Abstract
Objectives:
High-risk opioid-prescribing practices contribute to a national epidemic of opioid-related morbidity and mortality. The objective of this study was to determine whether the adoption of state-level opioid-prescribing guidelines that specify a high-dose threshold is associated with trends in rates of opioid overdose hospitalizations, for prescription opioids, for heroin, and for all opioids.
Methods:
We identified 3 guideline states (Colorado, Utah, Washington) and 5 comparator states (Arizona, California, Michigan, New Jersey, South Carolina). We used state-level opioid overdose hospitalization data from 2001-2014 for these 8 states. Data were based on the State Inpatient Databases and provided by the Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, via HCUPnet. We used negative binomial panel regression to model trends in annual rates of opioid overdose hospitalizations. We used a multiple-baseline difference-in-differences study design to compare postguideline trends with concurrent trends for comparator states.
Results:
For each guideline state, postguideline trends in rates of prescription opioid and all opioid overdose hospitalizations decreased compared with trends in the comparator states. The mean annual relative percentage decrease ranged from 3.2%-7.5% for trends in rates of prescription opioid overdose hospitalizations and from 5.4%-8.5% for trends in rates of all opioid overdose hospitalizations.
Conclusions:
These findings provide preliminary evidence that opioid-dosing guidelines may be an effective strategy for combating this public health crisis. Further research is needed to identify the individual effects of opioid-related interventions that occurred during the study period.
Keywords: analgesics, opioid, drug overdose, hospitalization, prescribing guidelines, policy evaluation
During the past 2 decades, changes in opioid-prescribing practices for chronic noncancer pain—including increases in average morphine-equivalent daily dose (MEDD)—contributed to a national epidemic of opioid-related hospitalizations and deaths.1-5 Numerous emerging state and national opioid-prescribing guidelines focused initially on best practices, such as opioid treatment agreements and patient risk evaluation, rather than on dosing guidance.6
High-dose opioid use is associated with substantial depression of central respiratory drive7,8 and higher opioid-related morbidity and mortality compared with lower doses.9-14 A large health plan–based study demonstrated a 9-fold increase in overdose risk at doses ≥100 mg MEDD compared with doses <20 mg MEDD.11 Although there is no manifest dose-response inflection point at which risk markedly increases,14 and consensus is lacking on the most appropriate high-dose threshold,15 opioid-prescribing guidelines that recommend avoiding high doses may decrease unintentional prescription opioid overdose.15 In 2007, Washington State became the first state to implement an opioid guideline specifying a high-dose threshold (120 mg MEDD) and associated clinical guidance.16 Other jurisdictions and organizations followed suit, including Utah in 2009 (120-200 mg MEDD) and Colorado in 2012 (120 mg MEDD).17 High-dose thresholds vary widely, from a high of 200 mg MEDD (eg, the 2009 American Pain Society/American Academy of Pain Medicine guideline) to a low of 50 mg MEDD (eg, the 2014 Centers for Disease Control and Prevention guideline) but have tended to become lower over time as more has been learned about the risks of high-dose opioids.6,15,18
Washington State workers’ compensation pharmacy data19 and Medicaid data20 showed reductions—of 50% and 16%, respectively—in the percentage of patients who were prescribed high-dose opioids (≥120 mg MEDD) after adoption of the 2007 guideline (2010 vs 2006). However, few studies have systematically evaluated the effects of opioid-prescribing guidelines on health outcomes such as mortality and opioid overdose. Prescription opioid-related mortality continued to rise in the Washington State workers’ compensation population through 2009, then dropped by about 50% in 2010.21 Studies of prescription opioid overdose using Washington State workers’ compensation data22 and Medicaid data23 were inconclusive regarding the effect of the 2007 guideline, in part because no rigorous comparison group was used. However, during the same period, national data showed a steady increase in opioid-related morbidity.22 A 2016 difference-in-differences study found that the combined implementation of a mandated prescription drug monitoring program (state-based electronic databases that collect dispensing data for controlled prescription drugs) review and pain clinic laws (which impose requirements such as state registration, physician ownership, prescribing restrictions, and record keeping) reduced prescribed opioids by 80 morphine equivalents per state resident per year and reduced annual prescription opioid overdose mortality by 1.2 per 100 000 state residents; however, the study did not assess opioid-dosing guidelines.24
Although the increase in the prescription opioid-related death rate slowed after 2014, the heroin-related death rate continues to increase.2 A 2013 study of national overdose hospitalizations during 1993-2009 showed strong bidirectional associations: each prescription opioid overdose admission predicted an increase in the subsequent year’s heroin overdose admissions by a factor of 1.26, and each heroin overdose admission predicted an increase in the subsequent year’s prescription overdose admissions by a factor of 1.57. On the basis of these findings, the authors suggested that policies restricting prescription opioid availability could have the unintended consequence of increasing heroin-related morbidity.25 However, Dowell et al24 found no evidence that mandated prescription drug monitoring program review and pain clinic laws resulted in increased heroin-related overdose death rates (ie, no association between restricted prescription opioid supply and consequent heroin substitution); rather, the authors suggested that such policies may reduce heroin initiation by reducing population exposure to prescription opioids. A 2017 study by Tedesco et al26 found that national emergency department and inpatient discharge rates for prescription opioid overdose began to decline around 2010, whereas discharge rates for heroin overdose began to increase around 2008. Although many factors drive opioid overdose trajectories, the pattern reported by Tedesco et al26 is more compatible with the hypothesis that exposure to high-risk opioid prescribing led to an increase in heroin misuse and morbidity than with the hypothesis that effective opioid guidelines and policies caused the increase via restriction of the prescription opioid supply. To support the latter hypothesis, the increase in heroin overdose rates should have occurred at or after the downturn in prescription opioid overdose trends. Finally, a review in 2016 found no consistent evidence of an association between prescription opioid policies and increases in heroin use or overdose. In most studies reviewed, increased rates of heroin use preceded implementation of prescription opioid policies.27
To reduce opioid-related morbidity and mortality, state agencies need better evidence to underpin and refine available policy approaches. In this study, we assessed associations between adoption of state-level opioid-dosing guidelines and subsequent trends in rates of opioid overdose hospitalizations—for prescription opioids, for heroin, and for all opioids.
Methods
Study Design
We compared postguideline trends in 3 guideline states with concurrent trends in 5 comparator states by using 14 years of state-level panel data on opioid overdose hospitalizations. (Panel refers to pooling data by both state and time, rather than only by time, resulting in trend estimates that account for individual state variation; using panel data and panel regression techniques minimizes aggregation bias.) Numerous factors may have played a role in reducing opioid-related morbidity, independent of state-based guidelines and policies: opioid-prescribing guidelines promulgated by national organizations (Table 1),36-38 changes in health services delivery (eg, shifts from inpatient care to outpatient care, improvements in emergency medical systems, widening naloxone distribution), increasing awareness of opioid-related risks among health care providers that might have led to improved standards of practice, and increasing public awareness of such risks that might have reduced demand for opioid prescriptions. Use of concurrent comparators was particularly important because national rates of prescription opioid overdose hospitalizations began to decline around 2010.26 The 3 guideline states adopted their opioid-dosing guidelines in different years (2007, 2009, and 2012), setting the stage for a multiple-baseline natural experiment.39 We used a difference-in-differences study design40 to compare postguideline trends in each guideline state with concurrent trends in the 5 comparator states, controlling for state-level baseline rates and for trends before the guideline adoption year. On the basis of the steep increases in rates of opioid overdose hospitalizations observed nationally during most of this period, we would expect average postguideline rates to remain higher than average preguideline rates. Therefore, this analysis focused on assessing changes in trends across periods: whether adoption of state-based guidelines and policies was associated with a flattening or a reversal of the rapidly increasing preguideline trends.
Table 1.
State and national opioid-dosing guidelines and policies, 2001-2014a
| Entity | Date | High-Dose Threshold and Related Guidance | Enforcement | Method of Publication/Dissemination |
|---|---|---|---|---|
| Guideline states | ||||
| Colorado Division of Workers’ Compensation | February 2012 | Increase clinical vigilance and consider pain consult at >120 mg MEDD; avoid >200 mg MEDD28 | Enforceable per state rules | Website |
| Colorado Department of Regulatory Agencies | July 2014 | Increase clinical vigilance and consider pain consult at ≥120 mg MEDD29 | Voluntary | Website |
| Utah Department of Health | March 2009 | Increase clinical vigilance and consider pain consult at >120-200 mg MEDD30 | Voluntary | Journal article; website |
| Washington State Agency Medical Directors’ Group | March 2007 | Document functional improvement or seek expert pain consult at >120 mg MEDD16 | Voluntary | Website |
| Washington State Agency Medical Directors’ Group | June 2010 | Guideline updated; >120 mg MEDD16 | Voluntary | Website |
| Washington State | July 2011 | Pain management rules for dentistry, podiatry, nursing, osteopathy; >120 mg MEDD31 | Enforceable per state rules | Website |
| Washington State | January 2012 | Pain management rules for medicine; >120 mg MEDD31 | Enforceable per state rules | Website |
| Washington State Department of Labor and Industries | July 2013 | Guideline updated; >120 mg MEDD32 | Enforceable via payer policies | Website |
| Comparator states | ||||
| Arizona Department of Health Services | November 2014b | Reevaluate therapy at >50-100 mg MEDD33 | Voluntary | Website |
| Medical Board of California | November 2014b | Increase clinical vigilance and consider specialist referral at ≥80 mg MEDD34 | Voluntary | Website |
| Michigan | NA | No qualifying guideline identified | NA | NA |
| New Jersey | NA | No qualifying guideline identified | NA | NA |
| South Carolina Boards of Medical Examiners, Dentistry, and Nursing | November 2014b | Reassess when ≥80 mg MEDD for >3 continuous months35 | Enforceable per state rules | Website |
| National organizations | ||||
| American College of Occupational and Environmental Medicine | December 2014 | Recommend maximum of 50 mg MEDD for acute or chronic pain36 | Voluntary | Journal article; membership-restricted website |
| American Pain Society/American Academy of Pain Medicine | February 2009 | Increased monitoring at >200 mg MEDD37 | Voluntary | Journal article; website |
| American Society of Interventional Pain Physicians | July 2012 | Consider pain management consult at >90 mg MEDD38 | Voluntary | Journal article; website |
Abbreviations: MEDD, morphine-equivalent daily dose; NA, not applicable.
aNonqualifying opioid-prescribing guidelines were excluded. A guideline or policy was considered as qualifying if it (1) was adopted before 2015, (2) was applicable statewide (although not necessarily to all payers), and (3) contained guidance related to any specified high-dose opioid threshold.
bHigh-dose guidelines were adopted by 3 states in November 2014 (Arizona, California, and South Carolina). These 3 states were identified as comparators because the timing of guideline adoption was considered too late to markedly affect opioid overdose rates during the study period.
Data Source and Samples
HCUPnet is a free online resource for data summaries from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (HCUP).41 Using HCUPnet, we downloaded annual state-level hospital discharge data summaries from the State Inpatient Databases (SID) for a convenience sample of 8 states that satisfied 2 selection criteria. The first criterion was that we had definitively identified timing and the presence or absence of statewide opioid guidelines with a high-dose threshold. (We had conducted this work in preparation for a separate planned study focused on injured workers; our SID sample was limited by criteria not relevant to the present study, such as workers’ compensation being a distinct payer category.) The second criterion was that SID data were publicly available via HCUPnet from 2001 through 2014 for each diagnosis code listed in the Opioid Overdose Hospitalizations section (ie, data had not been redacted by HCUPnet because of small numbers). Application of these criteria resulted in 3 guideline states (Colorado, Utah, and Washington) and 5 comparator states (Arizona, California, Michigan, New Jersey, and South Carolina).
Qualifying Opioid Guidelines and Policies
We searched peer-reviewed literature, gray literature, and the internet for qualifying opioid-prescribing guidelines or policies. A qualifying guideline or policy (1) was adopted before 2015, (2) was applicable statewide (but might apply only to a subset of patients, such as injured workers under state workers’ compensation rules), and (3) contained guidance related to any specified high-dose opioid threshold. We queried state agencies as needed to resolve ambiguity in policy details or timing. In addition, we queried workers’ compensation agencies in each state for which a qualifying workers’ compensation-specific guideline was not identified to confirm absence of such policies through December 2014. We identified Colorado, Utah, and Washington State as states that had implemented qualifying opioid-prescribing guidelines and policies with a high-dose threshold; threshold-related guidance varied by state (Table 1). We based postguideline periods on adoption date of the first qualifying guideline or policy in each guideline state, beginning in 2012 for Colorado,17,28 2009 for Utah,30 and 2007 for Washington State.16 Each guideline state adopted the initial qualifying guideline or policy in the first quarter of the postguideline period. In Colorado29 and Washington State,32 updates and implementation by different state agencies led to multiple adoption dates.
The 2012 Colorado guideline, adopted by the Division of Workers’ Compensation, was enforceable with respect to chronic pain treatment of injured workers and recommended clinical vigilance >120 mg MEDD and avoidance of doses >200 mg MEDD.28 The 2009 Utah guideline, adopted by the Utah Department of Health, was voluntary and recommended increasing clinical vigilance at daily doses higher than 120-200 mg MEDD.30 The 2007 Washington State guideline, adopted by the Washington State Agency Medical Directors’ Group (representing all publicly funded health insurance plans in Washington State), recommended avoiding doses >120 mg MEDD for patients who did not have clinically meaningful improvement in pain and function, without first obtaining a pain specialist consultation.16 This voluntary guideline was implemented as an educational pilot consisting of presentations to provider groups and free web-based continuing medical education trainings. The 2007 guideline was followed by legislation (effective June 10, 2010)31 mandating that new administrative opioid-prescribing rules be developed for professions that prescribe opioids; those rules took effect in July 2011 and January 2012.42
Three states implemented high-dose guidelines in November 2014 (Arizona,33 California,34 and South Carolina35). We classified these states as comparator states because their guideline adoption dates occurred too late to markedly affect overdose rates during the study period.
Opioid Overdose Hospitalizations
We classified opioid overdose hospitalizations by using the following International Classification of Diseases, Ninth Revision, Clinical Modification codes43: (1) prescription opioid overdose (965.00, 965.02, or 965.09), (2) heroin overdose (965.01), and (3) all opioids (prescription opioid overdose and heroin overdose combined). We used the principal diagnosis code rather than all available diagnosis codes (1) to ensure a consistent case definition, because differences in the number of available diagnosis fields—over time and across states—can affect the degree of case ascertainment, and (2) to avoid incidental inclusion of opioid-related events that occurred during the hospital stay, rather than being its principal cause.44,45 We calculated rates by using US Census Bureau estimates of resident population by state and year.46
Analytic Approach
We used negative binomial panel regression to model trends in annual opioid overdose hospitalizations, adjusting for resident population denominators and controlling for state-level baseline rates and for trends before the guideline adoption year.47-49 We bootstrapped bias-corrected and accelerated 95% confidence intervals (CIs), which correct for bias and skewness in the distribution of bootstrap estimates,50 by using 1000 replications (random sampling with replacement), while accounting for state-level panel structure. We conducted analyses by using Stata/SE 15.0 for Windows.51
For each of the 3 guideline states, we specified separate models for overdose hospitalizations resulting from (1) prescription opioids, (2) heroin, and (3) all opioids. Each model included 1 guideline state panel and 5 comparator state panels; trends for all 6 states in each model were interrupted at the relevant guideline adoption year (ie, Washington State preguideline period [2001-2006] and postguideline period [2007-2014], Colorado preguideline period [2001-2008] and postguideline period [2009-2014], Utah preguideline period [2001-2011] and postguideline period [2012-2014]). Thus, trends for the 5 comparator states were interrupted at 3 different years in separate models, enabling the multiple-baseline design. Each model included variables representing guideline state vs comparator state (binary), calendar year (continuous), pre-post guideline adoption year (binary), and saturated interaction terms. The 3-way interaction term (guideline state × year × pre-post guideline period) represented the postguideline trend for the guideline state relative to the 5 comparator states (ie, divergence in opioid overdose trend associated with dosing guideline implementation). Because of observed variation among comparator states, we conducted sensitivity analyses to assess each state’s contribution. For each regression model, we omitted 1 of 5 comparator states in turn (ie, using 5 sets of 4 comparators each). We also conducted post hoc sensitivity analyses for Washington State—specifying the guideline year as 2010 or 2012 instead of 2007—in alignment with later policy events in that state (Table 1).
Results
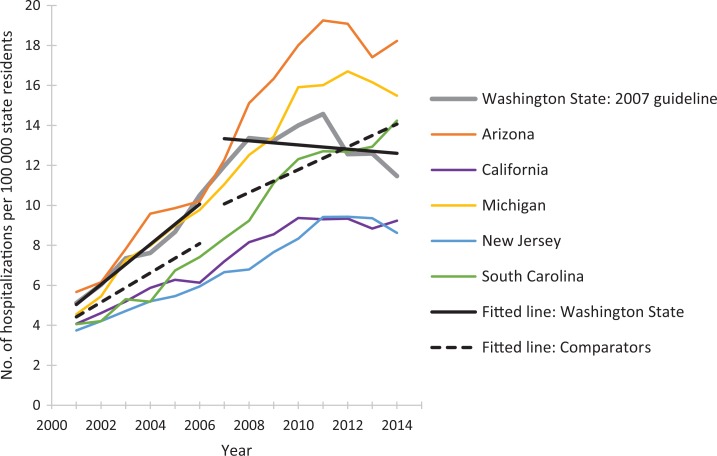
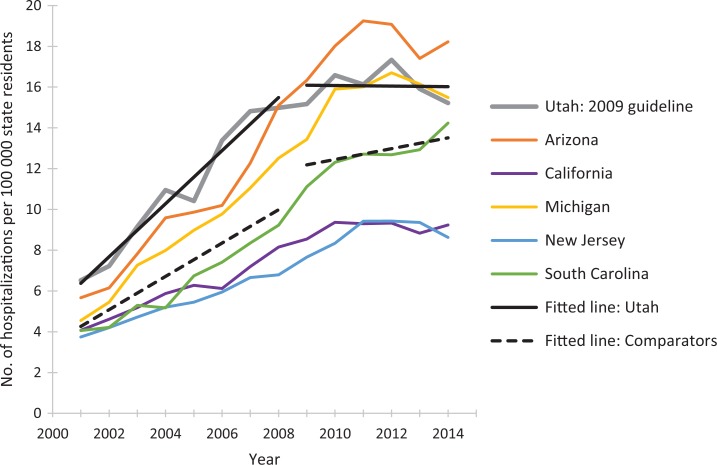
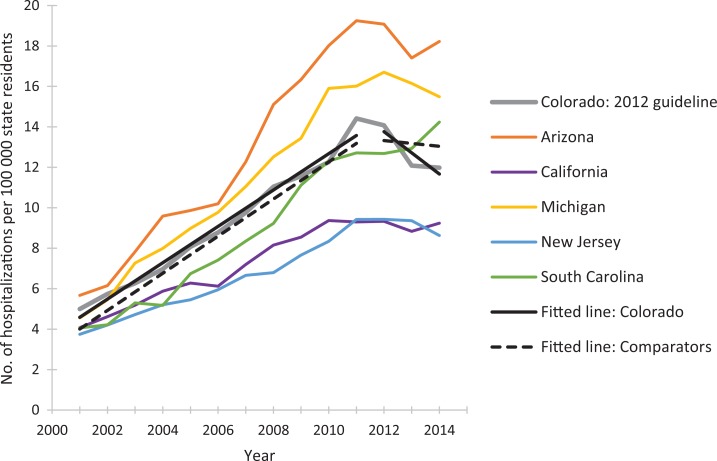
The postguideline fitted linear trend in rates of prescription opioid overdose hospitalizations for Washington State decreased compared with the 5 comparator states combined (Figure 1). We observed similar guideline vs comparator patterns during the postguideline periods for Utah (Figure 2) and Colorado (Figure 3). As the guideline years progressed (ie, across the baselines of 2007, 2009, and 2012), the trajectories for postguideline rates of prescription opioid overdose hospitalizations began to decrease for the comparator states collectively, yet trajectories for each guideline state decreased even more than for comparator states. These figures show linear regression lines fit to rate data and can be considered approximate representations of the negative binomial regression models; however, they do not incorporate control for state-level rates and preguideline trends, as the regression models do.
Figure 1.
Rates of prescription opioid overdose hospitalizations for Washington State and for 5 comparator states, 2001-2014. Linear-fitted trend lines were interrupted as of 2007, the year at which a guideline for opioid dosing was implemented in Washington State. The linear-fitted trend segments running from 2007 through 2014 represent postguideline trend divergence. Data source: Healthcare Cost and Utilization Project, HCUPnet.41
Figure 2.
Rates of prescription opioid overdose hospitalizations for Utah and for 5 comparator states, 2001-2014. Linear-fitted trend lines were interrupted as of 2009, the year at which a guideline for opioid dosing was implemented in Utah. The linear-fitted trend segments running from 2009 through 2014 represent postguideline trend divergence. Data source: Healthcare Cost and Utilization Project.41
Figure 3.
Rates of prescription opioid overdose hospitalization for Colorado and 5 comparator states, 2001-2014. Linear-fitted trend lines were interrupted as of 2012, the year at which a guideline for opioid dosing was implemented in Colorado. The linear-fitted trend segments running from 2012 through 2014 represent postguideline trend divergence. Data source: Healthcare Cost and Utilization Project.41
The results of the negative binomial regression models confirmed the impressions made by the figures, and all reported results were significant (Table 2). Sensitivity analyses, which omitted each of the 5 comparator states in turn, had no substantial effect on findings.
Table 2.
Mean annual trends in rates of opioid overdose hospitalizations for 3 states with opioid-prescribing guidelines and 5 states without opioid-prescribing guidelines, beginning in the guideline adoption year, United States, 2001-2014a
| State | Guideline Adoption Year |
Mean Annual Trend Divergence, %
(Bootstrapped BCa 95% CI) |
|---|---|---|
| Prescription opioid overdose | ||
| Washington State | 2007 | −7.5 (−8.7 to −5.1) |
| Utah | 2009 | −3.2 (−4.7 to −1.2) |
| Colorado | 2012 | −7.2 (−9.3 to −3.7) |
| Heroin overdose | ||
| Washington State | 2007b | 7.5 (0.2 to 15.2) |
| Utah | 2009 | −15.6 (−23.1 to −8.3) |
| Colorado | 2012 | −13.1 (−20.0 to −1.8) |
| All opioid overdose | ||
| Washington State | 2007 | −5.5 (−9.2 to −2.8) |
| Utah | 2009 | −5.4 (−11.8 to −2.9) |
| Colorado | 2012 | −8.5 (−10.7 to −4.1) |
Abbreviations: BCa, bias-corrected and accelerated; CI, confidence interval.
aData source: Healthcare Cost and Utilization Project.41 Each estimate is from a separate difference-in-differences negative binomial regression model. Each model included 1 guideline state panel and 5 comparator state panels (Arizona, California, Michigan, New Jersey, South Carolina); trends for all 6 states in each model were interrupted at the relevant guideline adoption year (ie, for Washington State, the preguideline time period was 2001-2006 and the postguideline time period was 2007-2014; for Colorado, the preguideline period was 2001-2008 and the postguideline period was 2009-2014; and for Utah, the preguideline period was 2001-2011 and the postguideline period was 2012-2014). To ease interpretation, coefficients were translated to mean annual trend divergence percentages (eg, −7.5% signifies that the postguideline trend for the specified guideline state diverged downward from that of the 5 comparator states in the amount of 7.5% per year on average [and would have been translated from an exponentiated 3-way interaction term of 0.925]). BCa 95% CIs were bootstrapped by using 1000 replications (random sampling with replacement), while accounting for state-level panel structure. BCa 95% CIs correct for bias and skewness in the distribution of bootstrap estimates.
bSee the Results section for results of sensitivity analyses specifying later pre-post interruption years.
For each guideline state, trends in postguideline rates of overdose hospitalizations for prescription opioids and for all opioids decreased significantly compared with trends in comparator states, adjusting for state-based differences in preguideline rates and trends. The mean annual relative percentage decrease ranged from 3.2% to 7.5% for trends in rates of prescription opioid overdose hospitalizations and from 5.4% to 8.5% for trends in rates of all opioid overdose hospitalizations (Table 2).
In parallel models for heroin overdose hospitalizations in Colorado and Utah, trends in postguideline rates of heroin overdose hospitalizations decreased significantly in guideline states compared with trends in the 3 comparator states, even more steeply than for prescription opioid overdoses (Table 2). In contrast, trends in postguideline rates of heroin overdose hospitalizations in Washington State increased significantly relative to trends in the 5 comparator states. To explore this finding, we conducted sensitivity analyses, specifying the guideline year as 2010 or 2012. During 2010 through 2014, the mean annual trend divergence for heroin overdose was not significantly different for Washington State compared with the comparator states (–0.02%; 95% CI, –11.2% to 9.0%). During 2012 through 2014—after the new professional rules for opioid prescribers had been implemented—trends in rates of heroin overdose hospitalizations in Washington State decreased significantly compared with trends in the comparator states (–13.1%; 95% CI, –22.4% to –3.1%), to a similar degree as for Colorado and Utah.
Discussion
We found that trends in rates of opioid overdose hospitalizations for prescription opioids and for all opioids decreased significantly in each of the guideline states compared with the 5 comparator states, after adoption of opioid-prescribing guidelines. These findings suggest a net beneficial effect on trends in rates of opioid overdose hospitalizations in all 3 guideline states.
The postguideline effect in Colorado was similar to that in other guideline states, even though Colorado’s guideline was promulgated by the Division of Workers’ Compensation and, thus, not applicable to patients covered by other payers. It may be that providers changed their opioid-prescribing practices for all patients as they changed them for injured workers.52
In Colorado and Utah, guideline adoption was associated with subsequent relative decreasing trends in rates of heroin overdose hospitalizations. In Washington State, although postguideline trends in rates of heroin overdose hospitalizations increased significantly compared with trends in comparator states, this pattern shifted when we conducted sensitivity analyses specifying 2010 or 2012 in place of 2007 as the guideline year. By 2012—when the Washington State guideline had become compulsory—trends in rates of heroin overdose hospitalizations in Washington State were decreasing significantly compared with trends in comparator states. This pattern suggests the importance of preguideline contributors to rising trends in rates of heroin overdose hospitalizations, including high-risk prescribing itself, and is not well-explained simply by restrictions in prescription opioid supply related to prescribing guidelines—if it were the latter, the effect of any such supply restrictions should have increased as prescribing guidelines became more effective. These findings comport with research showing that rates of heroin overdose hospitalizations began to increase in 2008, before rates of prescription opioid overdose hospitalizations began to decline.26,27
Opioid-prescribing guidelines can serve as (1) primary prevention by reducing unnecessary initial opioid exposure, (2) secondary prevention by minimizing the risks associated with high-dose opioid prescribing and certain concurrent medications, and (3) tertiary prevention by providing clear guidance for dosage tapering and treatment of opioid use disorder. If effective, prescribing guidelines may serve as an important primary prevention tool. Opioid-prescribing interventions may be less effective if targeted after a high-dose or chronic prescribing threshold has already been reached.53
States have experimented with various policy approaches to stem the tide of opioid-related morbidity and mortality. In addition to prescribing guidelines and policies, these efforts include pain clinic laws, naloxone distribution (intended to increase access to the medication used for reversal of opioid overdose), and prescription drug monitoring programs.24,54 Ongoing systematic evaluation of interventions aimed at reducing opioid-related morbidity and mortality is crucial to inform public health efforts. However, identifying which policy components are most effective and identifying suitable comparators can pose major challenges.54-56 A centralized catalog of all opioid-related state and national guidelines and policies, including features, intervention details, and implementation timing, may facilitate future evaluation efforts. Early efforts at such catalogs are promising57-59 but have limited scope or lack sufficient detail about intervention features and/or implementation timing to be adequate for the complex models required to isolate the individual effects of each policy intervention. Publicly available quarterly hospital discharge data would facilitate assessing lags in implementation and dissemination, testing effects of serial updates and improvements, and pinpointing changes in outcome trajectories.
Strengths and Limitations
This study had several strengths. First, to our knowledge, this study is the first to assess state-level opioid-dosing guidelines using comparator states to control for secular trends related to national guidelines, changes in health service delivery, and other factors that might affect opioid overdose trends across states. Second, guideline implementation dates differed across states, providing 3 baseline years for assessing guideline impact. This multiple-baseline approach allowed us to demonstrate repeated patterns of association between the policy intervention and outcome, at various points in time and in various jurisdictions, which adds to the weight of evidence that the policy intervention had an effect.39 Third, the inclusion of 8 states, representing various geographic regions and more than a quarter of the US population, enhanced the generalizability of our findings. Finally, our findings were robust to the specification of differing sets of comparator states.
This study also had several limitations. First, using hospital discharge data, we captured a large share of opioid-related morbidity but not the full scope. For example, >40% of injured workers with an opioid overdose covered by Washington State workers’ compensation were treated on an inpatient basis22; nationally, >50% of persons presenting to an emergency department with a prescription opioid overdose were admitted to the hospital.60 In emergency department data, unlike hospital discharge data, first-listed diagnosis is not equivalent to principal diagnosis.61 Hence, to enable a consistent case definition based on principal diagnosis, and comporting with surveillance recommendations, we did not include emergency department visits in this study. Basing our opioid overdose definitions on principal diagnosis presumably undercounted cases but should minimize bias in cross-state comparisons over time.
Second, trends in rates of opioid overdose hospitalizations are known to vary substantially by region62; we partially addressed this issue by including states from all 4 US Census regions. Opioid overdose rates for the comparator states were similar to rates for the guideline states in 2001, and they all trended steeply upward in roughly comparable fashion through at least 2007. The study design and statistical models adjusted for differences in state-level opioid overdose hospitalization rates and trends before each guideline year.
Third, although we found a strong association between guideline implementation and opioid-related morbidity trends, the observational study design could not rule out other causal factors, including national, state, and local opioid-related interventions. The difference-in-differences design mitigated the effects of national policies and secular trends, but other effective state-level opioid policies may interfere with identifying the effect of opioid-dosing guidelines. For example, the Utah Department of Health conducted a media campaign and launched a statewide provider education intervention during roughly the same time frame that the 2009 guideline was adopted. Guideline states may have internal characteristics or policy environments leading both to guideline adoption and to reduced opioid-related morbidity (ie, policy endogeneity63). By including 3 comparator states that adopted opioid-prescribing guidelines in November 2014 (Arizona, California, and South Carolina), we strengthened comparison group validity and at least partially mitigated potential policy endogeneity. Their classification as comparator states was also a conservative approach that would serve to shrink postguideline trend differences between comparator states and guideline states and reduce the ability to detect any actual guideline effect, because trends for these 3 comparison states would be subject to potential guideline effects at the end of 2014.
Fourth, opioid-prescribing guidelines may have a varying effect based on being voluntary or enforceable or based on their source. For example, guidelines adopted by a medical board may be disseminated primarily to physicians, and guidelines adopted by a workers’ compensation agency may not pertain to all opioid prescribers. Although we included policies imposed by state agencies, other payers may set their own high-dose thresholds or prior-authorization requirements, and we were not able to identify or control for such policies. Finally, other features of opioid-prescribing guidelines, such as recommendations against prescribing opioids concurrently with other medications that increase overdose risk (eg, sedatives, hypnotics, benzodiazepines, muscle relaxants),10,12 may be as important as the high-dose threshold.
Conclusions
We found that the adoption of opioid-prescribing guidelines by 3 states between 2007 and 2012 was associated with decreasing trends in rates of prescription opioid—and all opioid—overdose hospitalizations, relative to 5 concurrent comparator states. These findings provide preliminary evidence that opioid-dosing guidelines may be an effective state- and national-level strategy for combating this public health crisis. Further research is needed to identify the individual effects of the many opioid-related interventions that occurred during the study period.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jeanne M. Sears, PhD, MS, RN  https://orcid.org/0000-0002-7325-1279
https://orcid.org/0000-0002-7325-1279
References
- 1. Paulozzi L, Baldwin G, Franklin G. et al. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–13. [PubMed] [Google Scholar]
- 2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015 [published correction appears in MMWR Morb Mortal Wkly Rep. 2017;66(1):35]. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. [DOI] [PubMed] [Google Scholar]
- 3. Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R. Hospital Inpatient Utilization Related to Opioid Overuse Among Adults, 1993-2012. Statistical Brief No. 177 Rockville, MD: US Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 4. Franklin GM, Mai J, Wickizer T, Turner JA, Fulton-Kehoe D, Grant L. Opioid dosing trends and mortality in Washington State workers’ compensation, 1996-2002. Am J Ind Med. 2005;48(2):91–99. doi:10.1002/ajim.20191 [DOI] [PubMed] [Google Scholar]
- 5. Caravati EM, Grey T, Nangle B, Rolfs RT, Peterson-Porucznik CA. Increase in poisoning deaths caused by non-illicit drugs—Utah, 1991-2003. MMWR Morb Mortal Wkly Rep. 2005;54(2):33–36. [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Common elements in guidelines for prescribing opioids for chronic pain. 2014. https://stacks.cdc.gov/view/cdc/37319. Accessed March 29, 2019.
- 7. Walker JM, Farney RJ, Rhondeau SM. et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3(5):455–461. [PMC free article] [PubMed] [Google Scholar]
- 8. White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94(7):961–972. [PubMed] [Google Scholar]
- 9. Bohnert AS, Valenstein M, Bair MJ. et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi:10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 10. Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality [published correction appears in Pain Med. 2016;17(4):797-798. doi:10.1093/pn/pnw044]. Pain Med. 2016;17(1):85–98. doi:10.1111/pme.12907 [DOI] [PubMed] [Google Scholar]
- 11. Dunn KM, Saunders KW, Rutter CM. et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi:10.7326/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garg RK, Fulton-Kehoe D, Franklin GM. Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Med Care. 2017;55(7):661–668. doi:10.1097/MLR.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 13. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi:10.1001/archinternmed.2011.117 [DOI] [PubMed] [Google Scholar]
- 14. Coyle DT, Pratt CY, Ocran-Appiah J, Secora A, Kornegay C, Staffa J. Opioid analgesic dose and the risk of misuse, overdose, and death: a narrative review. Pharmacoepidemiol Drug Saf. 2018;27(5):464–472. doi:10.1002/pds.4366 [DOI] [PubMed] [Google Scholar]
- 15. Heins SE, Frey KP, Alexander GC, Castillo RC. Reducing high-dose opioid prescribing: state-level morphine equivalent daily dose policies, 2007-2017. Pain Med. 2019:pnz038. doi:10.1093/pm/pnz038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Washington State Agency Medical Directors’ Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy. 2010. http://www.agencymeddirectors.wa.gov/files/opioidgdline.pdf. Accessed March 29, 2019.
- 17. Nuckols TK, Anderson L, Popescu I. et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38–47. doi:10.7326/0003-4819-160-1-201401070-00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. doi:10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garg RK, Fulton-Kehoe D, Turner JA. et al. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J Pain. 2013;14(12):1620–1628. doi:10.1016/j.jpain.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 20. Sullivan MD, Bauer AM, Fulton-Kehoe D. et al. Trends in opioid dosing among Washington State Medicaid patients before and after opioid dosing guideline implementation. J Pain. 2016;17(5):561–568. doi:10.1016/j.jpain.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 21. Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325–331. [DOI] [PubMed] [Google Scholar]
- 22. Fulton-Kehoe D, Garg RK, Turner JA. et al. Opioid poisonings and opioid adverse effects in workers in Washington State. Am J Ind Med. 2013;56(12):1452–1462. doi:10.1002/ajim.22266 [DOI] [PubMed] [Google Scholar]
- 23. Fulton-Kehoe D, Sullivan MD, Turner JA. et al. Opioid poisonings in Washington State Medicaid: trends, dosing, and guidelines. Med Care. 2015;53(8):679–685. doi:10.1097/MLR.0000000000000384 [DOI] [PubMed] [Google Scholar]
- 24. Dowell D, Zhang K, Noonan RK, Hockenberry JM. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Aff (Millwood). 2016;35(10):1876–1883. doi:10.1377/hlthaff.2016.0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993-2009. PLoS One. 2013;8(2):e54496 doi:10.1371/journale.pone.0054496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tedesco D, Asch SM, Curtin C. et al. Opioid abuse and poisoning: trends in inpatient and emergency department discharges. Health Aff (Millwood). 2017;36(10):1748–1753. doi:10.1377/hlthaff.2017.0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154–163. doi:10.1056.BEHNra1508490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. State of Colorado Department of Labor and Employment, Division of Workers’ Compensation. Chronic pain disorder medical treatment guidelines. 2012. https://www.colorado.gov/pacific/sites/default/files/CPD_Guideline%20with%20in-text%20references.pdf. Accessed March 29, 2019.
- 29. Colorado Department of Regulatory Agencies, Division of Professions and Occupations. Policy for prescribing and dispensing opioids. 2014. https://drive.google.com/file/d/0B-K5DhxXxJZbd01vVXdTTklZLVU/view. Accessed March 29, 2019.
- 30. Rolfs RT, Johnson E, Williams NJ, Sundwall DN. Utah clinical guidelines on prescribing opioids for treatment of pain. J Pain Palliat Care Pharmacother. 2010;24(3):219–235. doi:10.3109/15360288.2010.503265 [DOI] [PubMed] [Google Scholar]
- 31. Engrossed Substitute House Bill 2876, chapter 209, Laws of 2010; effective date June 10, 2010.
- 32. Washington State Department of Labor and Industries. Guideline for prescribing opioids to treat pain in injured workers. 2013. http://www.lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALOpioidGuideline010713.pdf. Accessed March 29, 2019. [DOI] [PubMed]
- 33. Arizona Department of Health Services. Arizona opioid prescribing guidelines. 2014. http://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Accessed March 29, 2019.
- 34. Medical Board of California. Guidelines for prescribing controlled substances for pain. 2014. http://www.mbc.ca.gov/Licensees/Prescribing/Pain_Guidelines.pdf. Accessed March 29, 2019.
- 35. South Carolina Boards of Medical Examiners, Dentistry and Nursing. Joint revised pain management guidelines. 2014. http://www.llr.state.sc.us/POL/Medical/PDF/Joint_Revised_Pain_Management_Guidelines.pdf. Accessed March 29, 2019.
- 36. Hegmann KT, Weiss MS, Bowden K. et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143–e159. doi:10.1097/JOM.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 37. Chou R, Fanciullo GJ, Fine PG. et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi:10.1016/j.jpain.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manchikanti L, Abdi S, Atluri S. et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2—guidance. Pain Physician. 2012;15(suppl 3):S67–S116. [PubMed] [Google Scholar]
- 39. Hawkins NG, Sanson-Fisher RW, Shakeshaft A, D’Este C, Green LW. The multiple baseline design for evaluating population-based research. Am J Prev Med. 2007;33(2):162–168. doi:10.1016/j.amepre.2007.03.020 [DOI] [PubMed] [Google Scholar]
- 40. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401–2402. doi:10.1001/jama.2014.16153 [DOI] [PubMed] [Google Scholar]
- 41. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). HCUPnet 2001-2014 http://hcupnet.ahrq.gov. Accessed March 29, 2019. [PubMed]
- 42. Franklin G, Sabel J, Jones CM. et al. A comprehensive approach to address the prescription opioid epidemic in Washington State: milestones and lessons learned. Am J Public Health. 2015;105(3):463–469. doi:10.2105/AJPH.2014.302367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ingenix. ICD-9-CM for Hospitals—Volumes 1, 2, & 3 for Hospitals, Professional Edition. St Louis, MO: Elsevier; 2011. [Google Scholar]
- 44. Planning Comprehensive Injury Surveillance in State Health Departments Working Group. Consensus Recommendations for Injury Surveillance in State Health Departments. Atlanta, GA: State and Territorial Injury Prevention Directors Association; 2007. [Google Scholar]
- 45. Thomas KE, Johnson RL. State Injury Indicators Report: Instructions for Preparing 2016 Mortality Data. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2018. [Google Scholar]
- 46. US Census Bureau. Population and housing unit estimates tables. 2018. https://www.census.gov/programs-surveys/popest/data/tables.html. Accessed June 14, 2019.
- 47. Bailer AJ, Reed LD, Stayner LT. Modeling fatal injury rates using Poisson regression: a case study of workers in agriculture, forestry, and fishing. J Safety Res. 1997;28(3):177–186. doi:10.1016/S0022-4375(97)80006-0 [Google Scholar]
- 48. Liu W, Cela J. Count data models in SAS. 2008. https://pdfs.semanticscholar.org/55fe/536b4f0879b8f04d9e45b70d38a3a0b403a6.pdf?_ga=2.163271292.1599869234.1560182268-527972850.1560182268. Accessed June 10, 2019
- 49. Hilbe JM. Negative Binomial Regression. 2nd ed New York, NY: Cambridge University Press; 2011. [Google Scholar]
- 50. Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82(397):171–185. [Google Scholar]
- 51. StataCorp LLC. Stata Release 15 [computer software]. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 52. Elam K, Taylor V, Ciol MA, Franklin GM, Deyo RA. Impact of a worker’s compensation practice guideline on lumbar spine fusion in Washington State. Med Care. 1997;35(5):417–424. [DOI] [PubMed] [Google Scholar]
- 53. Buttorff C, Trujillo AJ, Castillo R, Vecino-Ortiz AI, Anderson GF. The impact of practice guidelines on opioid utilization for injured workers. Am J Ind Med. 2017;60(12):1023–1030. doi:10.1002/ajim.22779 [DOI] [PubMed] [Google Scholar]
- 54. Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi:10.1016/j.drugalcdep.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lagarde M. How to do (or not to do)…assessing the impact of a policy change with routine longitudinal data. Health Policy Plan. 2012;27(1):76–83. doi:10.1093/heapol/czr004 [DOI] [PubMed] [Google Scholar]
- 56. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109–118. doi:10.5055/jom.2016.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brandeis University. Prescription Drug Monitoring Program Training and Technical Assistance Center. https://www.pdmpassist.org. Accessed April 16, 2019.
- 58. Wickramatilake S, Zur J, Mulvaney-Day N, Klimo MC, Selmi E, Harwood H. How states are tackling the opioid crisis. Public Health Rep. 2017;132(2):171–179. doi:10.1177/0033354916688206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. National Alliance for Model State Drug Laws. https://namsdl.org. Accessed April 16, 2019.
- 60. Tadros A, Layman SM, Davis SM, Davidov DM, Cimino S. Emergency visits for prescription opioid poisonings. J Emerg Med. 2015;49(6):871–877. doi:10.1016/j.jemermed.2015.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Senathirajah M, Owens P, Mutter R, Nagamine M. Special Study on the Meaning of the First-Listed Diagnosis on Emergency Department and Ambulatory Surgery Records. HCUP Methods Series Report no 2011-03 Rockville, MD: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 62. Unick GJ, Ciccarone D. US regional and demographic differences in prescription opioid and heroin-related overdose hospitalizations. Int J Drug Policy. 2017;46:112–119. doi:10.1016/j.drugpo.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Besley T, Case A. Unnatural experiments? Estimating the incidence of endogenous policies. Econ J. 2000;110(467):F672–F694. [Google Scholar]