dicaprylyl Ether, Titanium Dioxide, Dimethicone, Methyl Methacrylate Crosspolymer, Cetyl Alcohol, Phenoxyethanol, Citric Acid, Sodium Citrate, Ethylhexylglycerin, Xanthan Gum, Silica, Disodium EDTA, Parfum, BHT, Hydroxycitronellal, Hexyl Cinnamal, Butylphenyl Methylpropional, Limonene. |
At baseline SC capacitance was measured using a Corneometer CM825 (Courage & Khazake electronic, Cologne, Germany), basal TEWL using an Aquaflux AF200 (Biox Systems, London, UK) and skin surface pH using a Skin‐pH‐Meter PH 905 (Courage & Khazake electronic, Cologne, Germany) on 30 predefined sites on the left‐hand side of the face (Fig. 2, Table 1). Skin capacitance was expressed as the mean value of three recordings, TEWL and skin surface pH were measured once. Two dimensional facial images were taken with the Visia‐CR imaging system, as described in the previous section and three dimensional (3D) digital images were taken with the Vectra m3 imaging system (Canfield Scientific Inc., Fairfield, NJ, USA). Capacitance was again evaluated after the 28‐day application phase. The mean data were transferred to the mean faces of each ethnicity. Baseline data were also taken for back of hands and volar forearms.
Capacitance mapping
At baseline poor hydration around the nasolabial fold area and the middle cheek in all three ethnic groups was shown and after the 4‐week treatment using a moisturising cream with high levels of bench mark humectants and keratinocyte differentiation enhancers, improved hydration was observed (Fig. 4). Remarkable capacitance gradients within short distances on selected areas of the face were once again recognised, which were distinctive among the different ethnic groups. Largely the Indian subjects had a more hydrated skin at baseline. However, a good correlation with poor hydration around the nasolabial fold area was found in all three ethnic groups. Interestingly these areas showed the most pronounced moisturising effect after the four‐week treatment. The sites that showed the biggest improvement were also the ones which remained poorly hydrated, particularly the sites around the nasolabial fold area and the middle cheek. Relative to other facial sites the moisturisation of the nasolabial folds remained to be inadequate in the three ethnicities and on the cheek of the Caucasian and Indian subjects.
Figure 4.
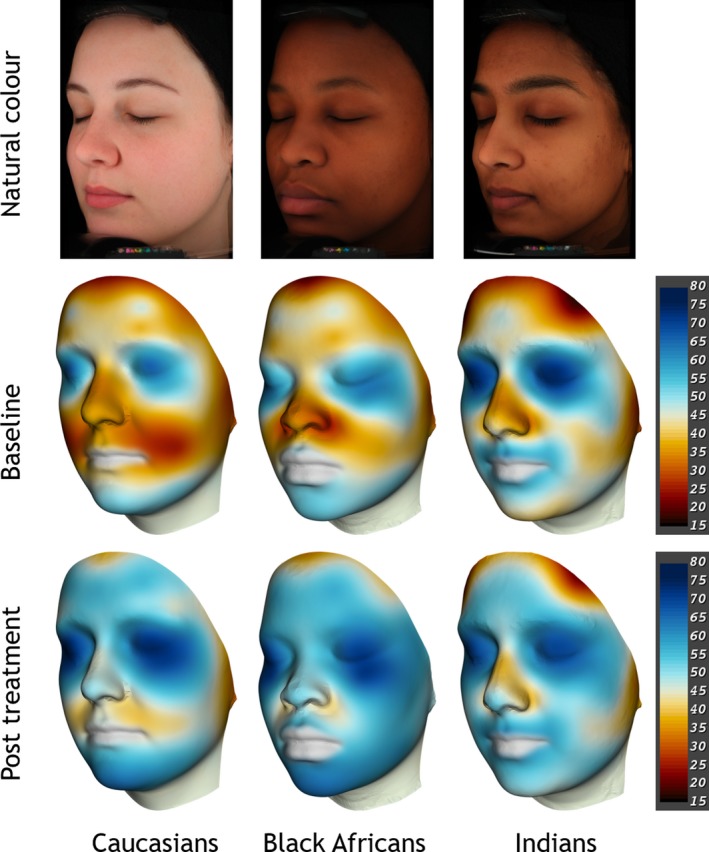
Skin hydration mappings displayed on a mean face per group, from left Caucasians, Black Africans, Indians. Top row: standard VISIA‐CR portraits in cross‐polarised light mode, natural skin colour. Middle and bottom row: 3D Vectra m3 images showing continuous capacitance colour maps before and after the 28‐day moisturiser treatment, mean values of each group. Colour code for Corneometer values (15–80 AU) shown on the scales on the right side (blue = good skin condition, red = impaired skin condition).
There were no statistically significant differences between the ethnicities when considering the overall baseline skin hydration values and all subject groups showed a significant improvement after four weeks of treatment (P < 0.05). Overall the moisturiser worked more effectively for the Black Africans (+12.2 ± 1.4 AU) ≥ Caucasians (+11.2 ± 2.1 AU) > Indians (+5.3 ± 2.1 AU). The Indian cohort showed the weakest overall moisturising effect which also is reflected in the colour maps.
Thus, from the first exploratory studies 39, the first interventional proof of concept study based on realistic 3D facial mapping was published a year later 48, 49, 50. The study was limited due to the small number of subjects and was not vehicle‐controlled. But even later studies published in 2018 had only used an intervention mapping study over 24 h use of a product for 12 subjects 51 with data projection onto a virtual 3D face.
Using a unique approach to visualising the statistical comparison of moisturisation on the different facial sites clear statistical improvements from baseline versus the 4‐week intervention were shown for the Caucasian and Black African subjects but not the Indian subjects (Fig. 5).
Figure 5.
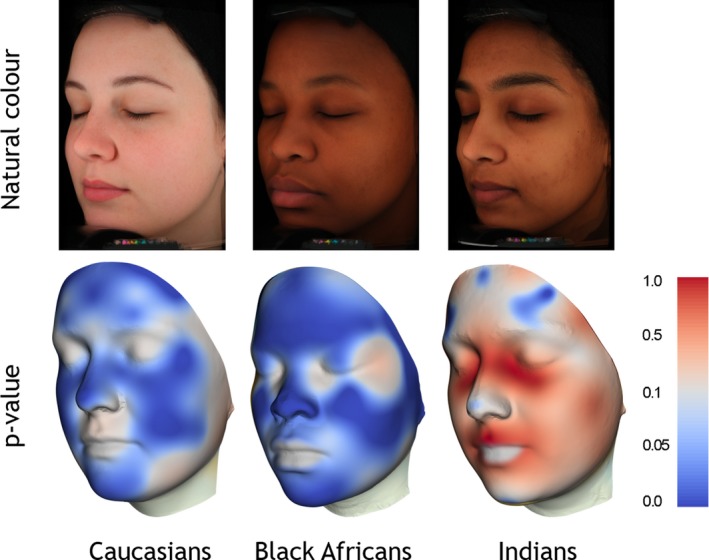
P‐value mapping displayed on a mean face per group, from left Caucasians, Black Africans, Indians. Top row: standard VISIA‐CR portraits in cross‐polarised light mode. Bottom row: 3D Vectra m3 images displaying continuous p‐value maps of intra‐ethnic comparisons before and after the moisturiser treatment, mean values of each group. Colour code for P‐values shown on the scale on the right side.
TEWL and skin surface pH mapping
The importance of an acidic skin surface pH for antimicrobial defence is well recognized 52, 53. However, this parameter may just be a surrogate measure of good epidermal differentiation e.g. increased NMF levels. Nevertheless, it has been reported that SC barrier function, recovery and desquamation were impaired at neutral/alkaline pH's with enhanced serine protease activity contributing to this effect, that was mitigated with acids or serine protease inhibitors 54, 55, 56, 57, 58, 59. A lower skin surface pH on volar forearm and dorsal hands of more pigmented subjects was correlated to superior SC barrier functionality. pH‐regulated mechanisms were proposed to account for pigment‐related differences in SC barrier function and the superior barrier function of darkly pigmented skin was attributed to the lower pH of the outer epidermis. However, others dispute this proposition as we do for facial skin sites 39, 46. Thus, our aim was to investigate the relationship between skin surface pH and TEWL on different body sites and globally across the face of differently‐pigmented ethnic groups and to correlate these results with their ITA°.
Generally a more acidic skin surface pH and lower TEWL values were measured on the Caucasian subjects (Fig. 6 and Table 4). On the facial sites, the Caucasian pH values were significantly lower compared to the Black African and Indian subjects but on the hands only compared to the Black Africans (Fig. S1). There were no differences on the volar forearms. However, TEWL was statistically significantly lower on the volar forearm for the Caucasian subjects compared to the Black African and Indian subjects and, on the hands only, lower compared to the Indians. Facial TEWL was similar between the Caucasian and Black African groups and both were lower than the Indian group. We found no correlations between basal TEWL and skin surface pH. Equally there were no significant correlations between ITA° values, TEWL and skin surface pH (data not shown). Again, the p‐value mapping approach highlighted areas of facial skin that are different between the different ethnic groups (Fig. 7).
Figure 6.
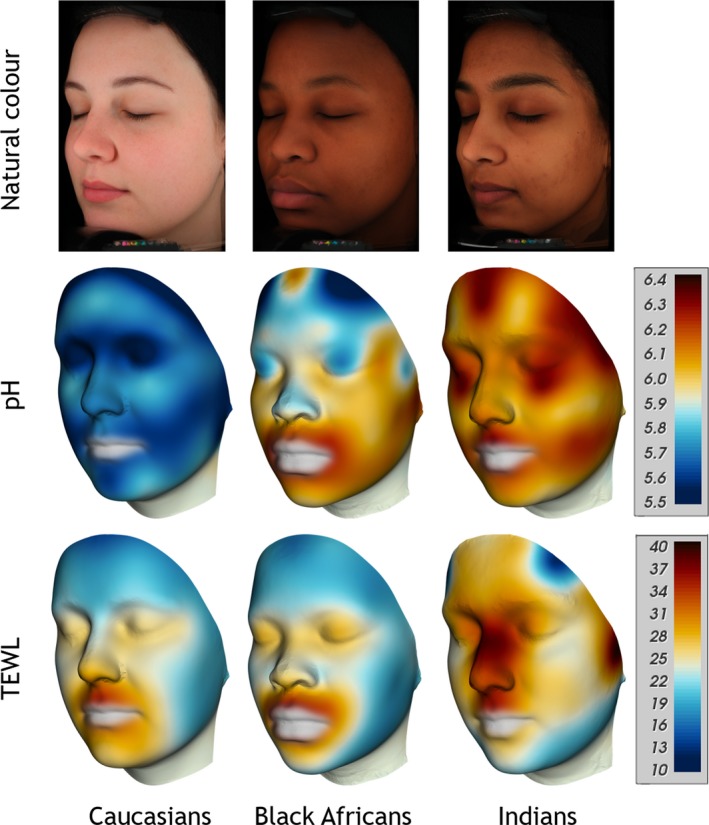
Colour mappings of skin surface pH and transepidermal water loss (TEWL) displayed on a mean face per group, from left Caucasians, Black Africans, Indians. Top row: standard VISIA‐CR portraits in cross‐polarised light mode, natural skin colour. Middle row: skin surface pH mappings, Bottom row: TEWL mappings. Colour code for pH values (5.5‐6.4) and TEWL values (10‐40 g m−2 h−1) shown on the scales on the right side.
Table 4.
Mean TEWL (g m−2 h−1), skin surface pH and ITA° values of the 30 facial test sites of three ethnic groups
| p‐value of comparison | ||||||
|---|---|---|---|---|---|---|
| Caucasians | Black Africans | Indians | Ca vs BA | Ca vs In | BA vs In | |
| TEWL | 22.3 ± 1.5 | 22.0 ± 1.7 | 27.0 ± 2.4 | n.s. | n.s. | n.s. |
| pH | 5.7 ± 0.1 | 6.0 ± 0.1 | 6.1 ± 0.2 | <0.05 | <0.05 | n.s. |
| ITA° | 31.5 ± 2.3 | −49.0 ± 2.7 | −21.5 ± 8.6 | <0.001 | <0.001 | <0.01 |
Results represent mean ± SEM.
Ca, Caucasians; In, Indians; BA, Black Africans; n.s., not significant.
Figure 7.
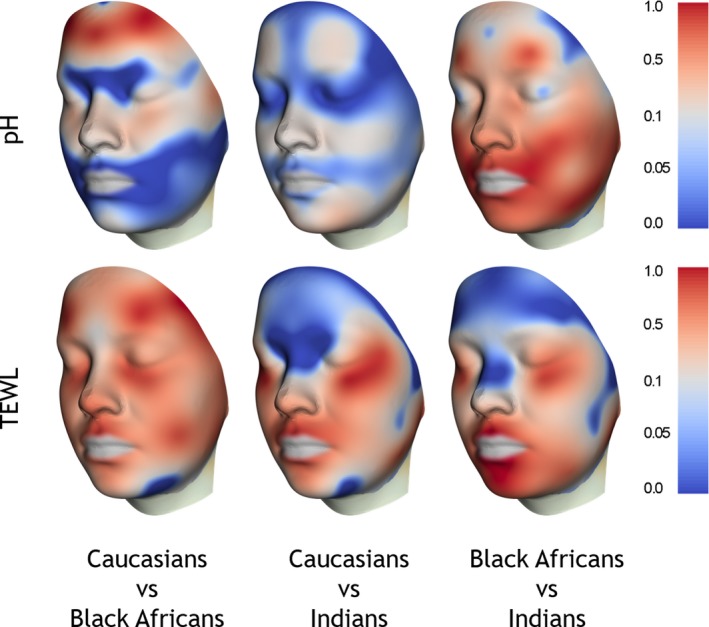
P‐value mapping of inter‐ethnic comparisons displayed on a mean face of all groups, based on 3D Vectra m3 images. Top row: skin surface pH, bottom row: TEWL. Colour code for P‐values shown on the scales on the right side.
The results of this study as well as earlier ones are discordant with the hypothesis that a lower pH of the outer epidermis leads to enhanced barrier function of darkly pigmented skin 7, 12, 13. There was no relationship of skin surface pH or pigmentation with superior basal barrier properties of the SC. On the contrary, more acid skin surface pH value was generally measured for the least pigmented Caucasian subjects, who also generally had the lowest TEWL. Moreover, although the Indian subjects had a significantly higher ITA° values than the Black African cohort, their SC barrier function is the most impaired one (Table 4).
Obviously factors other than skin surface pH and melanin levels regulate SC barrier properties. It is well known that excessive UV exposure compromises barrier function and this appears to be reflected in the elevated basal TEWL values of more exposed body sites, face > hand > forearm, although the subjects did not show visual signs of photoaging 60. Interestingly skin surface pH on more photoexposed sites is lower: face < hand < forearm.
Part 3: Facial sebum mapping, observational study on Caucasian subjects
It is well known, that sebum levels vary intensively on the different facial sites. Thus sebum mappings have already been demonstrated 36, 61. However, these approaches displayed sebum distribution only partially and not a fully continuously.
Twelve Caucasian females (44.3 ± 11.5 years old, Fitzpatrick skin phototype II‐III) were recruited for this exploratory observational study. The study took place in Switzerland in October 2018. Sebum casual level (CSL) measurements were performed in the late morning by means of a Sebumeter® SM 815 (Courage & Khazake electronic, Cologne, Germany) by taking duplicate samples on 30 predefined test sites on the left‐hand side of the face (Fig. 2, Table 1). The mean data were transferred to a 3D mean face and the colour mapping was applied as describe earlier (Fig. 8). The typical T‐zone pattern with highest sebum levels on the central forehead and the nasolabial region and the lowest values around the eyes, the posterior part of the cheek and the chin were found.
Figure 8.
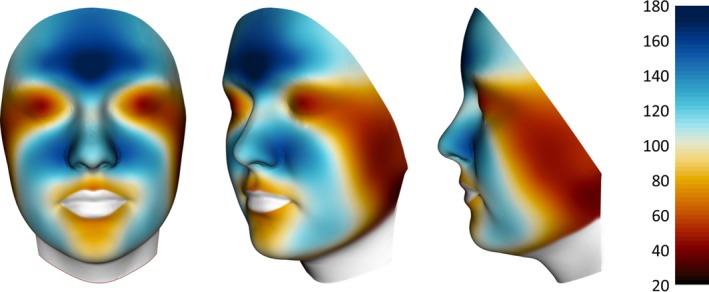
Colour mapping of sebum displayed on a 3D mean face based. Colour code for sebum level (20–180 mg cm−2) shown on the scale on the right side.
Part 4: Vehicle‐controlled moisturisation study: conductance measurements on Chinese subjects
In order to evaluate the efficacy of a test cream including 3% saccharide isomerate a blinded, vehicle‐controlled full face, parallel‐grouped study was conducted.
Chinese females (n = 62; 35.0 ± 0.9 years old, Fitzpatrick skin phototype II‐IV) living for more than 3 years in Beijing, participated in the study, which was composed of a 3‐day wash‐out phase and a 28‐day application phase. The study lasted from the beginning of April to the end of May, 2018. The subjects applied the vehicle or the test cream (Table 5) twice daily, once in the morning and once in the evening, under normal conditions of use. The subjects were acclimatised for 30 min before the measurements, and measurements were performed in a room at a temperature of 24 ± 2°C and 35 ± 10% relative humidity. Skin hydration via conductance was measured on 30 predefined facial sites (Fig. 2, Table 1) using a Skicon 200‐EX (I.B.S. Co., Hamamatsu, Japan) at baseline, 3 h after a single application and after the 28‐day application phase. The measurements were taken at the same time each day per subject, to minimise variations induced by the circadian rhythm. Digital facial images were taken with the Visia‐CR imaging system (Canfield Scientific Inc., Fairfield, NJ, USA) as described previously and mean faces were created. The evaluations on day 28 were taken at least 12 h after the last application of the test cream or the vehicle.
Table 5.
composition vehicle and test cream
| INCI name | Vehicle (%) | Test cream |
|---|---|---|
| Aqua | 85.24 | 82.35 |
| Cyclopentasiloxane | 8.00 | 8.00 |
| Cyclopentasiloxane; Dimethicone/Vinyl Dimethicone Crosspolymer | 3.00 | 3.00 |
| Stearyl Alcohol | 0.90 | 0.90 |
| Polyglyceryl‐3 Methylglucose Distearate | 0.90 | 0.90 |
| Phenoxyethanol; Ethylhexylglycerin | 0.80 | 0.80 |
| Ammonium Acryloyldimethyltaurate/Vp Copolymer | 0.75 | 0.75 |
| Sodium Lauroyl Glutamate; Aqua; Sodium Chloride | 0.20 | 0.20 |
| Chlorphenesin | 0.10 | 0.10 |
| Citric Acid; Aqua | 0.11 | 0.00 |
| Saccharide Isomerate; Aqua; Citric Acid; Sodium Citrate | 0.00 | 3.00 |
| Total | 100.00 | 100.00 |
At baseline the lower part of the face was significantly drier than the upper one in both groups, with the nasolabial region being the lowest hydrated site, similar to the previous studies. The forehead region was also dry but not as severe as the lower facial regions. The placebo showed a slight hydration trend at both time points (Fig. 9) (Fig. S2). In the group treated with the test formulation 3 hours after initial application, we found improved hydration which was statistically highly significant in all parts of the face after four weeks of administration.
Figure 9.
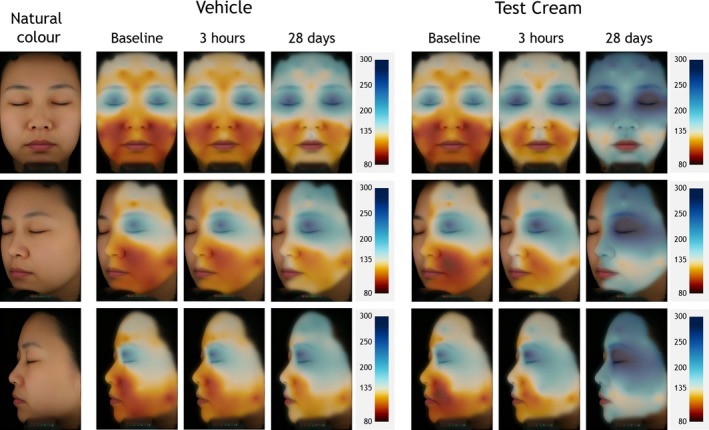
Skin hydration mappings displayed on a mean face based on standard VISIA‐CR portraits of female Chinese subjects in cross‐polarised light mode. Left column: natural skin colour. Middle columns: vehicle treated subjects. Right columns: subjects treated with the Test Cream. Colour code for conductance (80–300 µS) shown on the scales on the right side (blue = good skin condition, red = impaired skin condition).
Interestingly similar, but not identical hydration images, were observed for the baseline capacitance and conductance mapping despite the use of different Chinese subjects at different times of the year in different geographical location (Figs. 3 and 4).
Discussion
Over the last 5 years continuous 2D and 3D mapping approaches have been developed to measure a variety of skin parameters on the face among different ethnic groups. The 3D approach allows a more precise allocation of the measurements on the facial images. Since the landmark publications using these approaches, others have measured skin hydration and created colour mappings across the whole the body 62 while most recently facial hydration mapping studies have been compared with skin elasticity 51. The latter study confirmed the initial findings particularly that the cheeks are naturally less hydrated but in just one ethnic group of Caucasian subjects. Moreover, the group also concluded, as we did, that perhaps single point measurements do not describe the complexity of facial skin hydration and that use of surrogate testing sites such as the forearm may not be wise 63. We believe, however, that a gradient of blue colour being a good result and a bad result being a gradient of red colour is easier to interpret facial skin physiology complexity.
The studies reported here reveal clear ethnic skin differences in facial hydration, TEWL and skin surface pH for subjects living in South Africa. Despite the regional skin site complexity, facial skin barrier function was superior in the order of Caucasians > Black Africans > Chinese > Indians in two of the three studies. These results are inconsistent with the results and hypothesis of the studies of Elias et al. 2, 7, 12, 13 who showed superiority for more pigmented subjects albeit on forearms and hands. Further studies, however, also confirmed our facial data on these body sites that Caucasian skin generally has a stronger basal barrier function than Black African skin (Fitzpatrick skin phototypes V‐VI) albeit subjects living in South Africa. Moreover, it has been reported that the reason for the improved barrier function is due to lower skin surface pH values in more pigmented skin but again our results are diametrically opposed to these findings 7 and are consistent with the results of Hillebrand et al. 46. Indeed, no correlations with ITAº values were found. There may be other ethnic, climate, environment or dietary factors and/or body site differences that account for this discordancy. Nevertheless, the analysis of forearms and hand body sites in the current study is consistent with the original facial study and the more recent literature of other subjects living in South Africa 14. Moreover, the skin surface pH data does differ from that of Zlotogorski for Caucasian skin 64. The reported forehead to cheek differences are only apparent in the Black African subjects.
The power of our methodological approach, particularly for skin hydration, was highlighted in two intervention studies; one non‐vehicle‐controlled capacitance mapping study on Black Africans, Caucasians, Chinese and Indians living in Pretoria and one vehicle‐controlled conductance mapping study on Chinese subjects living in Beijing using saccharide isomerate as the primary humectant. Clear improvements in skin hydration were observed in the first study with the Black Africans having the bigger benefits. However, despite having slightly higher (non‐statistically) overall skin hydration values the average performance of the moisturiser on Indian facial skin was less than half of that for the other two ethnic groups. Clearly ethnic differences probably occur as a result of product application and/or underlying skin biology. In the conductance mapping study skin hydration values were the lowest on the lower parts of the face especially the cheek areas just like the capacitance studies. Clear improvements in skin hydration were observed for the saccharide isomerate‐containing moisturiser globally across the face whereas the lower parts of the face were still compromised for the vehicle treatment.
Similar hydration images were measured for the baseline capacitance and conductance mapping despite the use of different Chinese subjects at different times of the year in different geographical location implying that ethnic skin type is controlling this parameter rather than environmental condition. Nevertheless, more work is needed to come to concrete conclusions as others have found seasonal differences in cheek but not forehead hydration 23, 65.
Finally, the facial sebum gradients on Caucasians were determined and the characteristic T‐zone distribution was demonstrated.
Conclusion
Continuous colour mapping analysis of facial skin hydration, barrier function, skin surface pH and sebum has highlighted inter‐ethnic differences in these parameters not expected from other published single point measurement data on the face and surrogate test sites such as the volar forearm. A new p‐mapping approach adds further value to understanding ethnic skin differences. These studies show, unequivocally, that there is no relationship between skin pigmentation and barrier function on the face and that improved skin barrier function that is associated with lower skin surface pH values is not melanin‐driven, at least for subjects living in South Africa. Despite these differences, that still need to understand at the cellular and biochemical levels, validation of the hydration mapping approaches was shown in two intervention studies using the two most commonly measurement techniques. Again, clear ethnic differences were observed following moisturiser application. These studies possibly highlight why consumer facial skin moisturisation needs are currently not fully met.
Conflicts of interests
RV is an employee of DSM, AVR is a consultant to DSM and JG is an employee of Newtone. BS has no conflict of interest.
Funding information
All the studies were funded by DSM Nutritional Products Ltd., Basel, Switzerland.
Supporting information
Figure S1. Comparison of a) basal TEWL and b) skin surface pH values. Results represent means ± SEM, individual facial means have been averaged from 30 facial measurements, *p < 0.05, **p <0.01.
Figure S2. Conductance values averaged for the forehead, eye, cheek and jaw regions and whole face. Results represent mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. Vehicle data at different time points are shown in red bars, and the data of the Test Cream in blue bars.
Acknowledgements
We would like to thank the following colleagues, who made the studies possible with their enthusiasm and dedication: Pierre Seroul, Marie Cherel and Jean‐Michel Delaval of Newtone Technologies, Lyon, France, who developed the algorithms and technologies enabling the matchless colourful illustrations. Lebogang Kgatuke, Marlize Lategan, Caroline Moeletsi and Lee‐Ann Raaff of the Photobiology Laboratory, Sefako Makgatho Health Sciences University, Pretoria, South Africa for their indefatigability in conducting the multiethnic studies. Peter Kollias of Canfield Scientific Inc., Utrecht, The Netherlands for providing a Vectra m3 System and the corresponding education. Rotraut Schoop and Lorenzo Tanadini of DSM Nutritional Products Ltd, Kaiseraugst, Switzerland for their statistical consultancy. Dominik Imfeld and Remo Campiche of DSM Nutritional Products Ltd, Kaiseraugst, Switzerland for the planning and execution of the sebum mapping study. Anson Zhang of DSM Nutritional Products Ltd, Shanghai, China for monitoring the vehicle‐controlled skin hydration mapping study. The sebum mapping study was conducted at The Skin Test Institute, Neuchâtel, Switzerland and the vehicle‐controlled skin hydration mapping study at Landproof Testing Technology Co. Ltd., Guangzhou, China. All the studies were funded by DSM Nutritional Products Ltd., Basel, Switzerland.
References
- 1. Rawlings, A.V. Trends in stratum corneum research and the management of dry skin conditions. Int. J. Cosmet. Sci. 25, 63–95 (2003). [DOI] [PubMed] [Google Scholar]
- 2. Reed, J.T. , Ghadially, R. and Elias, P.M. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch. Dermatol. 131, 1134–8 (1995). [PubMed] [Google Scholar]
- 3. Warrier, A.G. , Kligman, A.M. , Harper, R.A. , Bowman, J. and Wickett, R.R. A comparison of black and white skin using noninvasive methods. J. Soc. Cosmet. Chem. 47, 229–40 (1996). [Google Scholar]
- 4. Berardesca, E. , Pirot, F. , Singh, M. and Maibach, H. Differences in stratum corneum pH gradient when comparing white Caucasian and black African‐American skin. Br. J. Dermatol. 139, 855–7 (1998). [DOI] [PubMed] [Google Scholar]
- 5. Grimes, P. , Edison, B.L. , Green, B.A. and Wildnauer, R.H. Evaluation of inherent differences between African American and white skin surface properties using subjective and objective measures. Cutis 73, 392–6 (2004). [PubMed] [Google Scholar]
- 6. Fotoh, C. , Elkhyat, A. , Mac, S. , Sainthillier, J.M. and Humbert, P. Cutaneous differences between Black, African or Caribbean Mixed‐race and Caucasian women: biometrological approach of the hydrolipidic film. Skin Res. Technol. 14, 327–35 (2008). [DOI] [PubMed] [Google Scholar]
- 7. Gunathilake, R. , Schurer, N.Y. , Shoo, B.A. , et al. pH‐regulated mechanisms account for pigment‐type differences in epidermal barrier function. J. Invest. Dermatol. 129, 1719–29 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muizzuddin, N. , Hellemans, L. , Van Overloop, L. , Corstjens, H. , Declercq, L. and Maes, D. Structural and functional differences in barrier properties of African American, Caucasian and East Asian skin. J. Dermatol. Sci. 59, 123–8 (2010). [DOI] [PubMed] [Google Scholar]
- 9. Mack, M.C. , Chu, M.R. , Tierney, N.K. , et al. Water‐holding and transport properties of skin stratum corneum of infants and toddlers are different from those of adults: studies in three geographical regions and four ethnic groups. Pediatr. Dermatol. 33, 275–82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu, M. and Kollias, N. Documentation of normal stratum corneum scaling in an average population: features of differences among age, ethnicity and body site. Br. J. Dermatol. 164, 497–507 (2011). [DOI] [PubMed] [Google Scholar]
- 11. Pappas, A. , Fantasia, J. and Chen, T. Age and ethnic variations in sebaceous lipids. Dermato‐Endocrinology. 5, 319–24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elias, P.M. , Menon, G. , Wetzel, B.K. and Williams, J. Barrier requirements as the evolutionary "driver" of epidermal pigmentation in humans. Am. J. Hum. Biol. 22, 526–37 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elias, P.M. and Williams, M.L. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: The barrier and metabolic conservation hypotheses revisited. Am. J. Phys. Anthropol. 161, 189–207 (2016). [DOI] [PubMed] [Google Scholar]
- 14. Young, M.M. , Franken, A. and du Plessis, J.L. Transepidermal water loss, stratum corneum hydration, and skin surface pH of female African and Caucasian nursing students. Skin Res. Technol. 25, 88–95 (2019). [DOI] [PubMed] [Google Scholar]
- 15. Proksch, E. Protection against dryness of facial skin: a rational approach. Skin Pharmacol. Physiol. 22, 3–7 (2008). [DOI] [PubMed] [Google Scholar]
- 16. Ya‐Xian, Z. , Suetake, T. and Tagami, H. Number of cell layers of the stratum corneum in normal skin – relationship to the anatomical location on the body, age, sex and physical parameters. Arch. Dermatol. Res. 291, 555–9 (1999). [DOI] [PubMed] [Google Scholar]
- 17. Hirao, T. , Denda, M. and Takahashi, M. Identification of immature cornified envelopes in the barrier‐impaired epidermis by characterization of their hydrophobicity and antigenicities of the components. Exp. Dermatol. 10, 35–44 (2001). [DOI] [PubMed] [Google Scholar]
- 18. Tagami, H. Location‐related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 30, 413–34 (2008). [DOI] [PubMed] [Google Scholar]
- 19. Pratchyapruit, W. , Kikuchi, K. , Gritiyarangasan, P. , Aiba, S. and Tagami, H. Functional analyses of the eyelid skin constituting the most soft and smooth area on the face: contribution of its remarkably large superficial corneocytes to effective water‐holding capacity of the stratum corneum. Skin Res. Technol. 13, 169–75 (2007). [DOI] [PubMed] [Google Scholar]
- 20. Mohammed, D. , Matts, P.J. , Hadgraft, J. and Lane, M.E. Variation of stratum corneum biophysical and molecular properties with anatomic site. AAPS J. 14, 806–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers, J. , Harding, C. , Mayo, A. , Banks, J. and Rawlings, A.V. Stratum corneum lipids: the effect of ageing and the seasons. Arch. Dermatol. Res. 288, 765–70 (1996). [DOI] [PubMed] [Google Scholar]
- 22. Masukawa, Y. , Narita, H. , Sato, H. , et al. Comprehensive quantification of ceramide species in human stratum corneum. J. Lipid Res. 50, 1708–19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishikawa, J. , Shimotoyodome, Y. , Ito, S. , et al. Variations in the ceramide profile in different seasons and regions of the body contribute to stratum corneum functions. Arch. Dermatol. Res. 305, 151–62 (2013). [DOI] [PubMed] [Google Scholar]
- 24. Koyama, J. , Horii, I. , Kawasaki, K. , et al. Free amino acids of stratum corneum as a biochemical marker to evaluate dry skin. J. Soc. Cosmet. Chem. 35, 183–95 (1984). [Google Scholar]
- 25. Raj, N. , Voegeli, R. , Rawlings, A.V. , Summers, B. , Munday, M.R. and Lane, M.E. Variation in the activities of late stage filaggrin processing enzymes, calpain‐1 and bleomycin hydrolase, together with pyrrolidone carboxylic acid levels, corneocyte phenotypes and plasmin activities in non‐sun exposed and sun‐exposed facial stratum corneum of different ethnicities. Int. J. Cosmet. Sci. 38, 567–75 (2016). [DOI] [PubMed] [Google Scholar]
- 26. McAleer, M.A. , Jakasa, I. , Raj, N. , et al. Early‐life regional and temporal variation in filaggrin‐derived natural moisturizing factor, filaggrin‐processing enzyme activity, corneocyte phenotypes and plasmin activity: implications for atopic dermatitis. Br. J. Dermatol. 179, 431–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voegeli, R. , Rawlings, A.V. , Doppler, S. , Heiland, J. and Schreier, T. Profiling of serine protease activities in human stratum corneum and detection of a stratum corneum tryptase‐like enzyme. Int. J. Cosmet. Sci. 29, 191–200 (2007). [DOI] [PubMed] [Google Scholar]
- 28. Voegeli, R. , Rawlings, A.V. , Doppler, S. and Schreier, T. Increased basal transepidermal water loss leads to elevation of some but not all stratum corneum serine proteases. Int. J. Cosmet. Sci. 30, 435–42 (2008). [DOI] [PubMed] [Google Scholar]
- 29. Takada, K. , Amano, S. , Kohno, Y. , Nishiyama, T. and Inomata, S. Non‐invasive study of gelatinases in sun‐exposed and unexposed healthy human skin based on measurements in stratum corneum. Arch. Dermatol. Res. 298, 237–42 (2006). [DOI] [PubMed] [Google Scholar]
- 30. Raj, N. , Voegeli, R. , Rawlings, A.V. , et al. Variation in stratum corneum protein content as a function of anatomical site and ethnic group. Int. J. Cosmet. Sci. 38, 224–31 (2016). [DOI] [PubMed] [Google Scholar]
- 31. Voegeli, R. , Monneuse, J.M. , Schoop, R. , Summers, B. and Rawlings, A.V. The effect of photodamage on the female Caucasian facial stratum corneum corneome using mass spectrometry‐based proteomics. Int. J. Cosmet. Sci. 39, 637–52 (2017). [DOI] [PubMed] [Google Scholar]
- 32. Guneri, D. , Voegeli, R. , Munday, M.R. and Rawlings, A.V. 12R‐lipoxygenase activity is reduced in photodamaged facial stratum corneum. A novel activity assay indicates a key function in corneocyte maturation. Int. J. Cosmet. Sci. 41, 274–280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guneri, D. , Voegeli, R. , Munday, M.R. and Rawlings, A.V. The importance of 12R‐lipoxygenase and transglutaminase activities in the hydration‐dependent ex vivo maturation of corneocyte envelopes. Submitted to Int. J. Cosmet. Sci. (2019). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voegeli, R. , Rawlings, A.V. and Summers, B. Facial skin pigmentation is not related to stratum corneum cohesion, basal transepidermal water loss, barrier integrity and barrier repair. Int. J. Cosmet. Sci. 37, 241–52 (2015). [DOI] [PubMed] [Google Scholar]
- 35. Schnetz, E. , Kuss, O. , Schmitt, J. , Diepgen, T.L. , Kuhn, M. and Fartasch, M. Intra‐and inter‐individual variations in transepidermal water loss on the face: facial locations for bioengineering studies. Contact Dermatitis. 40, 243–7 (1999). [DOI] [PubMed] [Google Scholar]
- 36. Lopez, S. , Le Fur, I. , Morizot, F. , Heuvin, G. , Guinot, C. and Tschachler, E. Transepidermal water loss, temperature and sebum levels on women's facial skin follow characteristic patterns. Skin Res. Technol. 6, 31–6 (2000). [DOI] [PubMed] [Google Scholar]
- 37. Marrakchi, S. and Maibach, H.I. Biophysical parameters of skin: map of human face, regional, and age‐related differences. Contact Dermatitis. 57, 28–34 (2007). [DOI] [PubMed] [Google Scholar]
- 38. Crowther, J. TEWL mapping of male face, Stratum Corneum VII Meeting, Cardiff (2012).
- 39. Voegeli, R. , Rawlings, A.V. , Seroul, P. and Summers, B. A novel continuous colour mapping approach for visualization of facial skin hydration and transepidermal water loss for four ethnic groups. Int. J. Cosmet. Sci. 37, 595–605 (2015). [DOI] [PubMed] [Google Scholar]
- 40. Berardesca, E. EEMCO guidance for the assessment of stratum corneum hydration: electrical methods. Skin Res. Technol. 3, 126–32 (1997). [DOI] [PubMed] [Google Scholar]
- 41. Rogiers, V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol. Appl. Skin Physiol. 14, 117–28 (2001). [DOI] [PubMed] [Google Scholar]
- 42. Parra, J.L. and Paye, M. EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol. Appl. Skin Physiol. 16, 188–202 (2003). [DOI] [PubMed] [Google Scholar]
- 43. Egawa, M. , Yanai, M. , Maruyama, N. , Fukaya, Y. and Hirao, T. Visualization of Water Distribution in the Facial Epidermal Layers of Skin Using High Sensitivity Near‐Infrared (NIR) Imaging. Appl. Spectrosc. 69, 481–487. (2015). [DOI] [PubMed] [Google Scholar]
- 44. Arimoto, H. , Yanai, M. and Egawa, M. Analysis of absorption and spreading of moisturizer on the microscopic region of the skin surface with near‐infrared imaging. Skin Res. Technol. 22, 505–12 (2016). [DOI] [PubMed] [Google Scholar]
- 45. Bookstein, F.L. Principal warps: Thin‐plate splines and the decomposition of deformations. IEEE Trans. Pattern Anal. Mach. Intell. 11, 567–85 (1989). [Google Scholar]
- 46. Hillebrand, G.G. , Levine, M.J. and Miyamoto, K. The age‐dependent changes in skin condition in African Americans, Asian Indians, Caucasians. East Asians and Latinos. IFSCC Magazine. 4, 259–66 (2001). [Google Scholar]
- 47. Diridollou, S. , de Rigal, J. , Querleux, B. , Leroy, F. and Holloway, Barbosa V. Comparative study of the hydration of the stratum corneum between four ethnic groups: influence of age. Int. J. Dermatol. 46(Suppl 1), 11–4 (2007). [DOI] [PubMed] [Google Scholar]
- 48. Voegeli, R. , Wikstroem, P. , Campiche, R. , et al. The presence of essential and non‐essential stratum corneum proteases: the vital need for protease inhibitors. IFSCC Magazine. 20, 111–8 (2017). [Google Scholar]
- 49. Voegeli, R. , Cherel, M. , Raaff, L.‐A. , Kollias, P. , Seroul, P. , Summers, B. , et al. Facial color mapping of stratum corneum hydration of different ethnic groups. 23rd IFSCC Conference. Zurich, Switzerland (2015).
- 50. ISBS . Johann‐Wilhelm‐Ritter Award 2016: Complexity of Facial Skin Hydration Visualized by 3D Color Mapping. Available from: http://www.jwr-award.org/winners/.
- 51. Pierre, J. , Francois, G. , Benize, A.M. , Rubert, V. , Coutet, J. and Flament, F. Mapping, in vivo, the uniformity of two skin properties alongside the human face by a 3D virtual approach. Int. J. Cosmet. Sci. 40, 482–7 (2018). [DOI] [PubMed] [Google Scholar]
- 52. Lambers, H. , Piessens, S. , Bloem, A. , Pronk, H. and Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 28, 359–70 (2006). [DOI] [PubMed] [Google Scholar]
- 53. Proksch, E. pH in nature, humans and skin. J. Dermatol. 45, 1044–1052 (2018). [DOI] [PubMed] [Google Scholar]
- 54. Mauro, T. , Holleran, W.M. , Grayson, S. , et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch. Dermatol. Res. 290, 215–22 (1998). [DOI] [PubMed] [Google Scholar]
- 55. Hachem, J.P. , Crumrine, D. , Fluhr, J. , Brown, B.E. , Feingold, K.R. and Elias, P.M. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J. Invest. Dermatol. 121, 345–53 (2003). [DOI] [PubMed] [Google Scholar]
- 56. Hachem, J.P. , Man, M.Q. , Crumrine, D. , et al. Sustained serine proteases activity by prolonged increase in ph leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J. Invest. Dermatol. 125, 510–20 (2005). [DOI] [PubMed] [Google Scholar]
- 57. Hachem, J.P. , Roelandt, T. , Schurer, N. , et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J. Invest. Dermatol. 130, 500–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Denda, M. , Kitamura, K. , Elias, P.M. and Feingold, K.K. trans‐4‐(Aminomethyl)cyclohexane Carboxylic Acid (T‐AMCHA), an anti‐fibrinolytic agent, accelerates barrier recovery and prevents the epidermal hyperplasia induced by epidermal injury in hairless mice and humans. J Invest. Dermatol. 109, 84–90 (1997). [DOI] [PubMed] [Google Scholar]
- 59. Voegeli, R. , Wikstroem, P. , Campiche, R. , et al. The effects of benzylsulfonyl‐D‐Ser‐homoPhe‐(4‐amidino‐benzylamide), a dual plasmin and urokinase inhibitor, on facial skin barrier function in subjects with sensitive skin. Int. J. Cosmet. Sci. 39, 109–20 (2017). [DOI] [PubMed] [Google Scholar]
- 60. Alhasaniah, A. , Sherratt, M.J. and O'Neill, C.A. The impact of ultraviolet radiation on barrier function in human skin: Molecular mechanisms and topical therapeutics. Curr. Med. Chem. 25, 5503–11 (2018). [DOI] [PubMed] [Google Scholar]
- 61. Han, B. , Jung, B. , Nelson, J.S. and Choi, E.H. Analysis of facial sebum distribution using a digital fluorescent imaging system. J. Biomed. Opt. 12, 014006 (2007). [DOI] [PubMed] [Google Scholar]
- 62. Cortes, H. , Mendoza‐Munoz, N. , Galvan‐Gil, F.A. , et al. Comprehensive mapping of human body skin hydration: A pilot study. Skin Res. Technol. 25, 187–193 (2018). [DOI] [PubMed] [Google Scholar]
- 63. Bazin, R. and Fanchon, C. Equivalence of face and volar forearm for the testing of moisturizing and firming effect of cosmetics in hydration and biomechanical studies. Int. J. Cosmet. Sci. 28, 453–60 (2006). [DOI] [PubMed] [Google Scholar]
- 64. Zlotogorski, A. Distribution of skin surface pH on the forehead and cheek of adults. Arch. Dermatol. Res. 279, 398–401 (1987). [DOI] [PubMed] [Google Scholar]
- 65. Black, D. , Del Pozo, A. , Lagarde, J.M. and Gall, Y. Seasonal variability in the biophysical properties of stratum corneum from different anatomical sites. Skin Res. Technol. 6, 70–6 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of a) basal TEWL and b) skin surface pH values. Results represent means ± SEM, individual facial means have been averaged from 30 facial measurements, *p < 0.05, **p <0.01.
Figure S2. Conductance values averaged for the forehead, eye, cheek and jaw regions and whole face. Results represent mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. Vehicle data at different time points are shown in red bars, and the data of the Test Cream in blue bars.


