Abstract
Aims
To ascertain the effects of improvements in diabetic foot services over 18 years on incidence of diabetic foot ulceration. We also compared survival time from first ulcer development with presence of neuropathy, peripheral vascular disease, age and healing.
Methods
Persons with new ulceration and those at high risk of ulcer development were referred to community podiatry from 1998. Their details were recorded, with verbal consent, on a central database. The effects of neuropathy, peripheral vascular disease, healing and age on survival were analysed by Cox proportional hazards ratios.
Results
The incidence of first ulcer presentation decreased from 11.1 to 6.1 per 1000 persons between 2003 to 2017 (P <0.0001). Recurrent ulceration incidence remained stable. Prevalence of chronic and new foot ulceration combined increased from 20.7 to 33.1 per 1000 persons (P <0.0001). Ten‐year survival was 85% for persons presenting with first ulcer and aged < 65 years, 50% for those aged 65–74 years and 25% for those aged 75–81 years (P < 0.0001). In those with peripheral vascular disease 5‐year survival was 35% (P <0.001).
Conclusions
Integrated care for the diabetic foot in one National Health Service (NHS) health service area over 18 years was associated with a reduction in first presentations of diabetic foot ulceration, but failed to reduce recurrent ulceration. Cumulative prevalence of all ulcers continues to increase. Monitoring ulceration incidence can inform audit and planning of diabetic foot care services. Survival is better than reported previously in persons < 65 years and in the absence of peripheral vascular disease.
What's new?
Prevention of diabetic foot ulceration has been difficult to acheive
Survival after diabetic foot ulceration has historically been poor
This study has shown reduction in first diabetes related foot ulceration with improved foot care services
Participant survival after development of first diabetic foot ulceation was longer than in recent reports especially for those under 65 years of age, where ulcers healed, and in those without peripheral vascular disease
Adequate commissioning of diabetic foot care services is necessary to prevent first foot ulceration
Counselling for younger patients with diabetic foot ulceration can be more posisitve
What's new?
Prevention of diabetic foot ulceration has been difficult to acheive
Survival after diabetic foot ulceration has historically been poor
This study has shown reduction in first diabetes related foot ulceration with improved foot care services
Participant survival after development of first diabetic foot ulceation was longer than in recent reports especially for those under 65 years of age, where ulcers healed, and in those without peripheral vascular disease
Adequate commissioning of diabetic foot care services is necessary to prevent first foot ulceration
Counselling for younger patients with diabetic foot ulceration can be more posisitve
Introduction
Five‐year survival in persons who present with diabetic foot ulceration has previously been shown to be as low as 50% 1, 2, 3, 4, 5, 6, 7 and to be worse in those with vascular disease 6. A recent large study of persons living with diabetes at high risk of foot ulceration in Scotland has shown associations between conventional cardiovascular risk factors, ulceration and 2‐year survival. Analysis in this large study of high risk, previously ulcerated and currently ulcerated persons did not focus on first presentation of diabetic foot ulceration 8. Persons living with diabetes and loss of protective foot sensation, foot deformity and or peripheral vascular disease are at high risk of diabetic foot ulceration, and those with previous ulceration are at even greater risk 9. Regular community podiatry review for persons at high risk of diabetic foot ulceration has been recommended by the National Institute for Health and Care Excellence (NICE) since 2004. However, attempts to reduce new and recurrent ulceration in high‐risk persons have required intensive intervention and follow‐up 1, 2, 10, 11, 12. The development of diabetic foot ulceration is associated with loss of protective sensation 13. This has been confirmed in the South Devon population 14. Incidence of amputation resulting from diabetic foot ulceration is also influenced strongly by ethnicity 15, 16. In South Devon, we have shown that integration of diabetes foot care including general practice, community podiatry and secondary care has resulted in a reduction in major amputation incidence from 2005 to 2017 in a high‐risk, predominantly white British population 17. In this geographical area, diabetes foot care has been rationalized since 1998 with a standardized operating procedure for foot examination in general practice and community podiatry. Community and hospital podiatry services were enhanced from 2007, and a NICE compliant multidisciplinary foot team (MDFT) was developed by 2010. Further increases in community podiatry staff numbers were introduced in 2012. These improvements enhanced promptness of access to treatment of diabetic foot ulcers in community podiatry and rotation of podiatrists into the MDFT. During this time, the indications for referral from general practice of those at high risk of foot ulceration and all with foot ulcers have remained unchanged. The incidence of first diabetes‐related foot ulcers, recurrent ulceration, 5‐ and 10‐year survival after first ulcer presentation have been analysed over the period of improvements in diabetic foot care service provision.
Participants and methods
Participants
All persons with high risk of diabetes related foot ulceration, and those with new foot ulcers were referred by general practice to community podiatry from 1998. This was reinforced by the introduction of a standard operating procedure (SOP) for foot annual review as part of regular meetings with the secondary care team. All practices were advised to refer persons living with diabetes who developed foot ulceration to community podiatry. A domiciliary podiatry service to access housebound persons living with diabetes was commenced in 2000. Verbal consent was obtained from all persons living with diabetic foot ulceration for their clinical details to be recorded on a database maintained by the community podiatry department from 2001. A diabetes‐related foot ulcer was defined as any break in the skin below the ankle in a person living with diabetes. We analysed the community podiatry database from 2001 to 2017 for trends in age, incidence and prevalence of diabetic foot ulceration, neuropathy and peripheral vascular disease and survival following first foot ulcer. It has also been possible to assess the Torbay Hospital MDFT database to ascertain the number of persons living with diabetes presenting with foot ulceration who were not previously known to community podiatry.
First diabetic foot ulcer was diagnosed from participant history, examination and confirmed by previous records over the preceding 5 years. Ulcer healing within first 2 years of follow‐up was recorded. Recurrent ulceration included presentations with breakdown of initial foot ulcer or any new ulcer on either foot. Reasons for discharge and death were recorded. To ensure completeness of follow‐up the South Devon community podiatry database was linked with the NHS digital Spine database 18. The Torbay Hospital MDFT database was interrogated to identify persons living with diabetes who were referred directly to the hospital for foot ulceration without community podiatry involvement. Status, alive or deceased was ascertained in all of the 1640 persons recorded as developing a first foot ulcer over the 18 years.
Clinical examination
Protective foot sensation was assessed with 10‐g monofilaments perception. Diabetic neuropathy was diagnosed as failure to perceive two or more 10‐g monofilament stimuli in both feet, with and without neuropathic symptoms. High risk for foot ulceration was confirmed if there was loss of perception of only one or two10‐g monofilament stimuli. Extensive callus, foot deformity, vascular disease or previous ulceration were also taken to confirm high risk. Peripheral vascular disease was identified by delay of > 5 s in great toe capillary refill time, absence of one or more foot pulses to palpation or monophasic or absent foot pulses with Doppler.
Statistical analysis
Numbers of diabetic foot ulcers per year and per 5‐year period were expressed as incidence and prevalence per 1000 persons living with diabetes recorded on the Quality Outcome Framework (QOF) register. The total number of persons per year with diabetes and foot ulcer disease, new, old and recurrent was measured to calculate cumulative prevalence of foot ulceration. The total number per year of persons with first ever diabetic foot ulceration, as described above, was taken as incidence of first ulceration. All are expressed per 1000 on the diabetes QOF register. Kaplan–Meier estimator and Cox proportional hazard ratios were generated for the effects of age, ulcer healing, gender, presence or absence of neuropathy and peripheral vascular disease on survival of persons with diabetes from the date of first development of foot ulceration. All statistical analysis was conducted in ‘R’.
Ethical consideratons
Persons living with diabetes who presented to community podiatry services in South Devon gave verbal consent for inclusion of their details on a centralised data base. The South Devon Healthcare ethics committee was consulted regarding retrospective audit of the data. As this was an audit of anonymised data it was not considered necessary to formally seek ethics committee approval.
Results
Hospital clinic only referrals
Some 83 persons with a diabetic foot ulcer who were unknown to community podiatry during the course of this study were identified through searches of the Torbay Hospital diabetic foot database. Thirty‐one had severe ischaemia and proceeded to vascular intervention. Twenty‐seven had chronic foot ulcers which were reviewed by the hospital podiatry team. Twenty‐five presented with first foot ulcer, of which nine healed within 2 years of follow‐up. They have not been included in this analysis.
Loss to follow‐up
Some 21 participants with healed ulcers and seven with unhealed ulcers were excluded from the analysis of healing and recurrent ulceration as they had less than 1 year of podiatry follow‐up.
Chronic ulceration, age and co‐morbidity
There was an increase in annual prevalence of all diabetic foot ulcers (first, recurrent and chronic combined) during the course of the study. The number of diabetes‐related foot ulcer participants in residential care or confined to home increased significantly from 17 in 2001 to 139 in 2017. The increase in chronic ulceration numbers per annum from 2012 onwards is linked to increased frequency of follow‐up facilitated by increase in community podiatry staffing. Percentages with neuropathy remained constant, whereas peripheral vascular disease decreased Table 1.
Table 1.
Demographic details of persons with diabetic foot ulcers, first ever, recurrent and chronic in Torbay and South Devon Health Care Trust 2003 to 2017
| Years | P‐value* | |||
|---|---|---|---|---|
| 2003–2007 | 2008–2012 | 2013–2017 | ||
| All ulcers, first, recurrent and chronic average /1000 year | 20.7 (1.3) | 24.9 (3.0) | 33.1 (6.2) | 0.007 |
| Mean age, years | 73.1 (12.2) | 74.0 (12.0) | 76.2 (12.2) | NS |
| % Female | 44.5 | 39.9 | 37.1 | NS |
| Home care per 1000 DM | 1.9 (0.3) | 2.6 (0.3) | 5.5 (1.9) | 0.0005 |
| % Neuropathy | 54.4 | 54.5 | 56.9 | NS |
| % Peripheral vascular disease | 19.8 | 14.2 | 14.7 | 0.0022 |
Significance levels shown are unpaired t‐tests 2003 to 2007 vs. 2013 to 2017(± sd). DM, diabetes mellitus; NS, not significant.
New and recurrent ulceration
There was a statistically significant fall in new diabetic foot ulcer incidence when analysed as an annual trend and in 5‐year periods. The incidence of recurrent foot ulceration remained stable over the same periods (Table 2). Annual numbers of new, recurrent, domiciliary and chronic diabetic foot ulcer participants from 2003 to 2017 are shown in the Table 3. One‐tenth of the total diabetic foot ulcers from 2003 to 2012 occurred in domiciliary care participants. This increased to one‐fifth from 2013 to 2017 (28.6% by 2017). There has been a sharp increase in annual chronic ulceration recorded from 2012; this is likely to have resulted from increased community podiatry staff numbers which has allowed more frequent review of participants with chronic ulceration. The percentage of participants with first ever diabetic foot ulceration who were alive and healed 12 weeks after presentation has not varied significantly between 2011 and 2017 (31.1, 30.1, 28.9, 32.4, 37.2 and 30.7%, respectively).
Table 2.
Five‐year incidences of first ever and recurrent diabetes related foot ulcers expressed as annual incidence/1000 persons living with diabetes
| Years | P‐value* | |||
|---|---|---|---|---|
| 2003–2007 | 2008–2012 | 2013–2017 | ||
| QOF diabetes numbers mean/year for each 5 year period | 10664 | 13940 | 16051 | 0.001 |
| First ever ulcers all in a 5‐year period | 543 | 604 | 493 | |
| Incidence of first ulcer mean/year over 5 years | 11.1 (1.1) | 12.5 (1.4) | 6.1 (1.5) | 0.0006 |
| Recurrent ulcers all in a 5‐year period | 382 | 570 | 614 | |
| Incidence of recurrent ulcers average/year over 5 years | 7.3 (1.2) | 8.4 (1.7) | 7.7 (2.4) | 0.35 |
Significance levels shown are unpaired t‐tests 2003 to 2007 vs. 2013 to 2017. Ulcer incidence expressed as mean (sd).
Table 3.
Categories of diabetic foot ulcers/year presenting to community podiatry in South Devon 2003 to 2017
| Category | 2003 | 2004 | 2005 | 2006 | 2007 | 5‐year 2003–2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 5‐year 2008–2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 5‐year 2013–2017 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic* | 35 | 30 | 24 | 28 | 57 | Total 174 | 127 | 167 | 61 | 55 | 98 | Total 508 | 245 | 333 | 308 | 364 | 303 | Total 1553 |
| Recurrent | 80 | 70 | 69 | 86 | 77 | Total 382 | 83 | 97 | 110 | 143 | 137 | Total 570 | 168 | 89 | 112 | 150 | 95 | Total 614 |
| New | 112 | 101 | 108 | 115 | 107 | Total 543 | 101 | 133 | 131 | 135 | 104 | Total 604 | 66 | 81 | 125 | 133 | 88 | Total 493 |
| Domiciliary† | 18 | 17 | 19 | 27 | 22 | Total 103 | 29 | 30 | 31 | 33 | 45 | Total 168 | 64 | 63 | 77 | 98 | 139 | Total 441 |
| All | 227 | 201 | 201 | 229 | 241 | Total 1099 | 311 | 397 | 302 | 333 | 339 | Total 1682 | 479 | 503 | 545 | 647 | 486 | Total 2600 |
| % of ulcers seen as domiciliary visits | 8 | 8 | 9 | 12 | 9 | Mean 9% | 9 | 8 | 10 | 10 | 13 | Mean 10% | 13 | 13 | 14 | 15 | 29 | Mean 17% |
| No. living with diabetes | 8997 | 9855 | 10640 | 11437 | 12292 | Total 10644 | 13110 | 13972 | 14100 | 14222 | 14297 | Total 13490 | 14784 | 15009 | 16243 | 16732 | 17191 | Total 15992 |
Chronic ulcers, cumulative from year to year.
Domiciliary ulcers are spread between the other categories of ulcer.
Survival trends
Survival was not influenced by gender, but older persons and those with peripheral vascular disease were found to have significantly reduced survival (Table 4 and Fig. 1). There was no effect of neuropathy (clinically diagnosed loss of protective sensation and symptoms) on survival. Figure 2 shows the reduced survival at 5 years for persons in whom foot ulcers remained unhealed for 2 years compared with those in whom the presenting diabetic foot ulcer had healed.
Table 4.
Kaplan–Meier analysis of ten year survival after first diabetic foot ulceration according to age at diagnosis
| Age group (years) | Time point (years) | No. at risk | Proportion alive | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| < 65 | 10 | 167 | 0.698 | 0.025 | 0.652 | 0.748 |
| 65–74 | 10 | 117 | 0.458 | 0.026 | 0.411 | 0.511 |
| 75–81 | 10 | 38 | 0.236 | 0.027 | 0.188 | 0.296 |
| ➢ 81 | 10 | 8 | 0.051 | 0.016 | 0.028 | 0.095 |
Figure 1.
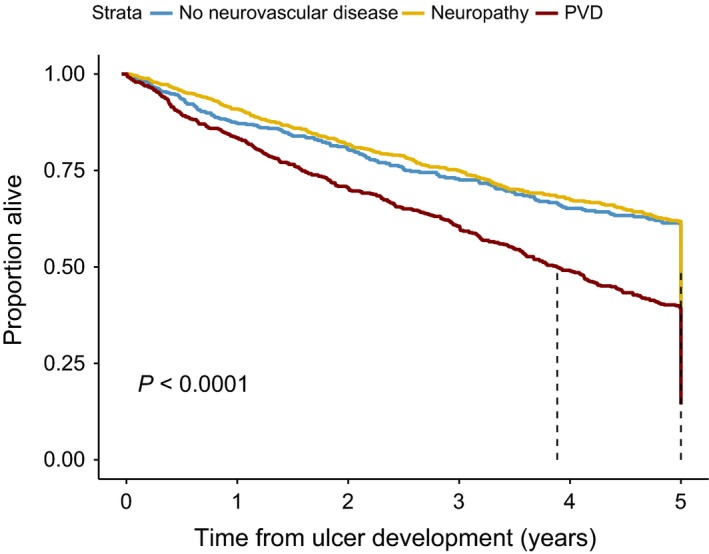
Survival over time (years) following recognition of first ulcer in diabetic population stratified by no neurovascular disease (blue), neuropathy (yellow), peripheral vascular disease (red).
Figure 2.
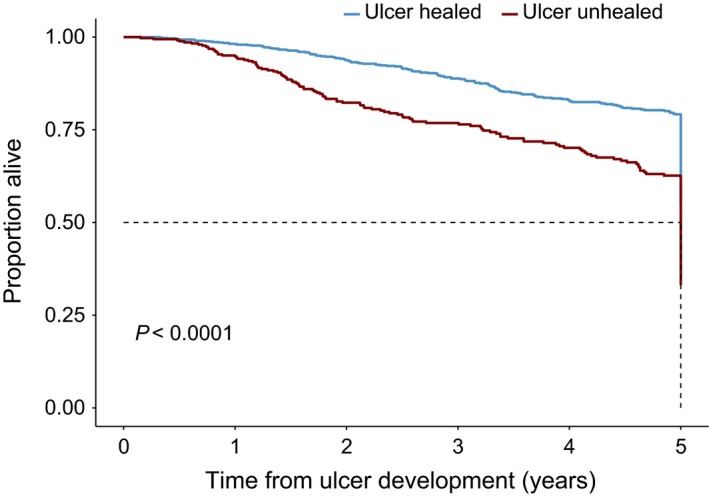
Comparison of survival over time in years following development of first ulcer stratified by ulcer healed on follow‐up (blue), or not (red).
Conclusions
Integration of diabetes foot care in South Devon from 2008, with improvements in podiatry staffing levels in 2008 and 2012, and introduction of a MDFT in 2009 have resulted in a sustained reduction in major amputation incidence up to 2017 17. This has been accompanied by a reduction in first‐time ulceration in persons living with diabetes at high risk of first diabetic foot ulcer. Annual incidence of recurrent ulceration remained stable during the follow‐up period. There was an increase in the cumulative prevalence of all diabetic foot ulceration associated with an increase in persons with long‐term chronic diabetic foot ulceration. Participants in domiciliary care comprised 28.6% of the total diabetic foot ulcer care population by 2017. This rising trend over 18 years is likely to continue and will place increased demands on podiatry and allied health professional services. Five‐year survival after first ulcer is reduced in those with peripheral vascular disease and older age, but is better than reported at 85% for persons presenting at age < 65 years and followed for 10 years. The reduced survival of older persons in this study is of interest. Although this may seem self‐evident and expected, it demonstrates that younger persons living with diabetes who develop diabetic foot ulcers in this cohort do not have such a high mortality as has been reported in a recent large 5‐year follow‐up study 7. That study also demonstrated that younger persons fared better. It categorized new foot ulceration from the THIN GP database in the UK as participants not seen for a diabetic foot ulcer in the previous 6 months. This group may well include an unknown number with recurrent ulceration. The study also recorded a worse survival rate in the South West of England, including South Devon. A single centre study has shown improved survival in a cohort of diabetic foot ulcer participants who were treated more intensively for cardiovascular risk 4. This cohort comprised hospital foot clinic data only and the definition of first diabetic foot ulcer is not clarified. Some researchers have suggested that all those presenting with diabetic foot ulceration should be informed that their life expectancy is poor. Our data would not support this view for younger persons presenting with first foot ulceration. This contrasts with the outcomes for prostate cancer where survival is similar across age ranges at presentation, and breast cancer where patients presenting at a younger age have worse survival 19, 20.
The most encouraging trend has been the significant decrease in incidence of new presentations of diabetic foot ulcers in previously non‐ulcerated persons. This may have resulted from a combination of improved practice care, strengthening of the foot protection service and development of a NICE‐compliant MDFT. Prevention of foot ulceration in those living with diabetes is critically dependant on their own foot care, annual foot review and access to podiatry. For success, there must be readiness of all healthcare professionals to offer education when the person is receptive, systematic and enthusiastic care in general practice, and fully staffed community podiatry services. Incidence of new foot ulceration is thus a key performance indicator of efficacy in diabetic foot care services and is likely to influence amputation rates. There is an urgent need to link practice and community nurses with community podiatrists and for this team to be seen as part of the multidisciplinary diabetes foot team.
There has been a reduction in peripheral vascular disease over time in this population which is likely to be related to the fall in smoking prevalence. Smoking rates in South Devon have mirrored those in England (33 and 38% of females and males, respectively in 1980, and 14.8% in sexes in 2017) 21, 22. Advanced age and peripheral vascular disease remain strong markers of reduced survival. These results are consistent with the National Diabetes Foot Audit (https://digital.nhs.uk/national‐diabetes‐foot‐care‐audit) 23 which has evaluated factors associated with ulcer free survival at 12 and 24 weeks in England.
Previous diabetic foot ulceration constitutes the highest risk for subsequent foot ulceration in diabetes 1, 9, 10, 12, 24. Effective foot protection to reduce re‐ulceration is challenging 25. Our data indicate that current advice and surveillance for previously ulcerated persons have not succeeded in reducing recurrent ulceration. Research is needed to explore new offloading techniques and early introduction of customized orthoses where early warning of shear stress has been identified such as redness, heat and callus formation. In this study, recurrent ulceration has included all new ulcers whether breakdown of the first presenting foot ulcer or a new ulcer on the same or other foot. This emphasizes the need to assess and treat all vulnerable areas of both feet once a diabetic foot ulcer has developed. Prospective trials of corrective orthopaedic surgery to reduce foot pressures are also needed.
The strengths of this study are that it derives from a stable population over 18 years that is at high risk of diabetic foot ulceration, and that the referral patterns from general practice have remained unchanged during this time. It is also of importance that the ulceration incidence and survival can be set in the context of improvements in the whole foot care pathway in the area, changes which have been associated with a sustained and significant reduction in major amputation incidence 17. The weaknesses are that it has not been possible to record all of the ulcer severity scores, or causes of death in every person. More intense screening for diabetes may have led to the increase in QOF‐registered persons living with diabetes with the possibility that this would diminish the severity of diabetes in that population. This would be expected to reduce the prevalence of all complications of diabetes related to duration of glucose intolerance. However, within this data set, the prevalence of all diabetic foot ulcer disease has increased in spite of possible inclusion of ‘early’ diabetes in the QOF numbers. A recent study found microvascular and macrovascular complications of Type 2 diabetes to be present in 30% of persons at diagnosis 26. Complications have also been shown to develop rapidly after diagnosis 27. Moreover, the constant incidence of recurrent ulceration, the fall in first time ulcers, and the longer survival of those < 65 years and those free from peripheral arterial disease are important to highlight.
The population studied is > 98% white British, in an area with pockets of high deprivation indices and where > 50% of the population living with diabetes is older than 65 years of age. Our findings may not be generalizable to persons from other ethnic groups living with diabetes.
In summary, this analysis shows that the incidence of first diabetic foot ulceration, but not recurrent ulceration is reduced in association with improvements in diabetes footcare. Peripheral vascular disease, failure of ulcer healing and age were associated with reduced survival. More intense follow‐up and bespoke orthoses may be necessary to reduce re‐ulceration. Careful auditing of diabetic foot ulcer numbers can form the basis for appropriate commissioning of foot protection services by clinical commissioning groups. These results can inform future development of the National Diabetes Foot Care Audit, in particular to analyse first time and recurrent ulceration incidences. Participation in the National Diabetes Foot Care Audit should be mandatory for providers of diabetes foot care in order to comprehensively measure how incidence and outcome are affected by ethnicity, site and severity of foot ulceration and service provision.
Contributors
The following podiatrists contributed to the clinical care and data collection: Rebecca Birch, Belinda Bowen, Roger Brown, Cheryl Clark, Richard Collings, Steve Cutts, Joanne Davies, Sean Ellin, Kerry‐Ann Evans, Rob Fisher, Sam Glasser, Martyn Hillstead, Gemma Hine, Sarah Levi, Amanda Martin, Lauren Mackintosh, Sadie Phillips, Ella Rawlinson, Anne‐Marie Shepperd, Kerrie Spicer, Su Stewart, Sam Stocker, Harriet Tailford, Justine Tansley, Hannah Uglow, Adam Widgery, Jen Williams, and Samuel Darke information analyst.
Torbay Hospital MDFT: Ruth Gornall, Gill Spyer, Jamie Smith. Robert McCarthy, James Davis, Ian Jakin.
Funding sources
None.
Competing interests
None declared.
Acknowledgements
We would like to thank participants with diabetic foot ulceration, their carers, the hospital transport service and South Devon primary care teams. We are grateful to Rosamund Paisey for proof reading and formatting of the document and Melvin Cowie for graphic design of the figures.
Ethical Approval
The study was approved by the Medical Ethics Committee of [INSTITUTION], and informed consent was obtained from all participants. This research study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Diabet. Med. 36: 1424–1430(2019)
References
- 1. Apelqvist J, Larsson J, Agardh CD. Long‐term prognosis for diabetic patients with foot ulcers. J Intern Med 1993; 233: 485–491. [DOI] [PubMed] [Google Scholar]
- 2. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE et al Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22: 382–387. [DOI] [PubMed] [Google Scholar]
- 3. Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer‐free survival following management of foot ulcers in diabetes. Diabet Med 2005; 22: 1306–1309. [DOI] [PubMed] [Google Scholar]
- 4. Young MJ, McCardle JE, Randall LE, Barclay JI. Improved survival of diabetic foot ulcer patients 1995–2008: possible impact of aggressive cardiovascular risk management. Diabetes Care 2008; 31: 2143–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winkley K, Sallis H, Kariyawasam D, Leelarathna LH, Chalder T, Edmonds ME et al Five‐year follow‐up of a cohort of people with their first diabetic foot ulcer: the persistent effect of depression on mortality. Diabetologia 2012; 55: 303–310. [DOI] [PubMed] [Google Scholar]
- 6. Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int Wound J 2016; 13: 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med 2016; 33: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 8. Vadiveloo T, Jeffcoate W, Donnan PT, Colhoun HC, McGurnaghan S et al; Scottish Diabetes Research Network Epidemiology Group . Amputation‐free survival in 17,353 people at high risk for foot ulceration in diabetes: a national observational study. Diabetologia 2018; 61: 2590–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crawford F, Cezard G, Chappell FM, the PODUS Group . The development and validation of a multivariable prognostic model to predict foot ulceration in diabetes using a systematic review and individual patient data meta‐analyses. Diabet Med 2018; 35: 1480–1493. [DOI] [PubMed] [Google Scholar]
- 10. Lincoln NB, Radford KA, Game FL, Jeffcoate WJ. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia 2008; 51: 1954–1961. [DOI] [PubMed] [Google Scholar]
- 11. Weiner RD, Hlad LM, McKenna DR. Recurrence of diabetic pedal ulcerations following tendo‐achilles lengthening. Diabet Foot Ankle 2011; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Örneholm H, Apelqvist J, Larsson J, Eneroth M. Recurrent and other new foot ulcers after healed plantar forefoot diabetic ulcer. Wound Repair Regen 2017; 25: 309–315. [DOI] [PubMed] [Google Scholar]
- 13. Monteiro‐Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis‐Ribeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Rev 2012; 28: 574–600. [DOI] [PubMed] [Google Scholar]
- 14. Paisey RB, Darby T, George AM, Waterson M, Hewson P, Paisey CF et al Prediction of protective sensory loss, neuropathy and foot ulceration in type 2 diabetes. BMJ Open Diabetes Res Care. 2016; 4: e000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holman N, Young RJ, Jeffcoate WJ. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia 2012; 55: 1919–1925. [DOI] [PubMed] [Google Scholar]
- 16. Robinson TE, Kenealy T, Garrett M, Bramley D, Drury PL, Elley CR. Ethnicity and risk of lower limb amputation in people with Type 2 diabetes: a prospective cohort study. Diabet Med 2016; 33: 55–61. [DOI] [PubMed] [Google Scholar]
- 17. Paisey RB, Abbott A, Levenson R, Harrington A, Browne D, Moore J et al Diabetes‐related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the South‐West of England. Diabet Med 2018; 35: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NHS Digital . Spine. Available at https://digital.nhs.uk/services/spine Last accessed 2 March 2019.
- 19. Pompe RS, Smith A, Bandini M, Marchioni M, Martel T, Preisser F et al Tumor characteristics, treatments, and oncological outcomes of prostate cancer in men aged ≤50 years: a population‐based study. Prostate Cancer Prostatic Dis 2018; 21: 71–77. [DOI] [PubMed] [Google Scholar]
- 20. Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong Y‐N, Edge SB et al Subtype‐dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 2016; 34: 3308–3314. [DOI] [PubMed] [Google Scholar]
- 21. NHS Digital . Statistics on Smoking –England, 2018. Available at https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-smoking/statistics-on-smoking-england-2018 Last accessed 2 March 2019.
- 22. Office for National Statistics . Adult Smoking Habits in the UK: 2017. Available at https://www.ons.gov.uk/releases/adultsmokinghabitsintheuk2017 Last accessed 2 March 2019.
- 23. Jeffcoate W, Holman N, Rayman G, Valabhji J, Young B. New national diabetes footcare audit of England and Wales. Diabet Med 2014; 31: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 24. Armstrong DG, Bharara M, White M, Lepow B, Bhatnagar S, Fisher T et al The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev 2012; 28: 514–518. [DOI] [PubMed] [Google Scholar]
- 25. Reiber GE, Smith DG, Wallace C, Sullivan K, Hayes S, Vath C et al Effect of therapeutic footwear on foot reulceration in patients with diabetes: a randomized controlled trial. JAMA 2002; 287: 2552–2558. [DOI] [PubMed] [Google Scholar]
- 26. Gedebjerg A, Almdal TP, Berencsi K, Rungby J, Nielsen JS, Witte DR et al Prevalence of micro‐ and macrovascular diabetes complications at time of type 2 diabetes diagnosis and associated clinical characteristics: a cross‐sectional baseline study of 6958 patients in the Danish DD2 cohort. J Diabetes Complicat 2018; 32: 34–40. [DOI] [PubMed] [Google Scholar]
- 27. Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]


