Abstract
Objective
To examine the behavioral functioning of children prenatally exposed to carbamazepine (CBZ), lamotrigine (LTG), levetiracetam (LEV), or valproate (VPA) monotherapy.
Methods
In collaboration with the European Registry of Antiepileptic Drugs and Pregnancy (EURAP), the Dutch EURAP & Development study was designed, a prospective observational study. Between January 2015 and March 2018, the Child Behavior Checklist and the Social Emotional Questionnaire were used to examine the nature and severity of behavioral problems. VPA‐exposed children were compared to children exposed to CBZ, LTG, or LEV, taking potential confounders into account. A direct comparison was also made between LTG and LEV, as these are first‐choice treatments for many women with epilepsy of childbearing potential.
Results
Of the 405 invited, 181 children were included; 26 were exposed to VPA, 37 to CBZ, 88 to LTG, and 30 to LEV. For most children, both parents completed the behavioral questionnaires. Across all four antiepileptic drug (AED) exposure groups, high percentages of children with clinically relevant behavior problems were found, with behavioral problems occurring in 32% of VPA‐exposed children, 14% of CBZ, 16% of LTG, and 14% of LEV. After controlling for potential confounders, VPA‐exposed children had significantly more social problems than those exposed to LTG (−2.8, 95% confidence interval [CI] = −5.2 to −0.4; P = 0.022) or LEV (−3.2, CI: −6.1 to −0.3; P = 0.028), and significantly more attention problems than LEV‐exposed children (−3.7, CI: −6.7 to −0.8; P = 0.013). LTG‐exposed children had significantly more attention deficit (−9.2, CI: −17.3 to 1.1; P = 0.026), but significantly less anxious behavior when compared to LEV‐exposed children (9.0, CI: 0.3‐17.6; P = 0.042).
Significance
Compared to population norms, a high proportion of children of mothers with epilepsy exposed prenatally to monotherapy with four common AEDs had clinical behavioral problems reported by parents. Different patterns were seen, with some but not all subscales raised for all AED exposure groups. It is important that prenatally AED‐exposed children are regularly screened for behavioral problems so that appropriate help can be provided.
Keywords: antiepileptic drugs, behavior, child development, EURAP & Development, pregnancy, teratogenicity
Key Points.
The use of AEDs in women of childbearing age is a concern due to possible behavioral teratogenic risks
High proportions of children with clinical behavioral problems were found in all four AED exposure groups
After controlling for potential confounders (eg, maternal behavioral problems), valproate‐exposed children were most affected
Based on parental reports, prenatally exposed children showed an increased risk of behavioral problems, which can have detrimental effects on daily lives
As these children bear multiple risks, it is important to consider in future studies other possible contributing factors such as the impact of maternal epilepsy
1. INTRODUCTION
The use of antiepileptic drugs (AEDs) in women of childbearing potential is of concern, as prenatal exposure is associated with increased risks of congenital malformations.1 Knowledge about long‐term effects on cognitive functioning has increased,2 but behavioral functioning of children of mothers with epilepsy has been less well studied.3
Behavioral problems, such as disruptive, inattentive, aggressive, anxious, or socially awkward behavior, can have detrimental effects on the daily lives of children. It can negatively affect their school functioning, relationships with peers, and parent‐child interactions.4 Children of mothers with epilepsy exposed to AEDs in utero have been associated with increased behavioral difficulties compared to unexposed children and children of mothers without epilepsy.5 Behavioral problems occur predominantly in valproate (VPA)‐exposed children3, 6, 7, 8, 9, 10, 11; they have a higher risk of autism spectrum disorders (ASDs)12, 13, 14 and attention‐deficit/hyperactivity disorder (ADHD).9, 13 Behavioral problems have also been reported in children exposed to carbamazepine (CBZ) and lamotrigine (LTG),15, 16 although other studies did not show increased risks.3, 6, 7, 9 The only study that examined behavioral functioning of school‐aged children exposed to levetiracetam (LEV) found no risk of behavioral problems.11 Because most studies, so far, used different methodologies, in children of different ages, using different rating systems, this may have led to different results.
Some studies were retrospective,7, 15, 17 with small sample sizes,6 made no distinction between AED types,5 or focused on adaptive functioning only.3, 7, 9 Symptoms of childhood psychiatric disorders have rarely been examined.18 Systematic screening will provide more insight into the behavioral development of exposed children. Controlling for potential confounders, such as epilepsy‐ and pregnancy‐related factors (eg, prenatal alcohol or nicotine exposure, folate use, and breast‐feeding), as well as more genetic and environmental components such as parental behavioral problems and educational level, is also important.5
We examined behavioral functioning in children of mothers with epilepsy prenatally exposed to VPA, CBZ, LTG, or LEV monotherapy, taking potential confounders into account. We compared VPA‐exposed children with the children exposed to the three other monotherapies and made a direct comparison between LTG and LEV, as these are first‐choice treatment options for women with epilepsy of childbearing potential. Based on previous findings, we hypothesized that VPA‐exposed children would demonstrate more behavioral problems.
2. MATERIALS AND METHODS
2.1. Study design and participants
In collaboration with the European Registry of Antiepileptic Drugs and Pregnancy (EURAP), we designed the Dutch EURAP & Development study, a prospective observational study of children of mothers with epilepsy. The current study is part of a larger longitudinal study in which long‐term effects of prenatal exposure to AEDs on neurocognitive and behavioral development are investigated from a family perspective.18
Participants were children of mothers with epilepsy identified from the EURAP‐NL database in The Netherlands, a national, single center pregnancy register that investigates the prevalence of major congenital malformations following prenatal exposure to AEDs. Women are enrolled by the EURAP‐NL center through self‐referral or by their health professional. Recruitment of women ideally occurs within the first 16 weeks of pregnancy, facilitating prospective information about health and well‐being during the pregnancy. Mother‐child pairs with risk factors assessed prenatally, after delivery, or up until 3 years of age were eligible. Inclusion criteria were maternal CBZ, LTG, LEV, or VPA monotherapy starting before conception and continuing during the entire pregnancy, and child age between 6 years and 7 years 11 months during the study period. Children were excluded if the mother was unable to take care of the child (eg, child was living in foster care), the child had a known chromosomal/genetic syndrome or prematurity (gestational age < 37 weeks), or there were factors other than AED exposure that significantly modified child development, such that reliable assessment was not possible. Both parents were invited to participate in the study. Parents received an invitation letter around the time of the child's sixth birthday. Families who did not respond received a reminder after 1 month. If no reply had been received after 3 months, families were contacted by telephone to ask whether they were willing to participate. Further detailed information on procedures was reported previously.18
2.2. Measures
2.2.1. General information
Parents completed an online questionnaire on demographic information, development, and child needs. Parents were also asked to indicate whether the child had a psychiatric diagnosis (eg, autism) or learning disability (eg, dyslexia).
2.2.2. Child behavioral problems
Mother and father completed the Child Behavior Checklist (CBCL)19 and the Social Emotional Questionnaire (SEV)20 online to screen for child behavioral problems. These provide well‐known standardized indicators with good validity and reliability.
The CBCL/6‐18 contains 118 items each scored as “not true” (0 points), “somewhat or sometimes true” (1 point), or “very true or often true” (2 points). Each item presents a problem behavior, such as “Acts too young for his/her age.” The CBCL has two broadband scales (Internalizing Problems and Externalizing Problems) and eight narrow‐band problem scales (Anxious/Depressed, Withdrawn, Somatic Complaints, Social Problems, Thought Problems, Attention Problems, Delinquent Behavior, and Aggressive Behavior). Raw scores are summed and converted to T scores for each scale (standardized for gender and age with mean T score = 50 and SD = 10).
The SEV contains 72 items with descriptions of problem behaviors, for example, “Is easily irritable or irritated.” The parent is asked to score the items using a 5‐point scale (from “the behavior does not occur” [0 points] to “the behavior occurs very often or daily” [4 points]). The specific social‐emotional problems that can be distinguished are: (1) ADHD, with subscales for attention deficit, hyperactivity, and impulsivity; (2) social problem behavior, consisting of opposition‐defiant behavior (ODD), aggressive behavior, and antisocial behavior (conduct disorder [CD]); (3) anxious behavior, with subscales for general anxiety, social anxiety, and anxious depressed behavior; and (4) autistic behavior. Raw scores are summed and converted to percentile scores for each (sub)scale (standardized for age and gender), with higher percentile score indicating more symptoms of behavioral problems.
Scores can fall in the normal range (meaning that parents report few or no problems), the borderline range (meaning that parents report minor or occasional problems), or the clinical range (meaning that parents report clear or frequent problems). Clinical scores are seen as problematic behaviors that can negatively affect the development and daily life of the child.
2.2.3. Adult behavioral problems
Mothers completed the Adult Self Report (ASR)21 on behavioral, emotional, and social problems (123 items, eg, “I am unhappy, sad, or depressed”). In an attempt to control for genetic factors of parental behavior that the child may have inherited, we controlled for maternal behavioral problems by using total behavior problems (ASR, T score) as a potential confounder in the analyses of child behavior.
2.3. Statistical analyses
Data were analyzed using IBM SPSS Statistics 24. Descriptive analyses were performed for each AED taken, to describe the sample and to examine the nature and severity of behavioral problems. Percentages of borderline and clinical scores were examined and compared to population norms (Binomial Proportion Test). To compare the four AEDs, we conducted multilevel regression analyses for each of the behavioral outcomes, including both mother and father report, with an indicator variable “father” to represent possible differences between parents. To account for dependencies between parents reporting on the same child and dependencies between siblings within the same family, we included both between‐family variance and within‐family variance in the multilevel regression analyses. We compared VPA with the three other AED monotherapies and made in addition a direct comparison between LTG and LEV.
Potential confounders were selected by assessing their relationships with the medication and outcome variables (through analysis of variance [ANOVA] with post hoc Tukey tests, chi‐square, Fisher's exact tests, and Pearson correlations). Variables included as potential confounders were maternal epilepsy type, tonic‐clonic seizures during pregnancy, periconceptional use of folic acid, alcohol and nicotine exposure during each trimester, breastfeeding, maternal age at delivery, maternal behavioral problems, and education. We included maternal education into the analyses instead of child intelligence quotient (IQ) because of missing child IQ scores (data available for 162 of 181 children): gestational age; age of the child at the time of the study; presence or absence of congenital malformations, and time of inclusion in the EURAP‐NL database (Table 1). Maternal education was significantly correlated with child IQ and was complete for all children (r = 0.39, P < 0.001). Variables showing a relationship (P < 0.15) with medication and outcome measure, or that were expected to influence child behavior (eg, maternal behavioral problems) were entered, each into a separate multilevel regression analysis. Variables that were related to AED use were maternal age at delivery, epilepsy type, alcohol use during first trimester, nicotine use during each trimester, age of the child, breast‐feeding, and presence of congenital malformations. Variables that were related to behavioral outcomes were maternal behavioral problems, maternal education, age of the child, breast‐feeding, gestational age, alcohol use during first trimester, nicotine use during second or third trimester, and epilepsy type.
Table 1.
Group demographic information by antiepileptic exposure group
| VPA | CBZ | LTG | LEV | P | |
|---|---|---|---|---|---|
| Sample size | 26 | 37 | 88 | 30 | |
| Maternal characteristics, and epilepsy and pregnancy information | |||||
| AED daily dose 1st trimester, mg/d, mean (range, min‐max) | 936.54 (500‐1500) | 637.84 (200‐1400) | 270.17 (50‐600) | 1183.00 (250‐3000) | NA |
| Maternal age at birth of the baby, y, mean (SD) | 33 (3) | 32 (5) | 31 (4) | 32 (4) | 0.022a |
| Maternal education, n (%) higher educationb | 13 (57%) | 17 (49%) | 44 (60%) | 16 (64%) | 0.647c |
| Maternal behavioral problems, mean (SD)b , d | 47.9 (9.6) | 49.8 (9.1) | 50.7 (9.0) | 50.3 (9.5) | 0.636a |
| Family type, n (%) two‐parent | 23 (89%) | 33 (89%) | 80 (91%) | 27 (90%) | 0.950e |
| Folate supplementation, n (%) yesf | 22 (88%) | 25 (71%) | 71 (81%) | 22 (73%) | 0.382e |
| Alcohol exposure, n (%) yes | |||||
| First trimester | 3 (12%) | 4 (11%) | 27 (31%) | 5 (17%) | 0.039e |
| Second and/or third trimester | 1 (4%) | 1 (3%) | 8 (9%) | 1 (3%) | 0.606e |
| Nicotine exposure, n (%) yes | |||||
| First trimester | 8 (31%) | 3 (8%) | 4 (5%) | 2 (7%) | 0.003e |
| Second and/or third trimester | 5 (19%) | 0 | 2 (2%) | 2 (7%) | 0.005e |
| Maternal epilepsy type, n (%) | |||||
| Generalized | 18 (69%) | 2 (5%) | 23 (26%) | 12 (40%) | 0.000e |
| Focal | 6 (23%) | 32 (87%) | 57 (65%) | 17 (57%) | |
| Unknown | 2 (8%) | 3 (8%) | 8 (9%) | 1 (3%) | |
| Tonic–clonic seizures, n (%) yes | 2 (8%) | 5 (14%) | 14 (16%) | 4 (13%) | 0.788c |
| Breast‐feeding, n (%) yes | 6 (23%) | 14 (38%) | 18 (21%) | 4 (13%) | 0.105e |
| Child characteristics | |||||
| Age, mo, mean (SD) | 81.7 (6.1) | 80.1 (6.1) | 82.3 (7.6) | 78.0 (5.3) | 0.024a |
| Gestational age, wk, mean (SD) | 40.4 (1.3) | 39.9 (1.3) | 39.8 (1.1) | 40.2 (1.2) | 0.161a |
| Child sex, n (%) male | 13 (50%) | 19 (51%) | 45 (51%) | 19 (63%) | 0.680c |
| Congenital malformations, n (%) yes | 6 (23%) | 4 (11%) | 4 (5%) | 2 (7%) | 0.034e |
| Sibling in the study, n (%) yes | 3 (12%) | 1 (3%) | 16 (18%) | 5 (17%) | |
| Inclusion time in EURAP‐NL, n (%) | |||||
| Before 16th week pregnancy | 17 (65%) | 25 (68%) | 67 (76%) | 21 (70%) | 0.725c |
| Between 16th week and birth | 7 (27%) | 7 (19%) | 11(13%) | 5 (17%) | |
| After birth | 2 (8%) | 5 (14%) | 10 (11%) | 4 (13%) | |
| Child diagnosis, n (%) | |||||
| Any diagnosisg | 8 (31%) | 4 (11%) | 10 (11%) | 1 (3%) | 0.015c |
| Diagnosis of autism spectrum disorder | 3 (12%) | 2 (5%) | 4 (5%) | 1 (3%) | 0.577c |
| Diagnosis of ADHD | 2 (8%) | 2 (5%) | 5 (6%) | 0 (0%) | 0.648c |
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; AED, antiepileptic drug; CBZ, carbamazepine; EURAP, European Registry of Antiepileptic Drugs and Pregnancy; LEV, levetiracetam; LTG, lamotrigine; NA, not applicable; VPA, valproate.
Analysis of variance (continuous data).
156 mothers.
Chi‐square.
Maternal behavioral problems measured with the Adult Self Report, T scores (mean = 50, SD = 10). Values were missing for five mothers.
Fisher's exact (dichotomous data).
Appropriate use of folic acid was defined as at least 4 weeks before conception with a minimum dose of 0.4 mg/d. Three values are missing because of unknown start date of folic acid.
Any diagnosis included: autism, ADHD, anxiety, trauma, conduct disorder, developmental language disorder, and dyslexia. Some children only had symptoms or a suspicion of an autism spectrum disorder or ADHD, but no official diagnoses. For ADHD, the age of the children may have played a role, because the majority of children were 6 years old and ADHD is often diagnosed later in life. Only one child of the 181 children was diagnosed with epilepsy.
As maternal age, nicotine use during first trimester, and presence of congenital malformations were not related to the outcome measures, they were excluded from further analyses. Potential confounders included in the multilevel regression analyses were maternal behavioral problems, maternal education, breast‐feeding, child age, gestational age, epilepsy type, alcohol use during first trimester, and nicotine use during second or third trimester. AED exposure type was entered into the model, with the VPA‐exposed group as the reference group. In additional analyses, to directly compare LTG‐exposed children with LEV‐exposed children, the LTG‐exposed group was set as reference. To guard against multicollinearity and overfitting, we applied a backward selection method. Predictors were removed until only related confounders (P < 0.15) remained in the multilevel regression models. If there were any noticeable occurrences in the intermediate steps, they were described. Correlation analyses were used to examine relationships between AED dose (daily dose of CBZ, LTG, LEV, or VPA) and outcome measures. To compare dosages of different AEDs, the dose was standardized, taking the percentage relative to the median dose (100 × [(dose first trimester − median AED dose)/median AED dose]). As dose did not correlate significantly with behavioral outcomes, it was not included in the regression analyses.
Full information maximum likelihood analyses were conducted using all available information, but without imputation for missing data on outcome variables. Five mothers did not complete the ASR. To include this variable as a potential confounder, we therefore conducted missing value analyses for the total behavioral problem score using expectation maximization.22 This method ensured that all available outcome variables could be included in the multilevel regression analysis, without changing the data.
Two children in the VPA‐exposed group had recently been assessed within a clinical setting. For these children, we included behavioral scores from the CBCL and/or SEV from the earlier psychological reports as well as parent information from the online questionnaire.
2.4. Standard protocol approvals, registration, and consents
The study was approved by the Medical Ethics Committee of the Academic Medical Center (NL 45505.018.13). Prior to enrollment of the first participant, the study was registered in the Dutch trial register (http://www.trialregister.nl, NTR4800). Parents provided written consent prior to the assessment. When the questionnaire was completed at home, parents consented by email or telephone.
3. RESULTS
3.1. Participants
Between January 2015 and March 2018, the behavioral questionnaires were completed for 183 children from 157 families (one set of twins and 25 sibling pairs; Figure 1). For most children (144), both parents completed at least one of the behavioral questionnaires. For 37 children only the mother reported and for two children only the father. The inclusion rate was around 45% of all initially invited mother‐child pairs.
Figure 1.
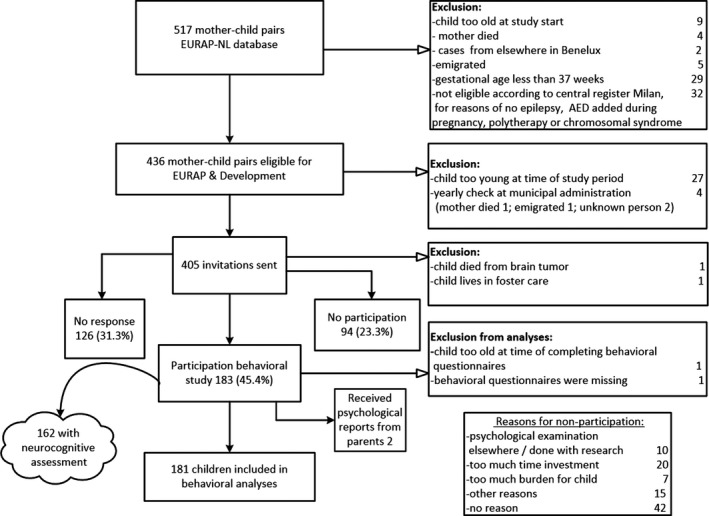
Flowchart Dutch EURAP & Development study—Behavioral domain. AED, antiepileptic drug
Two children were excluded from analyses, one because the child was too old when parents completed the questionnaire, and the second child (exposed to VPA) because her mother only completed general information, and behavioral outcomes were missing; this child was apparently diagnosed with autism (not counted as such in Table 1). Of the included children, 26 were prenatally exposed to monotherapy VPA, 37 to CBZ, 88 to LTG, and 30 to LEV.
Children from the four AED‐exposed groups were comparable across most demographic variables (Table 1). Children from the VPA‐exposed group were significantly more likely to have been exposed to nicotine. The mothers of children exposed to VPA were also slightly but significantly older at the child's birth. Mothers who used LTG were more likely to have consumed alcohol during the first trimester. Mothers who used VPA were significantly more likely to have generalized epilepsy (69%), whereas mothers who used CBZ were significantly more likely to have focal epilepsy (87%). Additionally, children exposed to LEV were significantly younger at the time of the study. VPA‐exposed children were significantly more likely to have congenital malformations.
Based on parental report of child diagnoses, valproate‐exposed children significantly more often had a (psychiatric) diagnosis (31%; Table 1). This was a combination of diagnoses of autism, ADHD, or developmental language disorder. When diagnosis was specified for autism or ADHD separately, there was no significant difference between exposure groups.
3.2. Nature and severity of behavioral problems
Overall, children had average behavioral scores (Table 2). Mothers reported more behavioral problems than fathers (for significant differences see Table 4 and Table S4a). The scores show few differences between the AED groups, with no specific AED‐exposure group having the most or fewest behavioral problems, and confirmative ANOVA (including post hoc Tukey tests) with unadjusted means showing no significant differences between the AED groups (Table 2).
Table 2.
Means and standard deviations (standard scores) of child behavior outcome measures
| CBCL | All children (181) | VPA (26) | CBZ (37) | LTG (88) | LEV (30) | P a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||||||
| Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | |||
| Sample size | 178 | 144 | 25 | 22 | 37 | 28 | 88 | 70 | 28 | 24 | 178 | 144 |
| Anxious/depressed | 56.2 (7.1) | 54.6 (6.4) | 56.4 (7.8) | 56.5 (8.2) | 56.1 (6.4) | 55.3 (6.6) | 56.1 (7.0) | 53.7 (5.6) | 56.7 (7.9) | 54.7 (6.2) | 0.977 | 0.290 |
| Withdrawn | 56.6 (6.9) | 56.0 (6.3) | 56.2 (7.8) | 56.5 (6.4) | 56.9 (6.3) | 54.4 (5.5) | 56.8 (7.0) | 56.1 (6.4) | 56.3 (7.0) | 57.3 (6.8) | 0.973 | 0.403 |
| Somatic complain | 57.3 (7.1) | 56.3 (6.8) | 56.8 (7.7) | 58.0 (7.0) | 57.3 (7.5) | 57.7 (8.3) | 57.85 (7.1) | 55.8 (6.5) | 55.9 (6.3) | 54.6 (4.6) | 0.616 | 0.217 |
| Social problems | 56.6 (6.9) | 55.8 (6.3) | 58.1 (8.1) | 58.2 (7.8) | 56.0 (5.9) | 55.8 (5.7) | 56.6 (6.9) | 55.5 (6.1) | 56.3 (7.6) | 54.6 (5.7) | 0.684 | 0.232 |
| Thought problems | 56.4 (6.7) | 55.4 (6.2) | 56.4 (6.7) | 56.5 (6.5) | 56.3 (7.0) | 55.9 (6.5) | 56.6 (6.9) | 54.9 (6.1) | 55.9 (6.4) | 55.6 (6.3) | 0.971 | 0.719 |
| Attention problems | 56.6 (7.1) | 55.9 (5.5) | 58.0 (7.0) | 57.3 (6.5) | 56.7 (4.7) | 56.5 (5.1) | 57.0 (8.3) | 55.7 (5.7) | 54.3 (5.4) | 54.2 (4.3) | 0.244 | 0.250 |
| Delinquent behavior | 55.5 (5.6) | 55.4 (6.1) | 55.3 (5.7) | 56.5 (7.0) | 56.6 (4.8) | 56.8 (6.0) | 55.5 (6.0) | 54.7 (5.7) | 54.4 (5.1) | 55.3 (6.4) | 0.454 | 0.382 |
| Aggressive behavior | 56.7 (8.0) | 55.4 (6.5) | 56.8 (7.9) | 56.1 (6.3) | 56.9 (6.1) | 55.9 (6.7) | 56.4 (8.2) | 55.2 (6.4) | 57.5 (9.8) | 55.0 (7.0) | 0.940 | 0.882 |
| Internalizing problems | 55.4 (9.8) | 52.9 (10.0) | 55.3 (10.3) | 55.1 (10.8) | 55.6 (8.9) | 53.4 (10.7) | 55.5 (10.1) | 51.9 (10.2) | 54.7 (9.8) | 53.4 (8.3) | 0.982 | 0.589 |
| Externalizing problems | 54.0 (10.2) | 52.8 (9.9) | 52.8 (12.3) | 53.0 (11.4) | 56.3 (6.9) | 54.3 (9.8) | 53.3 (10.7) | 51.9 (10.0) | 54.4 (9.9) | 53.2 (8.3) | 0.447 | 0.747 |
| Total problem behavior | 55.1 (9.8) | 53.3 (9.8) | 55.5 (11.7) | 55.4 (11.2) | 56.5 (7.8) | 54.7 (10.7) | 54.8 (10.1) | 52.3 (9.5) | 53.9 (9.5) | 52.4 (8.0) | 0.732 | 0.479 |
| SEV | Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | P a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | 177 | 143 | 26 | 23 | 36 | 28 | 87 | 68 | 28 | 24 | 177 | 143 |
| ADHD total | 59.5 (26.1) | 55.8 (25.2) | 64.9 (25.4) | 54.7 (25.9) | 64.5 (24.3) | 54.2 (23.1) | 58.1 (26.2) | 59.3 (24.8) | 52.2 (27.4) | 48.8 (27.6) | 0.180 | 0.349 |
| ADHD attention deficit | 55.1 (23.3) | 52.1 (22.8) | 61.2 (23.5) | 52.1 (25.0) | 56.0 (22.1) | 49.2 (18.7) | 56.0 (23.8) | 55.5 (23.9) | 45.4 (20.9) | 45.7 (21.0) | 0.073 | 0.273 |
| ADHD hyperactivity | 64.1 (25.6) | 59.6 (25.8) | 69.4 (25.3) | 57.0 (26.4) | 68.6 (24.0) | 59.6 (23.7) | 61.9 (26.5) | 63.1 (25.6) | 60.5 (24.6) | 51.8 (27.7) | 0.331 | 0.303 |
| ADHD impulsivity | 56.4 (25.8) | 56.7 (24.8) | 59.2 (28.2) | 56.8 (25.9) | 61.0 (24.1) | 57.7 (24.8) | 54.8 (25.1) | 57.0 (24.8) | 52.6 (27.8) | 54.8 (25.5) | 0.502 | 0.978 |
| Social problem behavior | 64.7 (25.6) | 63.2 (25.3) | 65.2 (28.5) | 58.0 (28.9) | 69.3 (23.2) | 64.9 (25.1) | 63.3 (25.0) | 63.5 (25.5) | 62.3 (28.3) | 65.3 (21.9) | 0.657 | 0.746 |
| ODD | 62.5 (26.0) | 60.4 (25.8) | 65.0 (27.4) | 58.1 (27.7) | 64.4 (23.0) | 61.1 (25.3) | 61.1 (25.9) | 60.3 (26.5) | 62.4 (29.6) | 62.0 (23.7) | 0.880 | 0.964 |
| CD aggression | 72.6 (13.5) | 69.7 (11.5) | 74.2 (14.7) | 69.5 (12.6) | 73.0 (13.6) | 72.5 (12.0) | 72.0 (13.0) | 69.1 (10.9) | 72.7 (14.1) | 68.5 (11.5) | 0.903 | 0.560 |
| CD antisocial | 71.6 (21.9) | 70.8 (22.1) | 70.1 (24.9) | 65.9 (24.5) | 74.9 (20.7) | 72.0 (22.3) | 71.7 (21.0) | 71.8 (21.5) | 68.2 (23.6) | 71.6 (21.8) | 0.662 | 0.712 |
| Anxiety total | 60.7 (24.5) | 53.9 (24.4) | 62.2 (23.6) | 56.5 (28.6) | 61.2 (24.0) | 49.2 (22.3) | 60.0 (25.2) | 52.0 (24.3) | 61.1 (25.2) | 62.1 (21.9) | 0.979 | 0.217 |
| General anxiety | 63.9 (20.7) | 59.1 (21.1) | 64.6 (20.6) | 63.8 (23.9) | 65.5 (20.1) | 58.1 (20.7) | 63.9 (21.1) | 58.3 (20.7) | 61.5 (21.4) | 57.8 (20.5) | 0.898 | 0.709 |
| Social anxiety | 61.7 (27.2) | 56.1 (25.6) | 59.8 (26.8) | 55.9 (26.6) | 61.1 (28.5) | 49.9 (23.9) | 61.1 (27.7) | 55.1 (26.7) | 66.3 (24.9) | 66.2 (21.7) | 0.807 | 0.138 |
| Anxious depressed | 63.7 (17.8) | 61.3 (16.4) | 64.0 (18.3) | 63.9 (18.9) | 62.9 (15.5) | 61.0 (16.2) | 63.8 (18.4) | 59.7 (15.4) | 64.2 (19.2) | 63.8 (17.2) | 0.991 | 0.625 |
| Autistic behavior | 68.2 (19.9) | 67.0 (19.2) | 71.0 (19.8) | 69.6 (20.5) | 69.0 (19.9) | 68.9 (18.7) | 67.3 (20.6) | 65.3 (18.6) | 67.1 (18.2) | 67.0 (21.1) | 0.853 | 0.746 |
CBCL mean T score = 50, SD = 10. Cutoff scores: narrow band (I, Anxious/depressed; II, Withdrawn; III, Somatic complaints; IV, Social problems; V, Thought problems; VI, Attention problems; VII, Delinquent behavior; VIII, Agresssive behavior), T score 65‐69 = borderline, ≥70 = clinical; broad band (internalizing problems, externalizing problems, total), T‐score 60‐63 = borderline, ≥ 64 = clinical. SEV percentile scores: higher scores indicate more problems. Cutoff scores: Autistic Behavior scale, percentile 95‐97 = subclinical, ≥98 clinical; all other scales, percentile 90‐94 = subclinical, ≥95 = clinical.
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; CBZ, carbamazepine; CD, conduct disorder; LEV, levetiracetam; LTG, lamotrigine; ODD, oppositional defiant disorder; SEV, Social Emotional Questionnaire; VPA, valproate.
Analysis of variance with post hoc Tukey tests showing no differences between the antiepileptic drug exposure groups.
However, a different pattern was found when examining the percentages of borderline (subclinical threshold) and clinical scores as reported by parents. Across all four AED exposure groups, high percentages of children within the borderline range and above the clinical cutoff were found (Table 3). For total behavioral problems, the range (clinical scores as reported by mothers) was between 14% and 32% (CBCL), with the highest percentage for VPA. VPA‐exposed children also showed a high percentage of social problems (16%, CBCL). Parental ratings from all the exposure groups placed between 6% and 23% of the children in the clinical range for symptoms of CD (SEV).
Table 3.
Percentages of children within the borderline range and above the clinical cutoff on behavior outcome measures
| CBCL | All children, n = 181 | VPA, n = 26 | CBZ, n = 37 | LTG, n = 88 | LEV, n = 30 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mother/father | Mother/father | Mother/father | Mother/father | Mother/father | ||||||
| Borderline | Clinical | Borderline | Clinical | Borderline | Clinical | Borderline | Clinical | Borderline | Clinical | |
| Anxious/depressed | 8.4%/6.3% | 7.3%/4.2% | 4%/9.1% | 12%/9.1% | 8.1%/14.3% | 5.4%/3.6% | 10.2%/4.3% | 6.8%/2.9% | 7.1%/0% | 7.1%/4.2% |
| Withdrawn | 16.9%/10.4% | 3.9%/4.2% | 28%/4.5% | 0%/9.1% | 13.5%/10.7% | 2.7%/0% | 18.2%/10.0% | 4.5%/4.3% | 7.1%/16.7% | 7.1%/4.2% |
| Somatic complaint | 9.0%/10.4% | 7.9%/3.5% | 8%/9.1% | 8%/4.5% | 8.1%/7.1% | 10.8%/10.7% | 10.2%/14.3% | 8.0%/1.4% | 7.1%/4.2% | 3.6%/0% |
| Social problems | 6.7%/9.0% | 6.7%/3.5% | 8%/9.1% | 16%/13.6 | 2.7%/10.7% | 5.4%/0% | 10.2%/10.0% | 4.5%/1.4% | 0%/4.2% | 7.1%/4.2% |
| Thought problems | 10.1%/6.3% | 6.7/4.2% | 8%/9.1% | 8%/4.5% | 8.1%/3.6% | 10.8%/7.1% | 11.4%/7.1% | 5.7%/2.9% | 10.7%/4.2% | 3.6%/4.2% |
| Attention problems | 8.4%/3.5% | 3.9%/1.4% | 20%/4.5% | 4%/4.5% | 5.4%/3.6% | 0%/0% | 5.7%/4.3% | 6.8%/1.4% | 10.7%/0% | 0%/0% |
| Delinquent behavior | 2.8%/6.3% | 3.4%/4.9% | 8%/9.1% | 0%/9.1% | 0%/7.1% | 2.7%/3.6% | 3.4%/4.3% | 4.5%/4.3% | 0%/8.3% | 3.6%/4.2% |
| Aggressive behavior | 6.7%/10.4% | 8.4%/3.5% | 8%/9.1% | 12%/0% | 10.8%/14.3% | 2.7%/7.1% | 5.7%/10.0% | 9.1%/2.9% | 3.6%/8.3% | 10.7%/4.2% |
| Internalizing problems | 13.5%/7.6% | 23.6%/18.1% | 16%/0% | 20%/27.3% | 13.5%/0% | 18.9%/21.4% | 14.8%/10.0% | 27.3%/15.7% | 7.1%/16.7% | 21.4%/12.5% |
| Externalizing problems | 10.7%/9.0% | 16.9%/14.6% | 4%/13.6% | 24%/18.2% | 24.3%/7.1% | 13.5%/21.4% | 9.1%/10.0% | 15.9%/11.4% | 3.6%/4.2% | 17.9%/12.5% |
| Total problem behavior | 15.2%/9.0% | 17.4%/17.4% | 8%/9.1% | 32%/27.3% | 27.0%/7.1% | 13.5%/21.4% | 14.8%/8.6% | 15.9%/15.7% | 7.1%/12.5% | 14.3%/8.3% |
| SEV | Subclinical | Clinical | Subclinical | Clinical | Subclinical | Clinical | Subclinical | Clinical | Subclinical | Clinical |
|---|---|---|---|---|---|---|---|---|---|---|
| ADHD total | 5.1%/3.5% | 5.1%/2.1% | 3.8%/8.7% | 3.8%/0% | 5.6%/7.1% | 2.8%/0% | 6.9%/2.9% | 5.7%/1.5% | 0%/4.2% | 7.1%/0% |
| ADHD attention deficit | 2.3%/0.7% | 4.0%/2.8% | 7.7%/0% | 3.8%/4.3% | 0%/3.6% | 2.8%/0% | 2.3%/0% | 4.6%/4.4% | 0%/0% | 3.6%/0% |
| ADHD hyperactivity | 7.3%/3.5% | 9.6%/4.9% | 11.5%/4.3% | 7.7%/0% | 5.6%/3.6% | 11.1%/7.1% | 6.9%/4.4% | 11.5%/5.9% | 7.1%/0% | 3.6%/4.2% |
| ADHD impulsivity | 3.4%/1.4% | 4.5%/2.8% | 3.8%/0% | 0%/0% | 5.6%/3.6% | 2.8%/7.1% | 3.4%/1.5% | 5.7%/1.5% | 0%/0% | 7.1%/4.2% |
| Social problem behavior | 5.1%/2.8% | 10.7%/6.3% | 11.5%/0% | 7.7%/4.3% | 5.6%/10.7% | 5.6%/0% | 3.4%/5.9% | 12.6%/5.9% | 3.6%/0% | 14.3%/4.2% |
| ODD | 6.2%/7.0% | 8.5%/4.2% | 11.5%/8.7% | 3.8%/0% | 8.3%/7.1% | 2.8%/3.6% | 4.6%/8.8% | 11.5%/5.9% | 3.6%/0% | 10.7%/4.2% |
| CD aggression | 8.5%/2.8% | 9.6%/7.0% | 3.8%/4.3% | 23%/8.7% | 13.9%/0% | 5.6%/10.7% | 8.0%/4.4% | 6.9%/4.4% | 7.1%/0% | 10.7%/8.3% |
| CD antisocial | 9.6%/8.4% | 11.3%/7.0% | 11.5%/17.4% | 11.5%/4.3% | 16.7%/3.6% | 5.6%/10.7% | 9.2%/8.8% | 12.6%/4.4% | 0%/4.2% | 14.3%/12.5% |
| Anxiety total | 7.3%/2.8% | 4.5%/1.4% | 3.8%/4.3% | 3.8%/4.3% | 5.6%/3.6% | 0%/0% | 9.2%/1.5% | 5.7%/1.5% | 7.1%/4.2% | 7.1%/0% |
| General anxiety | 5.6%/7.0% | 5.6%/0.7% | 3.8%/8.7% | 3.8%/4.3% | 8.3%/10.7% | 2.8%/0% | 5.7%/7.4% | 6.9%/0% | 3.6%/0% | 7.1%/0% |
| Social anxiety | 6.8%/2.1% | 6.8%/2.8% | 7.7%/0% | 0%/4.3% | 11.1%/3.6% | 5.6%/0% | 4.6%/1.5% | 8.0%/2.9% | 7.1%/4.2% | 10.7%/4.2% |
| Anxious depressed | 6.8%/6.3% | 5.1%/0.7% | 7.7%/13% | 7.7%/4.3% | 0%/3.6% | 0%/0% | 10.3%/2.9% | 4.6%/0% | 3.6%/12.5% | 10.7%/0% |
| Autistic behavior | 4.5%/4.9% | 4.0%/1.4% | 3.8%/4.3% | 7.7%/4.3% | 8.3%/7.1% | 0%/3.6% | 4.6%/2.9% | 4.6%/0% | 0%/8.3% | 3.6%/0% |
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; CBZ, carbamazepine; CD, conduct disorder; LEV, levetiracetam; LTG, lamotrigine; ODD, oppositional defiant disorder; SEV, Social Emotional Questionnaire; VPA, valproate.
Compared to Dutch population norms (SEV),20 LTG‐exposed children were found to have a significantly higher proportion of parent ratings of ODD (Table S3a). Significantly higher proportions of CD were found for VPA‐, LTG‐, and LEV‐exposed children. VPA‐ and LTG‐exposed groups had significantly higher proportions of children with clinical symptoms of autistic behavior. CBZ‐exposed children did not show higher proportions of clinical behavioral problems. No differences in proportions were found for parental report of ADHD or anxious behavior.
3.3. Comparison between children exposed to different AED types
After controlling for potential confounders, multilevel regression analyses showed that VPA‐exposed children had significantly more social problems than children exposed to LTG (−2.8, 95% CI = −5.2 to −0.4; P = 0.022) or LEV (−3.2, 95% CI = −6.1 to −0.3; P = 0.028; Table 4). Compared to LEV‐exposed children, VPA‐exposed children had significantly more attention problems (−3.7, 95% CI = −6.7 to −0.8; P = 0.013). VPA‐exposed children also had more symptoms of ADHD (total score; −13.2, 95% CI = −25.1 to −1.3; P = 0.030) and attention deficit than LEV‐exposed children (−11.7, 95% CI = −21.7 to −1.7; P = 0.022). No differences were found for other behavioral outcomes as reported by parents (Table S4a).
Table 4.
Multilevel regression analyses (VPA as reference group): Significant differences on child behavior problems
| CBCL | Social problems | Attention problems | ||||
|---|---|---|---|---|---|---|
| B (SE) | CI | P | B (SE) | CI | P | |
| Intercept | 47.0 (2.5) | 42.1 to 52.0 | 0.000 | 84.7 (14.0) | 57.0 to 112.3 | 0.000 |
| Fathera | −0.7 (0.4) | −1.6 to 0.2 | 0.107 | −0.4 (0.4) | −1.2 to 0.3 | 0.266 |
| CBZ | −2.5 (1.4) | −5.3 to 0.2 | 0.072b | −2.3 (1.4) | −5.1 to 0.5 | 0.106 |
| LTG | −2.8 (1.2) | −5.2 to −0.4 | 0.022* | −2.2 (1.3) | −4.7 to 0.3 | 0.078b |
| LEV | −3.2 (1.5) | −6.1 to −0.3 | 0.028* | −3.7 (1.5) | −6.7 to −0.8 | 0.013* |
| Maternal behavioral problems | 0.3 (0.04) | 0.2 to 0.4 | 0.000** | 0.2 (0.04) | 0.1 to 0.3 | 0.000** |
| Maternal education | −2.0 (0.8) | −3.6 to −0.4 | 0.016* | −3.3 (0.9) | −5.0 to −1.6 | 0.000** |
| Breast‐feeding | −2.1 (1.0) | −4.0 to −0.1 | 0.038* | — | — | — |
| Age of child | — | — | — | — | — | — |
| Epilepsy type | — | — | — | — | — | — |
| Gestational age | — | — | — | −0.9 (0.3) | −1.6 to −0.2 | 0.011 |
| Alcohol exposure 1st trimester | — | — | — | — | — | — |
| Nicotine exposure 2nd/3rd trimester | — | — | — | — | — | — |
| Random intercept: family variance | 0.0 (0.0) | — | — | 1.2 (7.6) | 0.874 | |
| Random intercept: child variance | 20.4 (3.2) | 15.1 to 27.7 | 0.000 | 21.2 (8.1) | 10.0 to 45.0 | 0.009 |
| SEV | ADHD total | ADHD attention deficit | ||||
|---|---|---|---|---|---|---|
| B (SE) | CI | P | B (SE) | CI | P | |
| Intercept | 110.2 (56.5) | −1.2 to 221.6 | 0.063 | 106.6 (50.4) | 7.2 to 206.0 | 0.036 |
| Fathera | −3.9 (1.9) | −7.6 to −0.3 | 0.036 | −3.1 (1.9) | −7.0 to 0.7 | 0.109 |
| CBZ | −3.4 (5.7) | −14.7 to 7.8 | 0.548 | −5.6 (4.8) | −15.0 to 3.8 | 0.242 |
| LTG | −6.2 (5.1) | −16.2 to 3.8 | 0.221 | −4.7 (4.2) | −12.9 to 3.6 | 0.266 |
| LEV | −13.2 (6.0) | −25.1 to −1.3 | 0.030* | −11.7 (5.1) | −21.7 to −1.7 | 0.022* |
| Maternal behavioral problems | 0.9 (0.2) | 0.5 to 1.3 | 0.000 | 0.6 (0.2) | 0.3 to 0.9 | 0.000** |
| Maternal education | — | — | — | — | — | — |
| Breast‐feeding | — | — | — | — | — | — |
| Age of child | — | — | — | 0.4 (0.2) | −0.04 to 0.8 | 0.073b |
| Epilepsy type | — | — | — | — | — | — |
| Gestational age | −2.2 (1.4) | 0.5 to 1.3 | 0.102 | −2.7 (1.2) | −5.0 to −0.4 | 0.020* |
| Alcohol exposure 1st trimester | — | — | — | — | — | — |
| Nicotine exposure 2nd/3rd trimester | — | — | — | — | — | — |
| Random intercept: family variance | 173.6 (84.7) | 66.7 to 452.0 | 0.041 | 47.3 (73.0) | 2.3 to 973.9 | 0.517 |
| Random intercept: child variance | 142.9 (80.8) | 47.1 to 433.0 | 0.077 | 121.6 (79.3) | 33.9 to 436.8 | 0.125 |
Maternal behavioral problems: measured with the Adult Self Report (interval); maternal education: received higher education (yes/no, dichotomous); breast‐feeding (yes/no, dichotomous); age of child at time of study (mo); epilepsy type: generalized epilepsy (yes, no, dichotomous); gestational age (wk); alcohol or nicotine exposure (yes, no, dichotomous).
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; B, unstandardized coefficients; CBCL, Child Behavior Checklist; CBZ, carbamazepine; CI, confidence interval; LEV, levetiracetam; LTG, lamotrigine; SE, standard error; SEV, Social Emotional Questionnaire; VPA, valproate.
As both parents are included in the analyses, this variable shows possible differences between mother and father report on child behavioral outcomes.
P <0.10.
*P < 0.05.
**P < 0.001.
On parental ratings of autistic behavior, there appeared to be a difference between VPA‐ and LTG‐exposed children, with VPA‐exposed children showing more autistic behavior, but after removing nonsignificant confounders (to limit overfitting) this result was no longer significant.
A direct comparison between LTG and LEV, while controlling for potential confounders, revealed few differences (Table S5). Parental ratings of LTG‐exposed children showed significantly more symptoms of ADHD attention deficit compared to LEV‐exposed children (−9.2, 95% CI = −17.3 to 1.1; P = 0.026). In comparison with LEV‐exposed children, parents of LTG‐exposed children reported significantly fewer symptoms of anxiety (total score; 9.0, 95% CI = 0.3‐17.6; P = 0.042). On other (sub)scales, no significant differences were found.
3.4. AED dose
We did not find a significant relationship with the daily dose of VPA, CBZ, LTG, or LEV during pregnancy and behavioral outcome measures.
4. DISCUSSION
Different patterns of behavioral problems were seen in prenatally AED‐exposed children, with some but not all (sub)scales being raised. Based on parental ratings, VPA‐exposed children were shown to be most affected, but parents of CBZ‐, LTG‐, and LEV‐exposed children also reported behavioral problems. Against expectation, parental ratings placed a high percentage of children from all exposure groups within the clinical range on the total behavioral problems scale (CBCL, 14%‐32%). For VPA‐exposed children, the proportion of behavioral problems was more than expected based on the worldwide prevalence of child psychiatric disorders (32% vs 13.4%),23 and much higher than LTG‐exposed (16%) or LEV‐exposed children (14%). For those exposed to LEV, LTG, or VPA, the proportion with parent‐reported CD symptoms was significantly higher than population proportions.20 LTG‐exposed children also had a higher proportion of ODD. On other scales, VPA‐exposed children displayed more behavioral problems than LTG‐ or LEV‐exposed children. The mean score comparisons did not, however, lead to significant differences between the groups.
As there are multiple factors involved in behavioral development, the finding of parental report of oppositional or aggressive behavior may not be directly attributable to the drug exposure. It is conceivable that, for LEV‐exposed children and, to a lesser extent, for LTG‐exposed children, there is an indirect or an interaction effect with drug exposure via a disharmonic intelligence profile. In our previous report, LEV exposure was associated with a disharmonic profile in favor of verbal functioning. Although our sample was relatively small and results need to be replicated, it is known that children with such a profile (verbal IQ > performance IQ) are at risk of being overestimated and may show more defiant behavior. They are verbally strong, but may have more problems with keeping a broad view (eg, planning or thinking about consequences), which is also important in behavior.24 It is also known that children with low verbal functioning, such as VPA‐exposed children, may be quick to express themselves physically and aggressively, in frustration at not being understood.4 It would be worthwhile to explore this further in future studies.
The use of LEV in people with epilepsy is associated with an increase in behavioral problems, including aggressive behavior.25, 26, 27 Future research could examine whether the symptoms of CD we observed in prenatally LEV‐exposed children have the same pharmacological mechanisms as those in individuals taking LEV for epilepsy. Alternatively, this could be related to exposure of the child to maternal behavioral problems at home.
Compared to LTG‐, LEV‐, and CBZ‐exposed children, those with VPA exposure showed more behavioral problems, with significant differences on social and attention problems and symptoms of ADHD. These results are in line with earlier research reporting behavioral problems,5 especially after VPA exposure.3, 6, 7, 9, 10, 13
Contrary to expectation, no significant difference was found on autistic behavior. When controlling for multiple potential confounders, there was a difference between VPA‐ and LTG‐exposed children, with those exposed to VPA showing more autistic behavior; this result could, however, be (partly) due to overfitting. It may also be due to small sample sizes of the groups. Compared to population proportions, VPA‐ and LTG‐exposed children showed significantly more clinical symptoms of autistic behavior. This agrees with an increase in autistic traits reported by parents of toddlers exposed to VPA or LTG.16 Other studies, based on diagnoses, have seen an increased risk of autism after prenatal VPA exposure, but not after prenatal LTG.13, 14
Based on parent information of child diagnoses, the proportion of children with a diagnosis of an ASD (Table 1) was higher than expected based on population prevalence (1%‐1.5%),28 and was also higher in the non‐VPA exposed children (3%‐5%). In our study, there seemed, however, to be a discrepancy between the number of children with a diagnosis of ASD and the number of children with a clinical score on the autistic behavior scale. On the basis of the behavioral questionnaire (SEV), fewer children had symptoms of autism. It is possible that parents with a child with autism have accepted the problem behavior, so that it is less reported as problematic.29
A direct comparison between LTG and LEV, which are the first choice of treatment for many women with epilepsy in their childbearing years, revealed some differences in behavioral functioning. Parents of LTG‐exposed children reported more attention deficit compared to LEV‐exposed children, and LEV‐exposed children were shown to have more anxiety as reported by parents. Children exposed to LTG and LEV, however, had average mean scores on attention and anxiety problems (Table S3a). On other behavioral outcome measures, LTG and LEV did not significantly differ.
Strengths of our study are (1) the prospective design, with recruitment of children through the national pregnancy register (EURAP‐NL); (2) the rigorous control for potential confounders, including maternal behavioral problems; (3) the use of reliable and valid standardized indicators to systematically examine symptoms of child psychiatric disorders; (4) mothers and fathers both reporting; and (5) the inclusion of LEV, which is increasingly prescribed in women with epilepsy.
There are also some limitations. We used parental reports of child behavior problems. Parents were not blinded to AED exposure and therefore concerns about teratogenic effects could be hypothesized to be inflating ratings. Parental report is subjective and does not directly correspond with a diagnosis made by professionals.16 It does, however, clearly reflect the child's behavior that parents experience at home. We did not include the teacher's perspective. Another possible limitation is that we did not exclude children who had experienced a head trauma or developed epilepsy themselves. Children with epilepsy frequently have behavioral problems.30, 31 Only one child with epilepsy was included, however.
In our study, there were some children with a psychiatric diagnosis or learning disability. It is possible that nonparticipating families whose child already had such a diagnosis were less likely to participate, which may have led to an underrepresentation of the problem. Follow‐up of children from early age on (before the time of child diagnosis) would therefore be recommended.
Equally, it is possible that families that participated had more suspicion of problems in their child. For example, the rates of congenital malformations in our study were higher in all of the AED exposure groups than traditionally reported from pregnancy registers.32, 33, 34 Parents might have been more anxious about the development of their child because of known teratogenicity and hence more willing to participate. Potential information bias in the rating of child behavior is a possibility.16 Controlling for maternal behavioral problems and educational level should have provided some control for this potential reporter bias.
We made a comparison between different types of AED monotherapy and compared results with population norms. A comparison with norms provides insight into the severity of behavioral problems in children of mothers with epilepsy, but such population norms were collected ahead of our study. We did not, however, include a control group of nonexposed children. Children who were exposed to AED polytherapy were also not included. More research is needed to answer related questions about a comparison with same‐time included nonexposed children, both from mothers with epilepsy and from the general population, and children exposed to different polytherapy combinations.
We did not correct the statistical threshold (P < 0.05) for the multitude of analyses. Therefore, some of the associations found might be spurious. The number of P values found to be significant was, however, greater than expected by chance. The sample size of some AED‐exposed groups was relatively small, so results should be interpreted with caution. Our findings are of clinical importance and require replication. As children of mothers with epilepsy bear multiple risks,35 it is of importance to consider other possible contributing factors (eg, impact of maternal epilepsy on the child) to behavioral outcomes. Further research into child development in the context of prenatal AED exposure is necessary to understand the (ir)reversibility of the exposure; to what extent is the behavioral teratogenic risk that occurred susceptible to adjustments? This may provide opportunities for interventions that help parents cope with, or ideally decrease child behavioral problems.
Based on parental ratings, this study showed that some but not all subscales of behavioral problems were raised for certain AED exposures. VPA‐exposed children were most affected, but parents of CBZ‐, LTG‐, and LEV‐exposed children also reported behavioral problems within the clinical range.
DISCLOSURE OF CONFLICT OF INTEREST
Y.H.‐M., F.J.O., and R.R. have no disclosures to report. D.L. has in the past (2000‐2002) received research grants from Janssen‐Cilag, GlaxoSmithKline, Pfizer, and the Netherlands Epilepsy Foundation to start up the basic EURAP study in The Netherlands. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
The study was funded by Stichting Panta Rhei and supported by the Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie (Nederland). Foundations were not involved in the study design, data collection, analysis and interpretation of the data, or manuscript preparation. We thank Eugène van Puijenbroek from the Netherlands Pharmacovigilance Center Lareb (coordinator EURAP‐NL); Dina Battino and colleagues at the Central Project Commission of EURAP (Chair: Torbjörn Tomson); all assessors who helped with data collection; Prof Ley Sander and Dr Gail Bell for critically reviewing the manuscript; and all participating children and their parents.
Huber‐Mollema Y, Oort FJ, Lindhout D, Rodenburg R. Behavioral problems in children of mothers with epilepsy prenatally exposed to valproate, carbamazepine, lamotrigine, or levetiracetam monotherapy. Epilepsia. 2019;60:1069–1082. 10.1111/epi.15968
REFERENCES
- 1. Pennell PB. Prescribing antiepileptic drugs to women of reproductive age. Lancet Neurol. 2018;17:485–6. [DOI] [PubMed] [Google Scholar]
- 2. Bromley RL, Baker GA. Fetal antiepileptic drug exposure and cognitive outcomes. Seizure. 2017;44:225–31. [DOI] [PubMed] [Google Scholar]
- 3. Deshmukh U, Adams J, Macklin EA, et al. Behavioral outcomes in children exposed prenatally to lamotrigine, valproate, or carbamazepine. Neurotoxicol Teratol. 2016;54:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wenar C, Kerig P. Developmental Psychopathology: From Infancy Through Adolescence. New York, NY: McGraw‐Hill; 2000. [Google Scholar]
- 5. Kjaer D, Christensen J, Bech BH, Pedersen LH, Vestergaard M, Olsen J. Preschool behavioral problems in children prenatally exposed to antiepileptic drugs—A follow‐up study. Epilepsy Behav. 2013;29(2):407–11. [DOI] [PubMed] [Google Scholar]
- 6. Viinikainen K, Eriksson K, Mönkkönen A, et al. The effects of valproate exposure in utero on behavior and the need for educational support in school‐aged children. Epilepsy Behav. 2006;9(4):636–40. [DOI] [PubMed] [Google Scholar]
- 7. Vinten J, Bromley RL, Taylor J, Adab N, Kini U, Baker GA. The behavioral consequences of exposure to antiepileptic drugs in utero. Epilepsy Behav. 2009;14(1):197–201. [DOI] [PubMed] [Google Scholar]
- 8. Cohen MJ, Meador KJ, Browning N, et al. Fetal antiepileptic drug exposure: motor, adaptive, and emotional/behavioral functioning at age 3 years. Epilepsy Behav. 2011;22(2):240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen MJ, Meador KJ, Browning N, et al. Fetal antiepileptic drug exposure: adaptive and emotional/behavioral functioning at age 6 years. Epilepsy Behav. 2013;29(2):308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rihtman T, Parush S, Ornoy A. Developmental outcomes at preschool age after fetal exposure to valproic acid and lamotrigine: cognitive, motor, sensory and behavioral function. Reprod Toxicol. 2013;41:115–25. [DOI] [PubMed] [Google Scholar]
- 11. Bromley RL, Calderbank R, Cheyne CP, et al. Cognition in school‐age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology. 2016;87(18):1943–53. [DOI] [PubMed] [Google Scholar]
- 12. Rasalam AD, Hailey H, Williams JH, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47(8):551–5. [DOI] [PubMed] [Google Scholar]
- 13. Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet. 2002;39(4):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veiby G, Daltveit AK, Schjølberg S, et al. Exposure to antiepileptic drugs in utero and child development: a prospective population‐based study. Epilepsia. 2013;54(8):1462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore SJ, Turnpenny P, Quinn A, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37(7):489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huber‐Mollema Y, van Iterson L, Sander JW, Oort FJ, Lindhout D, Rodenburg R. EURAP Study & Development: protocol of a Dutch prospective observational study into fetal antiepileptic drug exposure and long‐term neurocognitive, behavioral and family outcomes. Epilepsy & Behavior. 2018; 86:187–92. [DOI] [PubMed] [Google Scholar]
- 19. Verhulst F, Van der Ende J, Koot H. Handleiding Voor de CBCL/4‐18 (Nederlandse Versie) [Child Behavior Checklist 4–18 Dutch Edition Manual]. Rotterdam, the Netherlands: Sophia Kinderziekenhuis/Academisch Ziekenhuis/Erasmus Universiteit Rotterdam; 1996. [Google Scholar]
- 20. Scholte E, Van der Ploeg J. Handleiding Sociaal‐Emotionele Vragenlijst (SEV) [Social Emotional Questionnaire Manual]. Houten, the Netherlands: Bohn Stafleu van Loghum; 2007. [Google Scholar]
- 21. Achenbach T, Rescorla L. Manual for the ASEBA Adult Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- 22. Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147. [PubMed] [Google Scholar]
- 23. Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual Research Review: a meta‐analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345–65. [DOI] [PubMed] [Google Scholar]
- 24. Kaldenbach Y. WISC‐III—Profielhypothesen. 2017. [Cited 2018 July 1]. Available at: https://www.apollopraktijk.nl/ [Google Scholar]
- 25. White JR, Walczak TS, Leppik IE, et al. Discontinuation of levetiracetam because of behavioral side effects: a case‐control study. Neurology. 2003;61(9):1218–21. [DOI] [PubMed] [Google Scholar]
- 26. Halma E, de Louw AJ, Klinkenberg S, Aldenkamp AP, IJff DM, Majoie M. Behavioral side‐effects of levetiracetam in children with epilepsy: a systematic review. Seizure. 2014;23(9):685–91. [DOI] [PubMed] [Google Scholar]
- 27. Mula M, Agrawal N, Mustafa Z, et al. Self‐reported aggressiveness during treatment with levetiracetam correlates with depression. Epilepsy Behav. 2015;45:64–7. [DOI] [PubMed] [Google Scholar]
- 28. Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Twoy R, Connolly PM, Novak JM. Coping strategies used by parents of children with autism. J Am Acad Nurse Pract. 2007;19(5):251–60. [DOI] [PubMed] [Google Scholar]
- 30. Rodenburg R, Stams GJ, Meijer AM, Aldenkamp AP, Deković M. Psychopathology in children with epilepsy: a meta‐analysis. J Pediatr Psychol. 2005;30(6):453–68. [DOI] [PubMed] [Google Scholar]
- 31. Rodenburg R, Marie Meijer A, Deković M, Aldenkamp AP. Family predictors of psychopathology in children with epilepsy. Epilepsia. 2006;47(3):601–14. [DOI] [PubMed] [Google Scholar]
- 32. Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group . Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530–8. [DOI] [PubMed] [Google Scholar]
- 33. Hernandez‐Diaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692–9. [DOI] [PubMed] [Google Scholar]
- 34. Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry. 2014;85:1029–34. [DOI] [PubMed] [Google Scholar]
- 35. Huber‐Mollema Y, van Iterson L, Sander JW, Oort FJ, Lindhout D, Rodenburg R. Exposure to antiepileptic drugs in pregnancy: the need for a family factor framework. Epilepsy Behav. 2018;86:187–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


