Summary
Posaconazole is indicated for prophylaxis and treatment of invasive aspergillosis. Therapeutic drug monitoring (TDM) of posaconazole is used to optimise drug exposure. The aim of this study was to analyse and describe the TDM practices and exposure of posaconazole tablets. Patients who received posaconazole for treatment or prophylaxis of fungal infections were included in the study. The following therapeutic window was defined: if concentration was low (<0.7 mg/L for prophylaxis or < 1.5 mg/L for treatment) or high (>3.75 mg/L), the hospital pharmacist provided the physician with dosage advice, which implementation to patient care was analysed. A longitudinal analysis was performed to analyse if different confounding variables had an effect on posaconazole concentrations. Forty‐seven patients were enrolled resulting in 217 posaconazole trough concentrations. A median of 3 (IQR 1‐7) samples was measured per patient. The median concentration was 1.7 mg/L (IQR 0.8‐2.7) for prophylaxis and 1.76 mg/L (IQR 1.3‐2.3) for treatment. Overall, 78 posaconazole concentrations were out of the therapeutic window. For 45 (54%) of these concentrations, a dosage change was recommended. In the longitudinal analysis, the laboratory markers and patient baseline variables did not have an effect on posaconazole concentrations. Adequate posaconazole exposure was shown in 64% (affected 28 patients) of the measured concentrations. TDM practice of posaconazole can be improved by increasing the implementation rate of dose recommendation by a multidisciplinary antifungal stewardship team.
Keywords: clinical pharmacy, haematological malignancies, invasive fungal infections, longitudinal analysis, pharmacist, pharmacokinetics, posaconazole, therapeutic drug monitoring
1. INTRODUCTION
Invasive fungal infections (IFIs) are still the most common infection‐related causes for death among immunocompromised patients1, 2. Haematopoietic stem cell transplant recipients (HSCT), solid organ transplant recipients and other immunocompromised patients are at risk for fungal infections1. According to most recent Infectious Diseases Society of America (IDSA) Aspergillosis and Candidemia guidelines, azoles (voriconazole, posaconazole, fluconazole, isavuconazole and itraconazole), liposomal amphotericin B, micafungin and caspofungin are suggested for either treatment or prophylaxis of IFIs3, 4.
Posaconazole is active against a wide spectrum of pathogens including Candida species, Aspergillus species and zygomycetes5. This has led to posaconazole being used for prophylaxis and treatment of fungal infections6, 7, 8. However, posaconazole plasma concentrations may be influenced by other medications and diet, especially when posaconazole suspension is used9, 10, 11, 12. Additionally, related to the clinical condition of the patient, the physiological status of these patients can have an impact on pharmacokinetics of different drugs. For instance, there can be a change in the volume of distribution during fluid therapy and metabolism or clearance of drugs during hepatic and renal function disorders13. A significant variation of posaconazole concentrations has been reported between and within patients9, 10.
Therapeutic drug monitoring (TDM) is recommended in guidelines for treatment optimisation for posaconazole and other azoles like voriconazole and itraconazole2, 3. TDM can be recommended based on an exposure‐response relationship14 and association of higher drug concentrations with better outcome in daily practice6, 7, 15. For posaconazole, there is considered to be clinical benefit from TDM as posaconazole concentrations show large inter‐ and intra‐patient variability, especially when the suspension is used9, 16, 17.
In contrast to the suspension, currently used posaconazole tablets and intravenous infusion are expected to result in more stable posaconazole concentrations18, 19. TDM of posaconazole has been performed for several years20, 21, 22, 23, but the quality of TDM (application to clinical practice, dose alteration recommendations by pharmacists, optimal timing of measurements) and its implication to clinical practice has not been extensively addressed in studies as it has been for voriconazole24. Also, there is minimal information available on the potential benefit of TDM in clinical practice for the newer drug formulations. Therefore, TDM of posaconazole has continued to be a subject of debate25, 26. A recent study investigated the effect of inflammation reflected by C‐reactive protein (CRP) on posaconazole metabolism27. It was concluded that CRP does not affect posaconazole exposure. However, other laboratory markers may be associated with altered drug exposure. For instance, due to chemotherapy, concomitant medications can cause liver function disorders which affect the pharmacokinetic processes like absorption, distribution, elimination, metabolism, which can lead to changes in posaconazole exposure28. Analysing potential effect of routine laboratory markers can help defining the appropriate population for TDM of posaconazole.
The aim of this study was to evaluate the TDM practice in haematologic patients of posaconazole after the introduction of the new drug formulations and give recommendations for improvement of routine clinical practices of TDM. Additionally, we analysed if the routine laboratory measurements have effect on posaconazole concentrations.
2. MATERIAL AND METHODS
A post hoc analysis was performed from a prospective observational study conducted between August 2015 and June 2017 in the University Medical Center Groningen (UMCG), the Netherlands27. Patients (aged ≥ 18 years) with haematological malignancies, who received intravenous and/or oral posaconazole for treatment, or (primary and secondary) prophylaxis of fungal infections were included in the study.
The study was reviewed by the local ethics committee and received approval (Institutional Review Board 2013‐491). A written informed consent for collection of the medical data was obtained from each enrolled patient.
For every patient, information about posaconazole administration was recorded and included posaconazole dose, indication for posaconazole (treatment or prophylaxis), route of administration (oral or intravenous), time of administration, day after treatment initiation with posaconazole and posaconazole serum concentration. In addition, we collected laboratory analysis C‐reactive protein (CRP), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ‐GT (gamma‐glutamyltransferase) and bilirubin values. The blood samples for measuring posaconazole serum concentrations were collected for routine care, and concentrations were measured using a validated liquid chromatography‐tandem mass spectrometry assay29. In addition, other patient data including age, gender, height, underlying disease were collected.
During daily treatment with posaconazole, dosages were increased if predose trough concentrations were too low (ie < 0.7 mg/L for prophylaxis or < 1.5 mg/L for treatment) or decreased if predose trough concentrations were too high (>3.75 mg/L, both treatment and prophylaxis); however, no upper toxicity threshold for posaconazole levels is known15, 30. For this study, steady state was assumed on day 6 with a loading dose and on day 10 without a loading dose31. The concentrations obtained prior to steady state were not included in the longitudinal analysis. The samples that were not at steady state were used to analyse TDM practices of our hospital.
The recommendations including dosage advice given by the clinical pharmacist if the posaconazole concentrations were out of the therapeutic range were collected from the electronic prescribing and laboratory information systems. To determine intra‐patient variability in posaconazole plasma concentrations, patients who had more than one trough concentration measured were included in this subgroup analysis.
For the analysis of TDM practices, it was documented if a recommendation was provided when posaconazole concentrations were out of the therapeutic window. Additionally, the overall number of recommendations provided and how many of these required a dosage change were summarised. When a recommendation to change the dose was followed by an actual dose change, this was considered as a successful implementation into patient care.
For patients who received posaconazole for prophylaxis, occurrence of a breakthrough invasive fungal infection was documented. For all patients (receiving posaconazole for prophylaxis and treatment), 28‐day and 12‐week overall survival was documented, to analyse short‐ and long‐term survival. It was taken into account that optimum IFI treatment duration is 6‐12 weeks.3
Numerical variables were summarised with medians and interquartile range, while categorical variables were summarised by frequencies and percentages. The longitudinal data on posaconazole concentration were analysed with a random intercept model for subjects. For the longitudinal analysis, we included only steady‐state concentrations as defined in our prospective study27. The baseline variables gender, age, route of administration and dose, as well as the time‐varying variables ALP, ALT, AST, γ‐GT, bilirubin and CRP were included as independent variables. The Wald‐type test statistic was applied to test for the null hypothesis (α = 0.05) that the independent variables do not contribute to the posaconazole concentration. Multiple imputation, using predictive mean matching on all variables in the mixed model and 20 imputation data sets, was applied as sensitivity analysis. Pooled estimates were obtained using Rubin's rule. The analyses were conducted with SAS version 9.4.
3. RESULTS
3.1. Patient characteristics
Between August 2015 and June 2017, 47 patients with a median age of 62 (IQR 56‐67) were enrolled in this study and 217 posaconazole samples were available for analysis for TDM practices and 182 samples for longitudinal analysis. Seven samples were excluded for further analysis as posaconazole was not detectable (<0.1 mg/L) because the drug was stopped before that time, and one sample for one patient because of missing start date.
Most common underlying disease was acute myeloid leukaemia (AML, 61%), and the majority of patients (70%) received posaconazole for prophylaxis. Posaconazole modified release (MR) tablets were the main drug formulation used (89%), and five patients (11%) had treatment with both intravenous infusion which was followed by MR tablet throughout the study. Almost half (49%; 23/47) of the patients received a loading dose of 300 mg two times daily on the first day of treatment. The median daily dose for all measured concentrations was 4.1 mg/kg (IQR 3.5‐6.1), two patients were on dose 200 mg/day (prophylaxis), 33 patients were on dose 300 mg/day (26 prophylaxis, seven treatment), one patient was on dose 600 mg/day (treatment) and for 11 patients (four prophylaxis, seven treatment) the doses varied throughout treatment period. Other patient characteristics are described in Table 1. Figure 1 shows first and subsequent posaconazole concentrations.
Table 1.
Patient characteristics (n = 47)
| Characteristic | No. (%) of patients or median (IQR) | |
|---|---|---|
| Prophylaxis | Treatment | |
| Gender | ||
| Male | 17 (36) | 10 (21) |
| Age (years) | 62 (57‐68) | 60 (52‐67) |
| BMI (kg/m2) | 24.5 (23.5‐27.7) | 24.4 (21.7‐26.6) |
| Underlying conditions | ||
| AML | 19 (40) | 11 (24) |
| MDS | 7 (15) | 1 (2) |
| Othera | 7 (15) | 2 (4) |
| Stem cell transplantation | ||
| Allogeneic | 14 (30) | 5 (11) |
| Autologous | 2 (4) | 0 |
| No transplantation | 17 (36) | 9 (19) |
Abbreviations: AML,acute myeloid leukaemia; BMI, body mass index; MDS, myelodysplastic syndrome.
Other includes X‐linked gammaglobulinemia, T‐cell prolymphocytic leukaemia, follicular lymphoma, chronic myelomonocytic leukaemia, Burkitt's lymphoma, blastic plasmacytoid dendritic cell neoplasm, enteropathy‐associated T‐cell lymphoma type 2, systemic mastocytosis, primary cutaneous T‐cell lymphoma, aplastic anaemia, primary myelofibrosis and acute promyelocytic leukaemia.
Figure 1.
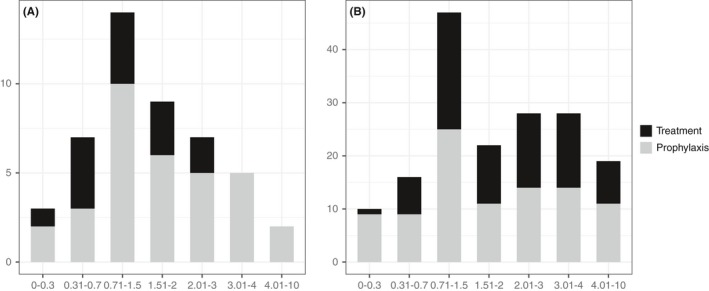
The distribution of initial posaconazole trough concentrations (n = 47 panel A) and the distribution of subsequent posaconazole trough concentrations (n = 170 panel B)
3.2. Analysis of TDM practices
For 212 (98%) posaconazole concentrations, a recommendation by a clinical pharmacist was given and made available to the physician in the electronic patient records. For 54 (25%) of these samples (31 prophylaxis, 23 curative treatment), a dosage change was recommended. However, dose recommendations were implemented in only 39% (10 prophylaxis, 11 treatment) of the cases. For six samples, we did not have follow‐up dosing.
The other dosages that were not changed (n = 27) can be explained by some suggestions given on a Friday or during weekend (n = 5), borderline concentrations 0.5‐0.7 mg/L for prophylaxis and 1.0‐1.5 mg/L for treatment (n = 8), concentrations over 3.75 mg/L as there is no upper toxicity concentration confirmed (n = 7), concentrations measured before day 6, the assumed steady state (n = 2) and other reasons (n = 5).
3.3. Prophylaxis with posaconazole
Thirty‐three patients received posaconazole for prophylaxis (126 posaconazole samples), and 32 of them were on MR tablets only. A median of 2 (IQR 1‐4) blood samples was taken per patient, and the median drug concentration was 1.7 mg/L (IQR 0.8‐2.7), the interpatient variance was 1.53 and standard deviation 1.24. Figure 2 shows intra‐ and interpatient variability for patients who had 5 or more samples measured while being on the same dose.
Figure 2.
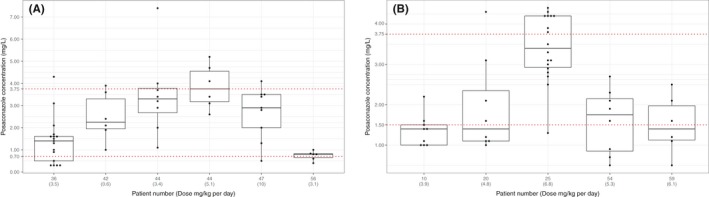
Intra‐and interpatient variability of posaconazole concentrations in prophylaxis (A) and treatment (B) groups, x‐axis presents patient number with the daily dose (mg/kg), y‐axis presents number of samples
Overall, 88 concentrations were within the therapeutic range (0.7‐3.5 mg/L) and 32 outside (16 samples < 0.7 mg/L, 16 samples > 3.75 mg/L). Table 2 presents the samples outside the predefined therapeutic window and posaconazole therapy.
Table 2.
Posaconazole concentrations during prophylaxis and treatment
| 33 (%) patients on prophylaxis | 14 (%) patients on treatment | |
|---|---|---|
| Route of administration | ||
| Oral | 32 (68) | 11 (24) |
| Intravenous and oral | 1 (2) | 3 (6) |
| Loading dose | 17 (36) | 6 (13) |
| Daily dose (mg/kg) | 3.5 (3.4‐4.3) | 5.3 (4.2‐6.8) |
| Number of samples taken per patient | 2 (1‐3.5) | 5.5 (2.75‐9) |
| Number of Posaconazole samples | 120 concentrations obtained for prophylaxis | 90 concentrations obtained for treatment |
| Posaconazole < 0.7 mg/L | 16 (# of samples) | |
| Posaconazole < 1.0 mg/L | 14 (# of samples) | |
| Posaconazole < 1.5 mg/L | 35 (# of samples) | |
| Posaconazole > 3.75 mg/L | 16 (# of samples) | 11 (# of samples) |
| Posaconazole < 0.7 mg/L with loading dose after/on day 6 / without loading dose after/on day 10 | 10 / 4 (# of samples) | |
| Posaconazole < 1.5 mg/L with loading dose after/on day 6/without loading dose after/on day 10 | 13/10 (# of samples) | |
From 33 patients who received posaconazole for prophylaxis, three patients (9%) developed a probable IFI and one (3%) received posaconazole as empiric treatment for IFI (suspected breakthrough IFI). These patients had adequate posaconazole concentrations—all samples measured were over 0.7 mg/L. The detailed description of these patients is presented in Table 3.
Table 3.
Clinical data of the patients who got a probable or possible breakthrough infection
| Pt | Demographic and clinical data | Initial posaconazole trough (mg/L) | Subsequent posaconazole troughs (mg/L) | IFI treatment | Chemotherapy/antimicrobial therapy | Diagnosis of IFI |
|---|---|---|---|---|---|---|
| 1 | 59‐year‐old woman with AML and had received a SCT | 2.37 mg/L | NI | Amphotericin B with caspofungin | Cytarabine (1000 mg/m2)/daunorubicin (60 mg/m2), prednisolone, piperacillin/tazobactam | HRCT: positive changes in the scan, galactomannan antigen serum index: 0.56, galactomannan antigene BAL index 0.35 |
| 2 | 46‐year‐old man with AML | 1.26 mg/L | 2.6 mg/L, 3.2 mg/L | Amphotericin B followed by caspofungin | Cytarabine (1000 mg/m2)/daunorubicin (60 mg/m2), colistin, piperacillin‐tazobactam, vancomycin | HRCT: positive masses in liver, galactomannan antigene serum index: 0.10 |
| 3 | 59‐year‐old man with systemic mastocytosis and had received a SCT | 1.2 mg/L | 2.4 mg/L, 1.8 mg/L | Amphotericin B with caspofungin | Ruxolitinib, cyclosporine, prednisolone, azithromycin | Galactomannan antigene BAL index 4.90 |
Abbreviation: NI, no information.
The mortality rate in the total prophylaxis group was 6% (two patients) after 28 days and 24% (8 patients) after 12 weeks. For the two patients who died after 28 days, adequate posaconazole concentrations (≥0.7 mg/L) were observed. For the eight patients who died after 12 weeks, 6 had adequate posaconazole concentrations (≥0.7 mg/L) and two patients both had one sample measured below 0.7 mg/L. Mortality was not attributed to a fungal infection.
3.4. Treatment with posaconazole
Fourteen patients received posaconazole MR tablets for treatment (91 posaconazole samples) and four received both posaconazole MR tablet followed by intravenous infusion or vice versa during the same treatment period. A median of 6 (IQR 3‐9) samples was taken per patient, and the median drug concentration was 1.76 mg/L (IQR 1.3‐2.3), the interpatient variance was 0.5 and standard deviation 0.71. Figure 2 shows intra‐ and interpatient variability of patients who had 5 or more samples taken.
Forty‐four posaconazole concentrations were within the therapeutic range (1.5‐3.75 mg/L) and 46 outside (35 samples < 1.5 mg/L, 11 samples > 3.75 mg/L). Table 2 presents the samples outside the predefined therapeutic window and posaconazole therapy.
The mortality rate in this group was 14% (two patients) after 28 days and 29% (four patients) after 12 weeks. For the two patients who died after 28 days, adequate posaconazole concentrations (≥1.5 mg/L) were observed. For the four patients who died after 12 weeks, two had adequate posaconazole concentrations (≥1.5 mg/L) and two had some concentrations under the predefined therapeutic concentration (≤1.5 mg/L).
3.5. Longitudinal analysis
The associations of the independent variables on posaconazole concentration together with their 95% confidence interval and the Wald‐type P‐value are provided in Table 4. The results on the original data (with missing data) as well as the pooled estimates from the imputation are given. The original data set contains 127 measurements (from the 182 measurements) with a complete data set.
Table 4.
Results of longitudinal analysis
| Variable | Original data set | Imputed data sets | ||
|---|---|---|---|---|
| Estimate [95%CI] | P‐value | Estimate [95%CI] | P‐value | |
| −0.172 [−1.341; 0.996] | 0.766 | 0.258 [−0.570; 1.084] | 0.542 | |
| Age | 0.032 [−0.020; 0.084] | 0.213 | 0.021 [−0.016; 0.058] | 0.258 |
| Route of administration | 0.322 [−0.848; 1.492] | 0.587 | 0.230 [−0.769; 1.229] | 0.652 |
| Dose | 0.387 [0.247; 0.527] | <0.001 | 0.296 [0.167; 0.425] | <0.001 |
| ALT | 0.006 [0.000; 0.012] | 0.040 | 0.004 [−0.003; 0.010] | 0.266 |
| AST | 0.004 [−0.013; 0.021] | 0.648 | 0.005 [−0.013; 0.023] | 0.575 |
| ALP | −0.001 [−0.008; 0.007] | 0.870 | 0.001 [−0.006; 0.007] | 0.815 |
| γ‐GT | −0.000 [−0.003; 0.003] | 0.939 | −0.000 [−0.003; 0.002] | 0.803 |
| Bilirubin | −0.009 [−0.035; 0.016] | 0.467 | −0.006 [−0.025; 0.012] | 0.506 |
| CRP | 0.001 [−0.004; 0.006] | 0.597 | −0.001 [−0.005; 0.004] | 0.691 |
It is obvious that the dose contributed to the posaconazole concentration. In the analysis of the original data set (with missing data), ALT seemed to contribute to the posaconazole concentration, but this association seemed to disappear when multiple imputation is being used. Multiple imputation showed that subjects who had missing data on ALT had on average a lower ALT value that the subjects from whom we observed ALT data (34.0 vs 51.6). This may suggest that the associations of the independent variables in the original data are somewhat biased.
4. DISCUSSION
The objective of this study was to analyse routine TDM practices of posaconazole. Our study showed that variability in drug exposure is still present. Posaconazole concentrations might be affected by treatment setting—some patients were treated in an outpatient setting. However, some of these patients suffered from graft‐vs‐host disease, which can compromise the absorption of posaconazole32. Variability of posaconazole Cmin (MR tablet) was also described in a recent study on lung transplant recipients33. However, we did see an increase of median posaconazole concentrations compared to a previous study done in our centre with posaconazole suspension. In that study, the median posaconazole concentration was 0.9 mg/L, and in our study, it was 1.7 mg/L (prophylaxis) and 1.76 mg/L (treatment). For most patients in van Elst et al study, the patients received mostly 600 mg/day (84%) for prophylaxis and 800 mg/day (80%) for treatment. In this study, 50% of the treatment group and 79% of the prophylaxis group received 300 mg of posaconazole per day9. So, we did see a better exposure with posaconazole tablet and intravenous formulation compared with the suspension. Lenczuk and colleagues also have shown that posaconazole concentrations are more likely to be in the therapeutic range when patients are being treated with posaconazole modified release tablet34.
Posaconazole concentrations have also been described to be affected by diarrhoea, body weight, male gender, use of PPIs and steroids35, 36. Our longitudinal analysis did not confirm the effect of weight and gender on posaconazole concentrations. We also did not see a change of AST levels, although posaconazole treatment is connected with liver function abnormalities37. On the other hand, it has also been presented previously that liver function markers like ƴ‐GT, ALP and ALT were not connected to higher posaconazole concentrations37. A limitation of our analysis is the fact that we did not analyse the effect of diarrhoea and use of PPIs and steroids and that we included patients of a previous study, which is a part of all measured posaconazole concentrations during the study period thus does not represent the whole patient population. On the other hand, the characteristics of our data set are somewhat similar to other studies describing posaconazole exposure in patients with haematological malignancies36, 37. The novelty of our study compared to earlier studies is the longitudinal analysis, which is taking into account the day of treatment and the time between measurements, also including all samples that have been collected for each patient34, 35, 36. The advantage of using longitudinal analysis over univariate and multivariate analysis that have been used by earlier studies is that this type of analysis better values the effect of measurements over time.
We cannot see a relationship between low posaconazole concentrations and mortality rates. Additionally, this data set is too small to show that low concentrations have an effect on outcomes, especially as we did not determine IFI‐attributable deaths. For treatment of IFIs, higher posaconazole plasma concentrations must be obtained26. In this study, over half of the concentrations measured for IFI treatment were below the therapeutic range (<1.5 mg/L). For patients receiving posaconazole as treatment, significantly more samples were taken per patient compared with patients receiving posaconazole as prophylaxis. On the other hand, in this study, the defined therapeutic concentration (≥1.5 mg/L) used for treatment of IFIs was higher than previously reported (≥1‐1.25 mg/L) to prevent antifungal resistance and to cover all strains15, 26. This caused more posaconazole concentrations to be out of the therapeutic window. If the therapeutic concentration of ≥ 1 mg/L was used, more concentrations would have been within the range (21 samples).
Our data set is too small to draw firm conclusions, although most patients receiving posaconazole as prophylaxis, and who had a breakthrough infection, had a therapeutic posaconazole concentration. However, three patients who received posaconazole for treatment did not have sufficient drug concentrations even when a loading dose was administered. Perhaps, administering a double dose for more than 1 day when posaconazole is used for treatment of IFIs should therefore be considered.
A suggestion for dose alteration was only followed for 39% of recommendations made. The reasons behind non‐implementation could have been due to borderline concentrations, and samples over 3.75 mg/L as posaconazole toxic concentration has not been confirmed in literature nor by the manufacturer31. Posaconazole practices were analysed before in conjunction with effect of concomitant medications, diet, concomitant chemotherapy and other variables.38 Additionally, in that study approximately for 20% of patients’ dosage changes were done, which led to more therapeutic concentrations. It was suggested that there is a benefit of TDM when using posaconazole suspension as it had insufficient exposure. In this analysis, we show that a quarter of posaconazole concentrations receive a suggestion for dosage change. Knowing that most of the patients received the oral formulation (tablet or suspension), we observed that TDM is still beneficial in this patient group. The overall results of this study and specific cases should be discussed in a multidisciplinary expert panel to avoid unnecessary orders and improve overall TDM practices taking into account different reasons behind non‐implementation. Currently, the recommendations are documented into an electronic system and retrieved by the attending physician. To improve the communication between physicians and pharmacists, an attending clinical pharmacist may be necessary, who would provide face‐to‐face consultations, thus aiding in preventing medication‐related errors and reducing costs39, 40, 41.
Furthermore, antimicrobial stewardship teams are widely initiated in hospitals worldwide and it has been suggested that these teams should also include a pharmacist39. The pharmacist could aid in choosing the best drug formulation to use, consult on appropriate empirical and prophylactic approaches, promote switching from intravenous to oral antimicrobials, analyse drug interactions and provide information about pharmacokinetics and TDM including prescribing of the new dose based on the TDM results42. Besides this, the team should be advising appropriate antimicrobial therapy taking the specific patient and condition, documenting and analysing resistance patterns into account40, 43.
5. CONCLUSIONS
Adequate posaconazole exposure was shown in 64% (affected 28 patients) of the measured concentrations. There was still an important variability present in posaconazole exposure; however, in the longitudinal analysis from all the confounders, only dose had a significant effect on posaconazole concentrations.
Even though posaconazole concentrations varied and recommendations were not always implemented to patient care, a large proportion of trough concentrations lied within the therapeutic range and did not need a recommendation at all. The communication between the clinical pharmacist and the attending physician should be enhanced to achieve better results in TDM practices. Close collaboration in a multidisciplinary antifungal stewardship team and further education of medical staff is needed to increase adherence to dosage alterations.
AUTHOR CONTRIBUTIONS
LFRS, DT, TSW and JWA designed the study, AGM, AV and MB collected the data, AGM and ERH performed the statistical analysis, AGM, AV, DT, ERH, TSW and JWA led the writing.
ACKNOWLEDGMENTS
This study was supported in part by a research grant from the Investigator Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this article do not necessarily represent those of Merck Sharp & Dohme Corp. Anne‐Grete Märtson was funded by Marie Skłodowska‐Curie Actions, Grant Agreement number: 713660—PRONKJEWAIL—H2020‐MSCA‐COFUND‐2015.
Märtson A‐G, Veringa A, van den Heuvel ER, et al. Posaconazole therapeutic drug monitoring in clinical practice and longitudinal analysis of the effect of routine laboratory measurements on posaconazole concentrations. Mycoses. 2019;62:698‐705. 10.1111/myc.12948
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
REFERENCES
- 1. Segal BH. Aspergillosis. N Engl J Med. 2009;360(18):1870‐1884. [DOI] [PubMed] [Google Scholar]
- 2. Drew RH, Townsend ML, Pound MW, Johnson SW, Perfect JR. Recent advances in the treatment of life‐threatening, invasive fungal infections. Expert Opin Pharmacother. 2013;14(17):2361‐2374. [DOI] [PubMed] [Google Scholar]
- 3. Patterson TF, Thompson George R III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1‐e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1‐e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torres HA, Hachem RY, Chemaly RF, Kontoyiannis DP, Raad II. Posaconazole: a broad‐spectrum triazole antifungal. Lancet Infect Dis. 2005;5(12):775‐785. [DOI] [PubMed] [Google Scholar]
- 6. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348‐359. [DOI] [PubMed] [Google Scholar]
- 7. Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft‐versus‐host disease. N Engl J Med. 2007;356(4):335‐347. [DOI] [PubMed] [Google Scholar]
- 8. Ullmann AJ, Cornely OA, Burchardt A, et al. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother. 2006;50(2):658‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Elst KCM, Brouwers CHS, van den Heuvel ER, et al. Subtherapeutic posaconazole exposure and treatment outcome in patients with invasive fungal disease. Ther Drug Monit. 2015;37(6):766‐771. [DOI] [PubMed] [Google Scholar]
- 10. Lindsay PJ, Bond SE, Norris R, Marriott DJE, Miyakis S. Posaconazole therapeutic drug monitoring in a regional hospital setting. Ther Drug Monit. 2016;38(6):804‐807. [DOI] [PubMed] [Google Scholar]
- 11. Alffenaar J‐WC, van Assen S, van der Werf TS, Kosterink JGW, Uges DRA. Omeprazole significantly reduces posaconazole serum trough level. Clin Infect Dis. 2009;48(6):839. [DOI] [PubMed] [Google Scholar]
- 12. Bruggemann RJM, Alffenaar J‐WC, Blijlevens NMA, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48(10):1441‐1458. [DOI] [PubMed] [Google Scholar]
- 13. Felton TW, Hope WW, Roberts JA. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis. 2014;79(4):441‐447. [DOI] [PubMed] [Google Scholar]
- 14. Andes D, Marchillo K, Conklin R, et al. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob Agents Chemother. 2004;48(1):137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2‐12. [DOI] [PubMed] [Google Scholar]
- 16. Dolton MJ, Ray JE, Marriott D, McLachlan AJ. Posaconazole exposure‐response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob Agents Chemother. 2012;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dolton MJ, Bruggemann RJM, Burger DM, McLachlan AJ. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob Agents Chemother. 2014;58(11):6879‐6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maertens J, Cornely OA, Ullmann AJ, et al. Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob Agents Chemother. 2014;58(7):3610‐3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kraft WK, Chang PS, van Iersel MLPS, Waskin H, Krishna G, Kersemaekers WM. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother. 2014;58(7):4020‐4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lebeaux D, Lanternier F, Elie C, et al. Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob Agents Chemother. 2009;53(12):5224‐5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neubauer WC, Engelhardt M, Konig A, et al. Therapeutic drug monitoring of posaconazole in hematology patients: experience with a new high‐performance liquid chromatography‐based method. Antimicrob Agents Chemother. 2010;54(9):4029‐4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dolton MJ, Ray JE, Chen SC‐A, Ng K, Pont L, McLachlan AJ. Multicenter study of posaconazole therapeutic drug monitoring: exposure‐response relationship and factors affecting concentration. Antimicrob Agents Chemother. 2012;56(11):5503‐5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shields RK, Clancy CJ, Vadnerkar A, et al. Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob Agents Chemother. 2011;55(3):1308‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luong M‐L, Al‐Dabbagh M, Groll AH, et al. Utility of voriconazole therapeutic drug monitoring: a meta‐analysis. J Antimicrob Chemother. 2016;71(7):1786‐1799. [DOI] [PubMed] [Google Scholar]
- 25. Stott KE, Hope WW. Therapeutic drug monitoring for invasive mould infections and disease: pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother. 2017;72(suppl 1):i12‐i18. [DOI] [PubMed] [Google Scholar]
- 26. Dekkers BGJ, Bakker M, van der Elst KCM, et al. Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep. 2016;10:51‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martson A‐G, Veringa A, Bakker M, et al. Posaconazole trough concentrations are not influenced by inflammation: a prospective study. Int J Antimicrob Agents. 2019;53:325‐329. [DOI] [PubMed] [Google Scholar]
- 28. Bϋdingen FV, Gonzalez D, Tucker AN, Derendorf H. Relevance of liver failure for anti‐infective agents: from pharmacokinetic alterations to dosage adjustments. Ther Adv Infect Dis. 2014;2(1):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alffenaar JWC, Wessels AMA, van Hateren K, Greijdanus B, Kosterink JGW, Uges DRA. Method for therapeutic drug monitoring of azole antifungal drugs in human serum using LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(1):39‐44. [DOI] [PubMed] [Google Scholar]
- 30. Cornely OA, Duarte RF, Haider S, et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother. 2016;71(3):718‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merck S, Dohme BV. Merck, Sharp & Dohme Ltd. Noxafil (posaconazole) ‐ summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000610/WC500037784.pdf. Published 2017. Accessed December 11, 2017.
- 32. Akpek G, Chinratanalab W, Lee LA, et al. Gastrointestinal involvement in chronic graft‐versus‐host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9(1):46‐51. [DOI] [PubMed] [Google Scholar]
- 33. Jeong W, Snell GI, Levvey BJ, et al. Single‐centre study of therapeutic drug monitoring of posaconazole in lung transplant recipients: factors affecting trough plasma concentrations. J Antimicrob Chemother. 2018;73(3):748‐756. [DOI] [PubMed] [Google Scholar]
- 34. Lenczuk D, Zinke‐Cerwenka W, Greinix H, et al. Antifungal prophylaxis with posaconazole delayed‐release tablet and oral suspension in a real‐life setting: plasma levels, efficacy, and tolerability. Antimicrob Agents Chemother. 2018;62(6):698‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses. 2015;58(7):432‐436. [DOI] [PubMed] [Google Scholar]
- 36. Cojutti PG, Candoni A, Lazzarotto D, et al. Co‐administration of proton pump inhibitors and/or of steroids may be a risk factor for low trough concentrations of posaconazole delayed‐released tablets in adult patients with haematological malignancies. Br J Clin Pharmacol. 2018;84(11):2544‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boglione‐Kerrien C, Picard S, Tron C, et al. Safety study and therapeutic drug monitoring of the oral tablet formulation of posaconazole in patients with haematological malignancies. J Cancer Res Clin Oncol. 2018;144(1):127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Elst KCM, Brouwers CHS, van den Heuvel ER, et al. Subtherapeutic posaconazole exposure and treatment outcome in patients with invasive fungal disease. Ther Drug Monit. 2015;37(6):66‐771. [DOI] [PubMed] [Google Scholar]
- 39. Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society . Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322‐327. [DOI] [PubMed] [Google Scholar]
- 40. Ruhnke M. Antifungal stewardship in invasive Candida infections. Clin Microbiol Infect. 2014;20(Suppl 6):11‐18. [DOI] [PubMed] [Google Scholar]
- 41. Cappelletty D, Jacobs D. Evaluating the impact of a pharmacist's absence from an antimicrobial stewardship team. Am J Health Syst Pharm. 2013;70(12):1065‐1069. [DOI] [PubMed] [Google Scholar]
- 42. Alhameed AF, Al Khansa S, Hasan H, Ismail S, Aseeri M. Bridging the gap between theory and practice; the active role of inpatient pharmacists in therapeutic drug monitoring. Pharmacy. 2019;7(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wickens HJ, Farrell S, Ashiru‐Oredope DAI, Jacklin A, Holmes A. The increasing role of pharmacists in antimicrobial stewardship in English hospitals. J Antimicrob Chemother. 2013;68(11):2675‐2681. [DOI] [PubMed] [Google Scholar]


