Abstract
Background
Spinal muscular atrophy (SMA) causes progressive respiratory muscle weakness but respiratory function (RF) in those using noninvasive ventilation (NIV) is not well described.
Objective
To describe RF in childhood SMA and assess differences between those using and not using NIV.
Methods
A cross‐sectional study of childhood SMA assessed polysomnography (PSG), spirometry, forced oscillation technique (FOT), lung clearance index (LCI), sniff nasal inspiratory pressures, peak cough flow, maximal inspiratory and expiratory pressure, and NIV use and indication.
Results
Twenty‐five children (median age [interquartile range], 8.96 [5.63] years; 10 F) with SMA 1 (n = 3), 2 (n = 15), and 3 (n = 7) were recruited. Spirometry and FOT testing was feasible in children as young as 3 years. Ten (40%) required NIV, 5 for sleep‐disordered breathing (SDB), and 5 initiated during lower respiratory tract infection (LRTI). Children requiring NIV were older (median, 10.52 vs 5.67 years; P < .02) with more abnormal forced vital capacity (FVC) z‐score (−5.70 vs −1.39, P < .02), Rsr8 z‐score (1.97 vs 0.50, P = .04), and LCI (8.84 vs 7.34, P = .01). Two had normal RF and SDB. For FVC z‐score less than −2.5 and LCI greater than 7.5, the odds ratio for NIV was 10.70 (95% confidence interval [CI], 1.39‐82.03) and 2 (95% CI, 0.40‐10.31), respectively. All children with LCI greater than 8 used NIV. FVC z‐score and LCI are associated with maximum transcutaneous carbon dioxide on PSG (r = 0.43, P < .001).
Conclusion
NIV is common in SMA. Normal RF does not exclude SDB. Children with more abnormal FVC and LCI should be considered at risk of starting NIV during/following an LRTI.
Keywords: neuromuscular disorders, noninvasive ventilation, respiratory function, sleep‐disordered breathing
Abbreviations
- AHI
apnea‐hypopnea index
- CHQ
Children's Health Queensland
- DMD
Duchenne muscular dystrophy
- FOT
forced oscillation technique
- FVC
forced vital capacity
- LCI
lung clearance index
- LRTI
lower respiratory tract infections
- MEP
maximal expiratory pressure
- MIP
maximal inspiratory pressure
- NIV
noninvasive ventilation
- PCF
peak cough flow
- PSG
polysomnography
- REM
rapid eye movement sleep
- RF
respiratory function
- Rrs8
respiratory resistance at 8 Hz
- SDB
sleep disordered breathing
- SMA
spinal muscular atrophy
- SNIP
sniff nasal inspiratory pressures
- TcCO2
transcutaneous carbon dioxide
- Xrs8
respiratory reactance at 8 Hz
1. INTRODUCTION
Spinal muscular atrophy (SMA) is a genetic neuromuscular disorder associated with progressive muscle weakness and eventual respiratory failure, which may be difficult to detect early in life. SMA has several types, with type 1 being the most severe. Children with type 1 have respiratory failure in the first year. Children with type 2 and 3 have a variable course, but most children with type 2 eventually progress onto respiratory failure, either intermittently during lower respiratory tract infections (LRTI) or chronically, requiring nocturnal noninvasive ventilation (NIV). The clinical course in type 3 is generally milder, although some children require intermittent or long term nocturnal NIV.
For some neuromuscular diseases, such as Duchenne muscular dystrophy (DMD), there are clear guidelines for performance of sleep studies, instigation of NIV and home base physiotherapy support, but the level of evidence in SMA is poor.1, 2, 3, 4 Spirometry has been the mainstay of respiratory assessment in SMA, but is volitional and young children may be unable to perform the test. Longitudinal changes in some respiratory function (RF) tests have previously been described5 but the relationship between RF and polysomnography (PSG) findings has not been extensively researched in SMA, particularly in younger children, some by this study group.6, 7 Need for respiratory assessment has become more relevant with the improved availability of NIV and newer disease‐modifying medications such as Nusinersen.
The aims of the study are to describe RF in childhood Nusinersen naive SMA and assess differences in those using NIV for sleep‐disordered breathing (SDB) or LRTI compared with those not requiring respiratory support. The authors hypothesize that children using NIV will have more abnormal RF than those not using NIV.
2. METHODS
This is a cross sectional cohort study. All children (aged 0‐18 years) with genetically confirmed 5q SMA type 1, 2, and 3 attending the Children's Health Queensland (CHQ), Brisbane, Australia during 2017 were included. This includes all children in Queensland and Northern New South Wales with SMA. The clinical records were reviewed for use of NIV and its indication.
The study was approved by the Children's Health Queensland Human Research Ethics Committee (reference no. HREC/17/QRCH/92, dated 2 April 2017).
2.1. Polysomnography
Full diagnostic PSG was performed for all subjects by standard methods (see the Supporting Information).8 Children requiring nocturnal NIV underwent NIV titration studies using a standard manual titration protocol to adjust the interface and ventilator settings and optimize breathing parameters and gas exchange. Where feasible, children requiring NIV periodically have a diagnostic component during their PSG (split study) to assess for changes in underlying PSG findings. Where this had occurred, the diagnostic only component of the sleep study was collected separately; otherwise, the last full diagnostic PSG was used as baseline data. SDB was defined as total apnea‐hypopnea index (AHI) greater than 5/h. No child in this cohort required daytime NIV.
2.2. Respiratory function tests
Multiple modalities were used to prospectively evaluate RF depending on age and cooperation of the child. Children who refused to perform a test were not forced. Respiratory function tests were reported as z‐scores where reference values exist, to allow for ready comparison across the pediatric age range.9, 10 The RF tests were spirometry, forced oscillation technique (FOT), lung clearance index (LCI), sniff nasal inspiratory pressures, peak cough flow (PCF), and maximal inspiratory and expiratory pressure. Standard methods were used (see Supporting Information).11, 12, 13, 14, 15, 16 All RF tests were performed a time distant to an LRTI.
2.3. Statistical analysis
Stata version 11 was used to analyse the results. Baseline and clinical characteristics and feasibility of testing are described. An association between PSG and RF parameters was sought using regression analysis and multivariate analysis using stepwise regression. the Mann‐Whitney U test was used to assess for differences in RF parameters for children requiring NIV and those not requiring NIV, and for those with SDB on PSG compared with those starting NIV during a chest infection. The odds ratio for needing NIV at various RF parameter cut‐offs were explored. Differences in PSG parameters during the diagnostic and NIV titration studies were assessed with the t test.
3. RESULTS
3.1. Baseline characteristics
Twenty‐five children (median age (interquartile range [IQR]) 8.96 (5.63); 10 F) were included in the analysis with a median age (IQR) of 8.96 (5.63) years. Three children had SMA type 1, 15 had SMA type 2, and 7 had SMA type 3. The baseline characteristics are summarized in Table 1. Ten children were using regular nocturnal NIV during the study period. The reason for starting NIV, age at initiation and duration of treatment before study inclusion are summarized in Table 2.
Table 1.
Summary of respiratory function tests and diagnostic sleep study findings
| n | Whole group | Type1 | Type 2 | Type 3 | P value* (types 2‐3) | |
|---|---|---|---|---|---|---|
| Age, y | 25 | 8.96 | 0.22 (5.44) | 9.30 (5.63) | 8.57 (4.67) | .90 |
| Male/female | 25 | 15/10 | 1 | 10/5 | 4/3 | .68 |
| FVC, L | 21 | 1.07 (1.01) | 0.69 | 0.87 (0.33) | 1.9 (0.63) | <.001 |
| FVC % predicted | 21 | 62 (53.2) | 57 | 51.25 (41.7) | 91.9 (12.00) | <.01 |
| FVC z‐score | 21 | −3.27 (4.74) | −3.4 | −4.27 (4.22) | −0.66 (1.09) | <.02 |
| Rsr8 z‐score | 18 | 0.77 (1.64) | – | 1.39 (1.92) | 0.29 (1.32) | <.02 |
| Xrs8 z‐score | 18 | 0.73 (1.46) | – | 1.28 (1.37) | −0.12 (0.62) | <.01 |
| LCI | 15 | 7.39 (1.74) | – | 8.06 (2.44) | 7.29 (0.55) | .13 |
| SNIP z‐score | 14 | −2.06 (2.87) | – | −2.53 (2.61) | −1.32 (2.47) | .33 |
| MIP z‐score | 12 | −0.36 (1.82) | – | −0.52 (1.57) | 0.19 (0.87) | .28 |
| MEP z‐score | 12 | −1.75 (2.2) | – | −1.76 (2.43) | −1.06 (2.22) | .53 |
| PCF, L/min | 14 | 231.9 (181.8) | – | 178.8 (246.0) | 277.8 (124.8) | .23 |
| Total AHI | 25 | 3.1 (4.9) | 7.6 (4.3) | 2.9 (2.4) | 3.1 (3.5) | .84 |
| REM AHI | 25 | 5.8 (9.1) | 20.0 (45.7) | 5.8 (9.9) | 4.6 (6.4) | .49 |
| Maximum TcCO2 | 25 | 47.8 (4.90) | 43.0 (6.20) | 48.2 (4.70) | 47.8 (6.70) | .49 |
Note: Data are presented as median (IQR).
Abbreviations: AHI, apnea‐hypopnea index; FVC, forced vital capacity; IQR, interquartile range; LCI, lung clearance index; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; PCF, peak cough flow; REM, rapid eye movement sleep; Rrs8, resistance at 8 Hz; SNIP, sniff nasal inspiratory pressure; TcCO2, transcutaneous carbon dioxide level; Xrs8, reactance at 8 Hz.
P values are calculated for the difference between type 2 and 3 spinal muscular atrophy patients.
Table 2.
Summary of NIV usage
| All | Type 1 | Type 2 | Type 3 | |
|---|---|---|---|---|
| Male/female | 6/4 | 0/1 | 5/3 | 1/0 |
| Reason for NIV (n) | ||||
| SDB | 5 | 0 | 4 | 1 |
| Chest infection (PICU) | 5 | 1 | 4 | 0 |
| Age NIV started, median (IQR), y | 7.54 (5.18) | 2.72 | 2.98 (5.21) | 7.69 |
| Duration of NIV, median (IQR), y | 3.86 (7.05) | 2.85 | 5.65 (6.53) | 0 |
Abbreviations: IQR, interquartile range; NIV, noninvasive ventilation; PICU, pediatric intensive care unit; SDB, sleep‐disordered breathing.
3.2. Feasibility
All children were able to undertake a full PSG. Not all children were able to perform the RF tests to the required standard. See Table 3. In young children, understanding, concentrating, and cooperating with testing were the limiting factors. However, preschool spirometry and FOT was feasible in children as young as 3 years, and all respiratory tests could be performed by children over 8 years. In older children with more advanced disease, weakness of the muscles of the mouth and face became more problematic for generating a good mouth seal and mouth opening could be limited due to contractures, which impeded usage of a mouthpiece, including a snorkel.
Table 3.
Summary of feasibility of lung function tests in children with Spinal muscular atrophy
| Total attempted, n | Achieved adequate test, % | Youngest age to achieve adequate test, y | Gender, males % | |
|---|---|---|---|---|
| Spirometry | 22 | 91 | 3.23 | 64 |
| FOT | 22 | 80 | 3.25 | 58 |
| LCI | 15 | 73 | 5.66 | 73 |
| SNIP | 14 | 93 | 7.19 | 79 |
| MIP | 14 | 86 | 8.17 | 83 |
| MEP | 14 | 86 | 8.17 | 83 |
| PCF | 14 | 50 | 7.12 | 71 |
Abbreviations: FOT, forced oscillation technique; LCI, lung clearance index; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; PCF, peak cough flow; SNIP, sniff nasal inspiratory pressure.
3.3. Respiratory function and PSG
The RF findings for type 1, 2, and 3 are summarized in Table 1. The time between diagnostic sleep study and performing the suite of RF tests had a median of 0.07 months (IQR, 26.76 months). Univariate regression analysis for a relationship between the RF tests and total AHI, rapid eye movement (REM) AHI, and maximum transcutaneous carbon dioxide (TcCO2) revealed the only significant relationships were for forced vital capacity (FVC) % predicted (P < .05) and FVC z‐score (P < .04) to maximum TcCO2. The LCI relationship to maximum TcCO2 was not significant (P = .17) for univariate analysis. Two outliers had normal RF, but significant SDB, see Figure 1. Stepwise multivariate regression analysis showed that the combination of FVC z‐score and LCI were more closely associated with maximum TcCO2 on PSG (r = 0.43, P < .001) than either test alone.
Figure 1.
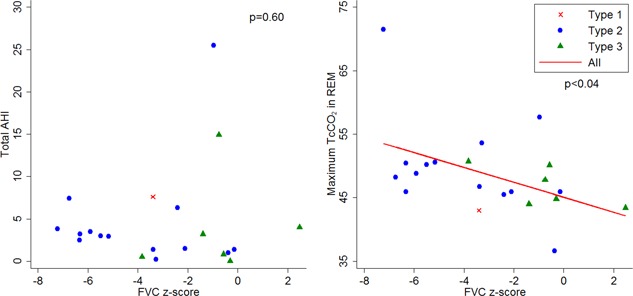
Relationship between PSG findings and FVC z‐score. AHI, apnea‐hypopnea index; FVC, forced vital capacity; PSG, polysomnography; TcCO2, transcutaneous carbon dioxide [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Noninvasive ventilation
Ten children were habitually using nocturnal NIV at the time of this study. Five began following a sleep study showing SDB, and 5 children were started during an LRTI that led to intensive care admission(s) for NIV. Three of these 5 children had contemporary PSG in the preceding 12 months that did not show significant SDB. The age of initiation and durations of NIV are summarized in Table 2.
Children with SMA requiring NIV were older (median, 10.52 vs 5.67 years; P < .02) with significantly more abnormal FVC z‐score (−5.70 vs −1.39; P < .02), Rsr8 z‐score (+1.97 vs +0.50, P = .04), and LCI (8.84 vs 7.34, P = .01) values than those not needing NIV, see Figure 2. Children needing NIV had higher total AHI (3.65 vs 1.50/h, P < .05), AHI in REM sleep (12.9 vs 4.6/h, P < .03) and maximum TcCO2 (49.5 vs 45.9 mm Hg, P < .05) than those not needing NIV. Two children had sleep hypoventilation (CO2 > 50 mm Hg for >2% total sleep time).
Figure 2.
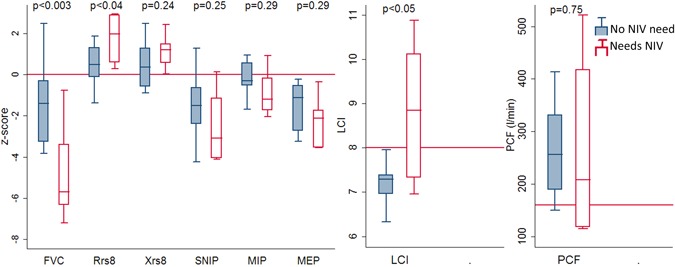
Respiratory function in children with spinal muscular atrophy who do not need noninvasive ventilation compared to those needing noninvasive ventilation. FVC, forced vital capacity; LCI, lung clearance index; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; NIV, noninvasive ventilation; PCF, peak cough flow; Rrs8, resistance at 8 Hz; SNIP, sniff nasal inspiratory pressure; Xrs8, reactance at 8 Hz [Color figure can be viewed at wileyonlinelibrary.com]
Children who were initiated on NIV during LRTI tend to have worse RF than those beginning NIV following a PSG showing SDB, but this did not reach statistical significance for any RF test value, see Figure 3.
Figure 3.
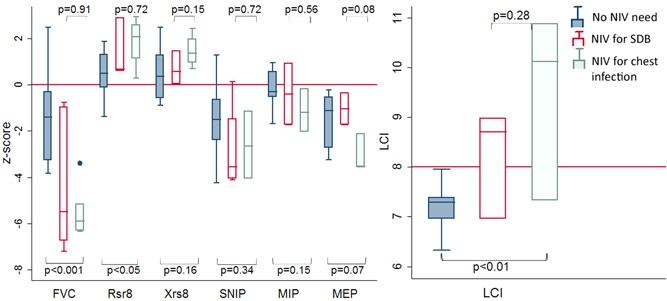
Respiratory function in children with spinal muscular atrophy who do not need NIV compared to those starting NIV for sleep‐disordered breathing and those starting during a chest infection. FVC, forced vital capacity; LCI, lung clearance index; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; NIV, noninvasive ventilation; SDB, sleep‐disordered breathing; Xrs8, reactance at 8 Hz [Color figure can be viewed at wileyonlinelibrary.com]
Some children who began NIV for SDB had a normal FVC z‐score, but all children who began NIV during an LRTI had an FVC less than −2 z‐scores. For FVC z‐score less than −2.5 the odds ratio (OR) is 10.70 (95% confidence interval [CI], 1.39‐82.03) for using NIV. All children not using NIV had LCI less than 8. All children using NIV had LCI greater than 7. and all children (n = 3) with LCI greater than 8 used NIV.
3.5. Effect of NIV on PSG
Diagnostic and NIV titration studies were compared for children treated with NIV, regardless of the reason NIV was started. There was a notable improvement in total AHI (3.65 vs 0.08, P < .04) AHI during REM sleep (12.9 vs 1.5, P < .05) and maximum TcCO2 (49.50 vs 45.65 mm Hg, P < .05) on NIV compared to the diagnostic sleep study, see Figure 4.
Figure 4.
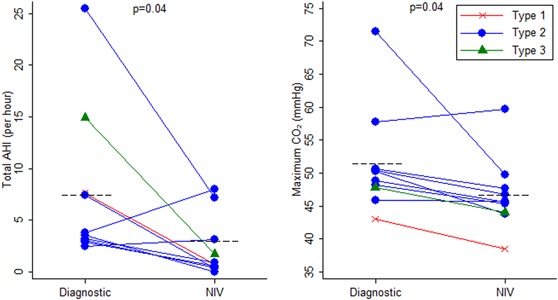
Change in total apnea‐hypopnea index and maximum transcutaneous carbon dioxide level from diagnostic sleep study to noninvasive ventilation titration study. AHI, apnea‐hypopnea index, NIV, noninvasive ventilation [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In childhood SMA, the need for nocturnal NIV in Queensland has been either SDB or severe LRTI requiring ventilator support, which is then difficult to wean. Children requiring ongoing NIV generally have more abnormal RF when compared with those not requiring NIV. These children tend to be older (median, 10.52 vs 5.67 years; P < .02) with significantly more abnormal FVC z‐score (−5.70 vs −1.39; P < .02), Rsr8 z‐score (+1.97 vs +0.50; P = .04) and LCI (8.84 vs 7.34; P = .01). In our cohort, we used multiple modalities for RF testing and correlated them to PSG parameters. In univariate analysis, only FVC z‐score and FVC % predicted was found to have a significant correlation with maximum overnight TcCO2 (P < .04). On multivariate analysis the combination of FVC z‐score and LCI were more closely associated with maximum TcCO2 on PSG (P < .001) than either test alone. FVC z‐score less than −2.5 z‐scores had a high correlation with NIV need (OR, 10.70 [95% CI, 1.39‐82.03]) and all children with LCI greater than 8 used NIV.
Respiratory function testing is feasible in children as young as 3 years; this group has demonstrated an ability to perform acceptable and repeatable spirometry, LCI and FOT testing as young as 3 years. However, time is required to achieve acceptable and repeatable results, and this may be challenging in a busy clinical setting. The series does not include any children aged 1 to 3 years, and it remains unknown if some of these children may also be capable of performing some RF tests, as is seen in the normal population. Testing became difficult again in children with advancing disease who had significant facial muscle weakness limiting lip seal, or contractures limiting the use of a snorkel mouthpiece. Additional interfaces may need further exploration in this group if RF tests are to be more heavily relied upon for clinical decision making. The authors note, however, that all older children with severe facial weakness or contractures already had an NIV requirement, so that the RF testing had a purely research focus.
Consensus statements for standards of care in SMA have suggested that all children with SMA who are symptomatic or unable to sit independently should begin treatment with NIV.2 However, in many parts of the world, including Australia and New Zealand, resource and funding mechanisms do not support this approach, and there is not universal agreement from parents to follow this approach, where no better indicators of NIV requirement (such as SDB) are present. Indeed, worse outcomes were reported in DMD patients who were started on NIV in the absence of SDB.17 Improved evidence for clinical indications, timing and benefits of NIV are required to avoid intensive care admissions and to provide evidence for NIV to funding bodies.
In our center, children with SMA are prospectively monitored for SDB and hypoventilation by performance of annual (or more regular if required) full PSG. As a result, SDB is generally detected early, when the AHI greater than 5, sometimes with REM rises in CO2. Children often do not fulfill the criteria for hypoventilation when SDB is first diagnosed and NIV is offered. As a result, only 2 children in this series met the criteria for hypoventilation. The level of peak in CO2 levels, generally seen in REM sleep does offer some insight into the progression of early SDB, and the peak CO2 was used in statistical analysis, rather than the percentage of time CO2 greater than 50 mm Hg. Had the recent consensus statement been followed, many more of these children would have been offered NIV.2 However, we believe that starting NIV for early SDB rather than hypoventilation is appropriate in the absence of better evidence to guide timing of NIV.
This study has included all children with SMA who reside in Queensland and Northern New South Wales, who are cared for clinically by CHQ. Its all‐inclusive nature is a strength of the study as the clinical care is “real‐world” and represents the breadth of disease severity seen in a large tertiary pediatric setting.
Two patients in this series were found to have significant SDB despite normal FVC z‐scores. The first is a 12‐year‐old boy with type 2 SMA who is obese, and had multiple admissions to intensive care in another state of Australia. He has required NIV on a number of occasions, but has been weaned during the recovery phase of his illness each time. He had a history of loud snoring and waking unrefreshed. His PSG showed mixed SDB with a combination of both central and obstructive events, but with an AHI of 25.5 (REM AHI 104.3) and a maximum TcCO2 of 57.7 mm Hg. He did not have adenotonsillar hypertrophy. The second child was a 7‐year‐old boy with type 3 SMA who is in the normal weight range. He stopped independent weight‐bearing at 3 years of age, and had never had a PSG previously. He had an easy sleep onset but frequent night waking and woke unrefreshed. He had purely central SDB with an AHI 11.0 overall and 10.7 in REM sleep. His maximum TcCO2 was 44 mm Hg. His SDB and symptoms have resolved on NIV.
One of the limitations of this study is that the PSG data were not collected prospectively for all participants. There were four patients who were unable to have their PSG started without NIV in situ, due to advanced disease state and intolerance of sleep initiation without NIV or absence of REM sleep without NIV. This resulted in a median delay between diagnostic PSG and the RF tests of 0.07 months but with IQR 26.76 months. In 6 of the 25 children, the diagnostic PSG was greater than 12 months before the RF tests. The authors believe this delay was unavoidable, given patient factors could not be overcome. The impact of a delay between diagnostic PSG and RF testing is likely to be an underestimation of the degree of SDB in these individuals, at the time of RF testing due to the progressive nature of the disease.
A previous study by this group has shown a linear relationship between respiratory reactance at 8 Hz and FVC (R 2, 0.54), unassisted PCF (R 2, 0.33), assisted PCF (R 2, 0.43), and AHI (R 2, 0.32).6 In that study, the PSG were all contemporary, however, subject numbers were smaller (12 compared to 25). None of the included patients had advanced disease state, and all were aged less than 10 years. Five of the 12 were using NIV habitually. It is possible that the relationship between RF and PSG parameters change in advanced disease state, or it may be that the current study did not detect associations with PSG findings as well due to the delay between diagnostic PSG and the RF testing.
With the recent availability of Nusinersen in many countries, it will be important to assess the influence of Nusinersen on RF carefully in children with SMA. Forced vital capacity and LCI should be considered important RF parameters to include in these studies. This study suggests that and FVC less than −2 z‐scores or and LCI greater than 7 should have a PSG to assess for SDB and should be considered at higher risk of needing NIV during an LRTI. In the absence of SDB, children with RF in this range should be considered at risk of significant LRTI needing NIV. The ability to predict the need for NIV is important in resource allocation for both PSG and NIV equipment allocation, and will also be important in development for guidelines for respiratory care in SMA where directing resources to those at highest clinical risk is essential.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors would like to acknowledge the children and families for their valuable assistance in this study. The research was funded by the Respiratory Department, CHQ and unfunded study conducted in Respiratory Medicine, CHQ.
Kapur N, Deegan S, Parakh A, Gauld L. Relationship between respiratory function and need for NIV in childhood SMA. Pediatric Pulmonology. 2019;54:1774‐1780. 10.1002/ppul.24455
This study has been presented at the Thoracic Society of Australia and New Zealand, 2018: TO 017, AP 011.
References
REFERENCES
- 1. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197‐207. [DOI] [PubMed] [Google Scholar]
- 3. Ragette R, Mellies U, Schwake C, Voit T, Teschler H. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax. 2002;57(8):724‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hukins CA, Hillman DR. Daytime predictors of sleep hypoventilation in Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2000;161(1):166‐170. [DOI] [PubMed] [Google Scholar]
- 5. Khirani S, Colell M, Caldarelli V, et al. Longitudinal course of lung function and respiratory muscle strength in spinal muscular atrophy type 2 and 3. Eur J Paediatr Neurol. 2013;17:552‐560. [DOI] [PubMed] [Google Scholar]
- 6. Gauld LM, Keeling LA, Shackleton CE, Sly PD. Forced oscillation technique in spinal muscular atrophy. Chest. 2014;146(3):795‐803. [DOI] [PubMed] [Google Scholar]
- 7. Czovek D, Shackleton C, Hantos Zn, et al. Tidal changes in respiratory resistance are sensitive indicators of airway obstruction in children. Thorax. 2016;71(10):907‐915. [DOI] [PubMed] [Google Scholar]
- 8. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 10. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gauld LM, Kappers J, Carlin JB, Robertson CF. Prediction of childhood pulmonary function using ulna length. Am J Respir Crit Care Med. 2003;168(7):804‐809. [DOI] [PubMed] [Google Scholar]
- 12. Hall GL, Sly PD, Fukushima T, et al. Respiratory function in healthy young children using forced oscillations. Thorax. 2007;62(6):521‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calogero C, Simpson SJ, Lombardi E, et al. Respiratory impedance and bronchodilator responsiveness in healthy children aged 2–13 years. Pediatr Pulmonol. 2013;48(7):707‐715. [DOI] [PubMed] [Google Scholar]
- 14. Beydon N, Davis SD, Lombardi E, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175(12):1304‐1345. [DOI] [PubMed] [Google Scholar]
- 15. Frey U, Frey U. Forced oscillation technique in infants and young children. Paediatr Respir Rev. 2005;6(4):246‐254. [DOI] [PubMed] [Google Scholar]
- 16. Domènech‐Clar R, López‐Andreu JA, Compte‐Torrero L, et al. Maximal static respiratory pressures in children and adolescents. Pediatr Pulmonol. 2003;35(2):126‐132. [DOI] [PubMed] [Google Scholar]
- 17. Raphael JC, Chevet S. Randomized trial of preventive nasl ventilation in Duchenne muscular dystrophy. Lancet. 1994;343:1600‐1604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


