Abstract
Aim
To investigate the efficacy of adalimumab treatment in an experimental rat sclerosing encapsulated peritonitis (SEP) model.
Methods
The study involved 40 Wistar albino rats divided into four groups: chlorhexidine (CH) group, control group, CH + adalimumab group, and CH + resting group. The control group received normal saline intraperitoneally (i.p.). Other groups received 0.1% CH gluconate, 15% ethanol, and normal saline mixture i.p. for three weeks in order to induce SEP. CH + adalimumab group received 5 mg/kg adalimumab i.p. at the beginning of week 4 and week 6, while CH + resting group was followed-up for three weeks without applying any procedure after the onset of SEP. Rats in groups CH and control group were sacrificed on day 21, and rats in group CH + adalimumab and CH + resting were sacrificed on day 42. All groups were evaluated for peritoneal thickness, inflammation, vascularization, and fibrosis.
Results
CH + adalimumab group showed a significant decrease in peritoneal thickness, fibrosis score, and vascular score compared with CH group and CH + resting group.
Conclusion
Adalimumab can prevent SEP development.
Sclerosing encapsulated peritonitis (SEP) is a rare complication of peritoneal dialysis, with high morbidity and mortality. The pathological findings of SEP include mesothelial denudation, increase in submesothelial thickness, interstitial fibrosis, and vasculopathy (1). Although currently there is no effective treatment for SEP, the discontinuation of peritoneal dialysis, corticosteroid and tamoxifen use, surgical treatments, and total parenteral nutrition can be applied (2). Therapies targeting vascular endothelial growth factor (VEGF), renin-angiotensin aldosterone system, or erythropoietin and immunosuppressive drugs have been tested in experimental peritoneal sclerosis models. Some of these therapies positively affected peritoneal thickness, vascularization, and fibrosis (3-7), whereas others produced no response or produced negative results (8,9).
A central role in the pathophysiology of SEP is played by transforming growth factor beta (TGF-β) (10), whose production is triggered by transmembrane TNF-α reverse signal in macrophages (11). This is why we hypothesized that adalimumab, as a monoclonal antibody against tumor necrosis factor-alpha (TNF-α) (12), could prevent SEP development. Adalimumab therapy has previously been shown to revert angiogenesis in patients with psoriasis (13), while anti-TNF therapy has led to clinical and endoscopic recovery in Crohn’s disease, both by inhibiting vascular proliferation and due to its anti-inflammatory effects (14). The aim of our study was to investigate the efficacy of adalimumab therapy in an experimental rat SEP model.
MATERIAL AND METHODS
Experimental animals
The study was performed in Canakkale Onsekiz Mart University Experimental Research Application and Research Center Laboratory between June and September 2016. It involved forty 6-8-month-old Wistar albino rats (20 males and 20 females) weighing between 200 and 250 g. The rats were kept in cages containing five rats under standard caging conditions at 24°C room temperature with 12 hours of dark/light cycle and standard feeding and water supply. At the end of the study, pain control was achieved before anesthesia by 5 mg/kg intramuscular (i.m.) lidocaine injection. Rats were then sacrificed by using 60 mg/kg i.m. ketamine hydrochloride injection on day 21 or on day 42, depending on the group. The rats with signs of infection and systemic reaction to adalimumab were excluded from study.
The study was approved by Canakkale Onsekiz Mart University animal ethics committee (Decision No:2016/01-03, Approval date: January 21, 2016).
Experimental design
Experimental SEP was induced according to Ishii et al (15). A mixture of 0.1% chlorhexidine (CH) gluconate (Drogsan Medicines Inc. Balgat, Ankara), 15% ethanol, and normal saline (NS) (10 mL/kg/d) was prepared and used aseptically. Adalimumab (Humira, 40 mg/0.8 mL [AbbVie, North Chicago, IL, USA]) was injected intraperitoneally (i.p.) at a dose of 5 mg/kg after having been dissolved in 40 mL of NS (1 mg/mL) (16). To eliminate the effects of direct damage to the peritoneum by repeated injections, daily injections were performed on the lower quadrant of the abdomen with a 21-G needle, and parietal peritoneum in the upper left quadrant was used for pathological examinations.
Four groups were formed with 10 rats in each. The duration of the study was 21 days for CH and control groups and 42 days for other groups. The groups were the following:
1. CH group (group 1) received a mixture of 0.1% CH gluconate, 15% ethanol, and NS (10 mL/kg/d) i.p. every day.
2. Control group (group 2) received NS (10 mL/kg/d) i.p. every day.
3. CH + adalimumab group (group 3) received a mixture of 0.1% CH gluconate, 15% ethanol, and NS (10 mL/kg/d) i.p. every day for 21 days. Adalimumab was administered biweekly at the fourth and sixth week at a dose of 5 mg/kg.
4. CH + resting group (group 4) received a mixture of 0.1% chlorhexidine gluconate, 15% ethanol, and NS (10 mL/kg/d) i.p. every day for 21 days. During the following 21 days, no intervention was performed.
Histopathological evaluation
All of the formaldehyde fixed parietal peritoneal tissue samples were embedded vertically in paraffin after routine tissue follow-up, and 5-mm thick sections were made. The sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome (MT) dyes. The stained sections were evaluated under light microscopy for parietal peritoneal thickness, inflammation, and fibrosis. In addition, 4-μm sections were prepared from all samples on negatively charged slides, and immunohistochemical analyses were performed with use of TGF-β1 (1/100, mouse monoclonal immunoglobulin G [IgG], Santa Cruz Biotechnology, Inc., Dallas, TX, USA), VEGF (1/100, rabbit polyclonal IgG, Spring Bioscience, Pleasanton, CA, USA), and alpha-smooth muscle actin (α-SMA, 1/100, mouse monoclonal IgG, Cell Marque, Rocklin, CA, USA) antibodies. All pathological examinations were performed by a single pathologist who was blinded to the characteristics of the groups.
To measure parietal peritoneal thickness, microscopic images obtained from H&E-stained sections were transferred to a computer using Nikon software, version 4.30 (Nikon Instruments Inc., New York, NY, USA). Parietal peritoneal thickness was measured at ten regions in micrometers, and mean thickness was recorded. Inflammation and fibrosis were semiquantitatively scored using H&E stain and MT stain, respectively (15). Inflammation score was classified as: 0 – no inflammation or the presence of isolated inflammatory cells; 1 – mild inflammation: the presence of a few scattered inflammatory cells; 2 – moderate inflammation: the presence of large numbers of inflammatory cells in small groups within one high magnification area; 3 – severe inflammation: the presence of inflammatory cells with a diffuse distribution or in large groups. Fibrosis score was classified as: 0 – no fibrosis; 1 – mild; 2 – moderate; 3 – severe fibrosis. Vascular score was obtained by counting vascular structures reacting to α-SMA immunohistochemical staining with use of 200 × magnification at ten regions for each section, and calculating their average (5).
TGF-β1 and VEGF score was obtained by counting fibroblast and macrophage cells reacting to TGF-β1 and VEGF immunohistochemical staining with use of 200 × magnification at ten regions for each section, and calculating their average (5). Vasculopathy was graded according to the degree of subendothelial hyalinization, luminal irregularity, and narrowing, as described by Williams et al (17).
Statistical analysis
All results are expressed as median (Q1-Q3). Due to the limited number of rats in each group, non-parametric methods were used. The difference in the distribution of categorical data was analyzed with the χ2 test. Peritoneal thickness, inflammation, fibrosis, and vascular score; and VEGF and TGF-β1 scores were compared between the groups with the Kruskal-Wallis test. In the post-hoc analysis, the Mann-Whitney U test with Bonferroni correction was used. Statistical significance level was P < 0.008 for the Mann-Whitney U test with Bonferroni correction and P < 0.05 for the other tests. The analyses were performed using the SPSS, version 20.0 for Windows (IBM Corp, Armonk, NY, USA).
RESULTS
All 40 rats completed the study. CH group showed thickening in the parietal peritoneum and adhesion in the abdominal wall during scarification, while these changes were absent in the control group. Control group had significantly lower median peritoneal thickness than CH group, CH + resting group, and CH + adalimumab group, (P = 0.0001, P = 0.0001, and P = 0.003, respectively). CH + adalimumab group had significantly lower median peritoneal thickness than CH group and CH + resting group (P = 0.0001 and P = 0.0001, respectively) (Table 1 and Figure 1).
Table 1.
Peritoneal thickness, inflammation, fibrosis, vascular score, vascular endothelial growth factor (VEGF), and transforming growth factor beta (TGF-β1) scores in different study groups
| Variables, median (Q1-Q3) | Chlorhexidine group (n = 10) | Control group (n = 10) | Chlorhexidine + adalimumab group (n = 10) | Chlorhexidine + resting group (n = 10) | P* |
|---|---|---|---|---|---|
| Peritoneal thickness (μm) |
394.4 (323.1-454.7) |
82.8 (66.9-121.9) |
181.7 (130.2-201.1) |
539.8 (381.6-649.5) |
0.0001 |
| Inflammation score |
1.0 (1-1) |
0.0 (0-0) |
1.0 (0-1) |
1.5 (1-2) |
0.0001 |
| Fibrosis score |
2.0 (1-2) |
0.0 (0-0.5) |
1.0 (1-1) |
2.0 (2-3) |
0.0001 |
| Vascular score |
8.0 (7-9) |
0.0 (0-0) |
5.0 (4.75-6) |
14.8 (15-16.3) |
0.0001 |
| VEGF |
5.0 (4.8-6) |
0.0 (0-0) |
3.0 (3-4) |
7.0 (6.75-8.25) |
0.0001 |
| TGF-β1 | 2.0 (1.8-2) | 0.0 (0-0) | 1.0 (0-1) | 2.0 (1.8-3) | 0.0001 |
*Kruskal-Wallis test.
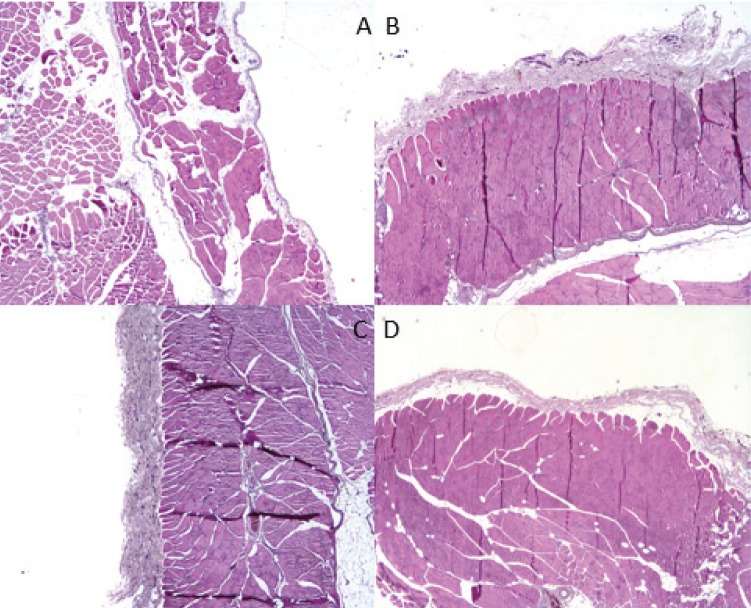
Figure 1.
Histopathological images of the parietal peritoneum thickness (hematoxylin and eosin ×10). (A) Control group; (B) Chlorhexidine group; (C) Chlorhexidine + resting group; (D) Chlorhexidine + adalimumab group.
Control group had significantly lower median inflammation score than CH group, CH + resting group, and CH + adalimumab group (P = 0.0001, P = 0.0001, and P = 0.002, respectively). CH + adalimumab group had significantly lower median inflammation score than CH + resting group (P = 0.005).
Control group had significantly lower median fibrosis score than CH group, CH + resting group, and CH + adalimumab group (P = 0.0001, P = 0.0001, and P = 0.001, respectively). CH + adalimumab group had significantly lower median fibrosis score than CH group and CH + resting group (P = 0.007 and P = 0.001, respectively).
Control group had significantly lower median vascular score than CH group, CH + resting group, and CH + adalimumab group (P = 0.0001, P = 0.0001, and P = 0.0001, respectively). CH + adalimumab group had significantly lower median vascular score than CH group and CH + resting group (P = 0.001, P = 0.0001, respectively).
Control group had significantly lower mean VEGF score than CH group, CH + resting group, and CH + adalimumab group (P = 0.0001, P = 0.0001, and P = 0.0001, respectively). CH + adalimumab group had significantly lower median VEGF score than CH group and CH + resting group (P = 0.0001). Parietal peritoneal images obtained by using VEGF immunohistochemical staining are shown in Figure 2.
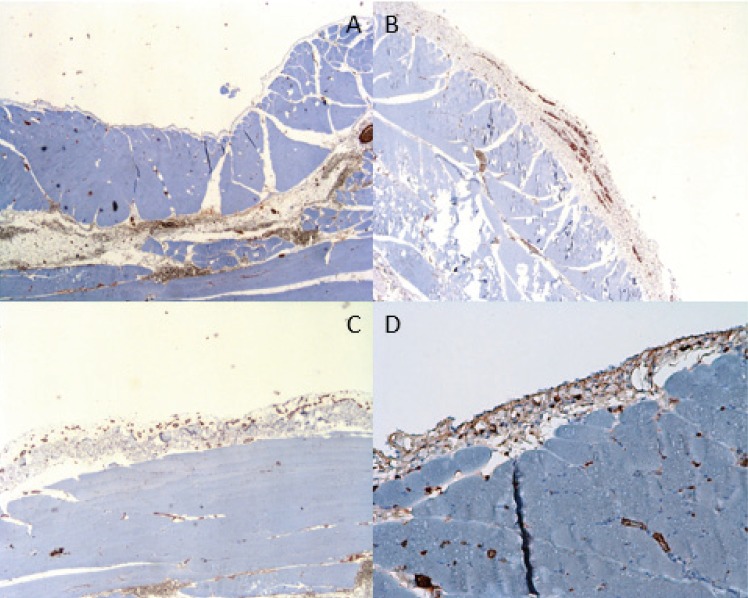
Figure 2.
Parietal peritoneal images obtained by using vascular endothelial growth factor immunohistochemical staining. (A) Control group ( × 10); (B) Chlorhexidine group ( × 10); (C) Chlorhexidine + resting group ( × 10); (D) Chlorhexidine + adalimumab group ( × 20).
Control group had significantly lower median TGF-β1 than CH group, CH + resting group, and CH + adalimumab group (P = 0.0001, P = 0.0001, and P = 0.002, respectively). CH + adalimumab group had significantly lower median TGF-β1 score than CH group and CH + resting group (P = 0.0001 and P = 0.001, respectively). Parietal peritoneal images obtained by TGF-β1 immunohistochemical staining are shown in Figure 3.
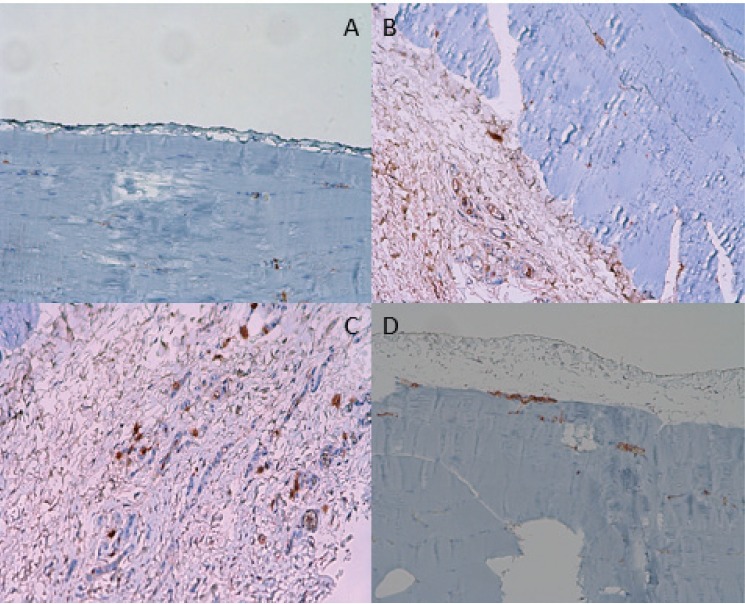
Figure 3.
Parietal peritoneal images obtained by transforming growth factor-β1 immunohistochemical staining. (A) Control group ( × 10); (B) Chlorhexidine group ( × 10); (C) Chlorhexidine + resting group ( × 20); (D) Chlorhexidine + adalimumab group ( × 10).
DISCUSSION
Our study found that adalimumab prevented the development of experimental SEP. Although our experimental SEP model was created according to Ishii et al (15), we studied the development of peritoneal fibrosis for 3 rather than for 8 weeks. In our study, CH group had significantly higher parietal peritoneal thickness, inflammation, and fibrosis scores compared with the control group, indicating that peritoneal fibrosis was effectively induced. In CH + resting group, morphological peritoneum changes were not reversed and they even progressed, suggesting that the peritoneum was still active.
In experimental SEP models, some therapies (VEGF inhibitor, renin-angiotensin system inhibitors, aliskiren, erythropoietin, and thalidomide) reduced peritoneal thickness, vascularization, and fibrosis (3-7). However, some therapies (calcium channel blocker and cyclosporine) yielded no positive results and may even pose a risk for the development of SEP (8,9).
TNF-α acts on innate immune cells and contributes to the development of fibrosis (18). However, it also suppresses fibrosis by down-regulating connective tissue growth factor (CTGF) in fibroblasts (19). In our study, the first to use adalimumab in an experimental SEP model, CH + adalimumab treated animals had significantly lower peritoneal thickness, inflammation, fibrosis, and vascular score compared with CH group and CH + resting group.
Factors that contribute to the pathogenesis of SEP are VEGF and TGF-β (10). Human peritoneal mesothelial cells play an important role in VEGF synthesis, and VEGF release increases in the presence of proinflammatory cytokines. VEGF can increase vascular permeability, vasodilatation, and neoangiogenesis in the peritoneal membrane (20). Some therapies (aliskiren, erythropoietin, and thalidomide) applied after inducing an experimental SEP model decreased TGF-β and VEGF release (5-7). In our study, CH + adalimumab significantly decreased TGF-β1 and VEGF compared with CH and CH + resting groups, probably because of anti-TNF effects of adalimumab. Anti-inflammatory and angiogenic activity of TNF-α inhibitors has been demonstrated in the treatment of immune-associated diseases. In addition, angiogenic effects of TNF are due to changed expression of VEGF and its endothelial receptor VEGF receptor 2 (21).
Other substances that play a role in the pathogenesis of SEP are fibroblast growth factor (FGF), matrix metalloproteinase (MMP), and myeloperoxidase (10). Serum FGF and VEGF levels in psoriatic patients were decreased by anti-TNF therapy (infliximab) (22). MMP-3 was significantly reduced by the addition of adalimumab therapy in patients with rheumatoid arthritis (23) and by TNF-α antagonist therapy (infliximab, etanercept, and adalimumab) in patients with spondyloarthritis (24,25). Adalimumab therapy also reduced myeloperoxidase activity (26,27). TNF-α inhibitors suppress nuclear factor-kappaB (NF-кB) activity (28), but one study showed that adalimumab therapy was not effective on the NF-кB activity of lymphocytes in patients with rheumatoid arthritis (29).
A limitation of our study is the fact that it did not investigate immunohistochemical localization of TNF-α and CTGF in peritoneal tissue, serum FGF and MMP-3 levels, and myeloperoxidase and NF-кB activities. In addition, it did not involve groups treated with different adalimumab doses.
In conclusion, this experimental SEP model indicates that adalimumab can prevent experimental SEP development. However, more extensive experimental and clinical studies are needed to find the optimal dose and duration of treatment.
Acknowledgments
Funding The study was supported by Canakkale Onsekiz Mart University Scientific Research Projects Unit (TTU-2016-840).
Ethical approval obtained from the Canakkale Onseliz Mart University animal ethics committee (Decision No:2016/01-03, Approval date: January 21, 2016).
Declaration of authorship all authors conceived and designed the study; all authors acquired the data; all authors analyzed and interpreted the data; all authors drafted the manuscript; YA and SeB critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- 1.Hansson JH, Watnick S. Update on Peritoneal Dialysis: Core Curriculum 2016. Am J Kidney Dis. 2016;67:151–64. doi: 10.1053/j.ajkd.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Habib SM, Betjes MG, Fieren MW, Boeschoten EW, Abrahams AC, Boer WH, et al. Management of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatment. Neth J Med. 2011;69:500–7. [PubMed] [Google Scholar]
- 3.Ada S, Ersan S, Sifil A, Unlu M, Kolatan E, Sert M, et al. Effect of bevacizumab, a vascular endothelial growth factor inhibitor, on a rat model of peritoneal sclerosis. Int Urol Nephrol. 2015;47:2047–51. doi: 10.1007/s11255-015-1116-8. [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt D, Cetin P, Sipahi S, Hur E, Nar H, Ertilav M, et al. The effects of renin-angiotensin system inhibition on regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(S5):S38–42. [PubMed] [Google Scholar]
- 5.Ke CY, Lee CC, Lee CJ, Subeq YM, Lee RP, Hsu BG. Aliskiren ameliorates chlorhexidine digluconate-induced peritoneal fibrosis in rats. Eur J Clin Invest. 2010;40:301–9. doi: 10.1111/j.1365-2362.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- 6.Mondello S, Mazzon E, Di Paola R, Crisafulli C, Italiano D, Buemi M, et al. Erythropoietin suppresses peritoneal fibrosis in rat experimental model. Eur J Pharmacol. 2009;604:138–49. doi: 10.1016/j.ejphar.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Mondello S, Mazzon E, Di Paola R, Crisafulli C, Mondello P, Buemi M, et al. Thalidomide suppresses sclerosing encapsulating peritonitis in a rat experimental model. Shock. 2009;32:332–9. doi: 10.1097/SHK.0b013e3181994974. [DOI] [PubMed] [Google Scholar]
- 8.Bozkurt D, Sipahi S, Cetin P, Hur E, Ozdemir O, Ertilav M, et al. Does immunosuppressive treatment ameliorate morphology changes in encapsulating peritoneal sclerosis? Perit Dial Int. 2009;29(S2):S206–10. [PubMed] [Google Scholar]
- 9.Nakamoto H, Imai H, Fukushima R, Ishida Y, Yamanouchi Y, Suzuki H. Role of the renin-angiotensin system in the pathogenesis of peritoneal fibrosis. Perit Dial Int. 2008;28(Suppl 3):S83–7. [PubMed] [Google Scholar]
- 10.Korte MR, Sampimon DE, Betjes MGH, Krediet RT. Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol. 2011;7:528–38. doi: 10.1038/nrneph.2011.93. [DOI] [PubMed] [Google Scholar]
- 11.Szondy Z, Pallai A. Transmembrane TNF-alpha reverse signaling leading to TGF-beta production is selectively activated by TNF targeting molecules: Therapeutic implications. Pharmacol Res. 2017;115:124–32. doi: 10.1016/j.phrs.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Mitoma H, Horiuchi T, Tsukamoto H, Tamimoto Y, Kimoto Y, Uchino A, et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008;58:1248–57. doi: 10.1002/art.23447. [DOI] [PubMed] [Google Scholar]
- 13.Campanati A, Moroncini G, Ganzetti G, Pozniak KN, Goteri G, Giuliano A, et al. Adalimumab modulates angiogenesis in psoriatic skin. Eur J Inflamm. 2013;11:489–98. doi: 10.1177/1721727X1301100218. [DOI] [Google Scholar]
- 14.Eder P, Lykowska-Szuber L, Iwanik K, Krela-Kazmierczak I, Stawczyk-Eder K, Majewski P, et al. The influence of anti-TNF theraphy on CD31 and VEGF expression in colonic mucosa of Crohn’s disease patients in relation to mucosal healing. Folia Histochem Cytobiol. 2016;54:75–80. doi: 10.5603/FHC.a2016.0008. [DOI] [PubMed] [Google Scholar]
- 15.Ishii Y, Sawada T, Shimizu A, Tojimbara T, Nakajima I, Fuchinoue S, et al. An experimental sclerosing encapsulating peritonitis model in mice. Nephrol Dial Transplant. 2001;16:1262–6. doi: 10.1093/ndt/16.6.1262. [DOI] [PubMed] [Google Scholar]
- 16.Börcek AÖ, Çivi S, Öcal Ö, Gülbahar Ö. Effects of tumor necrosis factor alpha blocker adalimumab in experimental spinal cord injury. J Korean Neurosurg Soc. 2015;57:73–6. doi: 10.3340/jkns.2015.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JD, Craig KJ, Topley N, von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–9. doi: 10.1681/ASN.V132470. [DOI] [PubMed] [Google Scholar]
- 18.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beddy D, Mulsow J, Watson RW, Fitzpatrick JM, O’Connell PR. Expression and regulation of connective tissue growth factor by transforming growth factor beta and tumour necrosis factor alpha in fibroblasts isolated from strictures in patients with Crohn’s disease. Br J Surg. 2006;93:1290–6. doi: 10.1002/bjs.5431. [DOI] [PubMed] [Google Scholar]
- 20.Mandl-Weber S, Cohen CD, Haslinger B, Kretzler M, Sitter T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 2002;61:570–8. doi: 10.1046/j.1523-1755.2002.00143.x. [DOI] [PubMed] [Google Scholar]
- 21.Murdaca G, Spanň F, Miglino M, Puppo F. Effects of TNF-α inhibitors upon the mechanisms of action of VEGF. Immunotherapy. 2013;5:113–5. doi: 10.2217/imt.12.151. [DOI] [PubMed] [Google Scholar]
- 22.Mastroianni A, Minutilli E, Mussi A, Bordignon V, Trento E, D’Agosto G, et al. Cytokine profiles during infliximab monotheraphy in psoriatic arthritis. Br J Dermatol. 2005;153:531–6. doi: 10.1111/j.1365-2133.2005.06648.x. [DOI] [PubMed] [Google Scholar]
- 23.Kanbe K, Chiba J, Nakamura A. Decrease of CD68 and MMP-3 expression in synovium by treatment of adalimumab for rheumatoid arthritis. Int J Rheum Dis. 2011;14:261–6. doi: 10.1111/j.1756-185X.2011.01643.x. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen SJ, Hetland ML, Sřrensen IJ, Ostergaard M, Nielsen HJ, Johansen JS. Circulating levels of interleukin-6, vascular endothelial growth factor, YKL-40, matrix metalloproteinase-3, and total aggrecan in spondyloarthritis patients during 3 years of treatment with TNFα inhibitors. Clin Rheumatol. 2010;29:1301–9. doi: 10.1007/s10067-010-1528-x. [DOI] [PubMed] [Google Scholar]
- 25.Wendling D, Cedoz JP, Racadot E. Serum levels of MMP-3 and cathepsin K in patients with ankylosing spondylitis: effect of TNFalpha antagonist theraphy. Joint Bone Spine. 2008;75:559–62. doi: 10.1016/j.jbspin.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz M, Tekekoglu S, Herek O, Ozmen O, Sahinduran S, Buyukoglu T. Ameliorative effect of adalimumab on experimentally induced acute pancreatitis in rats. Pancreas. 2010;39:1238–42. doi: 10.1097/MPA.0b013e3181dec1a6. [DOI] [PubMed] [Google Scholar]
- 27.Kurt A, Tumkaya L, Kalkan Y, Turut H, Cure MC, Cure E, et al. Is adalimumab protective in ischemia-reperfusion injury in lung? Iran J Basic Med Sci. 2015;18:1093–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YC, Lin YC, Huang MY, Kuo PL, Wu CC, Lee MS, et al. Tumor necrosis factor-alpha inhibitors suppress CCL2 chemokine in monocytes via epigenetic modification. Mol Immunol. 2017;83:82–91. doi: 10.1016/j.molimm.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Semmler M, Seeck U, Neustadt B, Schulz M, Dotzlaw H, Neeck G, et al. No effects of adalimumab theraphy on the activation of NF-kappaB in lymphocytes rheumatoid arthritis. Clin Rheumatol. 2007;26:1499–504. doi: 10.1007/s10067-007-0540-2. [DOI] [PubMed] [Google Scholar]