Abstract
Background
The traditional Japanese herbal medicine, daikenchuto (DKT), has been used to treat constipation and postoperative ileus. However, the precise mechanisms involved in the pharmacological effects of DKT remain uncertain. The aim of this study was to clarify the effect of DKT on motor patterns and transit activity in the isolated rat colon.
Methods
The entire colon or segments of the proximal colon in rats were isolated and placed in Krebs solution. The motility of the colon was evaluated by analyzing spatiotemporal maps of diameter derived from video imaging and measuring the intraluminal pressure in the anal end of the proximal colon, and the transit time of a plastic bead through the entire isolated colon.
Key Results
Several types of propagating contractions were observed in the isolated entire colon. When DKT was added to Krebs solution, the frequency of large‐extent anal propagating contractions increased. DKT treatment increased the intraluminal pressure in the isolated proximal colon, which was related to the propagating contractions. This effect was abolished by treatment with the neural blocker tetrodotoxin. These findings suggest DKT induced peristaltic contractions in the isolated colon. DKT accelerated colonic transit activity, which was related to peristaltic contractions induction in the colon. These effects were also observed in the colons treated with bethanechol and the active ingredient of DKT, hydroxy‐α‐sanshool.
Conclusions and Inferences
Daikenchuto could enhance colonic transit activity by inducing peristaltic contractions, which may be mediated by the activation of the enteric nervous system in the colon.
Keywords: Colon, daikenchuto, hydroxy‐α‐sanshool, peristaltic contraction, postoperative ileus
The colonic motor pattern on spatiotemporal maps of diameter of the entire isolated rat colon. Before drug treatment, various kinds of propagating contractions were observed. After 200 µg/mL daikenchuto (DKT) and 6 μmol/L bethanechol treatment, large‐extent anal propagating contractions were induced in the entire colon.

Key Points.
Daikenchuto (DKT) has been used to treat constipation and postoperative ileus. This study clarifies the effect of DKT on motor patterns and transits activity in the isolated rat colon.
Daikenchuto induced peristaltic contraction via enteric nervous system in rat colon and increased colonic transit activity.
The propulsive motor effect of DKT may contribute to the improvement of constipation and postoperative ileus in the colon.
1. INTRODUCTION
Colonic dysmotility presents with abdominal symptoms in diseases such as functional constipation and postoperative ileus (POI).1 Patients with constipation can exhibit slow small bowel and colonic transit, and delayed gastric emptying. However, gastrointestinal transit disorder in these patients primarily reflects delayed whole or segmental colon transit.2 Multiple drugs exist with therapeutic effects on colonic dysmotility in constipated patients. Laxatives and selective serotonin 4 receptor agonists may stimulate bowel movements. Novel drugs, including intestinal chloride channel activators and ileal bile acid transporter inhibitors, increase intestinal water content, causing accelerated colonic transit. However, these have limited efficacy because of undesirable side effects or no underlying pathophysiological targets.3
Herbal medicines have been used in Japan for nearly 1500 years. Currently, traditional Japanese herbal medicines (Kampo medicines) are completely integrated into the modern healthcare system in Japan. Daikenchuto (DKT) is a Kampo medicine used to treat constipation and POI.4 Numerous studies conducted in Japan and the United States have provided clinical evidence of DKT's effect on colonic transit and POI.4, 5, 6, 7 A randomized double‐blind placebo‐controlled study of 60 healthy subjects conducted at the Mayo Clinic revealed that DKT significantly accelerated ascending colonic emptying to a greater extent than a placebo.5 A systematic review and meta‐analysis of seven studies involving 1134 patients showed that DKT relieved POI in patients undergoing surgery for gastrointestinal cancer.4, 8
Regarding the prokinetic effect of DKT, in animal studies, DKT relieved POI,9 which is related to hydroxy‐α‐sanshool (HAS) contained in DKT. Our previous study demonstrated that HAS induced propulsive movements in the isolated rat proximal colon.10 The effect of HAS could be mediated by blocking the potassium channels, potassium two‐pore domain channel subfamily K member (KCNK) 3 in the longitudinal muscle layer, and KCNK 9 in the myenteric plexus of rat colons.10, 11 KCNK channels play crucial roles in maintaining resting membrane potentials in various cell types, suggesting that KCNK channel blockers could regulate the excitability of cells, such as smooth muscle cells and neurons, by triggering membrane depolarization.12 However, the effect of DKT on colonic motility remains unclear.
Colonic motor patterns in humans and animals are evaluated using several techniques. A high‐resolution manometry has been developed for recording motor patterns in the colon in vivo.13, 14, 15, 16 Meanwhile, motor patterns in isolated colon preparations of animals have been evaluated by spatiotemporal maps of changes in colonic diameter (DMaps) analysis.17, 18 Costa et al and others identified several types of propagating contractions in the isolated colon of animals using DMaps.18, 19, 20 Therefore, DMaps analysis could be useful to reveal the characteristic of colonic motor patterns induced by drug treatments. Hence, we examined the effect of DKT on motor patterns and transit activity in an isolated rat colon.
2. MATERIALS AND METHODS
2.1. Animals
Male Sprague Dawley (SD) rats aged 7‐14 weeks (Charles River Laboratories) were used (n = 63). The rats were housed in a room with controlled temperature and humidity under a 12‐hours (07:00‐19:00 hours) light/dark cycle, with free access to food and water. All experimental procedures were approved by the Experimental Animal Ethics Committee of Tsumura & Co. and were performed according to the institutional guidelines for the care and use of laboratory animals, which is in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Chemicals and drugs
Daikenchuto formulations consist of a mixture of a medicinal herbal extract powder (1.25 g) and maltose (10 g). We used the herbal extract powder included in DKT, which is obtained by spray drying a hot water extract of a mixture of three crude drugs: 5.0 g of processed ginger (Zingiberis processum rhizoma), 3.0 g of ginseng (Ginseng radix), and 2.0 g of Japanese pepper (Zanthoxylum fruit). HAS contained in the Japanese pepper was supplied by Tsumura & Co. (Tokyo Japan). Carbamyl‐β‐methylcholine chloride (bethanechol, Sigma‐Aldrich), tetrodotoxin (TTX, Wako Pure Chemical Industries, Ltd.), and the other chemicals were commercially obtained.
2.3. Experimental setup
Overnight‐fasted rats were killed by decapitation, and the colon was subsequently removed. Natural fecal contents in the preparations were removed by gentle flushing from the oral end of the colon with warmed Krebs solution. As reported in our previous study,10 the entire colon and proximal colon were placed in an organ bath (100 mL volume), which was continuously perfused with Krebs solution (5% CO2 and 95% O2 (v/v); pH 7.3‐7.4; 3.5 mL/min) at 34‐36°C. The oral and anal ends of the colon were cannulated by a flow tube filled with saline. Saline was infused from the oral side to the anal side at a rate of 0.15 mL/min. To initiate contractions, the colon was exposed to an intraluminal pressure load of approximately 4 cmH2O by elevating the drain tube on the anal side. Our previous study demonstrated that the increase in intraluminal pressure induced concomitantly with contractions were observed during the 2‐hour period after the beginning of the experiment; thereafter, with an accompanying decrease in the intraluminal pressure levels, the contractions were stably detected until 8 hours on our experimental setup.10 Therefore, to analyze the motor activity for a maximum time of 100 minutes in a stable condition, the colon was left to equilibrate for 120‐240 minutes before the experiment was started. Each experiment was recorded by a video camera, and the motility was observed via video images (DCR‐SR87 or HDR‐PJ670, SONY).
2.4. Sample preparation
Daikenchuto (20 mg) dissolved in a saline solution (0.1 or 0.2 mL) was added to the Krebs solution (100 mL) on the serosal side in the organ bath, because almost all of the ingredients of DKT administered orally are rapidly absorbed from the small intestine and not transported into the colonic lumen.10, 11 HAS (0.3 µmol) was dissolved in dimethyl sulfoxide (6 µL) and added to the Krebs solution (100 mL) on the serosal side in the organ bath. The saline solution of bethanechol (0.6 µmol/12 µL) and TTX (0.03 µmol/6 µL) were also added to the Krebs solution (100 mL) on the serosal side in the organ bath.
2.5. Experimental protocol
In 14 preparations of the proximal colon, after recording 15‐20 minutes of motor activity, DKT (200 µg/mL) was added to the organ bath and 15‐min recordings were continued. Five preparations were followed by a 30‐min recording after washout. In another study, it was followed by a 15‐min recording after treatment with TTX (0.3 µmol/L; n = 4) or a vehicle as sham treatment (n = 5). To analyze the characteristics of propagating contractions, the motor activity in 10 preparations of the entire colon was recorded for 20 minutes before and after DKT (200 µg/mL, n = 5) or bethanechol (6 µmol/L, n = 5) treatment. The effect of TTX (0.6 µmol/L) treatment on propagating contractions in three preparations of proximal colon was examined. Another set of experiments for bead transit were carried out in 36 preparations of the entire colon.
2.6. Construction of spatiotemporal maps of the colonic motion
To create images representing the motor activity of the colon, spatiotemporal maps of diameter (DMaps) were generated according to the method described by Hennig GW et al17 The still images of the colons were sampled every 5 seconds from a video movie. The diameter of the colon (in pixels) was calculated at each point and coded by image intensity using the free nature of NIH's ImageJ software. A contraction event was represented by a bright color, while a relaxation event was represented by a dark color. The intensity was displayed at each point along the length of the colon (image Y‐axis) with respect to the changes over time (image X‐axis), using Microsoft Excel.
2.7. Analysis of colonic motor patterns
In this study, we used DMaps to analyze colonic motor patterns. As in previous reports, 19, 20 a propagating contraction abolished by TTX was identified as a neurogenic contraction. Among these, the contractions extending over almost the entire length of the preparation, each accompanied by peaks in anal pressure, were identified as peristaltic contractions that are also called long distance contractions (LDCs).19 Propagating contractions unaffected by TTX were identified as myogenic ripples.19, 20
The characteristics of propagating contractions were analyzed by measuring the strength of contraction, and the frequency, length, and velocity of propagation. The strength of the contraction was calculated from the change in diameter to maximum diameter of colon. The frequency of propagating events was calculated from the number of propagating contractions that appeared during the 10‐ or 20‐minutes period before and after drug treatment. The propagation length was measured from the distance between the oral side and anal side of the region inducing propagating contractions. The velocity of the propagation calculated as the propagating colon length per time was obtained from least squares analysis.
2.8. Intraluminal pressure
To monitor the intraluminal pressure in cmH2O, the single pressure sensor, a Mikro‐Tip catheter transducer (SPR‐524, Millar Instruments), was placed in the lumen of the anal end of the proximal colon, while it was not used in the entire colon studies to focus on the DMaps analysis of the motor pattern. The intraluminal pressure waves were evaluated using a data acquisition and analysis system (MP100, BIOPAC System). The peak amplitude (PA) was calculated from the mean pressure of the peaks for 15 minutes before and after DKT (200 µg/mL) treatment. The peak frequency (PF) was calculated from the number of pressure peaks over the 15‐minutes period. The area under the curve (AUC) of the pressure waves during the allotted time period was also calculated. The effect of TTX treatment on the intraluminal pressure waves induced by DKT was estimated by the ratios of PA, PF, and AUC before and after TTX treatment.
2.9. Colonic transit
About 180 minutes after the isolated entire colon was placed into the organ bath, incisions approximately 6 mm in length were made in the oral and anal side colon segments. Plastic beads of 5.9 mm diameter were inserted into the oral side of the colon segments. The transit of the plastic beads from the oral to the anal end was monitored using a video image recorder. Colonic transit time was examined using a cutoff value of 100 minutes because there were specimens in which the pellet failed to travel along the length of the colon. The bead run was tested twice before and after drug treatment in 36 preparations of the entire colon. After adding DKT, bethanechol, and HAS to the organ bath, the mean transit time (sec/mm), measured as the time from the initial movement of the bead to when it reached the anal side, was compared with that of each preparation before drug treatment.
2.10. Data analysis
All values are expressed as means ± SE. The statistically significant difference between before and after the drug treatment was evaluated using the paired t test. Significant difference between the two groups was evaluated using the F test analysis of variance, followed by Student's t test or Aspin‐Welch's t test. A probability <.05 was considered statistically significant.
3. RESULTS
3.1. Motor activity in the isolated proximal colons
Representative serial photographs of motility in untreated and DKT‐treated proximal colons are shown in Figure 1A. The movie data are shown in Video S1. In the absence of treatment, distension with saline at 0.15 mL/min elicited regional constrictions in the direction of the circular muscle of the isolated proximal colon (Figure 1A, left). When DKT (final concentration, 200 μg/mL) was added to the Krebs solution on the serosal side, an overall constriction was induced, which appeared to coordinate from the oral to the anal side (Figure 1A, right).
Figure 1.
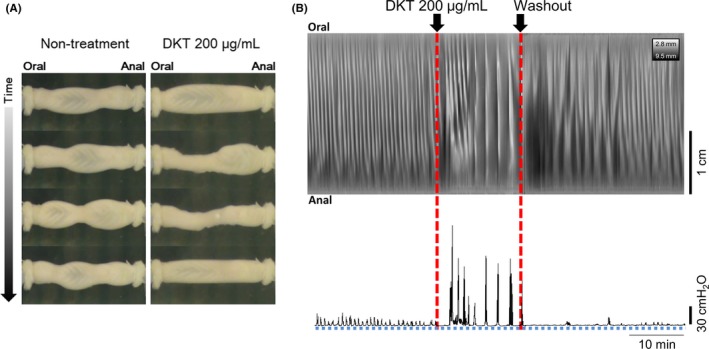
Changes in the motor activity after DKT treatment in the isolated rat proximal colons. A proximal colonic specimen was placed in the bath, and the motility and intraluminal pressure were recorded. A, Serial photographs of motility in the untreated (left) and DKT (200 μg/mL)‐treated (right) colons in the representative experiment. B, Relationship between the contraction pattern on the spatiotemporal map of diameter (DMap) and the intraluminal pressure chart before (no treatment) and after DKT treatment, and following washout, in the rat proximal colon. Propagating contractions with small pressure peaks were observed before DKT treatment, whereas propagating contractions with marked increases in internal pressure were induced by DKT treatment. The response disappeared after a washout of the DKT. The contraction or relaxation site is indicated by white or black colors on the DMap, respectively. DKT, daikenchuto
The intraluminal pressure measurement in the anal end of the proximal colon, which was assessed simultaneously with the DMaps construction, is shown in Figure 1B. Before DKT treatment, small pressure peaks were observed in the anal end of the proximal colon, which were consistent with the appearance of the white bands indicating the contraction site. In addition, the treatment with DKT (200 μg/mL) induced marked increases in internal pressure after a short delay for 2‐3 minutes, consistent with the contractions in the proximal colon. After washouts with fresh buffer, the internal pressure response with DKT treatment disappeared for 20‐30 minutes. It seems likely that the contraction of the proximal colon was gradually returned to the state prior to DKT treatment (Figure 1B).
The PA of the intraluminal pressure waves after treatment with DKT (200 μg/mL) increased significantly compared with that before treatment (before treatment: 3.8 ± 0.8 mmH2O; after DKT treatment: 59.6 ± 9.1 mmH2O; P = .0041; n = 5; Figure 2A). Conversely, the PF over 15 minutes was significantly decreased by the administration of DKT (before treatment: 21.6 ± 2.4; after DKT treatment: 7.6 ± 0.7; P = .0045; n = 5; Figure 2B). The AUC significantly increased after DKT administration (before treatment: 701 ± 91; after DKT treatment: 5103 ± 695; P = .0040; n = 5; Figure 2C).
Figure 2.
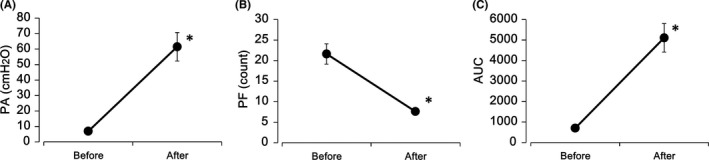
Effects of DKT on intraluminal pressure in isolated rat proximal colons. Significant increases in PA (A) and AUC (C) and a decrease in PF (B) of intraluminal pressure were observed in the proximal colons for 15 minutes following treatment with DKT (200 μg/mL). Each point is represented as mean ± standard error (n = 5). *: P < .01 for after vs before treatment, according to the paired t test. AUC, area under the curve of pressure waves; PA, mean peak amplitude; PF, peak frequency; DKT, daikenchuto
Figure 3A and 3 shows the changes in intraluminal pressure when a vehicle (n = 5) or TTX (0.3 μmol/L; n = 4) was added to the preparation with DKT treatment. TTX treatment reduced the intraluminal pressure. The levels of PA (vehicle: 85.2 ± 7.9%; TTX: 7.7 ± 3.5%; P = .0001; n = 4‐5; Figure 3C) and AUC (vehicle: 77.8 ± 7.9%; TTX: 24.5 ± 8.3%; P = .0024; Figure 3E) were significantly lowered with TTX in comparison with the PA and AUC on vehicle treatment, whereas a significant increase in PF was observed (vehicle: 97.4 ± 8.9%; TTX: 572.7 ± 118.4%; P = .0275; Figure 3D).
Figure 3.
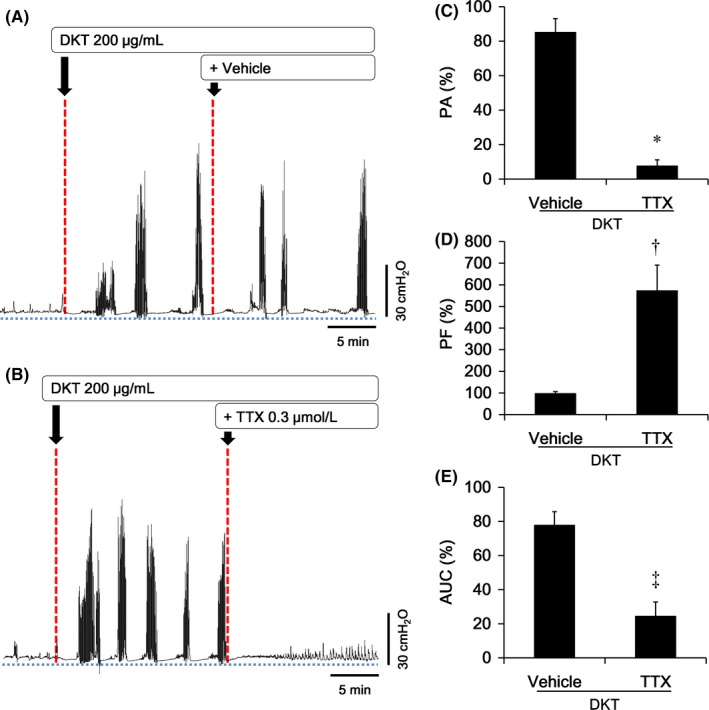
Influence of the neural blocker TTX followed by DKT treatment on intraluminal pressure in the isolated rat proximal colons. A‐B, A typical pattern of intraluminal pressure in the proximal colons after the addition of vehicle (A) and the neural blocker TTX (0.3 μmol/L) (B) under DKT (200 μg/mL) treatment. C‐E, The change in intraluminal pressure in the proximal colons treated with vehicle or TTX is expressed by the ratio of after and before treatment. Compared with vehicle treatment, TTX significantly decreased PA (A) and AUC (C), and increased PF (B) of the intraluminal pressure in the proximal colons that were pretreated with DKT. Each point is represented as mean ± standard error (n = 4‐5). †, ‡, *: P < .05, .01, .001 for TTX vs. vehicle treatment, according to the Student t test or Aspin‐welch t test. AUC: area under the curve of pressure waves; PA: mean peak amplitude; PF, peak frequency; DKT, daikenchuto; TTX, tetrodotoxin
3.2. Colonic motor pattern
The DMaps of the isolated colons are shown in Figure 4 and Figure S1. Distension with saline at 0.15 mL/min elicited the propagating contractions with a frequency of 1.93 ± 0.12 counts per minute (cpm) in the isolated proximal colons (n = 3) (Figure S1). Treatment with TTX (1 μmol/L) induced the motor patterns represented by the propagating contractions with a frequency of 3.20 ± 0.35 cpm (n = 3), identified as myogenic ripples (Figure S1). However, in the isolated entire colon as shown in Figure 4A, various kinds of propagating contractions in almost all preparations (n = 5) were observed. The propagating contractions occurred with a frequency of 2.53 ± 0.22 cpm and a strength of 26.2 ± 3.9% in the most proximal parts of the colon before DKT treatment. In the mid‐colon and distal colon, short‐extent anal propagating contractions were observed with a frequency of 0.29 ± 0.05 cpm. In addition, large‐extent anal propagating contractions, approximately 80% of the length of the colon, were observed. Before DKT treatment, these contractions were observed in 2 of 5 preparations and the frequency was low (0.03 ± 0.02 cpm), but it significantly increased with DKT (200 μg/mL) treatment (0.24 ± 0.02 cpm, P = .0004, n = 5). These contractions had a high strength of 70.9 ± 5.8% compared with that of contractions in the proximal parts of the colon before the DKT treatment (P = .0002), which could be identified as peristaltic contractions. There was no difference in the length and velocity of the peristaltic contractions (Table 1). These changes were also observed after bethanechol treatment (Figure 4B). The propagating contractions induced by bethanechol had a frequency of 0.14 ± 0.02 cpm, a length of 66.4 ± 3.7 mm, and a velocity of 1.09 ± 0.16 mm/sec in the preparations (n = 5).
Figure 4.
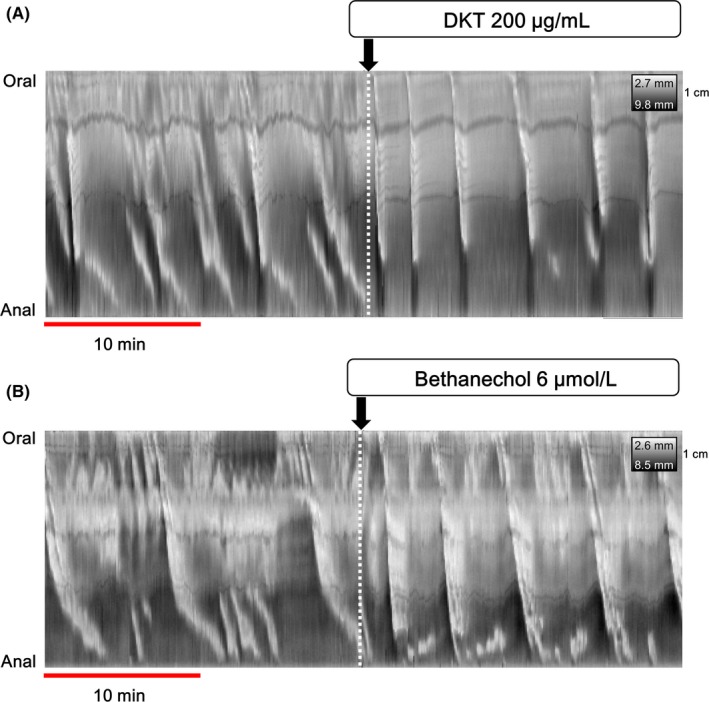
The colonic motor pattern on spatiotemporal maps of diameter (DMaps) of the entire isolated rat colon. Before drug treatment, various kinds of propagating contractions were observed. After 200 µg/mL DKT (A) and 6 µmol/L bethanechol (B) treatment, large‐extent anal propagating contractions were induced in the entire colon. DKT, daikenchuto
Table 1.
Characteristics of the peristaltic contraction in the isolated rat colons before and after DKT treatment
| Peristaltic contractions | DKT 200 μg/mL | |
|---|---|---|
| Before treatment | After treatment | |
| Length (mm) | 50.9 ± 2.9 | 58.0 ± 2.9 |
| Velocity (mm/sec) | 1.33 ± 0.50 | 1.88 ± 0.29 |
Mean length in five preparations of the entire isolated rat colons was 72.7 ± 5.1 mm. The characteristics of peristaltic contractions before treatment (n = 3) and after treatment (n = 24) were compared. The propagation length and velocity of peristaltic contractions were calculated from DMaps. All data are represented as mean ± standard error.
Abbreviation: DKT, daikenchuto.
3.3. Colonic transit
The plastic bead transport throughout the isolated rat colon is shown in Figure 5, Video S2, and Table 2. Figure 5 shows the transit time course using DMaps and typical photographs in the entire colons. The dark area on the DMaps indicates the movement trajectory of the bead in the colon. As shown in Figure S2 the bead failed to travel along the length of the colon in 20 of 36 preparations and the bead transit in 16 preparations was uninterrupted. Bead propulsion was interrupted in most untreated preparations of colon. However, the intermittent progress of the bead occurred by certain kinds of propagating contractions toward the anal end, whereas in the bethanechol‐, DKT‐, and HAS‐treated colons, the bead travelled by one or two large‐extent anal propagating contractions. Table 2 shows the average bead transit time through the colonic tract. The transit time was significantly reduced after treatment with bethanechol, a muscarinic 3 receptor agonist (6 μmol/L) [before treatment: 50.9 ± 6.0 sec/mm; bethanechol treatment: 12.4 ± 4.8 sec/mm; P = .0010; n = 5], compared with that in the colon before the treatment. Similarly, after treatment with DKT (200 μg/mL) [before treatment: 85.5 ± 15.7 sec/mm; DKT treatment: 6.3 ± 2.5 sec/mm; P = .0116; n = 5] or HAS (3 μmol/L) [before treatment: 68.6 ± 10.9 sec/mm; HAS treatment: 19.5 ± 3.9 sec/mm; P = .0037; n = 6], transit times were significantly shortened. The effect of HAS was significant less than that of DKT (P = .0248).
Figure 5.
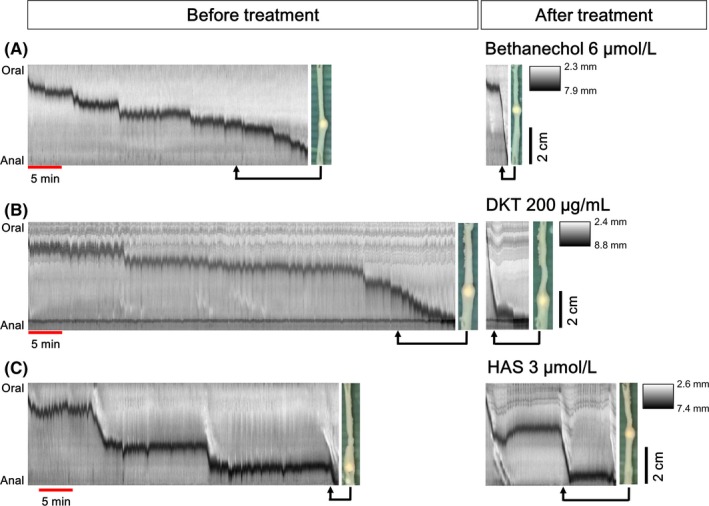
Intraluminal bead transit throughout the entire isolated rat colon. The transit time courses of the bead in the colons before and after drug treatments were analyzed based on the spatiotemporal maps of diameter (DMaps) made using video images. The y‐axis of the DMaps is indicative of the length of the colons from the oral to the anal side, and the x‐axis indicates the elapsed time. The dark area on the DMaps indicates the movement trajectory of the bead in the colon. The photograph displays the temporal movement of the bead at each point. Certain kinds of propagating contractions toward the anal end appeared in the colon before drug treatment (non‐treatment), whereas in bethanechol 6 μmol/L (A), DKT 200 μg/mL (B) and HAS 3 μmol/L (C)‐treated colons, peristaltic contraction was observed and the bead moved more quickly. DKT: daikenchuto; HAS: hydroxyl‐α‐sanshool
Table 2.
Bead transit time through the entire isolated rat colon
| Groups | Transit time (sec/mm) | |||
|---|---|---|---|---|
| Before treatment | After treatment | |||
| Bethanechol | 6 μmol/L | (n = 5) | 50.9 ± 6.0 | 12.4 ± 4.8† |
| DKT | 200 μg/mL | (n = 5) | 85.5 ± 15.7 | 6.3 ± 2.5* |
| HAS | 3 μmol/L | (n = 6) | 68.6 ± 10.9 | 19.5 ± 3.9† |
The bead transit time, defined as the time required for the bead to reach the anal side from the onset of movement in the oral side, was calculated from an analysis of video images of rat colons. Data are calculated by dividing time by transit distance. The muscarinic 3 receptor agonist bethanechol (6 μmol/L), DKT (200 μg/mL), and HAS (3 μmol/L) treatment significantly reduced bead transit time compared with that before treatment in isolated colons. Each point is presented as mean ± standard error (n = 5 or 6). *, †: P < .05, .01 after treatment vs before treatment according to the paired t test.
Abbreviations: DKT, daikenchuto; HAS, hydroxy‐α sanshool.
4. DISCUSSION
Our main result was that DKT could promote colonic transit through a propulsive movement pattern by the induction of peristaltic contractions.
Several types of propagating contractions have been observed to occur in the rat colon by spontaneous or fluid‐infusion induction.19, 20, 21 The analysis of DMaps in this study demonstrated that DKT induced large‐extent anal propagating contractions and increased the frequency of these contractions in the entire isolated colons. DKT‐induced propagating contractions were observed with increased intraluminal pressure in the anal end of the proximal colons, which was inhibited by TTX treatment. Moreover, according to the characteristics of the contractions, they were considered to be peristaltic contractions. HAS (1‐30 μmol/L), a constituent of DKT, has also been reported to induce LDC‐like contractions, which may indicate peristaltic contractions, and rhythmically constrict the proximal colon.10 The motor effect of DKT in the colon is considered to be mediated by the action of HAS. Single oral dosing of DKT in healthy volunteers from Japan and the United States was found to produce transient peak in the plasma concentration of HAS. These results indicate rapid absorption of DKT at high concentrations in the order of a few μmol/L, followed by their rapid elimination over a period of a few hours.22, 23 Among patients with liver or gastric cancer who underwent surgery, the time to first bowel movement was significantly shorter in the DKT group than in the placebo group, suggesting that DKT promotes early recovery of postoperative bowel function.6, 7
Daikenchuto promoted colonic transit by concomitantly inducing peristaltic contractions in the entire colon. In contrast, various kinds of propagating contractions, but few peristaltic contractions, were observed in untreated colons, and the colonic transit was very slow. The muscarinic 3 receptor agonist bethanechol has been reported to generate propulsive contractions by myogenic mechanisms.19, 20 Bethanechol induced shortening of the colonic transit time, suggesting a relationship between peristaltic contraction and colonic transit in the isolated rat colon. HAS also shortened the transit time in a manner similar to that by bethanechol, which could be mediated by the induction of peristaltic contractions.10 These findings suggest that DKT‐generated peristaltic contraction in the entire colon is involved in accelerating colonic transit.
Neurogenic propagating contractions have been reported to be inhibited by the neural blocker TTX,19, 20 which implies that cholinergic neurons, as well as nitrergic and serotonergic pathways in the myenteric plexus, are essential in the development of propulsive contractions.20 In the present study, the increase in intraluminal pressure induced concomitantly with propagating contraction by DKT treatment disappeared with TTX in the proximal rat colon. Therefore, DKT may induce peristaltic contraction by neural stimulation. The TASK‐1 (KCNK 3) and TASK‐3 (KCNK 9) channels within the two‐pore domain potassium channel family are expressed in the longitudinal muscles of the colon and/or myenteric plexus (Auerbach's plexus), and HAS is known to inhibit them.10, 11 Our previous study demonstrated that LDC‐like contractions resulting from the HAS contained in DKT might be mediated by KCNK channels,10 thereby suggesting that HAS could be principally involved as an active constituent in the action of DKT.
Although HAS also accelerated colonic transit, the effect was less than that of DKT in this study. DKT also contains a structural isomer of HAS, hydroxy‐β‐sanshool, which has been shown to generate LDC.10 In addition, [6]‐gingerol and [6]‐shogaol, which are constituents of DKT, are known to have transient receptor potential ankyrin 1 (TRPA1)‐stimulating activity.24 TRPA1 induces serotonin release from enterochromaffin cells,24 suggesting a relationship with the prokinetic effect of DKT.22, 23, 25 Therefore, it is possible that the function of DKT in colonic motility is mediated by synergism among the multiple constituents of DKT 26; however, the details of this action, the dose‐dependency, and the effect on the flow rate in isolated colons remain unclear. Validating these hypotheses will require further studies.
In conclusion, DKT induced peristaltic contractions in the isolated rat colon and enhanced colonic transit activity, which could be mediated by the enteric nervous system in the colon. These findings suggest that acceleration of propulsive motility in the colon is a critical pharmacologic mechanism of action of DKT. The DKT motor effect may be useful for developing future therapeutic strategies for patients with constipation and POI.
CONFLICT OF INTEREST
KK, AM, HM, NF, and MY are employed by Tsumura & Co. TK, AT, and MS have a financial interest in Tsumura & Co. relevant to this research.
AUTHOR CONTRIBUTIONS
KK initiated and directed the entire study, designed experiments, and wrote the manuscript; AM and HM supported the experiments and the analysis; NF, MY, and TK contributed to the experimental design, analyzed data, and wrote the manuscript; YM, MS, and AT co‐directed the entire study, analyzed data, and wrote the manuscript.
Supporting information
Kubota K, Mase A, Matsushima H, et al. Daikenchuto, a traditional Japanese herbal medicine, promotes colonic transit by inducing a propulsive movement pattern. Neurogastroenterol Motil. 2019;31:e13689 10.1111/nmo.13689
Funding information
This work was supported by a grant from Tsumura & Co.
REFERENCES
- 1. Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293‐e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stivland T, Camilleri M, Vassallo M, et al. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107‐115. [DOI] [PubMed] [Google Scholar]
- 3. Prichard DO, Bharucha AE. Recent advances in understanding and managing chronic constipation. F1000Res. 2018;7:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishizuka M, Shibuya N, Nagata H, et al. Perioperative administration of traditional Japanese herbal medicine daikenchuto relieves postoperative ileus in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta‐analysis. Anticancer Res. 2017;37:5967‐5974. [DOI] [PubMed] [Google Scholar]
- 5. Manabe N, Camilleri M, Rao A, et al. Effect of daikenchuto (TU‐100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298:G970‐975. [DOI] [PubMed] [Google Scholar]
- 6. Shimada M, Morine Y, Nagano H, et al. Effect of TU‐100, a traditional Japanese medicine, administered after hepatic resection in patients with liver cancer: a multi‐center, phase III trial (JFMC40‐1001). Int J Clin Oncol. 2015;20:95‐104. [DOI] [PubMed] [Google Scholar]
- 7. Yoshikawa K, Shimada M, Wakabayashi G, et al. Effect of daikenchuto, a traditional Japanese herbal medicine, after total gastrectomy for gastric cancer: a multicenter, randomized, double‐blind, placebo‐controlled, phase II trial. J Am Coll Surg. 2015;221:571‐578. [DOI] [PubMed] [Google Scholar]
- 8. Kono T, Shimada M, Nishi M, et al. Daikenchuto accelerates the recovery from prolonged postoperative ileus after open abdominal surgery: a subgroup analysis of three randomized controlled trials. Surg Today. 2019;49:704‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tokita Y, Yuzurihara M, Sakaguchi M, Satoh K, Kase Y. The pharmacological effects of daikenchuto, a traditional herbal medicine, on delayed gastrointestinal transit in rat postoperative ileus. J Pharmacol Sci. 2007;104:303‐310. [DOI] [PubMed] [Google Scholar]
- 10. Kubota K, Ohtake N, Ohbuchi K, et al. Hydroxy‐α sanshool induces colonic motor activity in rat proximal colon: a possible involvement of KCNK9. Am J Physiol Gastrointest Liver Physiol. 2015;308:G579‐G590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bautista DM, Sigal YM, Milstein AD, et al. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two‐pore potassium channels. Nat Neurosci. 2008;11:772‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enyedi P, Czirják G. Molecular background of leak K+ currents: two‐pore domain potassium channels. Physiol Rev. 2010;90:559‐605. [DOI] [PubMed] [Google Scholar]
- 13. Chen JH, Yu Y, Yang Z, et al. Intraluminal pressure patterns in the human colon assessed by high‐resolution manometry. Sci Rep. 2017;7:41436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinning PG, Wiklendt L, Maslen L, et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high‐resolution fiber‐optic manometry. Neurogastroenterol Motil. 2014;26:1443‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Costa M, Wiklendt L, Simpson P, Spencer NJ, Brookes SJ, Dinning PG. Neuromechanical factors involved in the formation and propulsion of fecal pellets in the guinea‐pig colon. Neurogastroenterol Motil. 2015;10:1466‐1477. [DOI] [PubMed] [Google Scholar]
- 16. Dinning PG, Arkwright JW, Costa M, et al. Temporal relationships between wall motion, intraluminal pressure, and flow in the isolated rabbit small intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G577‐G585. [DOI] [PubMed] [Google Scholar]
- 17. Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea‐pig small intestine using spatio‐temporal maps. J Physiol. 1999;517:575‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spencer NJ, Dinning PG, Brookes SJ, Costa M. Insights into the mechanisms underlying colonic motor patterns. J Physiol. 2016;594:4099‐4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costa M, Dodds KN, Wiklendt L, Spencer NJ, Brookes SJ, Dinning PG. Neurogenic and myogenic motor activity in the colon of the guinea pig, mouse, rabbit, and rat. Am J Physiol Gastrointest Liver Physiol. 2013;305:G749‐759. [DOI] [PubMed] [Google Scholar]
- 20. Chen JH, Zhang Q, Yu Y, et al. Neurogenic and myogenic properties of pan‐colonic motor patterns and their spatiotemporal organization in rats. PLoS ONE. 2013;8:e60474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M, Parsons S. Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci. 2011;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munekage M, Kitagawa H, Ichikawa K, et al. Pharmacokinetics of daikenchuto, a traditional Japanese medicine (kampo) after single oral administration to healthy Japanese volunteers. Drug Metab Dispos. 2011;39:1784‐1788. [DOI] [PubMed] [Google Scholar]
- 23. Munekage M, Ichikawa K, Kitagawa H, et al. Population pharmacokinetic analysis of daikenchuto, a traditional Japanese medicine (Kampo) in Japanese and US health volunteers. Drug Metab Dispos. 2013;41:1256‐1263. [DOI] [PubMed] [Google Scholar]
- 24. Tsuchiya K, Kubota K, Ohbuchi K, et al. Transient receptor potential ankyrin 1 agonists improve intestinal transit in a murine model of postoperative ileus. Neurogastroenterol Motil. 2016;12:1792‐1805. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe J, Kaifuchi N, Kushida H, et al. Intestinal, portal, and peripheral profiles of daikenchuto (TU‐100)'s active ingredients after oral administration. Pharmacol Res Perspect. 2015;3:e00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kono T, Shimada M, Yamamoto M, et al. Complementary and synergistic therapeutic effects of compounds found in Kampo medicine: analysis of daikenchuto. Front Pharmacol. 2015;4(6):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


