Abstract
Conservation of long‐distance migratory species poses unique challenges. Migratory connectivity, that is, the extent to which groupings of individuals at breeding sites are maintained in wintering areas, is frequently used to evaluate population structure and assess use of key habitat areas. However, for species with complex or variable annual cycle movements, this traditional bimodal framework of migratory connectivity may be overly simplistic. Like many other waterfowl, sea ducks often travel to specific pre‐ and post‐breeding sites outside their nesting and wintering areas to prepare for migration by feeding extensively and, in some cases, molting their flight feathers. These additional migrations may play a key role in population structure, but are not included in traditional models of migratory connectivity. Network analysis, which applies graph theory to assess linkages between discrete locations or entities, offers a powerful tool for quantitatively assessing the contributions of different sites used throughout the annual cycle to complex spatial networks. We collected satellite telemetry data on annual cycle movements of 672 individual sea ducks of five species from throughout eastern North America and the Great Lakes. From these data, we constructed a multi‐species network model of migratory patterns and site use over the course of breeding, molting, wintering, and migratory staging. Our results highlight inter‐ and intra‐specific differences in the patterns and complexity of annual cycle movement patterns, including the central importance of staging and molting sites in James Bay, the St. Lawrence River, and southern New England to multi‐species annual cycle habitat linkages, and highlight the value of Long‐tailed Ducks (Calengula haemalis) as an umbrella species to represent the movement patterns of multiple sea duck species. We also discuss potential applications of network migration models to conservation prioritization, identification of population units, and integrating different data streams.
Keywords: connectivity, eider, flyway, Long‐tailed Duck, migration, molt, network analysis, Scoter, sea duck, stopover
Introduction
Conservation of long‐distance migratory species requires understanding distribution and movement patterns across the annual cycle (Berger 2004). Migratory connectivity, generally defined as the extent to which groupings of individuals at breeding sites are maintained in wintering areas (Webster et al. 2002), is frequently used to evaluate population structure and identify key habitat areas for conservation (Martin et al. 2007, Ambrosini et al. 2009, Taylor and Norris 2010, Marra et al. 2011). While this traditional model of migratory connectivity may be sufficient to describe species that migrate back and forth between discrete breeding and non‐breeding locations, it fails to capture important aspects of migratory movements for species whose annual movement patterns are more complex (Seifert et al. 2016). Many species travel to pre‐ or post‐breeding staging sites separate from their principal breeding and overwintering areas or visit multiple breeding or wintering sites during a single season (Haig et al. 1998), and these movements play key roles in important demographic events such as pair formation (Weller and Batt 1988), attaining reproductive maturity (Bentzen and Powell 2015), or gathering information on habitat quality (Boulinier et al. 1996). Thus, movements that substantially influence population structure may fall outside the scope of many migratory connectivity assessments.
Migratory birds are a frequent focus of migratory connectivity research, since they travel greater distances than many terrestrial migrants and often have widely varying resource and habitat requirements during different periods of the annual cycle (Alerstam et al. 2003, Kirby et al. 2008). Consequently, the percentage of crucial habitat areas receiving some form of conservation is often lower for migratory birds than for non‐migratory avian species whose habitat requirements are met within more restricted areas (Runge et al. 2015). Among migratory birds, waterfowl are frequently targeted for conservation and management due to their value to both hunters and birders (Johnsgard 2010, Cooper et al. 2015, Rothe et al. 2015). However, understanding and conserving annual cycle habitat linkages in waterfowl is challenging due to their complex migration patterns: in addition to undertaking spring and fall migrations to and from breeding sites, members of many waterfowl species also migrate to specific post‐breeding sites to molt their flight feathers (Salomonsen 1968, Solovyeva et al. 2014) and may move between multiple sites during winter (Fox et al. 1994, Lindberg et al. 2007, Oppel et al. 2009). Although many waterfowl species display strong philopatry and site fidelity to breeding areas (Rohwer and Anderson 1988, Robertson and Cooke 1999), movements between and among non‐breeding sites may vary according to a suite of individual and environmental factors (Oppel et al. 2009). These additional non‐breeding movements are an important component of the annual cycle of some waterfowl, particularly sea ducks (Savard and Petersen 2015); however, they are not included in quantitative models of migratory connectivity that assume twice‐yearly movements between breeding and wintering habitats (Ambrosini et al. 2009). Fully representing complex migration patterns therefore requires a quantitative approach that accounts for movements among multiple habitat types, both between and within seasons.
Network analysis of landscape‐level habitat linkages offers a powerful tool for exploring complex interrelationships between habitat patches and quantitatively assessing the relative contributions of different areas to spatial networks (Bodin and Saura 2010). Mathematically, network analysis is a branch of graph theory that deals with pairwise relationships among a set of objects or entities. Integrating information about the connections (i.e., edges) between each pair of entities (i.e., nodes), allows for mathematical assessment of the properties of the community as a whole and the comparative roles of its individual elements. Although network analysis has been used in ecological studies for several decades to examine gene flow between individuals (Dyer et al. 2010), to assess theoretical movements of dispersing organisms across interconnected habitat patches (Bodin and Saura 2010), and to quantify effects of landscape structure on connectivity, dispersal, and demography (Minor and Urban 2008, Engelhard et al. 2017, Leonard et al. 2017, Wiederholt et al. 2017), its use in analysis of individual movement data has only recently begun to emerge (Jacoby and Freeman 2016). One of the challenges inherent in these types of applications is that the locations of nodes within the network has to be inferred from the data themselves, except in the case of telemetry studies with receivers in fixed locations (Jacoby et al. 2012, Stehfest et al. 2013). However, targeted applications of network analysis to individual movement data have provided insights into the spread of disease (Tian et al. 2015), landscape‐scale population structure (Knight et al. 2018), and the importance of stopover sites along migration routes (Shimazaki et al. 2004).
Telemetry studies that collect individual time series on movements of migratory species offer capacity to construct network models that incorporate movements of individual animals between discrete areas (Shimazaki et al. 2004, Rhodes et al. 2006, Knight et al. 2018). One such effort is the Atlantic and Great Lakes Sea Duck Migration Study (Sea Duck Joint Venture 2014), which has focused on the collection of satellite telemetry data from individual sea ducks (tribe: Mergini) across multiple species in eastern North America, in order to understand landscape‐scale movement patterns and migratory habitat linkages. Since 2002, the project and other associated tracking efforts have collected annual cycle movement data from 672 individual birds representing five species, including Common Eider (Somateria mollissima), Black Scoter (Melanitta americana), Surf Scoter (M. perspicillata), White‐winged Scoter (M. deglandi), and Long‐tailed Duck (Clangula hyemalis). To date, analyses of these data have focused on species‐specific movement patterns and habitat use (Loring et al. 2014, Beuth et al. 2017, Spiegel et al. 2017, Meattey et al. 2018). However, the complete dataset provides a unique opportunity to examine and compare movement patterns across several sympatric species tracked simultaneously. Multi‐species habitat analyses can provide insights into habitat use that transcend conclusions based on a single species and highlight landscape features with broad conservation importance (Block et al. 2011, Hindell et al. 2011, Raymond et al. 2015).
We developed a spatially explicit, multi‐species network model of habitat linkages to assess movement patterns of five species of sea ducks in eastern North America named in the paragraph above. We first derived a migration network from tracking data by using state‐space modeling to identify locations of residency and spatial cluster analysis to group resident points into a directed network of interconnected nodes. We then applied the quantitative tools of graph theory to distinguish key habitat locations and evaluate links between them. Our objectives included (1) evaluating the relative importance of key habitat sites within a network of interconnected migratory pathways, (2) determining whether site‐ or flyway‐specific subgroups exist within broader sea duck populations in the region, and (3) assessing annual cycle habitat linkages and overlap at the landscape scale for sympatric sea duck species.
Methods
Study area
We captured sea ducks in multiple areas along the Atlantic coast and Great Lakes of North America during the molting, staging, and wintering time periods (October–March) between 2002 and 2017 (Fig. 1a; Appendix S1: Table S1). In order to maximize capture efficiency, sampling locations were selected to represent locations and time periods of particularly high non‐breeding concentrations of each species. This approach resulted in lack of sampling efforts in less‐utilized winter and staging sites; however, given the tendency of waterfowl to form large aggregations during non‐breeding (Weller and Batt 1988), sampling known areas of high sea duck concentrations allowed us to efficiently target the majority of the study populations. Capture efforts for long‐tailed ducks, White‐winged Scoters and Surf Scoters focused primarily on wintering sites, with transmitter distribution allocated according to concentrations of birds observed during the Atlantic Winter Sea Duck Survey. Additional sampling of long‐tailed ducks on Lake Michigan was added late in the project to account for gaps in observed data. Capture efforts for Black Scoter focused on spring migration sites in the St. Lawrence River, with additional captures of the three scoter species during fall migration and molt. Sampling of Common Eider was limited to one of three eastern subspecies (S. m. dresseri) during breeding and wintering periods.
Figure 1.
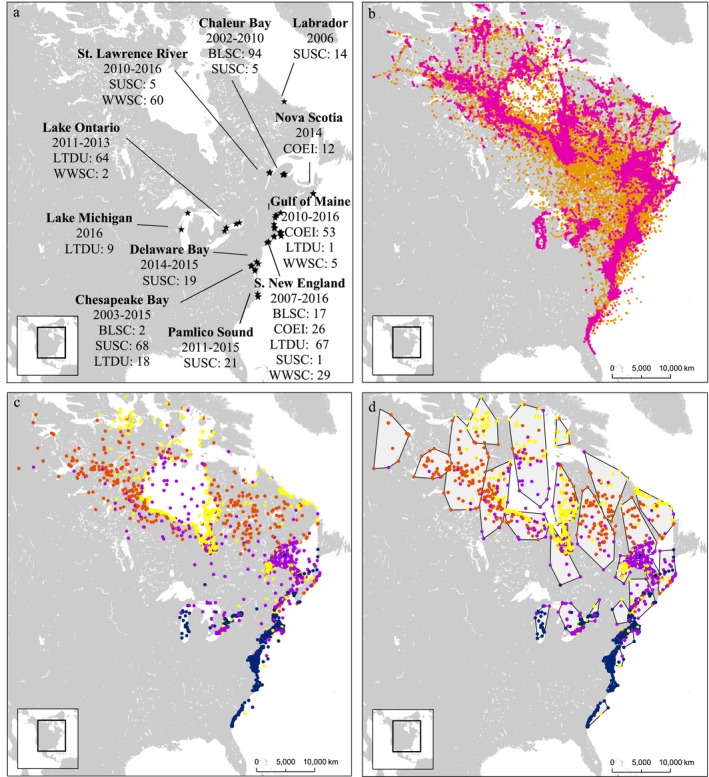
Network analysis workflow for telemetry locations. (a) Deployment of transmitters at wintering, staging, and molt sites across the eastern ranges of five sea duck species (BLSC, Black Scoter; COEI, Common Eider; LTDU, Long‐tailed Duck; SUSC, Surf Scoter; WWSC, White‐winged Scoter). (b) State‐space classification of location data (resident, magenta; transient, orange). (c) Geographic centroids for each residency period and assignment of centroids to seasonal categories (breeding, red; fall migration, yellow; winter, blue; spring migration, purple). (d) Spatial grouping of centroids using cluster analysis (i.e., nodes).
Transmitter deployment
We captured both sexes of subadult and adult ducks on water using a combination of decoys and netting. The majority of captures used floating nets (1.3 × 18 m2, 127‐mm mesh), which were positioned above water as mist nets to catch birds in flight (Brodeur et al. 2008), submerged as gillnets to catch birds during dives (Breault and Cheng 1990), or suspended horizontally underwater and lifted as birds swam over nets (Ware et al. 2013; Appendix S1: Table S1). Additional capture techniques included night‐lighting and dip‐netting for wintering birds roosting on the water, and net‐gunning. Female Common Eiders in Nova Scotia were also captured at nest sites using fishing nets. We determined age by measuring bursa depth and sex by examination of cloaca (Sea Duck Joint Venture 2015) and measured body mass with a Pesola spring scale (Pesola AG, Schindellegi, Switzerland; ±5 g) or digital hanging scale (UWE HS‐3000, Universal Weight Enterprise Co., Taipei, Taiwan; 2 g). Veterinarians experienced in avian surgery implanted 26–50 g coelomic‐implant Platform Transmitter Terminals (PTT; Microwave Telemetry, Columbia, Maryland, USA; Telonics, Mesa, Arizona, USA; Geotrak, Apex, North Carolina, USA; Appendix S1: Table S1) into the abdominal cavity following implantation techniques described by Korschgen et al. (1996). Individuals were selected for transmitter attachment based on body mass, such that transmitter mass represented less than 5% of overall body mass (Phillips et al. 2003). Under this criterion, all birds within the typical mass ranges of the target species were eligible for transmitter attachment; thus, individuals excluded were likely atypically small or in poor condition. Transmitters followed varying duty cycles consisting of 2–4 h “on” periods followed by 10–120 h “off” periods, resulting in one location every 0.5–5 d (for specific duty cycles by deployment event, see Appendix S1: Table S1). We excluded data collected during the first 14 d following surgeries to minimize potential biases in assessments of habitat use patterns and movement dynamics due to surgery (Esler et al. 2000). Argos location data were processed and disseminated through Collecte Localisation Satellites (CLS) America. PTT signals were received by equipment on polar‐orbiting National Oceanic and Atmospheric Administration and MetOp satellites. Data was transferred to the CLS America processing center in Lanham, Maryland, US where locations were estimated from the Doppler shift in the PTTs carrier frequency.
Data processing
To construct a spatial network from telemetry data, we used the following criteria: (1) filtered and refined locations using a state‐space model of underlying movement patterns, which used step lengths and turning angles between successive points to classify locations as either resident (short‐distance, tortuous movements) or transient (long distance, linear movements); (2) assigned each resident location to a season within the annual cycle, and calculated the geographic centroid of each distinct set of individual resident locations; and (3) spatially clustered individual habitat centroids to build a simplified global habitat network.
State‐space modeling
We used a switching state‐space model (Jonsen et al. 2005) to account for both variations in data quality and changes in the underlying movement patterns that generated observed locations. This approach allowed us to simultaneously model the observation error associated with each location due to variation in data quality and interpolate a temporally regular track from data with varying temporal gaps between locations. It also allowed us to classify locations based on their probability of assignment to one of two states: resident, in which movements between successive locations are characterized by short distances and frequent directional change, or transient, in which locations are widely spaced and directional change infrequent.
Raw satellite telemetry data varies in quality of location estimates based on the configuration and number of satellites used to obtain each location. Location estimates were acquired with Argos Standard Service Processing and assigned, in decreasing order of precision, to Argos Location Classes (LC 3, 2, 1, and 0) and Auxiliary Location Processing (LC A, B, and Z). Accuracy (i.e., one standard deviation) for location estimates with LC 3, 2, 1, and 0 were <250, 250–500, 500–1,500, and >1,500 m, respectively (CLS 2016). In subsequent modeling, each point was considered to represent the center of a probability distribution based on the error associated with its location class. To allow the model sufficient information to interpolate individual tracks, we removed all individuals with less than 50 locations in LC 1‐3 (typically, individuals with one month or less of location data) prior to analysis. During modeling, we interpolated tracking data to 1‐d intervals based on the most likely paths between locations. We did not interpolate over time periods of >7 d between successive locations, because longer temporal gaps produce unrealistic movement trajectories (Jonsen et al. 2005). Based on the duty cycles of the transmitters, the maximum programmed gap between locations for a correctly functioning unit was 5 d (120 h); thus, 92% of locations were separated by gaps of ≤7 d.
We ran all models in the bsam package (Jonsen et al. 2005, Jonsen 2016) in R (R Core Team 2018) using a switching first difference correlated random walk model with a 1‐d time step, 5,000 burn‐in samples for model training, and 5,000 posterior samples for analysis. We thinned posterior samples by selecting every fifth sample to reduce autocorrelation and computing time, and used a 0.1 smoothing parameter. While thinning is not a necessary step, it may be justified in cases where it substantially reduces computational efficiency or where extensive post‐processing is required (Link and Eaton 2012), and in this case was necessary to efficiently process a large number of individual tracks and locations. Model outputs included probable daily locations with 2.5%, 50%, and 97.5% confidence intervals, as well as a score from 1 to 2 (hereafter, b) indicating the average assignment of the location to either a transient (1) or resident (2) behavioral state across all retained samples (Fig. 1b).
Season assignment
We assigned each resident location (Fig. 1b) to one of four distinct seasons (wintering, spring staging, breeding, or flight feather molt/fall staging) based on its position within the annual cycle. Individuals that did not attend likely breeding sites (i.e., terrestrial locations near inland water bodies) generally occupied the same sites during both summer and fall staging, and were therefore considered to progress directly to fall staging. Because many individuals did not attend breeding sites, or occupied only a single breeding site per year (as opposed to multiple sites during migration and wintering periods), there were proportionally fewer sites assigned to the breeding state (12%) than to fall migration (30%), winter (32%), or spring migration (26%; Table 1).
Table 1.
Sample sizes for transmitter deployments on five species of sea ducks in eastern North America, 2002–2017
| Species | Transmitters deployed | Retained in network | Habitat centroids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total deployed | Locations per individual (mean) | Total retained | Males | Females | Total centroids | Winter | Spring migration | Breeding | Fall migration | |
| Black Scoter | 113 | 532 | 89 | 42 | 47 | 1,193 | 325 | 399 | 98 | 371 |
| Surf Scoter | 207 | 427 | 139 | 75 | 64 | 1,075 | 342 | 283 | 99 | 351 |
| White‐winged Scoter | 96 | 315 | 83 | 21 | 62 | 550 | 213 | 113 | 62 | 162 |
| Common Eider (dresseri) | 91 | 396 | 76 | 15 | 61 | 395 | 135 | 34 | 126 | 100 |
| Long‐tailed Duck | 165 | 187 | 89 | 30 | 59 | 510 | 164 | 146 | 78 | 122 |
| Total | 672 | 338 | 476 | 183 | 293 | 3,723 | 1,179 | 975 | 463 | 1,106 |
See Fig. 1 for specific deployment locations and years.
We defined a period of residency as a set of two or more resident locations (b > 1.5) separated before and after by two or more transient locations (b ≤ 1.5). Most resident points had high probabilities of assignment to the resident state: 82% of all resident locations had b values ≥ 1.9, and 95% had b values ≥ 1.7. For each residency period, we calculated the duration of residency as well as the geographic centroid of all locations (Fig. 1c). Locations with 1.5 < b < 1.7 typically occurred either just after an individual arrived at a resident site, or just before it departed, with high‐probability locations (b ≥ 1.9) forming the core of each resident centroid.
Spatial clustering
To define the spatial configuration of habitat areas represented by individual habitat centroids, we used an unsupervised clustering approach, which classifies data based on features of the data itself rather than using pre‐defined groupings. We first selected the optimal number of clusters using the NbClust package (Charrad et al. 2014) in R (R Core Team 2018), which compares clustering solutions across a suite of algorithms. We clustered habitat centroids based on latitude and longitude using complete linkages, allowing the total number of clusters (k) to vary from 20 ≤ k ≤ 50. We chose this interval by testing a larger range of cluster values (0 < k ≤ 200), and found that solutions where k < 20 tended to aggregate discrete habitat areas (e.g., Delaware and Chesapeake Bays), while solutions where k > 50 tended to subdivide areas of dense and relatively uniform habitat use (e.g., Long Island Sound). We chose to assign all locations to clusters, rather than allowing for outliers, to account for the varying densities of sea ducks across the annual cycle, Since individual centroids were farther apart during breeding than wintering, assigning all locations to clusters allowed us to identify loosely grouped breeding sites as nodes rather than discrete locations.
Because several different cluster solutions were recommended by multiple algorithms, we chose the largest number of clusters (k = 31) that resulted in a significantly improved D‐index value (i.e., minimized the distance between clusters relative to the distance between points within clusters) for the overall cluster solution. We then assigned centroids to clusters using a standard nearest‐neighbor clustering algorithm (Fig. 1d) and computed between‐cluster linkages by counting direct movements between each possible pair of clusters. We also tested the two cluster solutions with the second‐ and third‐highest D‐index values (k = 25 and k = 21) and found that that the same general areas scored highly in our analyses regardless of which solution was used to construct the network.
For each individual, we removed the first habitat centroid to avoid inflating the relative importance of capture locations. Since capture sites were selected to represent high‐use areas, this step may have resulted in down‐weighting the importance of sites used for capture; however, since individuals frequently used more than one habitat centroid within a node, overall node‐level importance values did not vary depending on whether the first centroid was removed or retained.
Network construction
Based on the clusters (nodes) and cluster pairs (edges) defined by cluster analysis, we constructed directed (i.e., edges could be incoming, outgoing, or both) global and species‐specific network graphs. To ensure equal contributions by each species to the global network, each habitat centroid and edge was weighted by the inverse proportion of the number of centroids/edges collected from that species (N sp) to the total number of centroids/edges across all species (N; Table 1). Within each species, we evaluated network use separately by sex. This allowed us to test how conclusions differed depending on which sex(es) were included, because female waterfowl are generally considered better representatives of use of breeding habitat than males. We constructed networks using the igraph R package (Csardi and Nepusz 2006, Csardi 2015) and conducted all subsequent analyses in igraph and Conefor 2.6 (Saura and Torne 2009).
The structure and analysis of network models can be influenced by the selection and treatment of weighting factors, both for nodes and for edges (Opsahl et al. 2010). For habitat networks, weights may be based on distance, area, and/or frequency of use (Bodin and Saura 2010). To evaluate the sensitivity of node importance to different weighting schemes, we calculated all centrality and importance metrics using four different weighting schemes: species‐weighted (all species contribute equally to the network), individual‐weighted (all individuals contribute equally to the network), species‐ and individual‐weighted (individuals contribute equally to each species, and each species contributes equally to the network), and duration‐weighted (each centroid is weighted by the number of consecutive days it was occupied as a proportion of the year). Specific details of calculation methods and final weights are included in Appendix S2: Table S1. Although absolute scores for centrality varied depending on node and edge weights, relative scores remained consistent regardless of weighting (Appendix S2: Table S1). We, therefore, chose to construct the model using species‐weighted frequencies for both nodes and edges. This meant that each species contributed equally to both importance of habitat locations and linkages among habitats in the global model, and that individuals with more location data contributed relatively more weight to the model than those with fewer locations. We also assessed the sensitivity of the model to the inclusion of data from the dresseri Common Eider, which occupies a more restricted range than the other populations included in the analysis. Since centrality results for the global model were not substantially altered by inclusion of data from this species (Appendix S2: Table S2), we chose to retain these data in multi‐species assessments.
Network analyses
Centrality
For both global and species‐specific models, we evaluated the importance of individual nodes within the network using the number of shortest pathways through the network that pass through a node (i.e., betweenness centrality; Freeman 1977). We further calculated the number of incoming and outgoing connections to a node (i.e., indegree and outdegree centrality, respectively; Freeman 1979) to measure specific aspects of the node's potential role in population structure. These three centrality measures provide complementary information: betweenness centrality evaluates the importance of habitat patches to various migratory pathways through the overall habitat network, while in‐ and out‐degree centrality show the extent to which the node serves as a mixing point among individuals from different portions of the species’ range. To account for the number of observed movements between locations (i.e., edge weights), we calculated node centrality measures at α = 0.5 (Opsahl et al. 2010), which adjusts scores of nodes so the number and strength of their links to other nodes contribute equally to overall importance values. The three centrality metrics we chose are relatively robust to differences in frequency of node use, allowing us to identify nodes that play key roles in linking other nodes together regardless of how often, or by how many individuals, they are used.
Connectivity
To evaluate the contribution of individual nodes to total habitat connectivity across the network, we conducted sequential removal of individual nodes from the network and evaluated the resulting change in overall probability of connectivity (dPC: Saura and Pascual‐Hortal 2007, Bodin and Saura 2010). Greater dPC values correspond to nodes whose removal resulted in greater decreases in network‐wide connectivity (i.e., nodes with greater dPC values contributed more than those with lesser values to total network connectivity). Like the centrality measures we chose, dPC can identify even a less‐used node as an important habitat linkage, providing its removal substantially impedes movement through the network.
Modularity
To identify sets of nodes with stronger linkages to one another than to the remaining nodes in the network (i.e., modules) and determine the overall degree of modularity across the network, we used the Girvan‐Newman algorithm (Girvan and Newman 2002). This allowed us to simultaneously find modules and calculate modularity without selecting the expected number of modules a priori. Using this algorithm, a network in which each group of nodes is completely connected within‐group, with no between‐group connections (i.e., a fully modular network) would receive a modularity score of one, whereas a fully connected network with no defined groups would receive a score of zero. In the context of migratory connectivity (Webster et al. 2002), a network with strong connectivity would be highly modular and score closer to 1, while a network with weak connectivity would score closer to zero.
Species‐specific networks
After defining the global network with equal contributions from each species and calculating multi‐species centrality and connectivity, we evaluated the use of the global network by individual species and by sex within species. We did this by removing all non‐target species and sexes, reweighting nodes and edges using only the movements of the target group, and using the new weights to recalculate centrality and dPC values. We then evaluated species‐level differences in node centrality and dPC relative to the global model and the other species, as well as within‐species differences between male and female network use. We also assessed the representativeness of individual‐species networks by calculating the percentage of global centrality contained in each species‐specific network.
Results
The global network for all sea duck species spanned ~26° of latitude and 15° of longitude and included 31 nodes (Table 2, Fig. 2), with eight nodes used by all five sea duck species; 19 nodes by two to four species; and 4 nodes by only one species. The highest density of habitat centroids occurred in nodes primarily used during winter and migration, while breeding areas showed much lower overall centroid densities (Fig. 2).
Table 2.
Proportional overlap between species‐specific networks in centrality and total network connectivity (dPC) for five sea duck species in eastern North America, 2002–2017
| Species | Global | Black Scoter | Common Eider (dresseri) | Long‐tailed Duck | Surf Scoter | White‐winged Scoter |
|---|---|---|---|---|---|---|
| Betweenness centrality | ||||||
| Black Scoter | 0.92 | 1.00 | 1.00 | 0.49 | 0.97 | 1.00 |
| Common Eider (dresseri) | 0.59 | 0.53 | 1.00 | 0.17 | 0.65 | 0.80 |
| Long‐tailed Duck | 0.98 | 0.97 | 0.97 | 1.00 | 0.97 | 1.00 |
| Surf Scoter | 0.96 | 1.00 | 1.00 | 0.71 | 1.00 | 1.00 |
| White‐winged Scoter | 0.93 | 0.98 | 1.00 | 0.64 | 0.84 | 1.00 |
| Total network connectivity (dPC) | ||||||
| Black Scoter | 0.98 | 1.00 | 1.00 | 0.54 | 1.00 | 0.99 |
| Common Eider (dresseri) | 0.78 | 0.40 | 1.00 | 0.08 | 0.66 | 0.86 |
| Long‐tailed Duck | 0.97 | 0.93 | 0.95 | 1.00 | 0.89 | 0.99 |
| Surf Scoter | 0.99 | 0.98 | 1.00 | 0.70 | 1.00 | 1.00 |
| White‐winged Scoter | 0.95 | 0.96 | 1.00 | 0.56 | 0.77 | 1.00 |
Figure 2.
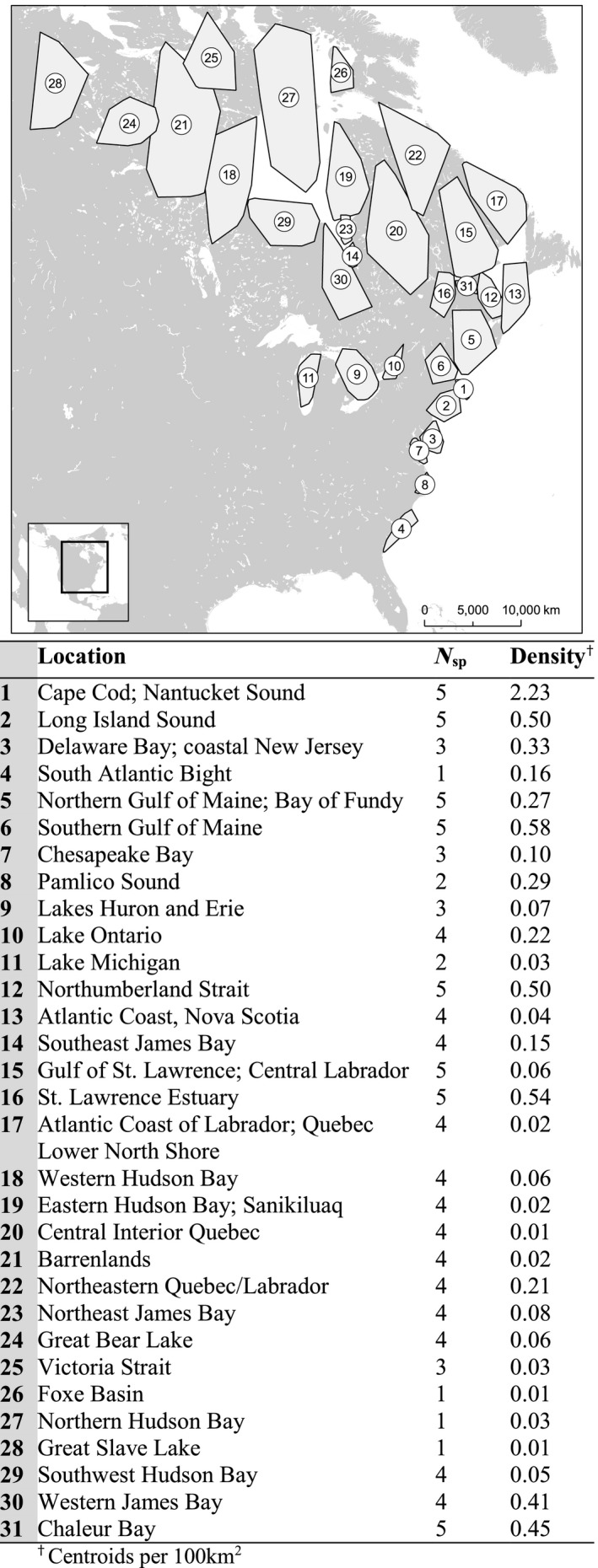
Node locations, footprints, and descriptions for the global movement network for five species of sea ducks (Black Scoter, Surf Scoter, White‐winged Scoter, Long‐tailed Duck, and dresseri Common Eider), 2002–2017.
Use of the global network varied among seasons (Fig. 3). Notably, while pre‐breeding staging and migration primarily occurred along the Atlantic Coast, post‐breeding molt and migration to winter sites tended to be associated more with inland sites and routes, with James Bay serving as an intermediate stopover between summerand winter sites. Individuals were tightly grouped in limited portions of the network during winter, spring staging, and spring migration, but occupied a broader and more dispersed network during breeding and post‐breeding molt, and migration to winter sites.
Figure 3.
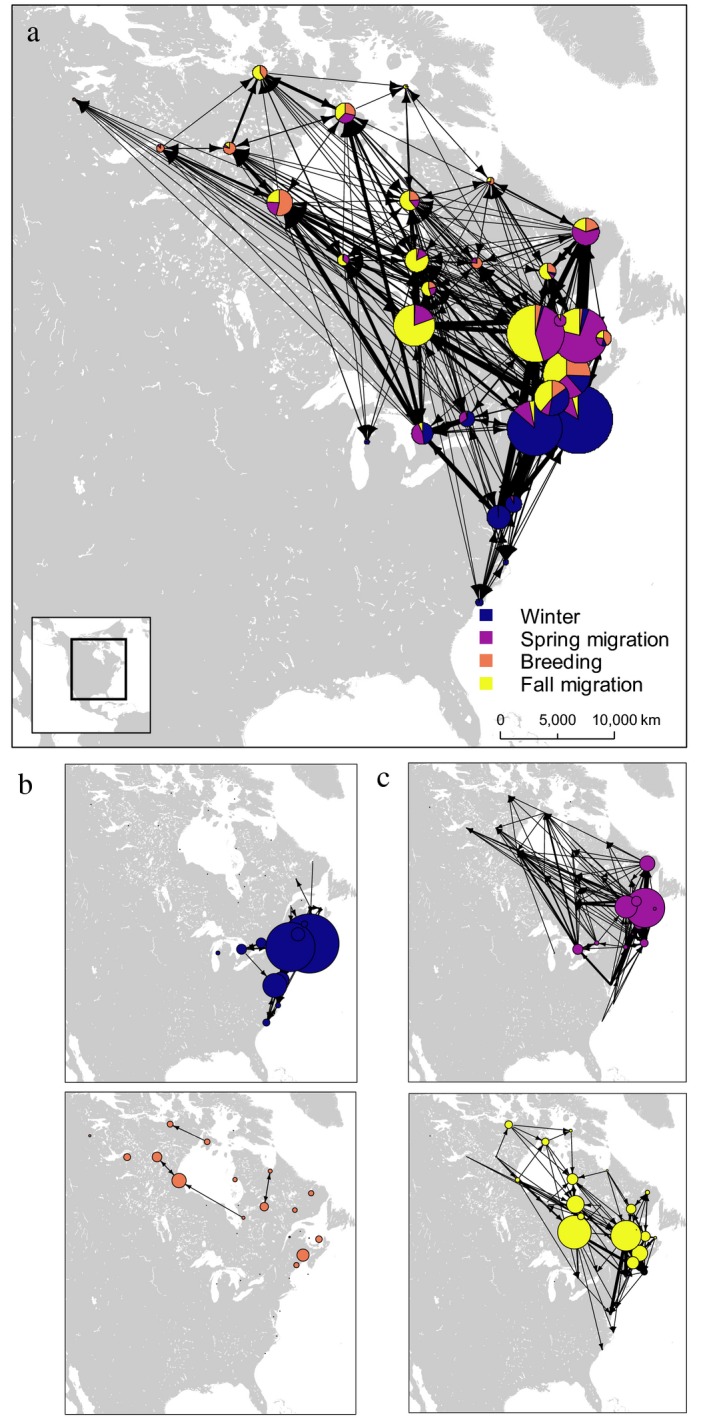
Movement network for five species of sea ducks (Black Scoter, Surf Scoter, White‐winged Scoter, Long‐tailed Duck, and dresseri Common Eider) by season within the annual cycle, 2002–2017. (a) All movements and proportional occupancy of nodes by season, (b) movements within winter (blue) and breeding (red) seasons, and (c) spring (purple) and fall (yellow) migration routes and stopover sites. Node size is proportional to the number of habitat centroids in each node. Edges represent number (width) and direction(s) (arrowheads) of individual movements between nodes.
Globally, network centrality values were greatest in the St. Lawrence River estuary, followed by Nantucket Sound and southeast James Bay (Fig. 4a–c). While absolute centrality values varied between the three centrality measures we examined, nodes with greater degree centrality generally also had greater betweenness. dPC values (i.e., total network connectivity) were greatest for Nantucket Sound, the St. Lawrence River estuary, and Long Island Sound (Fig. 4d). All other sites had lesser dPC values, particularly across breeding and southern wintering areas.
Figure 4.
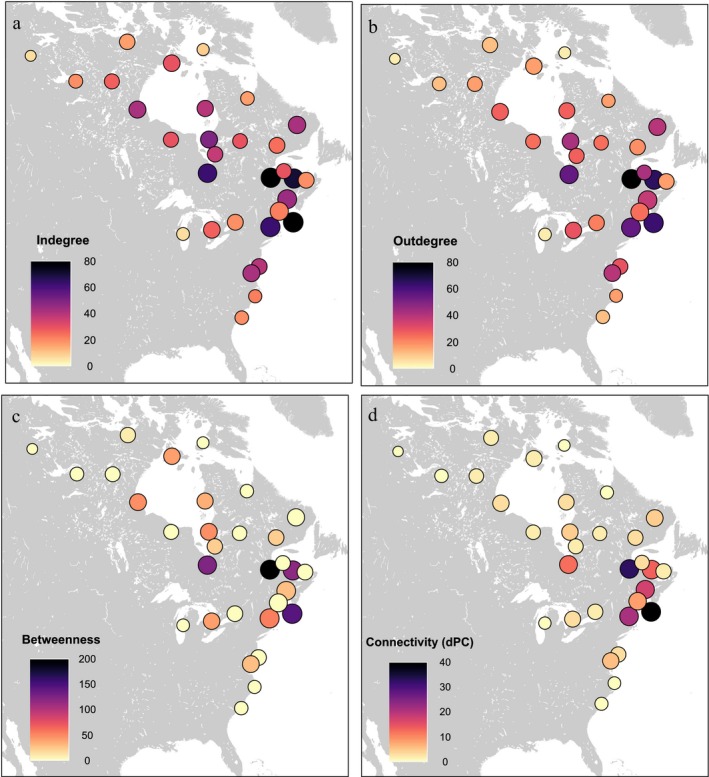
Four measures of centrality of the global network based on pooled data from five species of sea ducks (Black Scoter, Surf Scoter, White‐winged Scoter, Long‐tailed Duck, and dresseri Common Eider), 2002–2017. (a) Indegree centrality is based on the number of incoming connections to each node, (b) outdegree centrality is based on the number of outgoing connections, (c) betweenness centrality measures the number of shortest paths between other nodes that include each node, and (d) total network connectivity measures the decrease in network‐wide probability of connectivity (dPC) caused by removing a given node. Node size is proportional to the log number of habitat centroids in each node.
The Girvan‐Newman algorithm defined two modules within the global network, with one module containing 11 nodes distributed across the network (node numbers 2–8, 13, 15, 17, and 31) and the other containing the remaining 20 nodes. The two modules overlapped broadly in space, with no clear geographic boundaries between modules, and the modularity score (0.16) indicated weak modularity. Modularity scores for species‐specific networks ranged from 0.02 to 0.19 (0.13 ± 0.06 [mean ± SD]).
Network use varied among species, with no two species using identical subsets of the global network (Fig. 5). Black and Surf Scoters had the greatest degree of overlap among species, with 25 shared nodes. Key sites within their networks also differed between species (Fig. 6). For Surf and White‐winged Scoters, centrality values most exceeded multi‐species averages at winter sites (Chesapeake Bay and Nantucket Sound, respectively); while for Long‐tailed Ducks and Black Scoters, sites of unique importance included staging and molt sites (northern Hudson Bay and southeast James Bay, respectively; Fig. 6). Within species, network use was generally similar between males and females, with little variation in centrality values depending on sex, although breeding sites generally had slightly higher dPC values for females while molt sites had higher values for males (Fig. 7).
Figure 5.
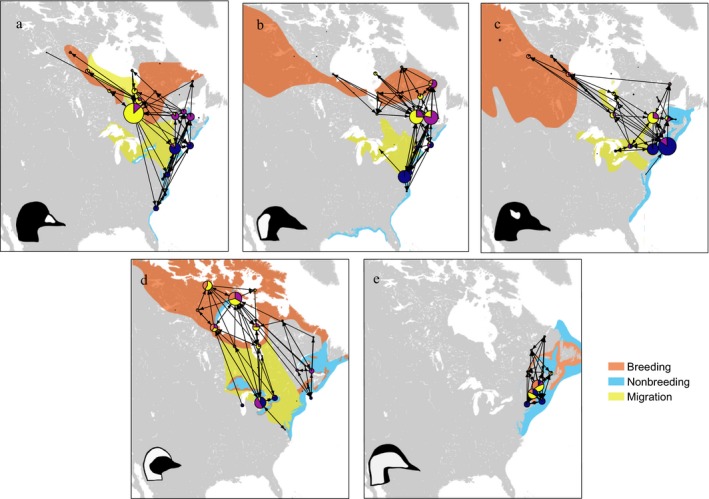
Species‐specific subnetworks overlaid on range maps for (a) Black Scoter (Eastern North American population), (b) Surf Scoter (Eastern and Western North American populations), (c) White‐winged Scoter (Eastern and Western North American populations), (d) Long‐tailed Duck (Eastern North American population), and (e) Common Eider (Eastern North American population, dresseri subspecies), 2002–2017. Range maps are adapted from BirdLife International species distributions (del Hoyo et al. 2014). Network nodes show proportional occupancy by season (red, breeding; yellow, fall migration; blue, winter; purple, spring migration). Node size is proportional to the number of habitat centroids in each node. Edges represent number (width) and direction (arrowheads) of individual movements between nodes. Only edges with weights >10 are shown.
Figure 6.
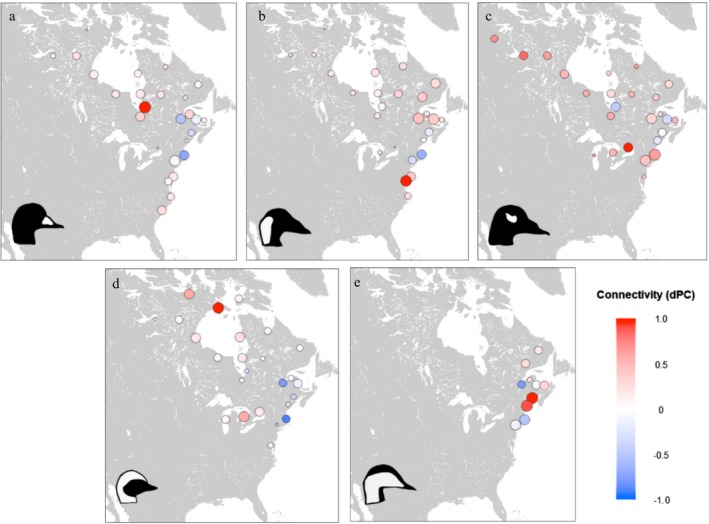
Species‐specific dPC values compared to global values for (a) Black Scoter, (b) Surf Scoter, (c) White‐winged Scoter, (d) Long‐tailed Duck, and (e) dresseri Common Eider, 2002–2017. Nodes with positive values (red) were more central to the single‐species network than the global network, nodes with negative values (blue) were less central compared to the global network, and nodes with zero values (white) were equally central. Node size is proportional to the log number of habitat centroids in each node.
Figure 7.
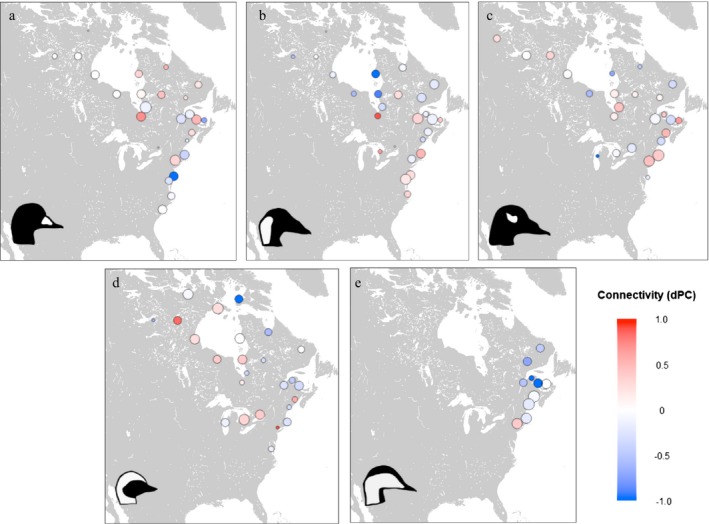
Differences in sex‐specific dPC values in single‐species networks for (a) Black Scoter, (b) Surf Scoter, (c) White‐winged Scoter, (d) Long‐tailed Duck, and (e) dresseri Common Eider, 2002–2017. Nodes with positive values (red) were more central for females than for males, negative values (blue) were more central for males than females, and zero values (white) were equally central for both sexes. Node size is proportional to the log number of habitat centroids in each node.
Among single‐species models, the sub‐network including only Long‐tailed Duck data was the most representative of the overall species assemblage, capturing ~98% and 97% of global network centrality and dPC values, respectively (Table 2). The Long‐tailed Duck sub‐network represented 96–100% of total centrality and 89–100% of total dPC for each of the other four species included in the global network. The surf scoter network was also effective in representing global network centrality (96%) and global network dPC (99%); however, it was not as effective as the Long‐tailed Duck network in representing important nodes for individual species (70–100%). The least representative network was based on common eider data, which captured 59% of global network centrality and 78% of global dPC.
Discussion
Applying network analysis to multi‐species telemetry data provides a basis for extrapolating population‐ and guild‐wide patterns of migration and habitat use, as well as examining species‐specific differences in migratory patterns. Our findings emphasize the conservation importance of staging and molt sites to linking annual cycle habitats for multiple species of sea ducks in eastern North America. From a conservation standpoint, network analysis suggests the need to integrate management efforts across the Atlantic and Mississippi flyways in order to better represent year‐round movements of the full multi‐species assemblage, and highlights a potential umbrella species (long‐tailed ducks) that effectively encompasses the habitat needs of multiple sympatric sea duck species.
Multi‐species network use
To compare the importance of different nodes within the multi‐species habitat network, we focused on degree centrality, betweenness centrality, and change in probability of connectivity following node removal (dPC). These metrics were generally in agreement about the relative importance of specific sites. Nodes with high degree centrality values represent locations used by individuals from multiple locations across the range of a species. From a population standpoint, nodes that concentrate individuals from multiple locations could be hotspots for genetic mixing, disease spread, and transfer of information between individuals (Talbot et al. 2015, Hollmen and Franson 2015, Ballard et al. 2017). Since sea ducks form pair bonds before migrating to breeding locations (Rohwer and Anderson 1988, Oring and Sayler 1992), overlap during non‐breeding periods is particularly relevant to population‐level genetic mixing. In addition, nodes that support large concentrations of individuals from throughout the network are likely to be resource rich and important for fueling energy‐intensive life stages (e.g., breeding, molt, migration), making them high‐impact targets for conservation and restoration efforts. Eight habitat nodes located in the Gulf of St. Lawrence, Gulf of Maine, and southern New England were used by all five species included in the model, indicating their value as multi‐species hubs (Fig. 2). The Saguenay‐Saint Laurent Marine Park, which encompasses about 1,250 km2 of marine habitat in the St. Lawrence River Estuary, is the only restricted marine protected area (IUCN category III or greater) in these key sites. Although other protected areas exist, including fisheries management zones in New England and designated Waterfowl Gathering Areas along much of the Quebec coastline, these designations do not imply any restrictions on activities or involve any monitoring of waterfowl populations.
Three nodes in particular, Northumberland Strait, the St. Lawrence River estuary, and southeast James Bay, scored at or near the top in all four importance metrics. These nodes were used during migration, with Northumberland Strait used primarily during spring, the St. Lawrence River estuary used during both spring and fall, and southeast James Bay used primarily during fall. These nodes supported high concentrations of individuals from throughout the global network, as stopover sites between more southerly wintering locations along the Atlantic coast (30–40° latitude), and high‐Arctic breeding and molt locations (50–70° latitude). Removing these nodes substantially reduced total network connectivity (i.e., high dPC), suggesting that they cannot be readily replaced by other nodes within the existing migratory network. Breeding and wintering areas generally had lower centrality and dPC values than staging and molt sites used during migration. The exceptions were Nantucket and Long Island sounds, located at the northern edge of the species’ winter ranges along the Atlantic coast. These nodes concentrated large numbers of individuals during winter, and also functioned as migratory stopovers for birds traveling to more southerly coastal wintering sites.
Both global and species‐specific network models showed highly interconnected habitat networks with no obvious modularity, suggesting that, within their eastern North America ranges, the populations of sea ducks included in this study cannot be divided into readily identifiable sub‐populations based on migratory patterns alone. Our analysis showed highly complex migration patterns, including considerable overlap at shared migration stopovers and considerable movement among sites during the migration and wintering periods. Since pair bonding in sea ducks occurs during non‐breeding, this extensive non‐breeding overlap is likely to facilitate a high degree of mixing in the overall population. An exception is Lake Michigan, which was not connected to other wintering sites. This may indicate potential isolation of this node from the rest of the eastern network, although individuals wintering on Lake Michigan occupied common breeding and migratory staging nodes to those used by individuals from other wintering sites. Although lack of evidence for spatial subgroups does not necessarily indicate that no barriers to gene flow exist (Friesen et al. 2007), the general lack of modularity and extensive overlap during all seasons suggests that these species likely function as a single population across their eastern ranges. In eastern North America, migratory sea ducks are managed according to a flyway‐based scheme that separates the population overwintering in the great lakes (Mississippi Flyway) from the Atlantic coast (Atlantic Flyway; Smith et al. 1989). While this system effectively represents the north‐south migration patterns of freshwater ducks, it is less representative of the east‐west migratory movements of sea ducks. Moreover, our analysis suggests that populations from these two flyways are highly connected across the annual cycle, including during winter, and rely on key shared sites year‐round. Consolidating management efforts across these two flyways could thus provide a more comprehensive and ecologically meaningful framework for fully representing habitat needs across the entire, interconnected population.
Variation in network use among species
Despite considerable between‐species overlap in network use, analysis of species‐specific subnetworks yielded insight into key differences in site use and importance between species. For example, while the three scoter species showed broadly similar migration patterns, they differed in use and importance of particular nodes. Compared to other species and to the global network, black scoters had greater dPC values in southeast James Bay, which served as a hub linking breeding and wintering sites during summer and fall staging. This confirms the results of previous aerial surveys documenting the importance of James Bay to molting (mostly male) Black Scoters (Ross 1983). Our findings support the importance of James Bay as a key facilitator of network‐scale habitat linkages and population interactions for Arctic‐breeding migratory birds. While the southeast James Bay node was particularly important for Black Scoters, it also had some of the greatest overall values for multi‐species centrality and total network connectivity (dPC), indicating that its importance extends across the suite of migratory sea ducks. In contrast, surf and white‐winged scoters were relatively less reliant on James Bay, but showed greater dPC values for the St. Lawrence River during spring staging. While dPC values of migratory routes and stopover sites were similar in these two species, their network use differed during winter and breeding. Winter sites along the mid‐Atlantic coast and breeding locations east of Hudson Bay were comparatively more important for total network connectivity in surf scoters than for other species, while white‐winged scoter network connectivity was comparatively greater at winter sites in southern New England and Lake Ontario and breeding sites west of Hudson Bay. Our results suggest that sympatry between these two scoter species is likely to be higher during migration than during other periods of the annual cycle.
Our analysis also provides insights into how effectively individual species represent multi‐species distribution patterns. Long‐tailed Ducks provided the most representative network, in that the subset of the network used by Long‐tailed Ducks captured ~98% of multi‐species network centrality. Long‐tailed Ducks used a more inland migratory route than the three scoter species, linking the mid‐Atlantic, Great Lakes, and northwestern Hudson Bay while largely bypassing the Gulf of Maine and Atlantic Canada. This pattern likely reflects the distribution of these species following the last glaciation (Talbot et al. 2015). Long‐tailed Ducks also occupied unique staging sites in northwestern Hudson Bay, which they used primarily during the period following spring arrival but preceding thaw at breeding sites (Mallory et al. 2006). These sites were a particularly important network hub for this species, but were rarely used by individuals of other species, and therefore did not rank highly in the global model. However, since the long‐tailed duck network included key areas of multi‐species overlap in addition to unique habitat areas, it encompassed the core of the other four species’ networks despite diverging in breeding and molt ranges. This may result in part from biological characteristics: while scoters and eiders feed primarily on bivalves (Perry et al. 2007), long‐tailed ducks are generalists that consume a wide variety of prey types (Bustnes and Systad 2001). From a conservation and monitoring standpoint, our results suggest that Long‐tailed Ducks can act as an umbrella species (Lambeck 1997) for representing habitat needs and landscape‐scale changes that affect the full suite of sympatric sea ducks in eastern North America, making them particularly high‐value targets for monitoring and conservation.
Sampling considerations
Movement patterns of sea ducks are known to vary based on sex, since females are more likely to attend breeding sites than males, while males may visit a greater variety of non‐breeding molt sites and stopover locations compared to females (Oring and Sayler 1992). Nevertheless, our results suggest that, in a network context, either sex provides similar information about overall range‐wide occupancy and habitat linkages. While some questions (e.g., breeding site fidelity, length of nest attendance) might be more appropriately answered by studying one sex than another, it is useful to note that studies of overall movement patterns are not likely to be biased by the sex ratio of the initial sample. However, it is important to note the relatively low proportion of breeding sites in our final sample (12% vs. 26–32% for other seasons). Although we attempted to account for this difference by selecting importance metrics that measure the role of nodes in movement pathways rather than their absolute use, differential attendance at breeding sites by males and females, as well as within sexes, likely contributed to the lower importance values we calculated for breeding nodes. Moreover, age of ducks may influence sex‐specific movements. Limited banding data from Long‐tailed Ducks breeding in Churchill, Manitoba, Canada indicate that second‐year males do not go to breeding areas, but second‐year females do (Alison 1975a, b). Of 143 Long‐tailed Ducks banded during June 2005–2012, no second‐year males were captured, but 13 second‐year females were (M. C. Perry, personal observation).
Another key consideration in designing movement networks is identifying potential biases in sampling that may affect the representativeness of the sample population. In this study, tracking of Common Eiders was limited to S. m. dresseri, one of three geographically distinct subspecies of Common Eider in eastern North America (Joint Working Group on the Management of the Common Eider 2004). While the data provide a useful representation of the subspecies’ range, they do not match the scale of the data collected from the other four species, complicating inter‐species comparisons. Since the dresseri Common Eider occupied a subset of the multi‐species network that was highly central for the other species included in the analysis, including or excluding the species from the global model did not substantially affect our results; however, it is important to consider the potential effects of mismatches in sampling range or scale when pooling multiple sympatric species for analysis. Chance sampling biases may have existed for other species, but because all were sampled across similar time periods and ranges, we concluded that data could reasonably be pooled and compared among species. In addition, node use represents a relatively coarse‐scale approximation of occurrence, and species that overlap in network analysis may still differ in fine‐scale habitat use. For example, an earlier analyses of telemetry locations of Surf and Black Scoters in breeding areas of Canada showed no significant differences between the latitude and longitude of the two species; however, there were significant differences between the habitat used in these areas by the two species (Perry et al. 2006).
Conservation and management implications
Our work provides a framework for using multi‐species telemetry data to prioritize sites within complex migratory networks. In the case of sea ducks, sites used during staging and molt, particularly southeastern James Bay, the Northumberland Strait of New Brunswick, the St. Lawrence Estuary, and southern New England, emerged as important locations in multi‐species analyses of centrality and total network connectivity. These sites represent particularly important targets for both population monitoring and conservation, since they concentrate individuals from various wintering and breeding sites, and cannot be easily replaced by other sites in the existing migration network if their suitability decreases. In southeastern James Bay, two coastal protected areas, the Boatswain Bay and Hannah Bay Migratory Bird Sanctuaries, lie within or adjacent to the node, although the majority of protected lands in the refuges are terrestrial (as opposed to the nearshore open‐water habitat used by sea ducks during stopovers). The St. Lawrence Estuary node includes an existing protected area, the Saguenay‐St. Lawrence Marine Park, which lies to the south of the primary use areas for sea ducks.
Our results also provide insights into strategies for effective management and monitoring of eastern North American sea ducks. The high levels of total network connectivity we found within both multi‐ and single‐species sea duck networks suggest that, with the possible exception of individuals wintering on Lake Michigan, populations of scoters and long‐tailed ducks from the Atlantic and Mississippi flyways are highly connected throughout the annual cycle and can be managed as a single population unit. Additionally, of the five species included in our analysis, we found that the long‐tailed duck network most effectively represented important habitats used by the full suite of species. This suggests that managers could obtain information on the status of key habitats and maximize multi‐species conservation benefits by focusing on this species when allocating limited resources for monitoring and restoration.
Supporting information
Acknowledgments
We thank Andrew Gilbert, who served as a primary data manager for much of the data used in this study. For the Lake Ontario Long‐tailed Duck captures, logistical and field assistance was provided by Bird Studies Canada/Long Point Waterfowl (T. Barney, J. Palframan, C. Moore, M. Illes, S. Gan, D. Okines); Canadian Wildlife Service (S. Meyer, C. Sharp, B. Campbell, J. Vanos, F. St. Pierre); Toronto Zoo (G. Crawshaw, D. Mihailovic); U.S. Geological Survey (USGS: C. Caldwell), and Western University (P. Wilson). Funding and in‐kind support was provided by Sea Duck Joint Venture, U.S. Fish and Wildlife Service (USFWS), Environment and Climate Change Canada/Canadian Wildlife Service, Toronto Zoo, USGS Patuxent Wildlife Research Center, Bird Studies Canada/Long Point Waterfowl, Bluff's Hunting Club, Ducks Unlimited Inc., Ontario Federation of Anglers and Hunters, Natural Sciences and Engineering Research Council of Canada, Ontario Ministry of Natural Resources, Wildlife Habitat Canada, TD Friends of the Environment Foundation, and Western University. For the Bureau of Ocean Energy Management (BOEM) diving bird captures, funding was provided by BOEM through Intra‐Agency Agreement M12PG00005 with USFWS, and by the U.S. Department of Energy, the Sea Duck Joint Venture, the Bailey Foundation, and USFWS. Data, field, and lab support, insight into project design, and other resources was provided by: Memorial University (B. Montevecchi, C. Burke), Biodiversity Research Institute (I. Stenhouse, W. Goodale), University of Maine (C. Gray), Cornell Lab of Ornithology (J. Fiely), USGS Patuxent Wildlife Research Center (C. Kilchenstein), Avian Specialty Veterinary Services (S. Ford), BOEM (J. Woehr, retired), North Carolina Wildlife Resources Commission (D. Howell), and Swansea University (S. Vandenabeele). Southern New England captures received funding and support from the Sea Duck Joint Venture, Wildlife Restoration, Rhode Island Department of Environmental Management, and the University of Rhode Island, with field assistance from P. Loring, J. Beuth, and D. Meattey. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Lamb, J. S. , Paton P. W. C., Osenkowski J. E., Badzinski S. S., Berlin A. M., Bowman T., Dwyer C., Fara L. J., Gilliland S. G., Kenow K., Lepage C., Mallory M. L., Olsen G. H., Perry M. C., Petrie S. A., Savard J.‐P. L., Savoy L., Schummer M., Spiegel C. S., and McWilliams S. R.. 2019. Spatially explicit network analysis reveals multi‐species annual cycle movement patterns of sea ducks. Ecological Applications 29(5):e01919 10.1002/eap.1919
Corresponding Editor: Dianne Brunton.
Data Availability
Data are available on Zenodo: https://doi.org/10.5281/zenodo.2646686
Literature Cited
- Alerstam, T. , Hedenström A., and Åkesson S.. 2003. Long‐distance migration: evolution and determinants. Oikos 103:247–260. [Google Scholar]
- Alison, R. M. 1975a. Breeding biology and behavior of the oldsquaw (Clangula hyemalis L.). Ornithological Monographs No. 18, American Ornithologists’ Union. Allen Press, Lawrence, Kansas, USA. [Google Scholar]
- Alison, R. M. 1975b. Capturing and marking oldsquaws. Bird‐Banding 46:248–250. [Google Scholar]
- Ambrosini, R. , Møller A. P., and Saino N.. 2009. A quantitative measure of migratory connectivity. Journal of Theoretical Biology 257:203–211. [DOI] [PubMed] [Google Scholar]
- Ballard, J. R. , Mickley R., Brown J. D., Hill N. J., Runstadler J. A., Clark D. E., Ellis J. C., Mead D. G., and Fischer J. R.. 2017. Detection of Wellfleet Bay Virus antibodies in sea birds of the Northeastern USA. Journal of Wildlife Diseases 53:875–879. [DOI] [PubMed] [Google Scholar]
- Bentzen, R. L. , and Powell A. N.. 2015. Dispersal, movements and site fidelity of post‐fledging King Eiders Somateria spectabilis and their attendant females. Ibis 157:133–146. [Google Scholar]
- Berger, J. 2004. The last mile: how to sustain long‐distance migration in mammals. Conservation Biology 18:320–331. [Google Scholar]
- Beuth, J. M. , McWilliams S. R., Paton P. W., and Osenkowski J. E.. 2017. Habitat use and movements of common eiders wintering in southern New England. Journal of Wildlife Management 81:1276–1286. [Google Scholar]
- Block, B. A. , et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475:86. [DOI] [PubMed] [Google Scholar]
- Bodin, Ö. , and Saura S.. 2010. Ranking individual habitat patches as connectivity providers: integrating network analysis and patch removal experiments. Ecological Modelling 221:2393–2405. [Google Scholar]
- Boulinier, T. , Danchin E., Monnat J. Y., Doutrelant C., and Cadiou B.. 1996. Timing of prospecting and the value of information in a colonial breeding bird. Journal of Avian Biology 27:252–256. [Google Scholar]
- Breault, A. M. , and Cheng K. M.. 1990. Use of submerged mist nets to capture diving birds. Journal of Field Ornithology 61:328–330. [Google Scholar]
- Brodeur, S. , Savard J.‐P. L., Robert M., Titman R. D., and Fitzgerald G.. 2008. Failure time and fate of harlequin ducks implanted with satellite transmitters. Waterbirds 31:183–187. [Google Scholar]
- Bustnes, J. O. , and Systad G. H.. 2001. Comparative feeding ecology of Steller's eider and long‐tailed ducks in winter. Waterbirds 24:407–412. [Google Scholar]
- Charrad, M. , Ghazzali N., Boiteau V., Niknafs A., and Charrad M. M.. 2014. NbClust: an R package for determining the relevant number of clusters in a data set. Journal of Statistical Software 61:1–36. [Google Scholar]
- CLS . 2016. Argos User's Manual. Last updated 15 June 2016. http://www.argos-system.org/manual/
- Cooper, C. , Larson L., Dayer A., Stedman R., and Decker D.. 2015. Are wildlife recreationists conservationists? Linking hunting, birdwatching, and pro‐environmental behavior. Journal of Wildlife Management 79:446–457. [Google Scholar]
- Csardi, G. 2015. igraph: Network analysis and visualization. R package version 1.0.1. http://igraph.org/nightly/get/r-win/igraph_1.0.1.zip
- Csardi, G. , and Nepusz T.. 2006. The igraph software package for complex network research. InterJournal, Complex Systems 1695:1–9. [Google Scholar]
- del Hoyo, J. , Collar N. J., Christie D. A., Elliott A. and Fishpool L. D. C.. 2014. HBW and BirdLife International Illustrated Checklist of the Birds of the World Volume 1: Non‐passerines. Lynx Edicions and BirdLife International, Barcelona, Spain: and Cambridge, UK. [Google Scholar]
- Dyer, R. J. , Nason J. D., and Garrick R. C.. 2010. Landscape modelling of gene flow: improved power using conditional genetic distance derived from the topology of population networks. Molecular Ecology 19:3746–3759. [DOI] [PubMed] [Google Scholar]
- Engelhard, S. L. , Huijbers C. M., Stewart‐Koster B., Olds A. D., Schlacher T. A., and Connolly R. M.. 2017. Prioritising seascape connectivity in conservation using network analysis. Journal of Applied Ecology 54:1130–1141. [Google Scholar]
- Esler, D. , Mulcahy D. M., and Jarvis R. L.. 2000. Testing assumptions for unbiased estimation of survival of radiomarked harlequin ducks. Journal of Wildlife Management 64:591–598. [Google Scholar]
- Fox, A. D. , Mitchell C., Stewart A., Fletcher J. D., Turner J. V. N., Boyd H., Shimmings P., Salmon D. G., Haines W. G., and Tomlinson C.. 1994. Winter movements and site‐fidelity of pink‐footed geese Anser brachyrhynchus ringed in Britain, with particular emphasis on those marked in Lancashire. Bird Study 41:221–234. [Google Scholar]
- Freeman, L. C. 1977. A set of measures of centrality based on betweenness. Sociometry 40:35–41. [Google Scholar]
- Freeman, L. C. 1979. Centrality in social networks conceptual clarification. Social Networks 1:215–239. [Google Scholar]
- Friesen, V. L. , Smith A. L., Gomez‐Diaz E., Bolton M., Furness R. W., González‐Solís J., and Monteiro L. R.. 2007. Sympatric speciation by allochrony in a seabird. Proceedings of the National Academy of Sciences USA 104:18589–18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvan, M. , and Newman M. E.. 2002. Community structure in social and biological networks. Proceedings of the National Academy of Sciences USA 99:7821–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig, S. M. , Mehlman D. W., and Oring L. W.. 1998. Avian movements and wetland connectivity in landscape conservation. Conservation Biology 12:749–758. [Google Scholar]
- Hindell, M. A. , Lea M. A., Bost C. A., Charrassin J. B., Gales N., Goldsworthy S., and Guinet C.. 2011. Foraging habitats of top predators, and areas of ecological significance, on the Kerguelen Plateau Pages 203–215 in Duhamel G., and Welsford D., editors. The Kerguelen Plateau: Marine Ecosystem and Fisheries. Societe Francaise d'Ichtyologie, Abbeville, France. [Google Scholar]
- Hollmen, T. E. , and Franson J. C.. 2015. Infectious diseases, parasites, and biological toxins in sea ducks Pages 97–124 in Savard J.‐P. L., Derksen D. V., Esler D. and Eadie J., editors. Ecology and conservation of North American Sea Ducks. CRC Press, New York, New York, USA. [Google Scholar]
- Jacoby, D. M. , Brooks E. J., Croft D. P., and Sims D. W.. 2012. Developing a deeper understanding of animal movements and spatial dynamics through novel application of network analyses. Methods in Ecology and Evolution 3:574–583. [Google Scholar]
- Jacoby, D. M. , and Freeman R.. 2016. Emerging network‐based tools in movement ecology. Trends in Ecology & Evolution 31:301–314. [DOI] [PubMed] [Google Scholar]
- Johnsgard, P. A. 2010. Waterfowl of North America: hunting and recreational values of North American waterfowl In Johnsgard P. A., editor. Waterfowl of North America, Revised Edition. University of Nebraska‐Lincoln Libraries, Lincoln, Nebraska, USA. [Google Scholar]
- Joint Working Group on the Management of the Common Eider . 2004. Québec Management Plan for the Common Eider Somateria mollissima dresseri. Special publication, Environment Canada, Sainte‐Foy, Québec, Canada. [Google Scholar]
- Jonsen, I. D. 2016. Joint estimation over multiple individuals improves behavioural state inference from animal movement data. Scientific Reports 6:20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsen, I. D. , Flemming J. M., and Myers R. A.. 2005. Robust state–space modeling of animal movement data. Ecology 86:2874–2880. [Google Scholar]
- Kirby, J. S. , Stattersfield A. J., Butchart S. H., Evans M. I., Grimmett R. F., Jones V. R., O'Sullivan J., Tucker G. M., and Newton I.. 2008. Key conservation issues for migratory land‐ and waterbird species on the world's major flyways. Bird Conservation International 18:S49–S73. [Google Scholar]
- Knight, S. M. , et al. 2018. Constructing and evaluating a continent‐wide migratory songbird network across the annual cycle. Ecological Monographs 88:445–460. [Google Scholar]
- Korschgen, C. E. , Kenow K. P., Gendron‐Fitzpatrick A., Green W. L., and Dein F. J.. 1996. Implanting intra‐abdominal radio transmitters with external whip antennas in ducks. Journal of Wildlife Management 60:132–137. [Google Scholar]
- Lambeck, R. J. 1997. Focal species: a multi‐species umbrella for nature conservation. Conservation Biology 11:849–856. [Google Scholar]
- Leonard, P. B. , Sutherland R. W., Baldwin R. F., Fedak D. A., Carnes R. G., and Montgomery A. P.. 2017. Landscape connectivity losses due to sea level rise and land use change. Animal Conservation 20:80–90. [Google Scholar]
- Lindberg, M. S. , Ward D. H., Tibbits T. L., and Roser J.. 2007. Winter movement dynamics of black brant. Journal of Wildlife Management 71:534–540. [Google Scholar]
- Link, W. A. , and Eaton M. J.. 2012. On thinning of chains in MCMC. Methods in Ecology and Evolution 3:112–115. [Google Scholar]
- Loring, P. H. , Paton P. W., Osenkowski J. E., Gilliland S. G., Savard J.‐P. L., and McWilliams S. R.. 2014. Habitat use and selection of black scoters in southern New England and siting of offshore wind energy facilities. Journal of Wildlife Management 78:645–656. [Google Scholar]
- Mallory, M. L. , Akearok J., North N. R., Weseloh D. V., and Lair S.. 2006. Movements of Long‐tailed Ducks wintering on Lake Ontario to breeding areas in Nunavut, Canada. Wilson Journal of Ornithology 118:494–501. [Google Scholar]
- Marra, P. P. , Hunter D., and Perrault A. M.. 2011. Migratory connectivity and the conservation of migratory animals. Environmental Law 41:317–354. [Google Scholar]
- Martin, T. G. , Chadès I., Arcese P., Marra P. P., Possingham H. P., and Norris D. R.. 2007. Optimal conservation of migratory species. PLoS ONE 2:e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meattey, D. E. , McWilliams S. R., Paton P. W., Lepage C., Gilliland S. G., Savoy L., Olsen G. H., and Osenkowski J. E.. 2018. Annual cycle of White‐winged Scoters (Melanitta fusca) in eastern North America: migration phenology, population delineation, and connectivity. Canadian Journal of Zoology 96:1353–1365. [Google Scholar]
- Minor, E. S. , and Urban D. L.. 2008. A graph‐theory framework for evaluating landscape connectivity and conservation planning. Conservation Biology 22:297–307. [DOI] [PubMed] [Google Scholar]
- Oppel, S. , Powell A. N., and Dickson D. L.. 2009. Using an algorithmic model to reveal individually variable movement decisions in a wintering sea duck. Journal of Animal Ecology 78:524–531. [DOI] [PubMed] [Google Scholar]
- Opsahl, T. , Agneessens F., and Skvoretz J.. 2010. Node centrality in weighted networks: generalizing degree and shortest paths. Social Networks 32:245–251. [Google Scholar]
- Oring, L. W. , and Sayler R. D.. 1992. The mating systems of waterfowl Pages 190–213 in Batt B. D. J., Afton A. D., Anderson M. G., Ankney C. D., Johnson D. H., Kadlec J. A. and Krapu G. L., editors. Ecology and management of breeding waterfowl. University of Minnesota Press, Minneapolis, Minnesota, USA. [Google Scholar]
- Perry, M. C. , Kidwell D. M., Wells A. M., Lohnes J. R., Osenton P. C., and Altmann S. H.. 2006. Characterization of breeding habitats for black and surf scoters in the eastern boreal forest and subarctic regions of Canada Pages 80–89 in Hanson A., Kerekes J. and Paquet J., editors. Limnology and Waterbirds 2003. The 4th Conference of the Aquatic Birds Working Group of the Societas Internationalis Limnologiae (SIL). Canadian Wildlife Service Technical Report Series No. 474. Sackville, New Brunswick, Canada. [Google Scholar]
- Perry, M. C. , Wells‐Berlin A. M., Kidwell D. M., and Osenton P. C.. 2007. Temporal changes of populations and trophic relationships of wintering diving ducks in Chesapeake Bay. Waterbirds 30:4–16. [Google Scholar]
- Phillips, R. A. , Xavier J. C., and Croxall J. P.. 2003. Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090. [Google Scholar]
- R Core Team . 2018. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- Raymond, B. , Lea M. A., Patterson T., Andrews‐Goff V., Sharples R., Charrassin J. B., and Goldsworthy S. D.. 2015. Important marine habitat off east Antarctica revealed by two decades of multi‐species predator tracking. Ecography 38:121–129. [Google Scholar]
- Rhodes, M. , Wardell‐Johnson G. W., Rhodes M. P., and Raymond B.. 2006. Applying network analysis to the conservation of habitat trees in urban environments: a case study from Brisbane, Australia. Conservation Biology 20:861–870. [DOI] [PubMed] [Google Scholar]
- Robertson, G. J. , and Cooke F.. 1999. Winter philopatry in migratory waterfowl. Auk 116:20–34. [Google Scholar]
- Rohwer, F. C. , and Anderson M. G.. 1988. Female‐biased philopatry, monogamy, and the timing of pair formation in migratory waterfowl Pages 187–221 in Johnson R. F., editor. Current ornithology. Volume 5. Springer, Boston, Massachusetts, USA. [Google Scholar]
- Ross, R. K. 1983. An estimate of the Black Scoter, Melanitta nigra, population moulting in James and Hudson bays. Canadian Field‐Naturalist 97:147–150. [Google Scholar]
- Rothe, T. C. , Padding P. I., Naves L. C., and Robertson G. J.. 2015. Harvest of sea ducks in North America: a contemporary summary Pages 417–469 in Savard J.‐P. L., Derksen D., Esler D. and Eadie J. M., editors. Ecology and conservation of North American sea ducks. CRC Press, New York, New York, USA. [Google Scholar]
- Runge, C. A. , Watson J. E., Butchart S. H., Hanson J. O., Possingham H. P., and Fuller R. A.. 2015. Protected areas and global conservation of migratory birds. Science 350:1255–1258. [DOI] [PubMed] [Google Scholar]
- Salomonsen, F. 1968. The moult migration. Wildfowl 19:5–24. [Google Scholar]
- Saura, S. , and Pascual‐Hortal L.. 2007. A new habitat availability index to integrate connectivity in landscape conservation planning: comparison with existing indices and application to a case study. Landscape and Urban Planning 83:91–103. [Google Scholar]
- Saura, S. , and Torne J.. 2009. Conefor Sensinode 2.2: a software package for quantifying the importance of habitat patches for landscape connectivity. Environmental Modelling & Software 24:135–139. [Google Scholar]
- Savard, J.‐P. L. , and Petersen M. R.. 2015. Remigial molt of sea ducks Pages 305–335 in Savard J.‐P. L., Derksen D. V., Esler D. and Eadie J., editors. Ecology and conservation of North American Sea Ducks. CRC Press, New York, New York, USA. [Google Scholar]
- Sea Duck Joint Venture Management Board . 2014. Sea Duck Joint Venture Strategic Plan 2014–2018. US Fish and Wildlife Service, Anchorage, Alaska, USA; Canadian Wildlife Service, Sackville, New Brunswick, Canada. [Google Scholar]
- Sea Duck Joint Venture . 2015. Atlantic and Great Lakes Sea Duck Migration Study: Progress Report June 2015. http://http//seaduckjv.org/science‐resources/atlantic‐and‐great‐lakes‐sea‐duck‐migration‐study/ [Google Scholar]
- Seifert, N. , Haase M., Van Wilgenburg S. L., Voigt C. C., and Schmitz Ornés A.. 2016. Complex migration and breeding strategies in an elusive bird species illuminated by genetic and isotopic markers. Journal of Avian Biology 47:275–287. [Google Scholar]
- Shimazaki, H. , Tamura M., and Higuchi H.. 2004. Network analysis of potential migration routes for Oriental White Storks (Ciconia boyciana). Ecological Research 19:683–698. [Google Scholar]
- Smith L. M., Pederson R. L., and Kaminski R. M., editors. 1989. Habitat management for migrating and wintering waterfowl in North America. Texas Tech University Press, Lubbock, Texas, USA. [Google Scholar]
- Solovyeva, D. , Newton J., Hobson K., Fox J. W., Afanasyev V., and Fox A. D.. 2014. Marine moult migration of the freshwater Scaly‐sided Merganser Mergus squamatus revealed by stable isotopes and geolocators. Ibis 156:466–471. [Google Scholar]
- Spiegel, C. S. , et al. 2017. Determining fine‐scale use and movement patterns of diving bird species in federal waters of the mid‐Atlantic United States Using Satellite Telemetry. OCS Study BOEM 2017‐069. U.S. Department of the Interior, Bureau of Ocean Energy Management, Sterling, Virginia, USA. [Google Scholar]
- Stehfest, K. M. , Patterson T. A., Dagorn L., Holland K. N., Itano D., and Semmens J. M.. 2013. Network analysis of acoustic tracking data reveals the structure and stability of fish aggregations in the ocean. Animal Behaviour 85:839–848. [Google Scholar]
- Talbot, S. L. , Sonsthagen S. A., Pearce J. M., and Scribner K. T.. 2015. Phylogenetics, phylogeography, and population genetics of North American sea ducks (Tribe: Mergini) Pages 29–62 in Savard J.‐P. L., Derksen D. V., Esler D. and Eadie J., editors. Ecology and conservation of North American Sea Ducks. CRC Press, New York, New York, USA. [Google Scholar]
- Taylor, C. M. , and Norris D. R.. 2010. Population dynamics in migratory networks. Theoretical Ecology 3:65–73. [Google Scholar]
- Tian, H. , et al. 2015. Avian influenza H5N1 viral and bird migration networks in Asia. Proceedings of the National Academy of Sciences USA 112:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, L. L. , Naumann B. T., Wilson P. L., Petrie S. A., and Schummer M. L.. 2013. A lift‐net method for capturing diving and sea ducks. Wildlife Society Bulletin 37:877–880. [Google Scholar]
- Webster, M. S. , Marra P. P., Haig S. M., Bensch S., and Holmes R. T.. 2002. Links between worlds: unraveling migratory connectivity. Trends in Ecology & Evolution 17:76–83. [Google Scholar]
- Weller, M. W. , and Batt B. D. J.. 1988. Waterfowl in winter: past, present, and future Pages 3–8 in Weller M. W., editor. Waterfowl in winter. University of Minnesota Press, Minneapolis, Minnesota, USA. [Google Scholar]
- Wiederholt, R. , et al. 2017. Estimating the per‐capita contribution of habitats and pathways in a migratory network: a modelling approach. Ecography 41:815–824. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on Zenodo: https://doi.org/10.5281/zenodo.2646686


