Abstract
Aim
Although treatment guidelines for pharmacological therapy for schizophrenia and major depressive disorder have been issued by the Japanese Societies of Neuropsychopharmacology and Mood Disorders, these guidelines have not been well applied by psychiatrists throughout the nation. To address this issue, we developed the ‘Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)’ integrated education programs for psychiatrists to disseminate the clinical guidelines. Additionally, we conducted a systematic efficacy evaluation of the programs.
Methods
Four hundred thirteen out of 461 psychiatrists attended two 1‐day educational programs based on the treatment guidelines for schizophrenia and major depressive disorder from October 2016 to March 2018. We measured the participants’ clinical knowledge of the treatment guidelines using self‐completed questionnaires administered before and after the program to assess the effectiveness of the programs for improving knowledge. We also examined the relation between the participants’ demographics and their clinical knowledge scores.
Results
The clinical knowledge scores for both guidelines were significantly improved after the program. There was no correlation between clinical knowledge and participant demographics for the program on schizophrenia; however, a weak positive correlation was found between clinical knowledge and the years of professional experience for the program on major depressive disorder.
Conclusion
Our results provide evidence that educational programs on the clinical practices recommended in guidelines for schizophrenia and major depressive disorder might effectively improve participants’ clinical knowledge of the guidelines. These data are encouraging to facilitate the standardization of clinical practices for psychiatric disorders.
Keywords: educational program, EGUIDE project, major depressive disorder, schizophrenia, treatment guideline
Treatment guidelines are standard tools for clinical practice. Various guidelines have been published for the treatment of psychiatric disorders.1, 2, 3, 4, 5, 6, 7, 8 In many countries, psychiatrists usually treat patients based on treatment guidelines. However, evidence‐based treatment guidelines for psychiatric disorders were not developed in Japan until 10 years ago; as a result, Japanese psychiatrists were likely to base clinical decisions on their own experiences. Consequently, pharmacotherapies for psychiatric disorders in Japan differed from those recommended by treatment guidelines in other countries.9, 10, 11, 12, 13, 14 For example, although most guidelines recommended antipsychotic monotherapy for schizophrenia, the number of antipsychotics used in Japan was higher than that used in other countries. In addition, the use of benzodiazepines as adjunctive treatment for major depressive disorder, which is not recommended in most guidelines, was higher in Japan than in the USA.6 To address these situations, the ‘Guideline for Pharmacological Therapy for Schizophrenia’ was published by the Japanese Society of Neuropsychopharmacology in 2015,15 and the ‘Treatment Guideline: Major Depressive Disorder’ was published by the Japanese Society of Mood Disorders in 201216 and revised as the ‘Treatment Guideline II: Major Depressive Disorder’ in 2016.17
Although treatment guidelines for schizophrenia and major depressive disorder have been published, pharmacological treatment for these disorders in Japan has still not changed.9, 10 One possible reason for this phenomenon is that there has been no official training program for learning the treatment guidelines by academic societies for psychiatrists and residents in Japan. Consequently, we realized the need to disseminate the guidelines to Japanese psychiatrists and provide education regarding the guidelines’ content. Therefore, we started the ‘Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment’ (EGUIDE) project in 2016 to disseminate the guidelines. The aims of the EGUIDE project were to disseminate the guidelines via education programs for psychiatrists that present the treatment guidelines for schizophrenia and major depressive disorder. In addition, we investigated the effectiveness of the guideline education programs by evaluating the participants’ clinical knowledge regarding the guidelines before and after the programs.
The aim of this study was to access the dissemination of the guidelines via educational programs for psychiatrists throughout Japan. Moreover, we evaluated the educational effect of the programs for each psychiatrist by comparing his or her knowledge of the treatment guidelines before and after the programs.
Methods
Design and participants
Psychiatrists were recruited from October 2016 to March 2018. Written informed consent was obtained for all participants after the procedures had been fully explained by a chief researcher at the facility. This study was approved by the ethics committees of the National Center of Neurology and Psychiatry (A2017‐105) and each participating university/hospital/clinic. The study procedures were conducted according to the Declaration of Helsinki. The protocol of this study was registered in the University Hospital Medical Information Network registry (UMIN000022645). Initially, the participants completed a self‐administered questionnaire that assessed their knowledge of clinical guidelines. The participants then attended 1‐day educational programs on schizophrenia and depression based on the ‘Guideline for Pharmacological Therapy for Schizophrenia’ published by the Japanese Society of Neuropsychopharmacology and the ‘Treatment Guideline II: Major Depressive Disorder’ published by the Japanese Society of Mood Disorders. Lectures on the guidelines and discussions of two clinical cases were included to present the guidelines and to describe how to implement them in practice. The participants then retook the self‐administered questionnaire at the end of the 1‐day program. The efficacy of each program was evaluated according to the changes in the scores of the self‐administered questionnaires between baseline and program completion.
Assessment measures
To evaluate the participants’ clinical knowledge of the ‘Guideline for Pharmacological Therapy for Schizophrenia,’ we created a self‐administered questionnaire consisting of 37 items (a total of 37 points) with seven subscale scores (Table S1). The participants’ clinical knowledge of the ‘Treatment Guideline II: Major Depressive Disorder’ was evaluated via another self‐administered questionnaire consisting of 37 items (a total of 37 points) with eight subscale scores (Table S1 and S2). All items were described in Japanese and required checking correct or incorrect in the square for each question. The participants were asked to answer all questions within 7 min. We excluded all subjects with any incomplete data for the two guideline tests (at baseline and after the program), such as missing checks in the squares for each question, which we regarded as missing data.
Statistical analysis
All statistical analyses were performed using Excel (Microsoft, Redmond, WA, USA) or spss 22.0 (spss, Chicago, IL, USA). The Kolmogorov–Smirnov test was used to evaluate the normality of the clinical knowledge scores and participants’ ages and professional experience. The data were analyzed with tests for matched pairs using statistical significance and effect size estimates. To compare the changes in the total clinical knowledge scores between baseline and post‐program for matched pairs, the Wilcoxon signed‐rank test was used. Effect sizes were determined with Z‐values divided by the square root of the number of subjects. The rates of correct answers at baseline and post‐program for each item were compared using χ2 tests. The relations among sex, age, professional experience, and clinical knowledge scores at baseline and post‐program were analyzed using Spearman's rank correlation coefficient. To identify associated factors for the total clinical knowledge scores in each program, multiple regression analysis was performed with three independent variables (age, sex, and professional experience).
The significance level was set at P < 0.05. The Bonferroni correction was applied for multiple testing.
Results
Participant demographics
A total of 443 participants attended the ‘Guideline for Pharmacological Therapy for Schizophrenia’ program, and 431 attended the ‘Treatment Guideline II: Major Depressive Disorder’ program over the 18‐month period. The participants represented over 90 medical institutions. Of 413 participants who participated in both programs, we used the data of 344 psychiatrists in the final analysis (Fig. S1). The demographics of the 344 psychiatrists are summarized in Table S3. A normal distribution was not observed for the participants’ ages and years of professional experience (Fig. S2).
Changes in the clinical knowledge scores before and after the program
The distribution of clinical knowledge scores before and after attending the educational program on the ‘Guideline for Pharmacological Therapy for Schizophrenia’ is shown in Figure 1a. The results showed that the vertex of the distribution of clinical knowledge scores shifted significantly to the right after the program (χ2 = 348.17, P = 1.9 × 10−66). Table 1 shows the means and statistical results for the total clinical knowledge score and subscale scores before and after the program. The accuracy rate of total clinical knowledge score increased significantly, from 90.3% (baseline) to 98.1% (after the program), as shown in Table 1 (Z = 15.02, P = 5.3 × 10−51, r = 0.81). Regarding the subscales of clinical knowledge, large and significant changes were observed in ‘Recommended pharmacotherapy for schizophrenia in general’ (Z = 9.45, P = 3.4 × 10−21, r = 0.51), ‘Management of recurrence or relapse of schizophrenia’ (Z = 12.48, P = 9.4 × 10−36, r = 0.67), ‘Pharmacotherapy during the maintenance phase’ (Z = 13.34, P = 1.3 × 10−40, r = 0.72.), and ‘Management of treatment‐resistant schizophrenia’ (Z = 9.73, P = 2.4 × 10−22, r = 0.52). In addition, significant and moderate changes were observed in ‘Other issues’ (Z = 6.21, P = 5.3 × 10−10, r = 0.34). A comparison of the correct answer rate for each item between baseline and post‐program is shown in Table S4. The accuracy rates were increased for most questions; the exceptions were F‐3 and G‐8.
Figure 1.
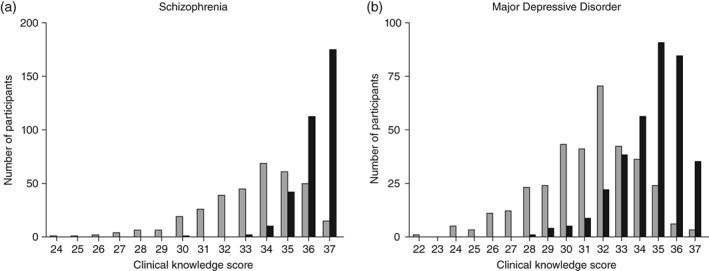
Distribution of clinical knowledge scores at ( ) baseline and (
) baseline and ( ) post‐program. (a) ‘Guideline for Pharmacological Therapy for Schizophrenia.’ (b) ‘Treatment Guideline II: Major Depressive Disorder.’
) post‐program. (a) ‘Guideline for Pharmacological Therapy for Schizophrenia.’ (b) ‘Treatment Guideline II: Major Depressive Disorder.’
Table 1.
Comparison of clinical knowledge scores at baseline and after the ‘Guideline for Pharmacological Therapy for Schizophrenia’ program
| Baseline | Post‐program | Statistic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | %† | Mean | SD | %† | Z ‡ | P | r | |
| Total clinical knowledge score | 33.4 | ±2.3 | 90.3 | 36.3 | ±0.9 | 98.1 | 15.02 | 5.3 × 10 −51 | 0.81 |
| Clinical knowledge subscale scores | |||||||||
| Recommended pharmacotherapy for schizophrenia in general | 3.6 | ±0.6 | 90.5 | 4.0 | ±0.1 | 99.9 | 9.45 | 3.4 × 10 −21 | 0.51 |
| Recommended pharmacotherapy for first‐episode psychosis | 4.9 | ±0.3 | 98.5 | 5.0 | ±0.1 | 99.8 | 3.82 | 1.3 × 10 −4 | 0.21 |
| Duration of pharmacotherapy for first‐episode psychosis in terms of relapse prevention | 2.8 | ±0.6 | 93.9 | 3.0 | ±0.1 | 99.9 | 5.49 | 4.1 × 10 −8 | 0.30 |
| Management of recurrence or relapse of schizophrenia | 4.1 | ±0.6 | 82.5 | 4.8 | ±0.5 | 95.0 | 12.48 | 9.4 × 10 −36 | 0.67 |
| Pharmacotherapy during the maintenance phase | 4.9 | ±0.9 | 82.0 | 5.8 | ±0.4 | 97.5 | 13.34 | 1.3 × 10 −40 | 0.72 |
| Management of treatment‐resistant schizophrenia | 5.3 | ±0.8 | 88.2 | 5.8 | ±0.4 | 96.7 | 9.73 | 2.4 × 10 −22 | 0.52 |
| Other issues | 7.7 | ±0.5 | 96.5 | 7.9 | ±0.3 | 98.9 | 6.21 | 5.3 × 10 −10 | 0.34 |
Percentage of correct answers.
The Wilcoxon signed‐ranks test was used for the statistical analysis as the Kolmogorov–Smirnov test did not indicate a normal distribution of clinical knowledge scores at baseline or after the program (P = 3.1 × 10−25, P = 1.0 × 10−83).
An effect size (r) of 0.5 or more indicates a large change, and an effect size (r) of 0.3 to 0.5 indicates a moderate change.
The significance level was set at two‐tailed P < 6.3 × 10−3 as the Bonferroni method was applied.
Significant P‐values are boldfaced.
The distribution of clinical knowledge scores before and after the ‘Treatment Guideline II: Major Depressive Disorder’ educational program is shown in Figure 1b. Similarly, the vertex of the distribution of clinical knowledge scores shifted to the right (χ2 = 279.37, P = 2.3 × 10−51). Table 2 shows the mean scores and the statistical results before and after the program for the total clinical knowledge and subscales. The correct answer rate for the total clinical knowledge score increased significantly, from 84.4% (baseline) to 93.5% (after the program; Z = 15.27, P = 1.3 × 10−52, r = 0.82). Regarding the subscales of clinical knowledge, significant and large changes were observed in ‘Diagnosis of major depressive disorder (DSM‐5)’ (Z = 12.39, P = 3.1 × 10−35, r = 0.67), ‘Management of mild depression’ (Z = 10.69, P = 1.1 × 10−26, r = 0.58), and ‘Management of psychotic depression’ (Z = 11.27, P = 1.9 × 10−29, r = 0.61). In addition, significant and moderate changes were observed in ‘Recommended treatment for moderate/severe depression’ (Z = 7.49, P = 6.7 × 10−14, r = 0.40), ‘Recommended treatment for moderate/severe depression if necessary’ (Z = 5.86, P = 4.6 × 10−9, r = 0.32), and ‘Management of depression in children and adolescents’ (Z = 7.41, P = 1.2 × 10−13, r = 0.40). A comparison of the correct answer rates for each item between the baseline and after the program is shown in Table S5. The accuracy rates were increased for most questions; the exceptions were B‐3 and C‐2.
Table 2.
Comparison of clinical knowledge scores at baseline and after the ‘Treatment Guideline II: Major Depressive Disorder’ program
| Baseline | Post‐program | Statistic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | %† | Mean | SD | %† | Z ‡ | P | r | |
| Total clinical knowledge score | 31.2 | ±2.7 | 84.4 | 34.6 | ±1.7 | 93.5 | 15.27 | 1.3 × 10 −52 | 0.82 |
| Clinical knowledge subscale scores | |||||||||
| Diagnosis of major depressive disorder (DSM‐5) | 4.6 | ±1.0 | 76.1 | 5.4 | ±0.6 | 90.3 | 12.39 | 3.1 × 10 −35 | 0.67 |
| Treatment of major depressive disorder | 4.2 | ±0.6 | 84.1 | 4.4 | ±0.6 | 87.6 | 4.86 | 1.2 × 10 −6 | 0.26 |
| Management of mild depression | 5.8 | ±0.9 | 82.1 | 6.4 | ±0.7 | 92.1 | 10.69 | 1.1 × 10 −26 | 0.58 |
| Recommended treatment for moderate/severe depression | 3.4 | ±0.7 | 85.3 | 3.7 | ±0.5 | 93.4 | 7.49 | 6.7 × 10 −14 | 0.40 |
| Recommended treatment for moderate/severe depression, if necessary | 2.8 | ±0.5 | 93.8 | 3.0 | ±0.1 | 99.5 | 5.86 | 4.6 × 10 −9 | 0.32 |
| Management of psychotic depression | 2.9 | ±1.1 | 72.5 | 3.7 | ±0.7 | 92.9 | 11.27 | 1.9 × 10 −29 | 0.61 |
| Management of depression in children and adolescents | 3.8 | ±0.5 | 94.0 | 4.0 | ±0.2 | 99.3 | 7.41 | 1.2 × 10 −13 | 0.40 |
| Management of sleep disorders associated with depression | 3.8 | ±0.4 | 95.8 | 4.0 | ±0.2 | 98.8 | 4.98 | 6.5 × 10 −7 | 0.27 |
Percentage of correct answers.
The Wilcoxon signed‐ranks test was used for the statistical analysis as the Kolmogorov–Smirnov test did not indicate normal distribution of clinical knowledge scores at baseline or after the program (P = 1.0 × 10−17, P = 6.6 × 10−37).
An effect size (r) of 0.5 or more indicates a large change, and an effect size (r) of 0.3 to 0.5 indicates moderate change.
The significance level was set at two‐tailed P< 6.3 × 10−3 as the Bonferroni method was applied.
Significant P‐values are boldfaced.
Relations between the clinical knowledge score and participant demographics
Table 3 shows the relations between the total clinical knowledge score (before and after the program) and the participant demographics for each guideline. In the ‘Guideline for Pharmacological Therapy for Schizophrenia’ program, there was no correlation between the total clinical knowledge score and the participant demographics. The distribution of clinical knowledge scores by years of professional experience is shown in Fig. S3a.
Table 3.
Relation between the clinical knowledge score and participant demographics
| Sex | Age | Professional experience | ||||
|---|---|---|---|---|---|---|
| ρ† | P | ρ† | P | ρ† | P | |
| Medical education program for the ‘Treatment Guideline for Pharmacological Therapy for Schizophrenia’ | ||||||
| Total clinical knowledge score at baseline | −0.09 | 0.11 | 0.02 | 0.74 | 0.07 | 0.17 |
| Total clinical knowledge score after the program | −0.04 | 0.48 | −0.06 | 0.29 | 0.01 | 0.83 |
| Medical education program for ‘Treatment Guideline II: Major Depressive Disorder’ | ||||||
| Total clinical knowledge score at baseline | −0.09 | 0.11 | 0.10 | 0.05 | 0.28 | 1.8 × 10 −7 |
| Total clinical knowledge score after the program | −0.05 | 0.38 | 0.05 | 0.39 | 0.18 | 7.7 × 10 −4 |
Spearman's rank correlation coefficient.
The significance level was set at two‐tailed P < 6.3 × 10−3 as the Bonferroni method was applied.
A correlation coefficient (ρ) of 0.2 to 0.4 indicates a weak correlation.
Significant P‐values are boldfaced.
For the ‘Treatment Guideline II: Major Depressive Disorder’ program, a weak positive correlation was found between the total clinical knowledge score at the baseline and the years of professional experience (ρ = 0.28, P = 1.8 × 10−7; Table 3). Additionally, a very weak positive correlation was found between the total clinical knowledge score after the program and the years of professional experience (ρ = 0.18, P = 7.7 × 10−4). There was no correlation between the total clinical knowledge score and the sex or age of the participants. The distribution of the clinical knowledge scores by years of professional experience is shown in Fig. S3b.
The results of multiple regression analysis revealed that there was no significant factor associated with total clinical knowledge in the ‘Guideline for Pharmacological Therapy for Schizophrenia’ program (Table 4). On the other hand, age and professional experience were significantly associated with total clinical knowledge scores both at baseline (beta = −0.22, P = 5.0 × 10−3 and beta = 0.41, P = 2.6 × 10−7, respectively) and after the program (beta = −0.21, P = 8.0 × 10−3 and beta = 0.35, P = 1.6 × 10−5, respectively) in the ‘Treatment Guideline II: Major Depressive Disorder’ program (Table 4).
Table 4.
Results of multiple regression analyses of the clinical knowledge scores
| Sex | Age | Professional experience | ||||||
|---|---|---|---|---|---|---|---|---|
| Beta† | P | Beta† | P | Beta† | P | Adjusted R 2 | ANOVA P | |
| Medical education program for the ‘Treatment Guideline for Pharmacological Therapy for Schizophrenia’ | ||||||||
| Total clinical knowledge score at baseline | −0.05 | 0.33 | −0.12 | 0.14 | 0.18 | 2.5 × 10−2 | 0.019 | 8.7 × 10−2 |
| Total clinical knowledge score after the program | −0.05 | 0.38 | −0.18 | 2.9 × 10−2 | 0.15 | 0.06 | 0.008 | 0.13 |
| Medical education program for ‘Treatment Guideline II: Major Depressive Disorder’ | ||||||||
| Total clinical knowledge score at baseline | −0.06 | 0.26 | −0.22 | 5.0 × 10 −3 | 0.41 | 2.6 × 10 −7 | 0.081 | 6.0 × 10 −7 |
| Total clinical knowledge score after the program | −0.02 | 0.79 | −0.21 | 8.0 × 10 −3 | 0.35 | 1.6 × 10 −5 | 0.048 | 1.8 × 10 −4 |
Standardized partial regression coefficient.
The significance level was set at two‐tailed P < 1.25 × 10−2 as the Bonferroni method was applied.
Significant P‐values are boldfaced.
ANOVA, analysis of variance.
Discussion
This is the first study to investigate the dissemination of treatment guidelines for schizophrenia and major depressive disorder and to evaluate the effectiveness of an educational program on the guidelines at the same time. Approximately half (n = 42) of the 82 university hospitals mainly responsible for psychiatric professional education in Japan participated in the EGUIDE project, and 344 psychiatrists attended educational programs aimed at ensuring an appropriate understanding of the guidelines (Fig. S4). Previous studies have suggested that there could be a huge gap between the development of guidelines based on research evidence and their uptake in clinical practice.18 Although the pathway from evidence to guideline is highly developed, the pathway from guideline to clinical practices is much less developed and has been examined in few studies.19, 20, 21 This situation suggests that a lack of awareness of and familiarity with guidelines, as well as lack of a supply system, might prevent the implementation of guidelines in clinical practice. To address these issues, the EGUIDE project created a supply system and provided an opportunity for clinicians to become aware of and familiar with the guidelines. Consequently, the EGUIDE project was used to disseminate and encourage the implementation of guidelines in clinical practice throughout Japan.
Regarding the effects of the educational program on the participants’ understanding of the guidelines, the results of this study showed that knowledge of the guidelines was significantly improved after the programs for schizophrenia and major depressive disorder. These results suggest that our educational programs can be useful for improving knowledge of treatment guidelines.
In this study, we performed multiple regression analysis for factors possibly associated with clinical knowledge score of each guideline before and after the program in independent variables (sex, age, and professional experience). As a result, clinical knowledge scores in the ‘Treatment Guideline II: Major Depressive Disorder’ program were negatively associated with age and positively associated with professional experience. However, it is difficult to explain these results with our limited data. There might be various potential confounding factors associated with the clinical knowledge scores of guidelines. Therefore, it is difficult to draw any conclusions regarding the relations observed in this study.
The ‘Guideline for Pharmacological Therapy for Schizophrenia’ emphasizes that antipsychotic monotherapy should be considered as a first‐line treatment for all patients with schizophrenia and that clozapine should be used for treatment‐resistant schizophrenia, similar to the guidelines of other countries.1, 4, 6, 7, 8 However, Japanese psychiatrists are more likely than psychiatrists in other countries to adjunctively prescribe multiple antipsychotics and other psychotropics, such as benzodiazepines, instead of antipsychotics monotherapy.11, 13, 14 In addition, fewer treatment‐resistant schizophrenia patients in Japan take clozapine.22 Taking these situations and the results of this study into consideration, significant improvement in total clinical knowledge scores, including recommendations regarding the appropriate use of antipsychotic monotherapy and clozapine, may lead to the implementation of appropriate pharmacological therapy for schizophrenia in Japan.
For ‘Treatment Guideline II: Major Depressive Disorder,’ the degree of knowledge about the guideline was significantly improved after the program. ‘Treatment Guideline II: Major Depressive Disorder’ recommends antidepressants as the first‐line treatment for major depressive disorder and does not recommended long‐term use of benzodiazepines. However, a majority of major depressive disorder patients in Japan are not treated according to guidelines, and many patients are treated with multiple drugs, including long‐term use of benzodiazepines.23 In addition, according to the study comparing the treatment choices for major depressive disorder between Japanese and US psychiatrists,12 the Japanese psychiatrists favored benzodiazepine monotherapy for the treatment of mild major depressive disorder, whereas the US psychiatrists favored antidepressant monotherapy. Given these situations, this study's findings of a significant increase in the total score for clinical knowledge of the guidelines, including the appropriate use of antidepressants and benzodiazepines, may have a positive influence on the clinical treatment of major depressive disorder in Japan.
Several limitations of this study should be considered when interpreting the results. First, it was difficult to evaluate the effect of the program because of the nature of the study, which was conducted as a single‐arm design without a control group. Second, because the questionnaires used were not validated, it was unclear whether the questionnaire could appropriately evaluate the knowledge of the guidelines. Third, it can be presumed that the participants’ background information was insufficient, and there may be multiple potential confounding factors related to improving understanding of the guidelines. Fourth, it is necessary not only to evaluate the knowledge of clinical guidelines but also to evaluate changes in quality indicators, such as the participants’ prescription patterns, to verify the effects of the program. Fifth, the distribution of the age of participants was relatively young. This could be due to the design of this clinical implementation research. Research and education programs are common in the university hospitals that participated in the EGUIDE project and young psychiatrists tend to outnumber older psychiatrists in university hospitals. It might be difficult to generalize the results to all psychiatrists in Japan due to the selection bias. Although this study was a preliminary survey, disseminating clinical guidelines and educating psychiatrists about clinical guidelines will lead to meaningful results in clinical settings. The EGUIDE project could provide not only for psychiatrists working for university hospitals but for all Japanese psychiatrists and residents in the future. To achieve this, the EGUIDE projects would need to collaborate with the official programs of academic societies in Japan. Moreover, comprehensive treatment guidelines, including psychosocial interventions and pharmacological treatment for achieving recovery, should be developed.24
In conclusion, the EGUIDE project, a dissemination and education program for the ‘Guideline for Pharmacological Therapy for Schizophrenia’ and ‘Treatment Guideline II: Major Depressive Disorder,’ could help to improve clinical knowledge regarding the guidelines among psychiatrists. Further study will be needed to clarify the effects of the EGUIDE project on the improvement of inappropriate pharmacological treatment in clinical settings.
Disclosure statement
The authors declare no financial or nonfinancial competing interests.
Author contributions
Y.T. was critically involved in data collection and data analysis and wrote the first draft of the manuscript. K.W. and K.Ina. were critically involved in the study design and contributed to the interpretation of the data and the writing of the manuscript. S.N., M.I., N.K., S.O., T.Tak., K.N., Y.Y., H.T., T.Tsu., N.T., N.H., Y.M., H.H., and H.Yamam. were involved in the data analysis and contributed to the interpretation of the data and the writing of the manuscript. N.S., T.S., T.K., A.H., M.U., R.F., K.Iwa., H.F., T.Nak., K.M., T.I., E.K., H.T., K.O., H.M., K.A., H.I., T.Nag., J.F., S.Y., T.O., A.M., Y.T., H.N., Y.M., K.T., J.I., K.Ich., K.O., and H.Yamad. were involved in the participant recruitment process and data collection and contributed to the interpretation of the data. R.H. supervised the entire project, collected the data and was critically involved in the design, analysis, and interpretation of the data. All authors contributed to and approved the final manuscript.
Supporting information
Fig. S1 Flow chart of the study participants. During the 18‐month study period, there were 445 participants in the ‘Guideline for Pharmacological Therapy for Schizophrenia’ and 433 participants in the ‘Treatment Guideline II: Major Depressive Disorder’ program. The participants represented over 90 medical institutions. Of these participants, we used the data of 344 in the final analysis.
Fig. S2 Distribution of age and years of professional experience. Distribution of demographics. (a) Age. (b) Years of professional experience. The Kolmogorov–Smirnov test was conducted. Normal distribution was not observed for (a) and (b) (A: P = 6.3 × 10−20, B: P = 5.5 × 10−59).
Fig. S3 The distribution of clinical knowledge scores for years of professional experience in the guidelines for schizophrenia and major depressive disorder. The distribution of clinical knowledge scores for each year of professional experience. (a) ‘Guideline for Pharmacological Therapy for Schizophrenia.’ (b) ‘Treatment Guideline II: Major Depressive Disorder.’ Error bars indicate standard deviation.
Fig. S4 Distribution of the facilities that participated in this study in Japan. Of a total of 82 university hospitals in Japan, approximately half (n = 42) joined the EGUIDE project (10 March 2019).
Table S1. Questions regarding knowledge about the pharmacotherapy of schizophrenia
Table S2. Questions regarding knowledge about the management of major depressive disorder
Table S3. Participant demographics
Table S4. Detailed comparison of clinical knowledge scores at baseline and after the ‘Guideline for Pharmacological Therapy for Schizophrenia’ program
Table S5. Detailed comparison of clinical knowledge scores at baseline and after the ‘Treatment Guideline II: Major Depressive Disorder’ program
Acknowledgments
We appreciate the cooperation of all the individuals who participated in this study. This study was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP16dk0307060, and AMED under Grant Number JP19dk0307083, the Health and Labor Sciences Research Grants (H29‐Seishin‐Ippan‐001, 19GC1201), the Japanese Society of Neuropsychopharmacology and the Japanese Society of Mood Disorders. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. American Psychiatric Association Work Group on Psychiatric Evaluation . The American Psychiatric Association Practice Guidelines for the Psychiatric Evaluation of Adults, 3rd edn. American Psychiatric Association, Arlington, VA, 2016. [Google Scholar]
- 2. Keating D, McWilliams S, Schneider I et al Pharmacological guidelines for schizophrenia: A systematic review and comparison of recommendations for the first episode. BMJ Open 2017; 7: e013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauer M, Severus E, Moller HJ, Young AH. WFSBP Task Force on Unipolar Depressive DisordersPharmacological treatment of unipolar depressive disorders: Summary of WFSBP guidelines. Int. J. Psychiatry Clin. Pract. 2017; 21: 166–176. [DOI] [PubMed] [Google Scholar]
- 4. Hasan A, Falkai P, Wobrock T et al World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia ‐ A short version for primary care. Int. J. Psychiatry Clin. Pract. 2017; 21: 82–90. [DOI] [PubMed] [Google Scholar]
- 5. Kennedy SH, Lam RW, McIntyre RS et al Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological Treatments. Can. J. Psychiatry 2016; 61: 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuipers E, Yesufu‐Udechuku A, Taylor C, Kendall T. Management of psychosis and schizophrenia in adults: Summary of updated NICE guidance. BMJ 2014; 348: g1173. [DOI] [PubMed] [Google Scholar]
- 7. Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can. J. Psychiatry 2017; 62: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt SJ, Schultze‐Lutter F, Schimmelmann BG et al EPA guidance on the early intervention in clinical high risk states of psychoses. Eur. Psychiatry 2015; 30: 388–404. [DOI] [PubMed] [Google Scholar]
- 9. Huang CY, Yang SY, Mojtabai R et al Trends of polypharmacy and prescription patterns of antidepressants in Asia. J. Clin. Psychopharmacol. 2018; 38: 598–603. [DOI] [PubMed] [Google Scholar]
- 10. Yang SY, Chen LY, Najoan E et al Polypharmacy and psychotropic drug loading in patients with schizophrenia in Asian countries: Fourth survey of research on Asian prescription patterns on antipsychotics. Psychiatry Clin. Neurosci. 2018; 72: 527–529. [DOI] [PubMed] [Google Scholar]
- 11. Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU. Prevalence and correlates of antipsychotic polypharmacy: A systematic review and meta‐regression of global and regional trends from the 1970s to 2009. Schizophr. Res. 2012; 138: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakagawa A, Williams A, Sado M et al Comparison of treatment selections by Japanese and US psychiatrists for major depressive disorder: A case vignette study. Psychiatry Clin. Neurosci. 2015; 69: 553–562. [DOI] [PubMed] [Google Scholar]
- 13. Ito H, Koyama A, Higuchi T. Polypharmacy and excessive dosing: Psychiatrists' perceptions of antipsychotic drug prescription. Br. J. Psychiatry 2005; 187: 243–247. [DOI] [PubMed] [Google Scholar]
- 14. Uchida H, Suzuki T, Mamo DC et al Survey of benzodiazepine and antidepressant use in outpatients with mood disorders in Japan. Psychiatry Clin. Neurosci. 2009; 63: 244–246. [DOI] [PubMed] [Google Scholar]
- 15. Japanese Society of Neuropsychopharmacology . Guideline for Pharmacological Therapy for Schizophrenia. Igakusyoin, Tokyo, 2015. (in Japanese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Japanese Sociaty of Mood Disorders . Treatment Guideline : Major Depressive Disorder. Igakusyoin, Tokyo, 2012. (in Japanese). [PubMed] [Google Scholar]
- 17. Japanese Sociaty of Mood Disorders . Treatment Guideline II: Major Depressive Disorder. Igakusyoin, Tokyo, 2016. (in Japanese). [Google Scholar]
- 18. Bighelli I, Ostuzzi G, Girlanda F et al Implementation of treatment guidelines for specialist mental health care. Cochrane Database Syst. Rev. 2016. 10.1002/14651858.CD009780.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabana MD, Rand CS, Powe NR et al Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 20. Yang J, Han C, Yoon HK et al Experiences and barriers to implementation of clinical practice guideline for depression in Korea. BMC Psychiatry 2013; 13: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herzog DP, Wagner S, Ruckes C et al Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: A naturalistic study. Eur. Arch. Psychiatry Clin. Neurosci. 2017; 267: 711–721. [DOI] [PubMed] [Google Scholar]
- 22. Bachmann CJ, Aagaard L, Bernardo M et al International trends in clozapine use: A study in 17 countries. Acta Psychiatr. Scand. 2017; 136: 37–51. [DOI] [PubMed] [Google Scholar]
- 23. Onishi Y, Hinotsu S, Furukawa TA, Kawakami K. Psychotropic prescription patterns among patients diagnosed with depressive disorder based on claims database in Japan. Clin. Drug Investig. 2013; 33: 597–605. [DOI] [PubMed] [Google Scholar]
- 24. Buonocore M, Bosia M, Baraldi MA et al Achieving recovery in patients with schizophrenia through psychosocial interventions: A retrospective study. Psychiatry Clin. Neurosci. 2018; 72: 28–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Flow chart of the study participants. During the 18‐month study period, there were 445 participants in the ‘Guideline for Pharmacological Therapy for Schizophrenia’ and 433 participants in the ‘Treatment Guideline II: Major Depressive Disorder’ program. The participants represented over 90 medical institutions. Of these participants, we used the data of 344 in the final analysis.
Fig. S2 Distribution of age and years of professional experience. Distribution of demographics. (a) Age. (b) Years of professional experience. The Kolmogorov–Smirnov test was conducted. Normal distribution was not observed for (a) and (b) (A: P = 6.3 × 10−20, B: P = 5.5 × 10−59).
Fig. S3 The distribution of clinical knowledge scores for years of professional experience in the guidelines for schizophrenia and major depressive disorder. The distribution of clinical knowledge scores for each year of professional experience. (a) ‘Guideline for Pharmacological Therapy for Schizophrenia.’ (b) ‘Treatment Guideline II: Major Depressive Disorder.’ Error bars indicate standard deviation.
Fig. S4 Distribution of the facilities that participated in this study in Japan. Of a total of 82 university hospitals in Japan, approximately half (n = 42) joined the EGUIDE project (10 March 2019).
Table S1. Questions regarding knowledge about the pharmacotherapy of schizophrenia
Table S2. Questions regarding knowledge about the management of major depressive disorder
Table S3. Participant demographics
Table S4. Detailed comparison of clinical knowledge scores at baseline and after the ‘Guideline for Pharmacological Therapy for Schizophrenia’ program
Table S5. Detailed comparison of clinical knowledge scores at baseline and after the ‘Treatment Guideline II: Major Depressive Disorder’ program


