Abstract
Skin ageing is a complex process involving the additive effects of skin's interaction with its external environment, predominantly chronic sun exposure, upon a background of time‐dependent intrinsic ageing. Here, using non‐invasive cutometry and ballistometry, we explore the consequences of ageing on the biomechanical function of skin in otherwise healthy White Northern European volunteers. Intrinsic skin ageing caused biomechanical decline; skin loses both resilience (P < 0.01) and elasticity (P < 0.001), which is characterised histologically by modest effacement of rete ridges (P < 0.05) and disorganisation of papillary dermal elastic fibres. At photoexposed sites, biomechanical testing identified significant loss of biomechanical function—particularly in the aged cohort. Photoaged forearm displayed severe loss of resilience (P < 0.001) and elasticity (P < 0.001); furthermore with repetitive testing, fatigue (P < 0.001), hysteresis (P < 0.001) and viscous “creep” (P < 0.001) were exacerbated. Histologically, both young and aged forearm displayed flattening of rete ridges and disruption to the arrangement of elastic fibres. We conclude that maintenance of skin architecture is inherently associated with optimal biomechanical properties. Modest perturbations to skin architecture—as exemplified by intrinsic ageing—result in moderate functional decline. Chronic sun exposure causes fundamental changes to the clinical and histological appearance of skin, and these are reflected by an extreme alteration in biomechanical function.
Keywords: ageing, biomechanics, collagen, extracellular matrix
1. BACKGROUND
Human skin offers protection against external mechanical trauma, via the reversible deformation of its structure.1 Following mechanical deformation, the fibrillar collagens and components of the dermal elastic fibre network work in concert to resist strain and enable the skin to return to its original shape.2 The biomechanical properties of human skin have been studied extensively during the past 20 years; this became possible after the development of non‐invasive devices that allow objective and quantitative measurement.3 During the human life course, increased laxity and decreased elasticity become clinically characteristic features of the skin and arise as a result of ageing.4 Skin ageing is a complex process involving the additive effects of skin's interaction with its external environment, predominantly chronic sun exposure—termed “photoageing”—upon a background of time‐dependent intrinsic ageing.5 Intrinsic ageing causes subtle changes to tissue structure and composition,6 whereas photoageing causes profound flattening of the dermal‐epidermal junction (DEJ),7 deposition of amorphous elastin—termed solar elastosis8—and disintegration of the well‐organised elastic fibre network.9 Recent studies have provided biologically meaningful data by realising the relationship between skin composition and biomechanical function in both health10 and disease.11, 12 However, the role of skin ageing on this relationship has not yet been studied.
1.1. Questions addressed
To further expand our knowledge of the relationship between biomechanical function and skin composition, we assessed the effect of intrinsic ageing and photoageing on these properties using cohorts of young and aged White Northern European individuals.
2. EXPERIMENTAL DESIGN
2.1. Participants
Healthy, White (Fitzpatrick skin phototypes I–II) young (mean: 24.5 years ± 3.3; M = 6; F = 14) and aged (mean: 75.7 years ± 5.3; M = 7; F = 13) volunteers were recruited to the study. Local ethical approval was obtained from the University of Manchester Research Ethics Committee (ref. 14161). Written informed consent was obtained from the participants, and the study adhered to the Declaration of Helsinki principles.
2.2. Measurement of skin biomechanical properties and biopsy procurement
Test sites were selected on the buttock and dorsal aspect of the forearm. The Cutometer® MPA580 (Courage + Khazaka Electronic) with a 4‐mm‐aperture probe (mode 1; 3‐second suction followed by 3‐second relaxation period, for a total of 10 cycles using a negative pressure of 450 mbar) and the Ballistometer (Dia‐Stron Ltd., Andover, UK) were applied to three adjacent but non‐overlapping areas at each test site (see Data S1, Figure S1, Table S1). Once all measurements had been completed, 6‐mm punch biopsies were obtained from the two body sites in a subset of young (mean: 22.7 years ± 3.6; M = 2; F = 4) and aged (mean: 72.2 years ± 4.1; M = 4; F = 1) participants. At the time of procurement, biopsies were snap‐frozen in liquid nitrogen and stored at −80°C. Biopsies were cryosectioned at 7 µm in a single run, using the same blade and the same cryostat settings.
2.3. Immunohistology, microscopy and statistical testing
Immunofluorescence staining was performed using mouse monoclonal antibodies to detect elastin (clone BA4, dilution 1:500; Sigma‐Aldrich) and fibrillin‐1 (clone 11C1.3, dilution 1:1000; Neomarkers). Picrosirius red histological staining was used for the detection of fibrillar collagens (see Data S1 for detailed protocols). Fluorescence and cross‐polarised images were captured using a BX53 microscope (Olympus Industrial), and image analysis was performed using ImageJ software.13 Statistical analysis was performed using GraphPad Prism 7.01 (GraphPad Software, Inc). Results were considered significant if P < 0.05 (95% confidence level).
3. RESULTS
The biomechanical properties of photoprotected buttock and photoexposed forearm skin were determined using the experimental methods of suction (cutometry) and indentation (ballistometry). Intrinsically aged buttock skin demonstrated significant decline for all cutometry parameters with differences identified for overall curve shape (F3 envelope; P < 0.001), total deformation (R0; P < 0.001) and immediate deformation (Ue; P < 0.001). In addition, intrinsically aged skin was less capable of returning to its original position following deformation (residual deformation [R1]; P < 0.01), had significantly reduced elasticity (R2, R5, R6 and R7; P < 0.001 for all parameters) and exhibited signs of skin fatigue (R4; P < 0.001) and hysteresis (R9; P < 0.01) as compared to young buttock skin. Similarly, ballistometry demonstrated significantly reduced indentation of the skin (P < 0.01), reduced elasticity from increased alpha values (P < 0.001) and decreased coefficient of restitution (CoR; bounce height relative to start height; P < 0.001) with reduced area of bounce profile (P < 0.001) compared to young buttock skin (Figure 1A,B; Table S2).
Figure 1.
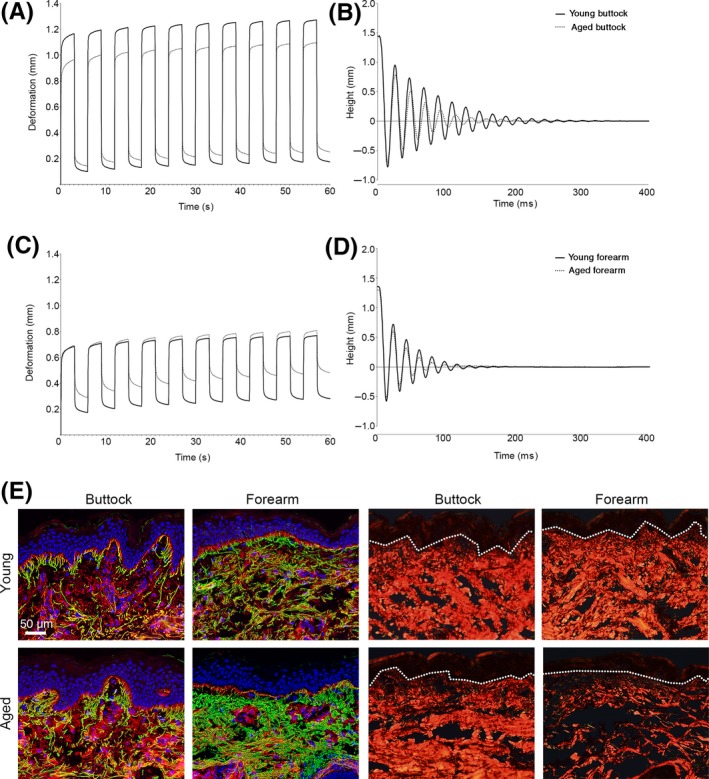
The biomechanical properties of young and aged skin were determined using the Cutometer® (A & C) and the Ballistometer (B & D). Immunofluorescence staining of elastin (green) and fibrillin‐1 (red) in photoprotected buttock and photoexposed forearm of young and aged individuals (E). Picrosirius red staining for organised fibrillar collagens in photoprotected buttock and photoexposed forearm of young and aged individuals (F). Scale bar = 50 µm
Biomechanical properties were next determined for extensor forearm, an anatomical site often chronically photoexposed. For both young and aged forearm skin, cutometry revealed a marked decline in biomechanical function as compared to photoprotected buttock skin, exemplified by a reduction in curve area (young: −54%; aged: −57%) and dramatically impaired skin deformation. The severity of this biomechanical decline was particularly exacerbated in the aged forearm; where there was a decline in the ability of skin to return to its original position (R1; P < 0.001), elasticity was markedly reduced (R2, R5 and R7; all P < 0.001), and both fatigue (R4; P < 0.001) and hysteresis (R9; P < 0.001) were significantly increased compared to young forearm. Of particular note was that despite no difference in height of the first deformation, after repetitive cycles, aged forearm exhibited an incremental increase in deformation—or viscous “creep”—a phenomenon not reported for young forearm (R6; P < 0.001). Similarly, ballistometry of aged forearm revealed a decline in skin elasticity (alpha: P < 0.001; CoR: P < 0.001), whilst indentation and area were unchanged compared with young forearm skin (Figure 1C,D; Table S2).
Histologically, young buttock skin was characterised by strong interdigitation of the rete ridges at the DEJ and a highly organised arrangement of candelabra‐like arrays of elastic fibres connecting the papillary, reticular and deep dermis. Perturbation to this highly ordered skin structure was evident in intrinsically aged buttock skin, with reduced interdigitation of rete ridges and a disorganisation of papillary dermal elastic fibres, accompanied by an accumulation of truncated elastin. Further disruption to elastic fibres and flattening of rete ridges at the DEJ was apparent in photoexposed skin from both young and aged cohorts. Here, fibrillin‐rich microfibrils at the DEJ were severely truncated and unable to form cascades connecting the layers of the dermis, whilst an accumulation of amorphous elastin (solar elastosis) was identified in the dermis (Figure 1E). Picrosirius red staining and polarised light microscopy further demonstrated differences in the composition of the dermis. Both young buttock and forearm skin contained highly organised, abundant fibrillar collagen. Intrinsic skin ageing reduced the abundance of organised collagen, whilst the additive effect of chronic photoexposure caused a further, profound reduction (Figure 1F).
4. CONCLUSIONS
In this study, we established that flattening of the DEJ, accumulation of solar elastosis, disruption to elastic fibre arrangement and reduced collagen organisation appear to be severely detrimental to skin's biomechanical behaviour.
Cutometry and ballistometry are useful methods that describe related, but not identical, aspects of skin biomechanics14; cutometry predominantly measures skin elasticity, whilst ballistometry predominantly measures stiffness.15 Cutometry curves for photoprotected buttock demonstrate a moderate decline in biomechanical function of intrinsically aged skin as compared to young buttock skin. In contrast, cutometry curves for photoexposed forearm revealed significant deterioration of skin's biomechanical properties is evident even in young skin that display little or no clinical signs of photodamage, whilst chronic photoexposure further exacerbates this decline. Solar elastosis appears to be a significant driver of altered biomechanical function in lightly pigmented skin; the deposition of dystrophic elastin is absent in intrinsically aged skin, and consequently, biomechanical function is much better preserved. That said, the disorganisation of both fibrillin‐1 and elastin within the superficial papillary dermis also appears to impact skin's elastic function and resilience.9, 16 Similarly, the decrease in skin elasticity parameters that occurs after 70 years of age and at photoexposed forearm might, in part, be the consequence of DEJ flattening, resulting in a more fragile epidermal‐dermal interface and an epidermis that is less resistant to shearing forces.17 The role of the fibrillar collagens in influencing skin's biomechanical function appears to be less well defined. Both young buttock and forearm skin contained highly organised, abundant fibrillar collagen, yet the biomechanical properties at these anatomical sites were vastly different. Thus, it is unlikely that the fibrillar collagens were responsible for these functional differences. In contrast, photoaged forearm displayed a marked loss of organised fibrillar collagen and a severe decline in biomechanical function—over and above that seen in young photoexposed forearm. The Ballistometer alpha parameter was significantly increased in photoaged skin, which is indicative of the tissue being stiff and energy dampening—properties that are usually attributed to a loss of organised collagen and increased collagen cross‐linking.18, 19
The results presented here provide a detailed insight into the interplay between skin architecture and its effect on biomechanical function; an appreciation of these properties is important for our understanding of skin health.
CONFLICT OF INTEREST
The authors have declared no conflicting interests.
AUTHOR CONTRIBUTIONS
AKL, CEMG and REBW conceived the study and designed the research study. AKL and HKG performed the research. AKL analysed and interpreted the data. AKL wrote the manuscript. All authors have read and approved the final manuscript.
Supporting information
Supplementary Fig 1. Measurement of biomechanical properties of the skin. Graphical representation of the biomechanical properties obtained from application of the ballistometer to the skin (a). Application of the Cutometer® in mode 1 generates time–strain curves (b).
Supplementary Table 1. Ballistometer and Cutometer® parameters.
Supplementary Table 2. Biomechanical properties of buttock and forearm skin.
ACKNOWLEDGEMENTS
Professor Christopher Griffiths is an NIHR Senior Investigator. Professor Griffiths and Professor Rachel Watson are supported in part by the NIHR Manchester Biomedical Research Centre. This study was funded by a programme grant from Walgreens Boots Alliance.
Langton AK, Graham HK, Griffiths CEM, Watson REB. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp Dermatol. 2019;28:981–984. 10.1111/exd.13980
REFERENCES
- 1. Edwards C, Marks R, Clin Dermatol. 1995, 13, 375. [DOI] [PubMed] [Google Scholar]
- 2. Hussain SH, Limthongkul B, Humphreys TR, Dermatol. Surg. 2013, 39(2), 193. [DOI] [PubMed] [Google Scholar]
- 3. Serup J, Jemec GB, Grove GL, Handbook of Non‐Invasive Methods and The Skin, 2nd ed. CRC Press, Boca Raton, FL: 2006. [Google Scholar]
- 4. Escoffier C, de Rigal J, Rochefort A, Vasselet R, Leveque JL, Agache PG, J Invest Dermatol. 1989, 93, 353. [PubMed] [Google Scholar]
- 5. Fenske NA, Lober CW, J Am Acad Dermatol. 1986, 15, 571. [DOI] [PubMed] [Google Scholar]
- 6. Montagna W, Carlisle K, J Invest Dermatol. 1979, 73, 47. [DOI] [PubMed] [Google Scholar]
- 7. Allan AK, Anat Rec (Hoboken). 1958, 131, 717. [Google Scholar]
- 8. Kligman AM, JAMA 1969, 210, 2377. [PubMed] [Google Scholar]
- 9. Watson R, Griffiths C, Craven NM, Shuttleworth CA, Kielty CM, J Invest Dermatol. 1999, 112, 782. [DOI] [PubMed] [Google Scholar]
- 10. Langton AK, Alessi S, Chien AL, Kang S, Griffiths C, Watson R, J Invest Dermatol. 2017, 137, S302. [DOI] [PubMed] [Google Scholar]
- 11. Dobrev H, Acta Derm Venereol. 2000, 80, 263. [DOI] [PubMed] [Google Scholar]
- 12. Dobrev HP, J Am Acad Dermatol. 1999, 40, 436. [DOI] [PubMed] [Google Scholar]
- 13. Abramoff MD, Magelhaes PJ, Ram SJ, Biophotonics Int. 2004, 11, 36. [Google Scholar]
- 14. Woo MS, Moon KJ, Jung HY, Park SR, Moon TK, Kim NS, Lee BC, Skin Res Technol. 2014, 20, 422. [DOI] [PubMed] [Google Scholar]
- 15. Jemec GB, Selvaag E, Agren M, Wulf HC, Skin Res Technol. 2001, 7(2), 122. [DOI] [PubMed] [Google Scholar]
- 16. Langton AK, Graham HK, McConnell JC, Sherratt MJ, Griffiths C, Watson R, Br J Dermatol. 2017, 177, 818. [DOI] [PubMed] [Google Scholar]
- 17. Lavker RM, Zheng PS, Dong G, J Invest Dermatol. 1987, 88, 44s. [DOI] [PubMed] [Google Scholar]
- 18. Lovell CR, Smolenski KA, Duance VC, Light ND, Young S, Dyson M, Br J Dermatol. 1987, 117, 419. [DOI] [PubMed] [Google Scholar]
- 19. Boyer G, Laquieze L, Le Bot A, Laquieze S, Zahouani H, Skin Res Technol. 2009, 15, 55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1. Measurement of biomechanical properties of the skin. Graphical representation of the biomechanical properties obtained from application of the ballistometer to the skin (a). Application of the Cutometer® in mode 1 generates time–strain curves (b).
Supplementary Table 1. Ballistometer and Cutometer® parameters.
Supplementary Table 2. Biomechanical properties of buttock and forearm skin.


