Abstract
Objectives
To measure serum leptin concentration in dogs with pituitary‐dependent hyperadrenocorticism and varying degrees of cholestatic disease and determine whether serum levels differed between dogs with pituitary‐dependent hyperadrenocorticism and those with gall bladder mucocoele.
Materials and Methods
Client‐owned healthy dogs (n=20), dogs diagnosed with gall bladder mucocoele (n=20) and dogs diagnosed with pituitary‐dependent hyperadrenocorticism (n=60) were enrolled. Only dogs of normal body condition score were included. Dogs with pituitary‐dependent hyperadrenocorticism were divided into three groups according to the severity of cholestatic disease: normal gall bladder (n=20), cholestasis (n=20) and gall bladder mucocoele (n=20). Serum leptin levels were measured using sandwich enzyme‐linked immunosorbent assay.
Results
Serum concentrations of leptin were similar between dogs with gall bladder mucocoele and those with pituitary‐dependent hyperadrenocorticism accompanied by gall bladder mucocoele; these concentrations were significantly higher than those in healthy control dogs. In dogs with pituitary‐dependent hyperadrenocorticism, circulating leptin concentration significantly increased with the severity of cholestasis: higher in the cholestasis group than the normal gall bladder group and higher in the gall bladder mucocoele group than the cholestasis group.
Clinical Significance
Elevated circulating leptin concentration was associated with canine pituitary‐dependent hyperadrenocorticism and gall bladder mucocoele. Homeostatic imbalance of leptin concentration might be associated with severity of cholestatic disease in pituitary‐dependent hyperadrenocorticism.
INTRODUCTION
Hyperadrenocorticism (HAC), is a common endocrine disorder in dogs, characterised by excessive production of cortisol by the adrenal cortices. The cause of HAC is either overproduction of adrenocorticotropic hormone (ACTH) by the pituitary gland, or autonomous cortisol secretion from the adrenal gland, either of which result in chronically high circulating cortisol levels (Ettinger & Feldman 2009). Pituitary‐dependent hyperadrenocorticism (PDH) accounts for 85% of canine HAC, while functional adrenocortical tumour (FAT) accounts for 15% (Cho et al. 2014). HAC is typically chronic and progressive, causing classical clinical symptoms such as polyuria, polydipsia, polyphagia, abdominal distention (pot belly) and endocrine alopecia (Ettinger & Feldman 2009). The treatment selection for canine HAC depends on several factors, such as the presence of other diseases, HAC complications or whether the cause of HAC is attributed to FAT or PDH. HAC can be treated with medication, surgery, radiation therapy or a combination of these options (Mauldin & Burk 1990, Hara et al. 2010, Ramsey 2010, Behrend et al. 2013).
In dogs, gall bladder mucocoele (GBM) is an immobile accumulation of excessive and inspissated bile and mucus, accompanied by a distended gall bladder (Besso et al. 2000). Although most dogs with GBM are asymptomatic, and GBM may be observed incidentally during diagnostic examinations, it can often lead to life‐threatening conditions, such as extra‐hepatic biliary obstruction. Although the aetiopathogenesis of GBM in dogs remains unclear, breed predisposition (Besso et al. 2000, Pike et al. 2004), hyperlipidaemia (Kutsunai et al. 2014) and endocrine disorders, notably HAC (Mesich et al. 2009), have been suggested as risk factors. Additionally, disordered gall bladder motility (Tsukagoshi et al. 2012), biliary obstruction itself (Bernhoft et al. 1983) and altered bile composition (Kakimoto et al. 2017) are known to be associated with the pathogenesis and progression of GBM.
Since the discovery of leptin over 20 years ago, research has associated it with nutrition and fat metabolism and suggested its role as a pathophysiological regulatory factor in various diseases, such as endocrinopathy (Cho et al. 2014, Kim et al. 2015) and gall bladder disease (Lee et al. 2017a,2017b). In our previous study, by examining the expression of leptin and leptin receptor, we confirmed that the canine gall bladder is a leptin‐affected tissue (Lee et al. 2016). Furthermore, dysregulation of leptin expression is associated with the pathogenesis of GBM, and serum leptin levels are indicators of the severity of GBM (Lee et al. 2017a). Another report on the relationship between PDH and leptin has suggested that serum leptin concentrations are related to the pathogenesis of canine PDH, as well as the response of PDH to therapeutics (Cho et al. 2014).
Although previous studies in human and animals, including dogs, have revealed multiple roles of leptin in different diseases, little is known about the relationships among PDH, GBM and leptin in dogs. Previous investigations into the connections between PDH and GBM (Mesich et al. 2009, Kim et al. 2017), PDH and leptin (Cho et al. 2014) and GBM and leptin (Lee et al. 2017a) indicate some association between the three factors, but the overarching structure of this relationship remains unclear. Therefore, this study aimed to elucidate the relationships and interdependencies of leptin, PDH and cholestatic disease, including GBM, in dogs. To achieve this, we compared serum leptin concentrations using an enzyme‐linked immunosorbent assay (ELISA) in dogs with GBM, dogs with PDH and healthy dogs. In addition, dogs with PDH were further divided into three groups according to the severity of the cholestatic disease, and their serum leptin was measured.
MATERIALS AND METHODS
Ethical approval
Informed consent for this study was obtained from all the dog owners, and all procedures were approved by the Seoul National University Institutional Animal Care and Use Committees (SNU‐170518‐1).
Sample preparation
Twenty client‐owned healthy control dogs, 20 dogs with newly diagnosed GBM and 60 dogs with PDH recruited from the Veterinary Medical Teaching Hospital, Seoul National University were enrolled in this study. Of the 60 dogs with PDH, 33 were newly diagnosed with PDH and had not received any medical treatment, while 27 had been treated with trilostane for at least 3 months and shown good clinical response, according to owner evaluation. All dogs underwent a physical examination, examinations for serum biochemical profiles, electrolytes, complete blood counts and diagnostic imaging, including abdominal sonography and radiography (thoracic and abdominal). Only dogs with a normal body condition score (BCS) (5 to 6 out of 9) were selected and the BCS of each dog was recorded by a single investigator, based on a 1 to 9 scoring scale (Laflamme 1997). The healthy control dogs exhibited a lack of abnormalities in all medical examinations, including gall bladder ultrasonography.
In dogs with gall bladder disorders, the severity of cholestasis was characterised as “normal”, “cholestasis” or “GBM”, determined by multiple radiologists using abdominal ultrasonography applying the same criteria. HAC diagnosis was based on clinical signs (polyuria/polydipsia, polyphagia, pot belly and alopecia) and the results of endocrine tests, including the urine corticoid to creatinine ratio (UCCR) and either a low‐dose dexamethasone screening test (LDDST) or an ACTH stimulation test (Behrend et al. 2013). To discriminate dogs with PDH from those with FAT, ultrasonography, LDDST or high‐dose dexamethasone suppression test (HDDST) were performed. Dogs diagnosed with PDH were further divided into three groups, based on the results of the gall bladder ultrasonography: normal gall bladder group (n=20), cholestasis group (n=20) and GBM group (n=20).
For all dogs, blood samples for biochemical analysis were collected from the jugular vein. All dogs underwent fasting for 12 hours before blood collection. Serum samples were separated from the collected blood by centrifugation (10 minutes at 1000×g). A portion of each serum sample was used for serum biochemistry analysis, and the remainder was frozen at −80°C to be used for ELISA.
Diagnostic evaluation
In general, the diagnosis of cholestatic disease including GBM is typically based on the results of a combination of laboratory tests, imaging data and observation of clinical signs, such as abdominal pain, lethargy, vomiting and anorexia. Among these, diagnostic imaging (especially abdominal ultrasonography) is known to exhibit the highest sensitivity for the detection of cholestatic disease. A thorough abdominal ultrasonography, including evaluation of the liver and gall bladder, was performed in all dogs at the time of diagnosis for grouping, and patients showing evidence of biliary obstruction were excluded. The normal gall bladder group displayed no evidence of bile precipitation in the gall bladder. Dogs in the cholestasis group showed characteristics of gravity‐dependent gall bladder sludge or mobile precipitate. Dogs in the GBM group exhibited immobile sludge and a stellate or kiwi fruit‐like pattern on the images (Besso et al. 2000, Pike et al. 2004, Ettinger & Feldman 2009).
The diagnosis of HAC was made following analysis of the history, clinical signs, laboratory test results and abdominal ultrasonography. If HAC was suspected, UCCR was performed as a screening test to rule this out. If UCCR did not exclude HAC, either LDDST or an ACTH stimulation test was conducted to confirm a diagnosis of HAC. For UCCR, the owners were requested to collect a sample of the first urination of the morning at home and HAC was ruled out by a UCCR <60×10−6. For the ACTH stimulation test, serum samples were collected and cortisol level was measured before and 1 hour after intravenous (iv) administration of synthetic ACTH (5 mcg/kg). Diagnosis of HAC was confirmed if the post‐ACTH cortisol concentration was >20 mcg/dL. For LDDST, serum cortisol concentrations were evaluated before and 4 and 8 hours after dexamethasone administration (0.01 mg/kg, iv), and HAC diagnosis was confirmed if there was failure of serum cortisol suppression at 8 hours (>1.4 mcg/dL). Discrimination of PDH from FAT was based on ultrasonographic characteristics of the adrenal gland and on results of the LDDST and HDDST. Dogs with evidence of FAT based on ultrasonography, such as unilateral or asymmetrical adrenal gland enlargement (maximum diameter of adrenal gland >2 cm) with atrophied contralateral gland (<5 mm), and invasion or compression of adjacent structures, were excluded from the investigation. LDDST or HDDST (when suppression was not found in LDDST) were performed to diagnose PDH. Demonstrated cortisol suppression (<1.4 mcg/dL) at 4 hours was considered diagnostic for PDH, and allowed us to rule out FAT (Ettinger & Feldman 2009, Behrend et al. 2013).
All patients in the PDH treatment group were treated with trilostane once or twice daily. These patients were re‐evaluated 2 and 4 weeks after medication for dosage adjustment. After 1 month, the re‐evaluation interval was adjusted for each individual. At each evaluation, owners were asked about clinical signs and potential adverse effects of trilostane treatment. In addition, physical examination, serum biochemistry and ACTH stimulation test were performed on every visit; ACTH stimulation tests were performed 4 to 6 hours after the administration of trilostane. A post‐ACTH cortisol concentration of 1.5 to 5.5 mcg/dL was considered adequate. The population of the PDH treatment group included only patients that had been managed for 3 months with an adequate dosage of trilostane, as evaluated by their clinical response and the results of routine and endocrine testing (Ramsey 2010).
Sandwich ELISA
Serum leptin concentrations in all samples were measured in duplicate using a commercial canine‐specific leptin sandwich ELISA (canine leptin ELISA) kit (Canine Leptin ELISA; Millipore), according to the manufacturer's protocol. The intra‐ and inter‐assay coefficients of variation were 5 and 7%, respectively. The absorbance of each well was measured with an automated microplate spectrophotometer (Epoch; BioTek Instruments Inc.) at 450 nm.
Statistical analysis
Statistical analyses were performed using SPSS statistical software, version 23.0 (IBM). The Shapiro–Wilk method was used to determine whether the obtained data were normally distributed. The Kruskal–Wallis test was performed to analyse differences in circulating leptin levels among the following groups, respectively: controls, GBM dogs and PDH dogs with GBM; controls and PDH patients classified according to the severity of cholestatic disease; controls and dogs with PDH with or without treatment. If a significant difference was detected, post hoc comparisons were performed using the Mann–Whitney test for follow‐up pair‐wise comparisons with Bonferroni–Holm multiple comparison adjustments. The normally distributed data are presented as the mean value and standard deviation, while other data are presented as the median and range or interquartile range (IQR). A P‐value of less than 0.05 was considered to represent statistical significance.
RESULTS
Cases
Basic information on the 20 healthy control dogs, 20 dogs with GBM and 20 dogs with PDH and GBM are summarised in Table 1. The 60 dogs with PDH enrolled in this study were divided into three groups, depending on the severity of the cholestatic disease, as follows: normal gall bladder group (n=20), cholestasis group (n=20) and GBM group (n=20). Detailed information regarding these groups is summarised in Table 2.
Table 1.
Comparison of demographic characteristics among healthy dogs (controls), dogs with GBM and dogs with PDH exhibiting GBM
| Healthy dogs | Dogs with GBM | Dogs with PDH exhibiting GBM | |
|---|---|---|---|
| n | 20 | 20 | 20 |
| Age (years) | 9.65 (2.91) | 12.15 (2.35) | 12.60 (3.03) |
| Sex (n) | Males (1) | Males (2) | Castrated males (5) |
| Castrated males (6) | Castrated males (8) | Female (5) | |
| Female (4) | Female (2) | Spayed females (10) | |
| Spayed females (9) | Spayed females (8) | ||
| Breed (n) | Poodle (2) | Poodle (4) | Poodle (2) |
| Maltese (3) | Maltese (5) | Maltese (7) | |
| Cocker spaniel (2) | Cocker spaniel (1) | Cocker Spaniel (3) | |
| Schnauzer (1) | Schnauzer (1) | Schnauzer (2) | |
| Pomeranian (2) | Pomeranian (2) | Pomeranian (3) | |
| Shih‐tzu (3) | Shih‐tzu (4) | Mixed breed (3) | |
| Spitz (1) | Pug (1) | ||
| Yorkshire terrier (3) | Spitz (1) | ||
| Miniature pinscher (1) | Yorkshire terrier (1) | ||
| Pekingese (2) | |||
| BW (kg) | 4.45 (3.30) | 5.32 (3.55) | 3.65 (3.25) |
| BCS (out of 9) | 5 (5 to 6) | 5 (5 to 6) | 5 (5 to 6) |
PDH Pituitary‐dependent hyperadrenocorticism, GBM Gall bladder mucocele, BW Bodyweight, BCS Body condition score, n Number of patients
Continuous variables are presented as the mean and standard deviation, except for BW (which are presented as the median and interquartile range); BCS, which is a discontinuous variable, is presented as the median and range
Table 2.
Comparison of demographic characteristics among healthy dogs and subgroups in the PDH group
| Healthy dogs | PDH patients group | |||
|---|---|---|---|---|
| With normal gall bladder | With cholestasis | With GBM | ||
| n | 20 | 20 | 20 | 20 |
| Age (years) | 9.00 (4.00) | 13.50 (1.75) | 12.50 (1.00) | 13.00 (3.50) |
| Sex (n) | Males (1) | Males (2) | Males (1) | Castrated males (5) |
| Castrated males (6) | Castrated males (5) | Castrated males (12) | Female (5) | |
| Female (4) | Female (2) | Female (1) | Spayed females (10) | |
| Spayed females (9) | Spayed females (11) | Spayed females (6) | ||
| Breed (n) | Poodle (2) | Poodle (3) | Poodle (1) | Poodle (2) |
| Maltese (3) | Maltese (6) | Maltese (5) | Maltese (7) | |
| Cocker spaniel (2) | Shih‐tzu (5) | Cocker spaniel (1) | Cocker spaniel (3) | |
| Schnauzer (1) | Yorkshire terrier (2) | schnauzer (2) | Schnauzer (2) | |
| Pomeranian (2) | Pekingese (1) | Pomeranian (2) | Pomeranian (3) | |
| Shih‐tzu (3) | Mixed breed (3) | Shih‐tzu (6) | Mixed breed (3) | |
| Spitz (1) | Mixed breed (3) | |||
| Yorkshire terrier (3) | ||||
| Miniature pinscher (1) | ||||
| Pekingese (2) | ||||
| BW (kg) | 4.45 (3.30) | 4.57 (1.86) | 6.18 (4.13) | 3.65 (3.25) |
| BCS (out of 9) | 5 (5 to 6) | 5 (5 to 6) | 5 (5 to 6) | 5 (5 to 6) |
PDH Pituitary‐dependent hyperadrenocorticism, BW, bodyweight, BCS Body condition score, n, Number of patients
Continuous variables are presented as the median and interquartile range; BCS, which is a discontinuous variable, is presented as the median and range
Analysis of serum concentrations of leptin
The serum concentration of leptin in both dogs with GBM [median 7.12 ng/mL; IQR 3.70 to 9.31]) and those with PDH exhibiting GBM (median 9.18 ng/mL; IQR 5.06 to 12.47) were significantly higher than that of the healthy control dogs (median 2.46 ng/mL; IQR 1.23 to 4.03) (P=0.001 and P<0.001, respectively), but similar (P=0.130) to each other (Fig 1). Interestingly, analysis of serum leptin concentration in the PDH patient sub‐groups [normal gall bladder (median 4.30 ng/mL; IQR 2.34 to 6.28), cholestasis (median 6.95 ng/mL; IQR 3.46 to 7.34) and GBM (median 9.18 ng/mL; IQR 5.06 to 12.47)] suggested that leptin concentrations increased with the severity of cholestasis, as indicated by comparisons between the normal gall bladder and cholestasis groups (P=0.027) and between cholestasis and GBM groups (P=0.030) (Fig 2). Furthermore, in dogs with PDH, serum leptin levels were significantly higher in the non‐treated (median 4.68 ng/mL; IQR 2.89 to 7.26) compared with the treated group (median 7.14 ng/mL; IQR 4.55 to 10.42) (P=0.034) (Fig 3).
Figure 1.
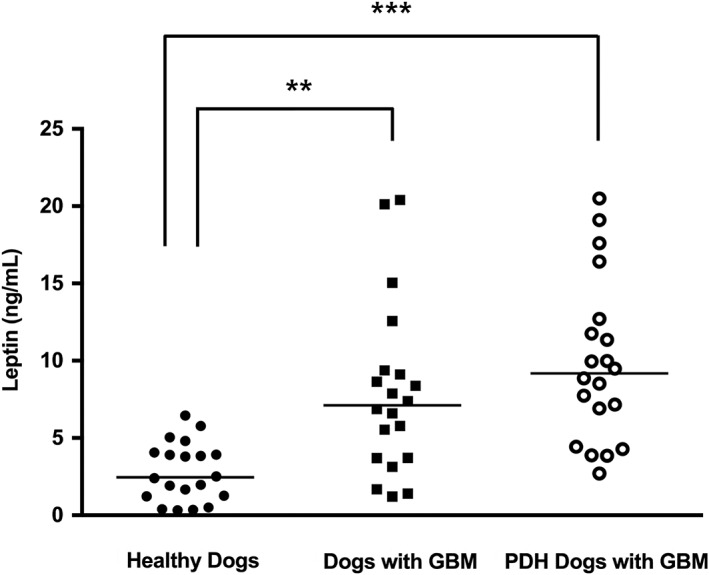
Comparison of serum leptin concentrations among healthy dogs (n=20), dogs with gall bladder mucocoele (GBM, n=20), and dogs with pituitary‐dependent hyperadrenocorticism (PDH) exhibiting GBM (n=20). The horizontal bars indicate the median values. **P<0.01, ***P<0.001 between groups
Figure 2.
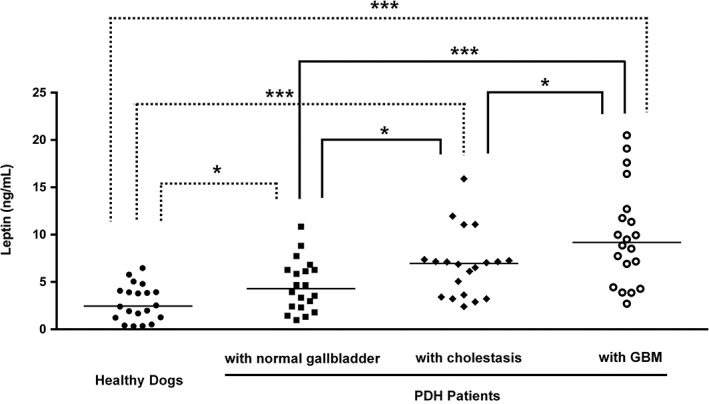
Comparison of serum leptin concentrations among healthy dogs, and pituitary‐dependent hyperadrenocorticism (PDH) patients (n=60) classified according to the degree of cholestatic disease: normal gall bladder (n=20), cholestasis (n=20) and gall bladder mucocoele (GBM, n=20). The horizontal bars indicate the median values. *P<0.05, ***P<0.001 between groups
Figure 3.
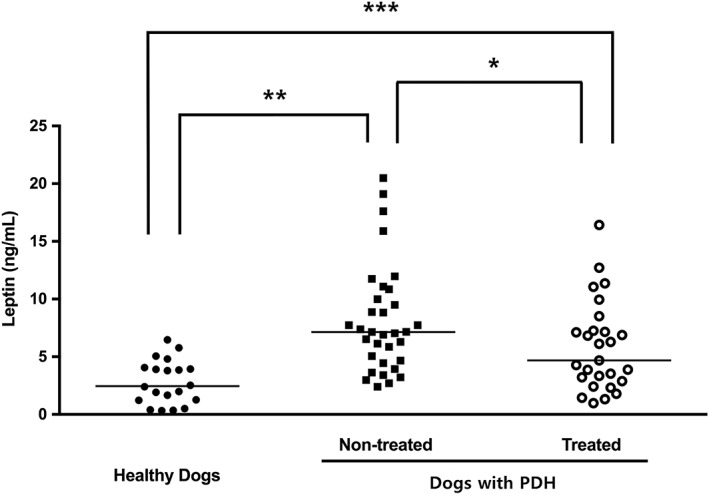
Comparison of serum leptin concentrations among healthy dogs (n=20) and dogs with pituitary‐dependent hyperadrenocorticism (PDH, n=60), with or without treatment (non‐treated group, n=33; treated group, n=27). The horizontal bars indicate the median values. *P<0.05, **P<0.01, ***P<0.001 between groups
DISCUSSION
In previous studies, plasma concentration of leptin was found to be elevated in dogs with higher BCS values, but no differences were associated with sex, age or breed (Ishioka et al. 2007, Piantedosi et al. 2016). These results suggest that plasma leptin concentration may be a reliable parameter for measuring adiposity independent of variations in sex, age and breed (Ishioka et al. 2007). Based on these reports, we limited our study to dogs with similar, normal, BCS.
Previous investigations have suggested a relationship between PDH and GBM in dogs. One retrospective case–control study showed that a considerable number of dogs with GBM exhibit endocrinopathies, such as PDH and hypothyroidism, and that the odds of GBM in dogs with HAC are 29 times that of dogs not having HAC (Mesich et al. 2009). Another retrospective study reported that dogs with PDH and cholestatic disease, including GBM, have higher post‐ACTH stimulation cortisol levels and more severe clinical symptoms than those without GBM. These findings indicate that cholestatic diseases, including GBM, are critical complications of PDH in dogs, suggesting that dogs with PDH require cautious monitoring for the risk of cholestasis and GBM formation (Kim et al. 2017). In addition, other reports comparing circulating leptin concentrations between healthy controls and dogs with PDH or GBM confirmed that leptin levels were significantly higher in both disease groups than in the healthy group (Cho et al. 2014, Lee et al. 2017a). Based on this evidence, we hypothesised that serum leptin concentration would be even higher in dogs diagnosed with both diseases than those with either PDH or GBM alone. Our results suggest that this is incorrect, instead being consistent with previous indications of no difference in serum leptin concentration between GBM dogs with or without endocrinopathies (Lee et al. 2017a).
Factors such as changes in the absorption and secretion of bile, hypersecretion of mucin and decreased motility of gall bladder may affect the development of cholestatic disease, including GBM, and several reports discuss the relationship between these factors and leptin concentration. A previous study showed that leptin was associated with decreased sodium concentration and pH of the bile and, also, decreased gall bladder volume through regulation of gall bladder genes associated with absorption and secretion, suggesting that leptin dysregulation can lead to alterations in the properties of bile (Graewin et al. 2008). Furthermore, leptin deficiency has been suggested to affect sensitivity of the gall bladder smooth muscle and the sphincter of Oddi to neuropeptide Y and cholecystokinin, thereby altering its contractility (Goldblatt et al. 2002). Dysregulation of leptin may also be associated with mucin over‐secretion (Lee et al. 2017a). Our current study further supports a relationship between leptin and the severity of cholestatic disease in dogs with PDH, by demonstrating an increase in serum leptin concentration corresponding with cholestatic status (normal, cholestasis and GBM). These results are supported by our previous study, which shows that patients with more severe GBM exhibited higher serum leptin levels and higher expression of leptin and its receptor in gall bladder tissue than both healthy controls and dogs with less severe GBM (Lee et al. 2017a). However, in order to further analyse the relationships between leptin and the factors associated with GBM, more in‐depth investigations are required.
Serum leptin concentrations are elevated in proportion to serum cortisol levels in dogs with PDH (Cho et al. 2014) and, in Cushing's syndrome patients, leptin secretion from visceral fat was proposed to contribute to the elevation of serum leptin concentration (Leal‐Cerro et al. 1996, Ishioka et al. 2002, Robaczyk et al. 2003). In our study, serum leptin concentration was found to be significantly higher in the untreated PDH group than in the PDH group treated with trilostane. In addition, patients in the treated group presented with a higher leptin concentration than healthy controls. These results are consistent with those of a previous study in which serum leptin concentration was higher in patients with PDH than controls, despite having undergone long‐term treatment, due to a large persistent visceral fat mass (Barahona et al. 2009).
There are several limitations to this study. First, this was an exploratory / discovery study to investigate differences in leptin concentration in dogs with HAC according to cholestatic status and our results should be considered preliminary. Second, the analysis was performed on a relatively small sample population of 20 dogs per group; analyses with a greater number of samples would have provided more robust data. Third, control over the assigned analysis groups and histories of each patient may be limited due to a lack of complete medical records, including detailed diagnostic and treatment histories. A previous study has also shown that treatment of PDH in dogs could attenuate alterations in serum leptin concentrations (Cho et al. 2014). If this is the case, misclassification of patients may have affected our results. Finally, changes in serum leptin levels in relation to the treatment response in patients with PDH could not be evaluated definitively, as the groups were defined based on current treatment status, without evaluation of the serial changes in leptin concentration over the course of treatment in individual patients.
In conclusion, increased circulating leptin concentration appears associated with PDH and GBM in dogs. Circulating leptin concentration may also serve as a useful marker for indicating the likely severity of cholestatic status in dogs with PDH.
Conflict of interest
No conflict of interest has been declared.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2017R1D1A1B03032499), and partially supported by the Research Institute for Veterinary Science, Seoul National University.
References
- Barahona, M. J. , Sucunza, N. , Resmini, E. , et al (2009) Persistent body fat mass and inflammatory marker increases after long‐term cure of Cushing's syndrome. The Journal of Clinical Endocrinology & Metabolism 94, 3365‐3371 [DOI] [PubMed] [Google Scholar]
- Behrend, E. , Kooistra, H. , Nelson, R. , et al (2013) Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). Journal of Veterinary Internal Medicine 27, 1292‐1304 [DOI] [PubMed] [Google Scholar]
- Bernhoft, R. A. , Pellegrini, C. A. , Broderick, W. C. , et al (1983) Pigment sludge and stone formation in the acutely ligated dog gallbladder. Gastroenterology 85, 1166‐1171 [PubMed] [Google Scholar]
- Besso, J. , Wrigley, R. , Gliatto, J. , et al (2000) Ultrasonographic appearance and clinical findings in 14 dogs with gallbladder mucocele. Veterinary Radiology & Ultrasound 41, 261‐271 [DOI] [PubMed] [Google Scholar]
- Cho, K. D. , Paek, J. , Kang, J. H. , et al (2014) Serum adipokine concentrations in dogs with naturally occurring pituitary‐dependent hyperadrenocorticism. Journal of Veterinary Internal Medicine 28, 429‐436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger, S. J. & Feldman, E. C. (2009) In: Textbook of Veterinary Internal Medicine. 7th edn. Ed Ettinger S. J. Elsevier Health Sciences, Philadelphia, PA, USA: [Google Scholar]
- Goldblatt, M. I. , Swartz‐Basile, D. A. , Svatek, C. L. , et al (2002) Decreased gallbladder response in leptin‐deficient obese mice. Journal of Gastrointestinal Surgery 6, 438‐444 [DOI] [PubMed] [Google Scholar]
- Graewin, S. J. , Kiely, J. M. , Lu, D. , et al (2008) Leptin regulates gallbladder genes related to gallstone pathogenesis in leptin‐deficient mice. Journal of the American College of Surgeons 206, 503‐510 [DOI] [PubMed] [Google Scholar]
- Hara, Y. , Teshima, T. , Taoda, T. , et al (2010) Efficacy of transsphenoidal surgery on endocrinological status and serum chemistry parameters in dogs with Cushing's disease. Journal of Veterinary Medical Science 72, 397‐404 [DOI] [PubMed] [Google Scholar]
- Ishioka, K. , Soliman, M. M. , Sagawa, M. , et al (2002) Experimental and clinical studies on plasma leptin in obese dogs. Journal of Veterinary Medical Science 64, 349‐353 [DOI] [PubMed] [Google Scholar]
- Ishioka, K. , Hosoya, K. , Kitagawa, H. , et al (2007) Plasma leptin concentration in dogs: effects of body condition score, age, gender and breeds. Research in Veterinary Science 82, 11‐15 [DOI] [PubMed] [Google Scholar]
- Kakimoto, T. , Kanemoto, H. , Fukushima, K. , et al (2017) Bile acid composition of gallbladder contents in dogs with gallbladder mucocele and biliary sludge. American Journal of Veterinary Research 78, 223‐229 [DOI] [PubMed] [Google Scholar]
- Kim, A. Y. , Kim, H. S. , Kang, J. H. , et al (2015) Serum adipokine concentrations in dogs with diabetes mellitus: a pilot study. Journal of Veterinary Science 16, 333‐340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. H. , Han, S. M. , Jeon, K. O. , et al (2017) Clinical relationship between cholestatic disease and pituitary‐dependent hyperadrenocorticism in dogs: a retrospective case series. Journal of Veterinary Internal Medicine 31, 335‐342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsunai, M. , Kanemoto, H. , Fukushima, K. , et al (2014) The association between gall bladder mucoceles and hyperlipidaemia in dogs: a retrospective case control study. The Veterinary Journal 199, 76‐79 [DOI] [PubMed] [Google Scholar]
- Laflamme, D. (1997) Development and validation of a body condition score system for dogs. Canine Practice 22, 10‐15 [Google Scholar]
- Leal‐Cerro, A. , Considine, R. , Peino, R. , et al (1996) Serum immunoreactive‐leptin levels are increased in patients with Cushing's syndrome. Hormone and Metabolic Research 28, 711‐713 [DOI] [PubMed] [Google Scholar]
- Lee, S. , Lee, A. , Kweon, O. K. , et al (2016) Presence and distribution of leptin and leptin receptor in the canine gallbladder. Acta Histochemica 118, 674‐678 [DOI] [PubMed] [Google Scholar]
- Lee, S. , Kweon, O. K. & Kim, W. (2017a) Increased leptin and leptin receptor expression in dogs with gallbladder mucocele. Journal of Veterinary Internal Medicine 31, 36‐42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Kweon, O. K. & Kim, W. H. (2017b) Associations between serum leptin levels, hyperlipidemia, and cholelithiasis in dogs. PloS One 12, e0187315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin, G. & Burk, R. (1990) The use of diagnostic computerized tomography and radiation therapy in canine and feline hyperadrenocorticism. Problems in Veterinary Medicine 2, 557‐564 [PubMed] [Google Scholar]
- Mesich, M. L. , Mayhew, P. D. , Paek, M. , et al (2009) Gall bladder mucoceles and their association with endocrinopathies in dogs: a retrospective case‐control study. The Journal of Small Animal Practice 50, 630‐635 [DOI] [PubMed] [Google Scholar]
- Piantedosi, D. , Di Loria, A. , Guccione, J. , et al (2016) Serum biochemistry profile, inflammatory cytokines, adipokines and cardiovascular findings in obese dogs. The Veterinary Journal 216, 72‐78 [DOI] [PubMed] [Google Scholar]
- Pike, F. S. , Berg, J. , King, N. W. , et al (2004) Gallbladder mucocele in dogs: 30 cases (2000‐2002). Journal of the American Veterinary Medical Association 224, 1615‐1622 [DOI] [PubMed] [Google Scholar]
- Ramsey, I. K. (2010) Trilostane in dogs. Veterinary Clinics of North America. Small Animal Practice 40, 269‐283 [DOI] [PubMed] [Google Scholar]
- Robaczyk, M. , Krzyzanowiska‐Swiniarska, B. , Andrysiak‐Mamos, E. , et al (2003) Plasma leptin levels in relation to body composition and body fat distribution in patients with Cushing's syndrome. Polskie Archiwum Medycyny Wewnetrznej 110, 1299‐1308 [PubMed] [Google Scholar]
- Tsukagoshi, T. , Ohno, K. , Tsukamoto, A. , et al (2012) Decreased gallbladder emptying in dogs with biliary sludge or gallbladder mucocele. Veterinary Radiology & Ultrasound 53, 84‐91 [DOI] [PubMed] [Google Scholar]


