Abstract
Insulin is present in most maintenance media for human embryonic stem cells (hESCs), but little is known about its essential role in the cell survival of individualized cells during passage. In this article, we show that insulin suppresses caspase cleavage and apoptosis after dissociation. Insulin activates insulin‐like growth factor (IGF) receptor and PI3K/AKT cascade to promote cell survival and its function is independent of rho‐associated protein kinase regulation. During niche reformation after passaging, insulin activates integrin that is essential for cell survival. IGF receptor colocalizes with focal adhesion complex and stimulates protein phosphorylation involved in focal adhesion formation. Insulin promotes cell spreading on matrigel‐coated surfaces and suppresses myosin light chain phosphorylation. Further study showed that insulin is also required for the cell survival on E‐cadherin coated surface and in suspension, indicating its essential role in cell–cell adhesion. This work highlights insulin's complex roles in signal transduction and niche re‐establishment in hESCs. stem cells 2019;37:1030–1041
Keywords: hESC, Cell survival, Cell adhesion, Focal adhesion, Niche, Individualization, Insulin, Integrin, Caspase, IGF, AKT
This study demonstrates the essential and unique role played by insulin in cell survival and adhesion in human embryonic stem cells after individualization. The insulin regulation is independent of rho‐associated protein kinase and is the prerequisite for the rho‐associated protein kinase inhibitor‐dependent cell survival. As the only growth factor required for cell survival in the maintenance media, insulin activates IGF1R/PI3K/AKT pathway and inhibits caspase activation. Insulin also stimulates integrin activation and promotes cell–matrix and cell–cell adhesion. This study reveals insulin/insulin‐like growth factor pathway as the central player in cell survival and niche re‐formation during human embryonic stem cell passaging.
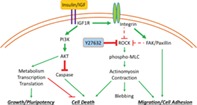
Significance Statement.
This study demonstrates the essential and unique role played by insulin in cell survival and adhesion in human embryonic stem cells after individualization. The insulin regulation is independent of rho‐associated protein kinase (ROCK) and is the prerequisite for the ROCK inhibitor‐dependent cell survival. As the only growth factor required for cell survival in the maintenance media, insulin activates IGF1R/PI3K/AKT pathway and inhibits caspase activation. Insulin also stimulates integrin activation and promotes cell–matrix and cell–cell adhesion. This study reveals insulin/insulin‐like growth factor pathway as the central player in cell survival and niche re‐formation during human embryonic stem cell passaging.
Introduction
Insulin is an essential hormone and it regulates glucose metabolism, gene transcription, and protein translation in our body 1, 2, 3. Insulin is present in most maintenance media for human embryonic stem cells (hESCs) 4, 5, 6, 7. However, it is rarely discussed how hESCs would behave in the absence of insulin, and it is unknown what molecular mechanisms are associated with insulin functions. Here, we report that insulin plays essential roles in the survival and cell adhesion in individualized hESCs during expansion and stem cell manipulations.
As a common serum component, insulin is widely used in most cell culture‐based studies 6, 7, 8. For hESC cultures, the majority of culture systems contain insulin 6, 7. We previously reported that individualized hESCs die without insulin during passaging; hence, insulin is deemed as an essential component to assemble E8 hESC medium 7. In the rare cases where insulin is not used, insulin‐like growth factor (IGF) is present in the medium. It is suggested that insulin and IGF support hESC self‐renewal in similar pathways 3. Insulin shares sequence homology with insulin‐like growth factor 1 (IGF1) and insulin‐like growth factor 2 (IGF2). They all belong to the insulin superfamily of peptide hormones 9. Insulin and IGFs can all bind to insulin receptor (INSR) family members including INSR, insulin‐like growth factor 1 receptor (IGF1R), and IGF2 receptor (IGF2R) 10. The ligand binding to these receptors activates phosphoinositide 3‐kinase (PI3K) and subsequently protein kinase B (AKT) kinases to promote cell proliferation 11. In hESCs, the PI3K/AKT activation is essential for pluripotency by suppressing FOXO3a expression 11, 12. PI3K inhibitors promote the exit of self‐renewal and drive lineage‐specific cell differentiation 13, 14, 15.
In addition to insulin and other signal stimuli from medium, hESCs also require extracellular matrix (ECM) to survive and for self‐renewal 16, 17. The soluble growth factors, the ECM, and the surrounding cells form the stem cell niche, which is necessary for pluripotency and cell survival 4, 16, 18, 19, 20. Integrin and E‐cadherin coordinate to maintain hESC adhesion, cytoskeleton organization, and the integrity of stem cell colonies 21, 22. hESCs have been successfully cultured on various ECMs including matrigel, vitronectin, E‐cadherin, and bioactive peptides 7, 23. Matrigel, vitronectin, and bioactive peptides activate integrin receptors to regulate the organization of actin stress fibers through focal adhesion kinase (FAK) activation. Focal adhesions (also known as adhesomes) are formed through activated integrin cytosolic domains that assemble a complex of proteins including FAK, Paxillin, Talin, Kindlin, and Vinculin 24. Focal adhesion complexes interact with actin fibers to regulate cytoskeletal structure and promote cell attachment and migration 24, 25. Meanwhile, endogenous E‐cadherin is activated by E‐cadherin from neighboring cells or culture supplement, and then it organizes actin stress fibers through β‐catenin 26, 27. Both integrin and E‐cadherin have been reported to promote cell survival and proliferation through kinase cascades such as PI3K/AKT and MAPK pathways 28, 29. Interference of integrin or E‐cadherin pathways usually leads to cell death and differentiation 18, 30. Even though both cell adhesion and insulin are essential components of the stem cell culture, it is unclear whether these two pathways interact to affect pluripotency and cell survival.
hESCs thrive in colonies and expand quickly. However, their niches have to be routinely disrupted for practical reasons. hESCs need to be frequently passaged every few days and the cells are often individualized for gene targeting and differentiation as well 31, 32, 33. Individualization disintegrates niche structures and the hESCs lose both cell adhesion and focal adhesion. In the process, rho‐associated protein kinase (ROCK) is activated to initiate actinomyosin contraction and severe cell death of individualized cells is usually observed after being replated to ECM‐coated surfaces 7, 8, 34. With higher plating density, a significant portion of cells survives after they manage to re‐establish cell–cell and cell–ECM adhesions in time 35, 36, 37. When ROCK/actinomyosin axis is inhibited by small chemicals such as Y‐27632, nicotinamide, or blebbistatin, cell survival is significantly improved in either ECM‐containing or ECM‐free conditions 38, 39. Caspase‐dependent apoptosis is often considered a downstream event of ROCK activation in cell death after individualization 4. However, there is no report on whether alternative pathways regulate caspase activity during the passaging process.
In order for the individualized cells to survive after passaging, the hESCs need to re‐establish their stem cell niches 37. We are particularly curious why hESCs die so quickly without insulin after individualization 7. We hypothesize that insulin could play essential roles for hESCs to re‐establish niches. In this study, we dissected the culture components contributing to cell survival and explored molecular mechanisms of insulin in the survival of individualized hESCs. We demonstrated that insulin is required for cell survival in the first 2–4 hours after individualization. Insulin activates the IGF receptor and PI3K/AKT pathways and suppresses caspase cascades independent of ROCK pathway. We also found that the constitutive activation of IGF1R/AKT pathway only partially rescued cell survival. Further analysis shows that insulin stimulates integrin activation and promotes cell adhesion and niche formation. IGF receptor colocalizes with focal adhesions and promotes the reorganization of focal‐adhesion proteins and cytoskeleton proteins independent of PI3K/AKT activation. After individualization, insulin and ROCK inhibitor synergistically promote cell survival. This study suggests that insulin not only activates the IGF1R/AKT pathway but also stimulates cell adhesion to promote cell survival. The study reveals the molecular mechanism of insulin's essential roles in hESCs and establishes hESCs as a novel platform to study cell adhesion and niche formation.
Materials and Methods
hESC Culture
hESCs (H1 and H9) and human‐induced pluripotent cell line NL‐1 were maintained using matrigel‐coated tissue culture plates in E8 medium 7. The cells were passaged every 3 days using EDTA method in the presence of Y‐27632 as previously described 35. Medium was changed daily. hESCs with normal karyotype were used from passage P27 to a maximum of P50.
Survival Assay
hESCs (H1) were dissociated with TrypLE (Gibco, Waltham, Massachusetts, USA) for 10 minutes at 37°C, collected, and washed twice with Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco) to remove all traces of insulin. Then the cells were plated with different treatments on a matrigel‐coated surface for the survival experiments. The cells were then harvested with TrypLE at specified time points and cell counts were determined using a BD Accuri C6 flow cytometer. Cell counts were normalized to the original cell number that was plated.
Annexin V Assay
Annexin V assays were carried out using Dead Cell Apoptosis Kit (ThermoFisher, Waltham, Massachusetts, USA) following manufacturer's protocols. Briefly, cells were harvested, washed with 1× phosphate‐buffered saline (PBS; Gibco) and resuspended in 1× annexin‐binding buffer. Cells were stained with FITC‐Annexin V and propidium iodide (PI) at room temperature (RT) for 15 minutes and then analyzed on BD Accuri C6 flow cytometer.
Caspase 3/7 Assay
Caspase 3/7 activation was measured using CellEvent Caspase‐3/7 Green Detection Reagent (ThermoFisher) following manufacturer's protocols. Briefly, the cells were plated on a matrigel‐coated surface with different treatments for 1.5 hours. The Caspase 3/7 detection reagent was added to the cells and incubated for an additional half‐hour at 37°C. Then the cells were harvested and analyzed using a BD Accuri C6 flow cytometer.
Western Blot
For Western blot of soluble proteins, cells were collected using a 1:1 mixture of RIPA buffer and 2× laemmli buffer supplemented with benzonase 1:2,000, protease inhibitors, and phosphatase inhibitors. Samples were sonicated to solubilize the proteins. The protein concentration was measured by Pierce BCA Protein Assay Kit (ThermoFisher). After SDS‐PAGE using 8% to 15% gel, proteins were transferred onto a PVDF membrane. The membrane was blocked using 10% milk in 1× TBST buffer for an hour, incubated with the primary antibody in bovine serum albumin (BSA) 2% (Sigma, Saint Louis, Missouri, USA) for 2 hours, and then with the secondary antibody‐HRP in BSA 2% for 1 hour, all three at RT with shaking, and washed with 1× TBST for 3 × 10 minutes at the end of each step. Chemiluminescence was developed using SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher) and detected on ChemiDoc Imaging System (Bio‐Rad, Hercules, California, USA).
Lentiviral Constructs Expressing Constitutively Active IGF1R or AKTs
| Primer name | Sequence |
|---|---|
| MyrAkt1_5′_SpeI | GCCTCGACTAGTATGGGGAGTAGCAAGAGCAAGCCTAAGGACCCCAGCCAGCGCATGAGCGACGTGGCTATTGTG |
| Akt1_3′_NsiI | CAGTCCACATGCATCTCAGGCCGTGCCGCTG |
| MyrAkt2_5′_SpeI | CCACGCTACTAGTATGGGGAGTAGCAAGAGCAAGCCTAAGGACCCCAGCCAGCGCATGAATGAGGTGTCTGTCATC |
| Akt2_3′_NsiI | GCGTGGATGCATTGCTCACTCGCGGATGCTG |
| CD8_5′_SpeI | TGGGGAACTAGTCATGGCCTTACCAGTGACC |
| CD8_3′_EcoRI | TTGCACAGAATTCGACGTATCTCGCCGAAAGG |
| Igf1R_5′_EcoRI | AGAAAGGAATTCAACAGCAGGCTGGGGAATGG |
| Igf1R_3′_NsiI | TTCAGGATGCATGGATCAGCAGGTCGAAGACTGG |
pSIN4 plasmid was used as a backbone to generate lentiviral overexpression constructs 40. In order to constitutively activate AKT1 and AKT2, a myristoylation signal sequence was fused to the N‐terminus of AKT1 and AKT2 41. The AKT1 and AKT2 were introduced to pSIN4 plasmid through the restriction sites of SpeI and NsiI (New England Biolabs, Ipswich, Massachusetts, USA).
For the constitutively active IGF1R, the extracellular/transmembrane domain of human CD8a (amino acids 1–218) was fused with the intracellular domain of IGF1R (amino acids 964–1,367) 42. CD8 and IGF1R fragments were inserted together into pSIN4 vector through the restriction sites of SpeI and NsiI.
Lentivirus Packaging and hESC Transduction
293FT cells were maintained in DMEM/10% fetal bovine serum medium until 60% confluence. For lentiviral transfection, AKT1, AKT2, and IGF1R overexpression constructs (see above) were combined with the packaging plasmids psPAX2 and pMD2.G (Addgene, Watertown, Massachusetts, USA) and used to transfect 293FT cells using the polyethylenimine method. Viral supernatant was collected at 48 hours and used to transduce the H1 cells (30%–40% confluence) with polybrene. Transduced cells were selected with puromycin.
Cell Cycle Analysis
After EdU is added to the cells for 1 hour at 37°C, cells are harvested with TrypLE and washed with PBS 1×. A second wash with ice‐cold 1× PBS is done before fixing the cells with cold 70% ethanol and storing them 24 hours at −20°C. Cells were centrifuged, washed with cold PBS 1×, and incubated in PI/Triton X‐100 staining solution for 15 minutes at 37°C. Samples were transferred to ice and analyzed using a BD Accuri C6 flow cytometer.
Immunostaining
Cells were cultured on coverslips that have been sterilized with ethanol 75% and coated with matrigel 1 mg/ml previously. After the length of the treatment, the cells were washed with 1× PBS (Gibco) and fixed with paraformaldehyde 4% for 20 minutes at RT. Cells were then washed with 1× PBS, permeabilized for 15 minutes with Triton 0.5%, and incubated with primary antibodies in BSA 2% for 2 hours at RT. After washing, the cells were incubated with secondary antibodies in BSA 2% for 1 hour at RT in the dark. Cells were washed twice with 1× PBS and the nuclei were stained with Hoechst‐455 nm for 10 minutes in darkness at RT. Samples were mounted on slides and imaged using Carl Zeiss Confocal LSM710 microscope.
Statistical Analysis
Statistical analysis was made using two‐tailed Student's t test. Statistical significance is described as p‐value <.05 (*), p‐value <.01 (**), and p‐value <.001 (***).
Error bars are calculated according to the standard variation of the data. Each experiment is represented as mean ± SEM of three biological repeats, unless specified. Each phenotype has been repeated at least three times, showing a representative graph of each phenotype.
Antibodies
| Antibody name | Brand | Catalog number |
|---|---|---|
| Caspase 3 | Santa Cruz | SC‐1225 |
| Cleaved Caspase 3‐D175 | Cell Signaling | 9664S |
| INSR Blocking Ab | Millipore | MAB1137Z |
| IGF1R Blocking Ab | BD Pharmingen | 555998 |
| IGF2R Blocking Ab | R&D Systems | AF2447 |
| TNNT2 | DSHB | CT3 |
| Integrin β1 Blocking Ab | DSHB | AIIB2 |
| Integrin α6 Blocking Ab | DSHB | P5G10 |
| GAPDH | Santa Cruz | SC‐25778 |
| AKT | Cell Signaling | 9272 |
| Phospho‐AKT‐S473 | Cell Signaling | 9271S |
| FAK | Millipore | 05‐537SP |
| Phospho‐FAK‐Y397 | Millipore | MAB1144 |
| Paxillin | Millipore | 05‐417 |
| Phospho‐Paxillin‐Y118 | ThermoFisher | 44‐722G |
| IGF1R | Santa Cruz | SC‐713 |
| Phospho‐INSR/IGF1R‐Y1158/1162/1163 | Millipore | 07‐841 |
| Integrin β1 | Cell Signaling | 9699 |
| Phospho‐Integrin β1‐T788/789 | Millipore | AB1929 |
| Myosin Light Chain | Sigma | M4401 |
| Phospho‐Myosin Light Chain 2‐S19 | Cell Signaling | 3671l |
Results
Insulin Inhibits Apoptosis During Passaging
Chemically defined E8 medium can efficiently support hESC self‐renewal 7; hence, we examined the E8 medium components for their function in H1 hESC survival during passaging. We showed that insulin alone in base medium DMEM/F12 was sufficient to support the survival of individualized cells after being replated onto matrigel‐coated plates and the survival rate was comparable to full E8 medium containing insulin, FGF2, and TGFβ (Fig. 1A). Insulin also promoted cell survival on vitronectin‐coated plates (Fig. 1B). Meanwhile, FGF2 and TGFβ did not improve cell survival in the absence of insulin (Supporting Information Fig. S1A). The insulin effect on cell survival was repeatable in other hPSCs such as H9 and NL‐1 (Supporting Information Fig. S1B). However, this phenomenon is cell type‐specific. For example, fibroblasts survive effectively in the absence of insulin after dissociation (Supporting Information Fig. S1C). Taken together, these data indicate that insulin is the only growth factor necessary for hPSC survival after dissociation, both on matrigel and on vitronectin. For simplicity of discussion, matrigel was the main coating material used for this study, unless otherwise specified.
Figure 1.
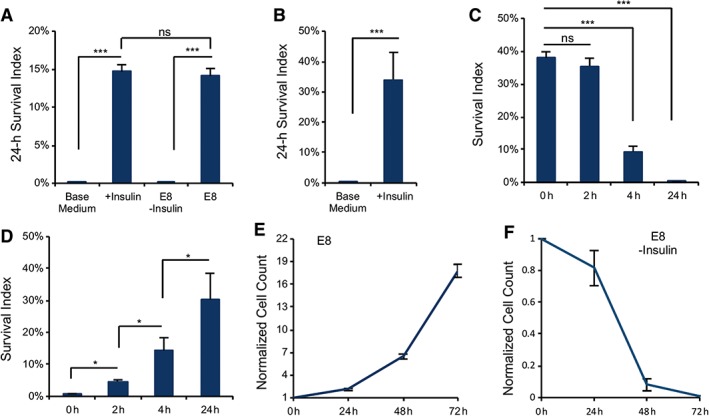
Insulin is required for cell survival after dissociation. (A): Plots showing the survival of dissociated H1 embryonic stem (ES) cells 24 hours after plating on matrigel‐coated surface with or without insulin compared with E8 medium (***, p < .001, n = 3 biological repeats; data are normalized to the number of cells plated). Base medium, Dulbecco's modified Eagle's medium/F12. (B): Twenty‐four hour survival of dissociated cells on vitronectin‐coated surface with and without insulin (***, p < .001, n = 3). (C): Survival of individualized cells on matrigel when insulin was added at different time points after plating (***, p < .001, n = 3). (D): Plots showing the survival of dissociated ES cells after 24 hours on matrigel when insulin was removed at different time points after cell plating (*, p < .05, n = 3). (E, F): Cell proliferation on matrigel‐coated surface in E8 medium with or without insulin (n = 3 biological repeats for each time point; data are normalized to time zero cell count).
Insulin not only improved cell survival in a dose‐dependent manner (Supporting Information Fig. S1D) but also affected cell survival in a time‐dependent fashion. Insulin was most effective for cell survival when applied within the first 2 hours after replating and most cells died when insulin was added later than 4 hours (Fig. 1C). In parallel, transient exposure to insulin in the first 4 hours significantly improved cell survival (Fig. 1D). These data indicate that the first few hours are the critical time window for insulin to promote the survival of individualized cells after replating. In contrast to dissociated cells, undissociated cells respond differently to the removal of insulin. Even though the cell growth was arrested without insulin compared with regular culture containing insulin (Fig. 1E), it took a few days for the cells to die out (Fig. 1F). Cell death occurs in significantly shorter time in individualized cells without insulin and it indicates that insulin could play an additional role in the individualized cells that need to re‐establish their niches.
ROCK and actinomyosin inhibitors are beneficial for the survival of individualized cells 37. However, we found that ROCK inhibitor, Y‐27632 did not sufficiently rescue cell survival in the absence of insulin (Fig. 2A). Most Y‐27632‐treated cells died without insulin, but insulin and Y‐27632 synergistically improved cell survival. This result suggests that insulin plays an essential role in parallel with ROCK pathway.
Figure 2.
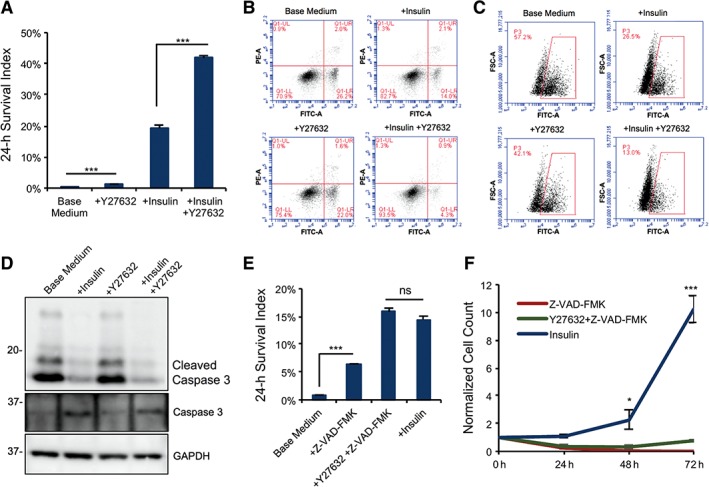
Insulin inhibits apoptosis during passaging. (A): Cell survival of dissociated cells 24 hours after plating on matrigel with or without insulin and rho‐associated protein kinase (ROCK) inhibitor (Y‐27632; ***, p < .001, n = 3). (B): Annexin V assay showing the percentage of apoptotic cells 4 hours after dissociation and plating on matrigel‐coated surface, with or without insulin or Y‐27632. (C): Flow cytometry analysis of Caspase 3/7 activity in dissociated embryonic stem (ES) cells 4 hours after plating on matrigel with or without insulin or Y‐27632 (Caspase 3/7, FITC‐A channel; FSC‐A, forward scattering). (D): Western blot showing the cleavage of Caspase 3 at Asp‐175 in cells cultured 4 hours on matrigel after dissociation with or without insulin and Y‐27632. Quantification is shown in Supporting Information Figure S2A. (E): Plots showing the cell survival 24 hours after plating on matrigel‐coated surface with or without the Caspase inhibitor Z‐VAD‐FMK, ROCK inhibitor Y27632, or insulin (***, p < .001, n = 3). (F): Cell proliferation of dissociated H1 ES cells during 72 hours after plating on matrigel‐coated surface comparing insulin effect to the Caspase inhibitor Z‐VAD‐FMK (*, p < .05; **, p < .01, n = 3; data are normalized to time zero cell count). Abbreviation: PI, propidium iodide (Annexin V, FITC‐A channel; PI, PE‐A channel).
The exposure to insulin in the first few hours was critical for cell survival (Fig. 1C, 1D); hence, we examined whether insulin has any effect on apoptosis in dissociated cells with Annexin V assay. Without insulin, more than 25% of cells were Annexin V‐positive, but insulin significantly decreased the Annexin V‐positive population. In contrast, Y‐27632 was not as effective as insulin. However, insulin and Y‐27632 together suppressed the apoptotic phenotype most effectively (Fig. 2B). At the same time, Caspase 3/7‐activation assay demonstrated similar phenomena. Without insulin, caspase activity was detected in more than a 50% of individualized cells. The caspase activity was significantly suppressed by insulin, especially along with Y‐27632 (Fig. 2C). The impact of insulin on caspase was further confirmed by Western blot and insulin suppressed caspase cleavage in individualized cells (Fig. 2D and Supporting Information Fig. S2A). These data indicate that insulin suppresses caspase activation and apoptosis and the function is parallel to ROCK pathway. Interestingly, although pan‐caspase inhibitor Z‐VAD‐FMK increased the number of adherent cells 24 hours after passaging (Fig. 2E), those adherent cells could not grow up in the next few days even with insulin added later (Fig. 2F). We also observed that the caspase inhibitor helps to improve the cell adhesion to the ECM, but cells did not migrate and form colonies (Supporting Information Fig. S2B). These data indicate that under the insulin‐free condition, caspase inhibition only suppressed the cell lysis but not cell death.
Insulin Activates IGF Receptor to Promote Cell Survival
Insulin can activate both INSR and IGF receptors (IGF1R and IGF2R) 10. In order to understand which receptor is involved in the insulin‐dependent survival, specific receptor neutralizing antibodies were applied to the cells after passaging. Only anti‐IGF1R antibody significantly suppressed cell survival, whereas anti‐INSR and anti‐IGF2R antibodies had no significant effect (Supporting Information Fig. S3A). The result indicates that IGF1R is a main target for insulin to promote cell survival. Considering that IGF1R is also activated by IGF proteins, we tested IGF1 and IGF2 in the cell survival assay without insulin. IGF1 and IGF2 fully rescued cell survival in the absence of insulin (Fig. 3A). IGFs promoted cell survival at a significantly lower concentration (50 ng/ml) than insulin does (10 μg/ml). It is consistent with the notation that IGF1R, not INSR, is the major receptor through which insulin promotes hPSC survival after dissociation.
Figure 3.
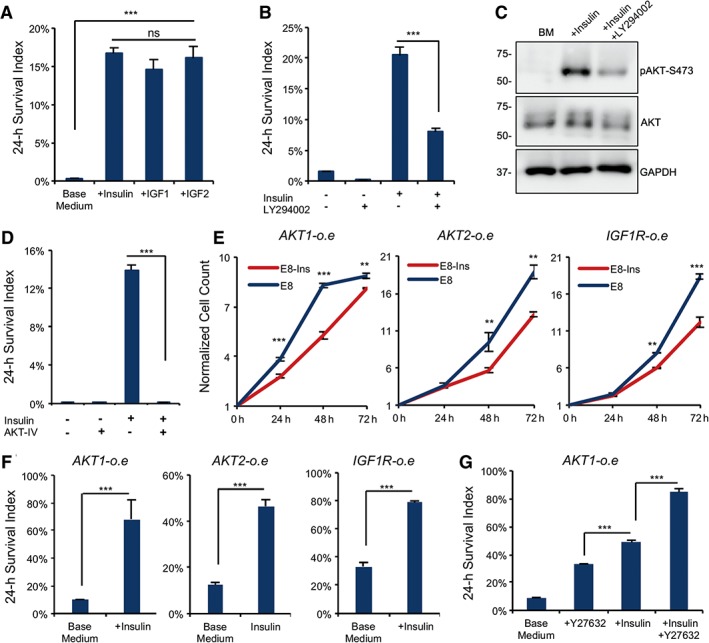
Insulin activates insulin‐like growth factor (IGF) receptor to promote cell survival. (A): Plots comparing cell survival 24 hours after plating on matrigel in the presence or absence of insulin (10 μg/ml), IGF1 (50 ng/ml), and IGF2 (50 ng/ml; ***, p < .001, n = 3). (B): Plots showing 24‐hour survival of dissociated embryonic stem (ES) cells with or without the PI3K inhibitor LY294002 (10 μM) or insulin (***, p < .001, n = 3). (C): Western blot showing levels of AKT and phospho‐AKT‐S473 in the presence or absence of insulin and LY294002 on matrigel‐coated surface 2 hours after plating (n = 3 technical repeats). Quantification is shown in Supporting Information Figure S3B. (D): Plots showing 24‐hour survival of dissociated ES cells with or without AKT inhibitor IV (5 μM; ***, p < .001, n = 3). (E): Cell proliferation of AKT1, AKT2, and IGF1R overexpression cell lines during 72 hours after plating on matrigel‐coated surface in E8 media with and without insulin (***, p < .001; **, p < .01, n = 3; data are normalized to time zero cell count). (F): Plots showing 24‐hour survival of AKT1, AKT2, and IGF1R overexpression cells after plating on matrigel with or without insulin (***, p < .001, n = 3). (G): Plots showing 24‐hour survival of AKT1 overexpression cells after plating on matrigel comparing Y‐27632 and insulin (***, p < .001, n = 3).
The PI3K/AKT pathway is the main downstream pathway of insulin/IGF receptors and hence we examined the PI3K/AKT cascade after plating the cells. The cell survival was significantly lower under the influence of PI3K inhibitor LY294002 with the number of surviving cells decreased by about half compared with control (Fig. 3B). AKT phosphorylation was increased by insulin but was suppressed by LY294002 (Fig. 3C and Supporting Information Fig. S3B) and AKT inhibitor IV killed all the cells in the presence of insulin (Fig. 3D). The cell cycle of the surviving cells was also arrested at G1 phase in 24 hours by the presence of LY294002 (Supporting Information Fig. S3C). These data indicate that PI3K/AKT pathway is essential for cell survival and insulin brings beneficial effect through the stimulation of PI3K and AKT activities.
Overexpression of PI3K/AKT Partially Rescues Insulin Phenotype
In order to confirm the function of IGF1R and AKT, we overexpressed constitutively active IGF1R, AKT1, and AKT2 in hESCs. Compared with the cell death phenotype in adherent cells shown in Figure 1E, each overexpression construct sustained cell proliferation in the absence of insulin and the growth trend is similar to the ones with insulin in medium (Fig. 3E). It suggests that IGF1R and AKT are probably the main downstream effectors of insulin activation for cell proliferation, so the constitutive active proteins are able to sustain cell proliferation without insulin in adherent hESCs.
Next, we examined the effect of constitutively active IGF1R and AKT in individualized hESCs. Each ectopically expressed protein significantly rescued the cell survival of individualized hESCs in the absence of insulin (Fig. 3F) in comparison with cell survival without insulin in H1 cells (Fig. 1A). It indicates that IGF1R/AKT cascade contributes to cell survival under the influence of insulin. Surprisingly, each overexpressed protein only partially rescued the cell survival because the presence of insulin still led to significantly higher cell survival rate in those cells even in the presence of Y‐27632 (Fig. 3G). This result suggests that other critical pathway besides PI3K/AKT is regulated by insulin in supporting cell survival.
Insulin Promotes Focal Adhesion Formation
Due to insulin's indispensable role in individualized cells compared with adherent cells, we hypothesize that insulin could probably play some role in niche formation; hence, we examined whether insulin affects the re‐establishment of cell adhesion. We showed that cell survival was inhibited by both anti‐integrin β1 and anti‐integrin α6 blocking antibodies even in the presence of insulin (Fig. 4A). It suggests that integrin activation was required for cell survival. We then demonstrated that insulin significantly enhanced integrin β1 expression and its phosphorylation (Fig. 4B and Supporting Information Fig. S4A) indicating that insulin promotes integrin activation after passaging.
Figure 4.
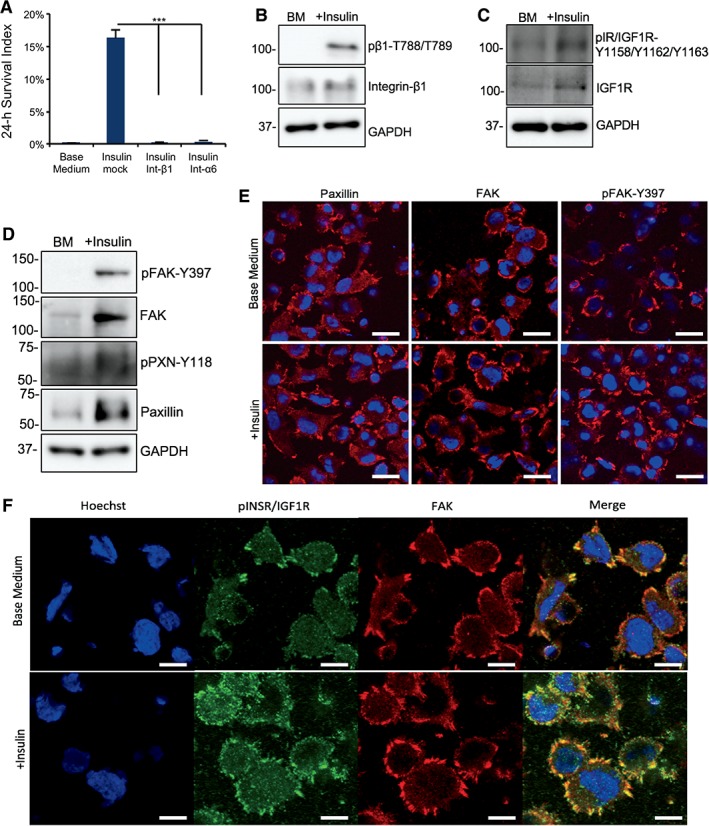
Insulin promotes focal adhesion formation in dissociated cells. (A): Plots showing 24‐hour cell survival of insulin with integrin β1 and α6 blocking antibodies compared with Insulin+TNNT2 antibody (mock) on matrigel (***, p < .001, n = 3). (B): Western blot showing the level of integrin β1 and phosphorylated integrin β1‐788‐789 2 hours after plating on matrigel‐coated surface in base medium with or without insulin. Quantification is shown in Supporting Information Figure S4A. (C): Western blot showing the protein expression of IGF1R and phospho‐IGF1R 2 hours after plating on matrigel in base medium with or without insulin. Quantification is shown in Supporting Information Figure S4B. (D): Western blot showing the protein expression of focal adhesion kinase (FAK), phospho‐FAK‐Y397, Paxillin, and phospho‐Paxillin‐Y118 2 hours after plating on matrigel in base medium with or without insulin. Quantification is shown in Supporting Information Figure S4C. (E): Immunofluorescent image showing Paxillin, FAK, and phospho‐FAK‐Y397 expression in focal adhesions 2 hours after plating on matrigel‐coated surface with or without insulin. Scale bar = 20 μm. Quantification is shown in Supporting Information Figure S4E. (F): Immunostaining showing FAK (red) and phospho‐INSR/IGF1R‐Y1158‐Y1162‐Y1163 (green) expression in focal adhesions 2 hours after plating on matrigel‐coated surface with or without insulin. Nuclei were stained with Hoechst (blue). Scale bar = 10 μm.
Because insulin was most effective for survival in the first few hours after seeding (Fig. 1C, 1D), we focused on insulin's impact on cell adhesion during this period. Consistent with its role in cell survival (Supporting Information Fig. S3A), insulin significantly increased phosphorylated and total IGF1R in the cell (Fig. 4C and Supporting Information Fig. S4B). Insulin also promoted the expression and phosphorylation of focal adhesion proteins FAK and Paxillin (Fig. 4D and Supporting Information Fig. S4C). After hESCs were plated on matrigel, cells attached to the surface normally with or without insulin (Supporting Information Fig. S4D) and there were no significant phenotypical differences under microscope. Immunostaining showed that focal adhesions were present with or without insulin. However, more focal adhesions per cell were observed in the presence of insulin according to the staining of FAK, Paxillin, and phospho‐FAK (Fig. 4E and Supporting Information Fig. S4E). Similar phenotype was observed in the constitutively active AKT‐IGF1R cells where the overall number of focal adhesions per cell was higher than in H1 cells, but insulin still promoted a more intense and homogeneous distribution of focal contacts (Supporting Information Fig. S4F). These results suggest that insulin is involved in focal contact formation in individualized cells. We then show that insulin also increased the colocalization of IGF1R and FAK (Fig. 4F), which is consistent with protein expression levels shown previously (Fig. 4C, 4D). Taken together, our results suggest that insulin promotes integrin activation and stimulates focal adhesion formation.
Synergistic Effect of Insulin and ROCK Inhibition in Different Culture Platforms
The formation of adhesomes is associated with the organization of the cytoskeleton and hence we examined whether insulin could affect cytoskeleton organization. During individualization by TrypLE, membrane blebbing emerges within 5 minutes of the TrypLE treatment. Immunostaining showed that Paxillin was sequestered in the membrane blebs just 5 minutes after the treatment with dissociation reagent TrypLE (Fig. 5A). It indicates that individualization disrupted adhesome structure and caused protein relocation.
Figure 5.
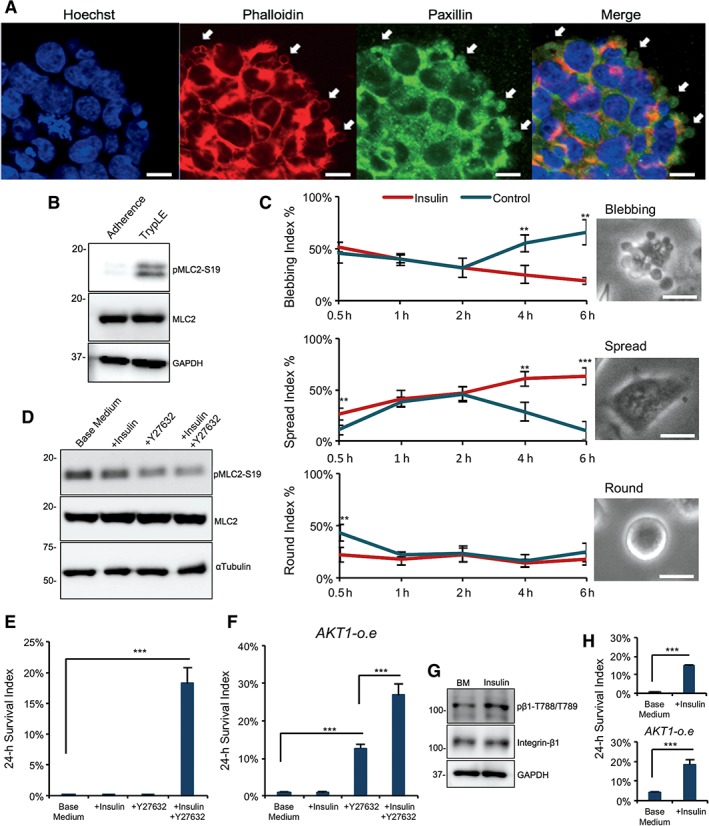
Synergistic insulin and rho‐associated protein kinase (ROCK) inhibition suppresses MLC2 phosphorylation to improve cell survival. (A): Micrographs showing the expression of Paxillin (green) and Phalloidin (red) in undissociated H1 embryonic stem (ES) cells after 5 minutes of TrypLE treatment. Nuclei were stained with Hoechst (blue). Scale bar = 10 μm. (B): Western blot showing the protein expression of MLC2 and phospho‐MLC2‐S19 after dissociating the cells with TrypLE for 10 minutes. Quantification shown in Supporting Information Figure S5A. (C): Left, quantification of cells displaying each kind of phenotype 6 hours after dissociation and plating on matrigel‐coated surface with and without insulin (***, p < .001, n = 3; **, p < .01, n = 3). Right, phase contrast images showing blebbing phenotypes. Scale bar = 10 μm. (D): Western blot showing the protein expression of MLC2 and phospho‐MLC2‐S19 30 minutes after plating on matrigel‐coated surface in base medium with or without insulin or Y‐27632. Quantification is shown in Supporting Information Figure S5B. (E): Survival of H1 ES cells in suspension culture 24 hours after dissociation (***, p < .001, n = 3). (F): Twenty‐four‐hour survival of AKT1‐overexpression cells in suspension culture (***, p < .001, n = 3). (G): Western blot showing the protein expression of integrin β1 and phospho‐integrin β1‐788‐789 30 minutes after dissociation with or without insulin in suspension culture. Quantification is shown in Supporting Information Figure S5D. (H): Survival of H1 cells (top) and AKT1o‐e cells (bottom) 24 hours after plating on E‐cadherin‐coated surface (***, p < .001, n = 3).
After individualization, MLC2 phosphorylation was significantly increased indicating that the membrane blebbing is caused by actinomyosin contraction (Fig. 5B and Supporting Information Fig. S5A). When the individualized cells were replated onto a matrigel‐coated surface, the morphology changed through time. Within 30 minutes after plating, hESCs displayed three morphologies: blebbing, round, and spreading. In the presence of insulin, blebbing cells continuously decreased, whereas spreading cells increased. In contrast, without insulin, spreading decreased and blebbing cells increased (Fig. 5C). Meanwhile, round cells displayed different percentages initially but later reached similar ratio independent of insulin. It indicates that insulin promoted focal adhesion for spreading and inhibited membrane blebbing caused by actinomyosin contraction.
Membrane blebbing is often associated with increased phosphorylation of myosin light chain (MLC) and we showed that insulin significantly decreased MLC2 phosphorylation in comparison to cells without insulin within 30 minutes after passaging (Fig. 5D and Supporting Information Fig. S5B). It is possible that insulin stimulates focal adhesion formation, which indirectly decreases MLC2 phosphorylation in the reorganization of cytoskeleton. In comparison, Y‐27632 was more efficient in suppressing MLC2 phosphorylation (Fig. 5D), even though cells did not survive without insulin (Fig. 2A). The dual treatment of insulin and Y‐27632 led to the lowest MLC2 phosphorylation and the best cell survival (Figs. 5D, 2A). We also observed that integrin blocking antibodies decreased cell survival in the presence of insulin and Y27632 (Supporting Information Fig. S5C). It suggests that maximal cell survival requires the signals from insulin, integrin, and ROCK inhibition.
Besides insulin's function for the cell survival on matrigel, we also explored its role in ECM‐free and E‐cadherin based conditions. In suspension without ECM, hESCs form aggregates as embryoid bodies to survive. Without insulin, cells failed to form aggregates and all of them died even in the presence of ROCK inhibitor. Both insulin and ROCK inhibitor were required for the cell survival in embryoid body formation (Fig. 5E). The constitutively active AKT1 partially rescued the cell survival in suspension without insulin. However, the maximum cell survival can only be achieved with both insulin and ROCK inhibitor (Fig. 5F). Insulin also increased integrin β1 in suspension indicating that insulin can regulate integrin and focal adhesion even in the absence of exogenous ECM (Fig. 5G and Supporting Information Fig. S5D). These data suggest that insulin supports hESC survival as aggregates through AKT pathway and cell adhesion.
E‐cadherin is required for hESC cell–cell adhesion to form their niche in cell aggregates and hence we examined whether insulin is necessary for the cell survival on E‐cadherin coated surfaces. Without insulin, all cells died on E‐cadherin coated plates. The cell survival was partially rescued by the constitutively active AKT1 (Fig. 5H). This phenotype is similar to what we observed on matrigel‐coated surfaces (Fig. 3F). These data suggest that insulin is also essential for the E‐cadherin mediated cell–cell adhesion and it contributes to the embryoid body formation through E‐cadherin. In summary, insulin is indispensable for the maximum survival of hESCs on all culture platforms tested and it plays important roles in cell adhesion that supports cell survival.
Application of Insulin‐Free Passaging in Cell Culture Practice
Based on our study of insulin's role in cell survival after dissociation, we proposed a model where insulin activates both cell adhesion and PI3K/AKT pathways to promote cell survival (Fig. 6A) and applied this model to improve daily cell culture practices. When hPSCs are differentiated to specific cell types, it is often necessary to remove undifferentiated hPSCs from the cell population. Conventionally, small chemicals are used to kill hPSCs 43, 44. Here, we examined whether insulin‐free passaging can be used to eliminate undifferentiated cells.
Figure 6.
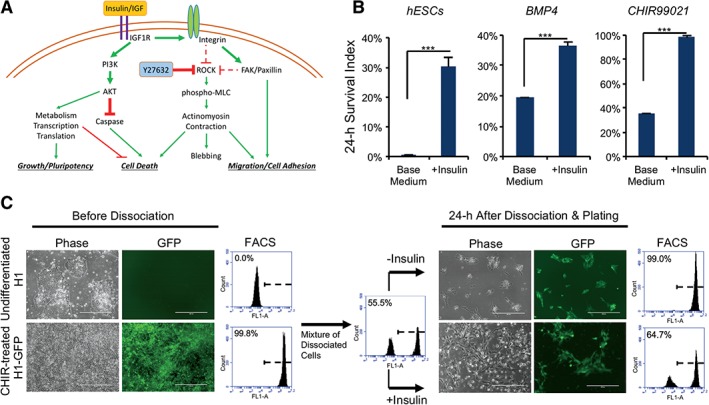
Insulin effect working model and application. (A): Working model of insulin function in human embryonic stem cell survival after dissociation. (B): Plots showing the survival of dissociated H1 ES cells 24 hours after plating on matrigel‐coated surface with or without insulin, in comparison to survival of differentiated cells induced by 4 days of treatment with BMP4 (20 ng/ml) or CHIR99021 (5 μM; ***, p < .001, n = 3). (C): Enrichment of differentiated cell population by insulin‐free passaging. H1 cells and CHIR‐differentiated AAVS1‐GFP H1 cells (left panel) were individualized, mixed, and plated on matrigel‐coated plate. Twenty‐four‐hour survival of undifferentiated H1 (unlabeled) versus differentiated cells (green) was shown by micrographs and flow cytometry analysis (right panel). Scale bar = 200 μm (FL1 = green channel).
Similar to fibroblasts (Supporting Information Fig. S1C), we observed that following induction of differentiation by Bone Morphogenetic Protein 4 (BMP4) or the GSK3 inhibitor CHIR99021, differentiated hPSCs survived without insulin after individualization (Fig. 6B). A green fluorescent protein (GFP)‐labeled H1 hESC line was induced to differentiate by CHIR99021 for 4 days and then mixed with undifferentiated GFP‐negative H1 cells. This mixed cell population was passaged with or without insulin. When insulin was present, the surviving cells maintained a ratio similar to the mixture before passaging. In contrast, differentiated cells were highly enriched when insulin was not added during passaging because almost all the undifferentiated hESCs died after plating (Fig. 6C). These results suggest that insulin‐free passaging can eliminate unwanted hPSCs without drug treatment in the production of specific cell types. It could serve as an effective alternative to chemical methods in improving the purity of differentiated cell types.
Discussion
Our results demonstrate that insulin plays essential roles in hESC maintenance and expansion practices. Insulin activates PI3K/AKT pathway, suppresses caspase activity, and promotes the re‐establishment of the stem cell niche after dissociation. Together with ROCK inhibitor, insulin/IGF help achieve optimal cell survival after dissociation. These essential functions of insulin have not been elucidated in hESCs previously.
ROCK inhibitor is considered the most important promoter for cell survival in individualized hESCs 37, 45. However, all the ROCK inhibitor studies were conducted in insulin‐containing media, so insulin's vital role has long been overlooked. By analyzing insulin function in defined culture, we show that insulin is the prerequisite for ROCK inhibitor‐dependent cell survival in all culture platforms. Insulin is essential for both individualized cells and undissociated cells in colonies. Firstly, insulin activates IGF1R to initiate PI3K/AKT cascade for cell survival. PI3K/AKT pathway has essential functions in metabolism, transcription, and translation, so it is not surprising to observe that the ectopic expression of IGF1R and AKTs rescued the proliferation and survival of both undissociated and individualized cells. Secondly, we found that insulin is the primary suppressor of caspase activity and apoptosis and its function is independent of ROCK pathway. It is reported that caspase cascades are activated by ROCK and actinomyosin contraction after individualization 38. Our study shows that caspase activity is mainly suppressed by insulin and ROCK inhibitor plays a supportive role. This finding enhances our understanding of cell survival mechanism in individualized hESCs. It is important to notice that caspase inhibitor alone failed to rescue cell survival. It suggests that other functions downstream of PI3K/AKT are also essential for cell survival in addition to caspase inhibition (Fig. 2F).
Insulin plays more roles than PI3K/AKT activation and caspase inhibition. We showed that insulin was required for hESC survival in the first few hours after individualization, whereas undissociated cells survived without insulin for more than 2 days. Overexpression of IGF1R and AKT only partially rescued the cell survival. These findings led us to establish a model on how insulin promotes cell survival of individualized cells through re‐establishing their niche. Insulin induced phosphorylation of integrin β1, increased colocalization of IGF1R and FAK, and enhanced the formation of adhesomes. The adhesome assembly then suppresses actinomyosin contraction and increases cell spreading and cell survival. It is reported in some specific cell types that IGF1R directly interacts with integrins and the activated IGF1R stimulates integrin‐mediated focal adhesions 46, 47, 48, 49. IGF is also required for integrin‐IGF1R‐based ternary complex formation in anchorage‐independent survival 48. In hESCs, it is reported that focal adhesion suppresses detachment and apoptosis 50. Our data suggest that insulin and IGF sustain focal adhesions and suppress apoptosis. Based on these findings, we propose a working model to describe how insulin regulates cell survival through PI3K/AKT and cell adhesion pathways (Fig. 6A).
hESC passaging involves niche disintegration and reformation and it could serve as a good platform to study functions of stem cell niche. In defined stem cell culture, we are able to choose specific ECM and medium components to study their interactions. In this study, we found that insulin is essential not only for cells on matrigel‐coated surfaces but also for those cultured on E‐cadherin or in suspension. In all the cases, IGF1R/AKT could not fully rescue the cell survival. It indicates that there are still more to explore about insulin/IGF function in the cell adhesion. We believe that the adhesion and survival after individualization could become an excellent model system to study niche formation.
Insulin‐dependent cell survival is specific to pluripotent stem cells and differentiated cells lose the reliance on insulin. This unique characteristic allows us to eliminate undifferentiated hPSCs while enriching differentiated cell types. Eliminating hPSCs from cell products is a necessary step in cell therapy before transplantation. Small chemicals were previously used to remove stem cells from differentiated cell derivatives. However, it is unknown whether the chemical treatment would bring any complications to the cell products. Our approach provides an effective alternative to eliminate residual stem cells through a simple cell culture manipulation.
In summary, this study elucidates one important aspect of insulin as an essential growth factor for hESCs. Our results show that insulin plays multiple roles to promote cell survival and the re‐establishment of stem cell niche in individualized hESCs. Insulin works as a central molecule to coordinate PI3K/AKT kinase cascade, caspase inhibition, and the formation of cell adhesion and ultimately promotes cell survival after individualization. This study helps people better understand the molecular regulation by insulin and IGF in hESCs and provides a model to study niche formation in hESCs.
Conclusion
In this report, we highlight insulin/IGF pathway as the most important factor in cell survival in parallel with the ROCK pathway. Our results suggest that insulin is the main regulator on caspase activity hESCs and it activates IGF1R/PI3K/AKT pathway to promote cell survival. Meanwhile, insulin stimulates cell adhesion during niche re‐formation and is essential for optimal cell survival after passaging.
Author Contributions
C.G.‐P., G.C.: conception and design, conducted experiments, manuscript writing; C.D.: conducted experiments; W.L.: conducted experiments, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Figure S1 (Related to Fig. 1). Insulin improves hESC survival.
(A) Plot showing the effects of E8 medium components on cell survival 24 hours after plating on Matrigel‐coated surface (n = 3 biological repeats). (B) Plots showing the survival of dissociated H9 and NL‐1 cells 24 hours after plating on Matrigel‐coated surface with or without insulin (n = 3 biological repeats). (C) Plots showing the survival of dissociated fibroblasts 24 hours after plating on Matrigel‐coated surface with or without insulin (n = 3 biological repeats). (D) Plot showing the dose effect of insulin on the cell survival on a matrigel‐coated surface 24 hours after plating (***p < .001, n = 3).
Figure S2 (Related to Fig. 2). Caspase inhibition by insulin.
(A) Quantification of the western blot shown in Figure 2D, normalized to GAPDH (*p < .05, n = 3 technical repeats). (B) Micrographs showing the phenotypes achieved with base medium, pan‐caspase inhibitor Z‐VAD‐FMK, insulin and Y‐27632 + Z‐VAD‐FMK on Matrigel after 24 hours of cell culture. Scale bar = 100 μm.
Figure S3 (Related to Fig. 3). Insulin promotes cell survival through IGF1R/PI3K pathway
(A) Plots showing the survival of dissociated H1 ES cells 24 hours after plating on Matrigel‐coated surface with or without neutralizing antibodies (**p < .01, n = 3 biological repeats). (B) Quantification of the western blot shown in Figure 3C. Levels of AKT and phospho‐AKT‐S473 were normalized to GAPDH and total AKT, respectively (*p < .05, n = 3 technical repeats). (C) Flow Cytometry analysis showing the cell cycle status with or without insulin and the PI3K inhibitor LY294002 after 24 hours treatment of undissociated H1 cells.
Figure S4 (Related to Fig. 4). Impact of insulin on cell attachment and adhesome formation
(A) Quantification of the western blot for Integrin β1 and phospho‐Integrin β1‐788‐789 shown in Figure 4B (*p < .05, n = 3 technical repeats). (B) Quantification of the western blot for IGF1R and phospho‐INSR/IGF1R‐Y1158‐1162‐1163 shown in Figure 4C (*p < .05, n = 3 technical repeats). (C) Quantification of the western blot for FAK, phospho‐FAK‐Y397, Paxillin and phospho‐Paxillin‐Y118 shown in Figure 4D (*p < .05, n = 3 technical repeats). (D) Phase contrast image showing hPSCs 2 hours after plating on Matrigel with or without Insulin. Scale bar = 100 μm. (E) Quantification of the immunostaining for Paxillin, FAK and phospho‐FAK‐Y397‐positive adhesomes per cell 2 hours after plating, corresponding to Figure 4E (**p < .01, *p < .05, n = 3 biological repeats). (F) Left panel: Immunostaining showing expression of Paxillin (green) in focal adhesions formed during 2 hours on Matrigel‐coated surface with or without insulin in AKT2 and IGF1R‐overexpression cell lines. Nuclei were stained with Hoechst (blue). Scale bar = 20 μm. Right panel: adhesomes/cell quantification of the image on the left (*p < .05, n = 3 biological repeats).
Figure S5 (Related to Fig. 5). Synergistic effect of insulin and ROCK inhibition on cell survival
(A) Quantification of the western blot for MLC2 phospho‐MLC2‐S19 shown in Figure 5B (*p < .05, n = 3 technical repeats). (B) Quantification of the western blot for MLC2 and phospho‐MLC2‐S19 shown in Figure 5D (*p < .05, n = 3 biological repeats). (C) Cell survival on matrigel in the presence of Insulin+Y27632 with Integrin β1 and α6 blocking antibodies, compared to Insulin+Y27632 + TNNT2 antibody (mock) (***p < .001, n = 3). (D) Quantification of the western blot for Integrin β1 and phospho‐Integrin β1‐788‐789 shown in Figure 5G (*p < .05, n = 3 technical repeats).
Acknowledgments
This work was supported by the Science and Technology Development Fund of Macau SAR (FDCT/131/2014/A3 and FDCT/056/2015/A2) and University of Macau Multiyear Research Grant (MYRG2015‐00228‐FHS and MYRG2018‐00135‐FHS).
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans 2006;34:213–216. [DOI] [PubMed] [Google Scholar]
- 2. Seltzer HS, Allen EW, Herron AL et al. Insulin secretion in response to glycemic stimulus: Relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967;46:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang L, Schulz TC, Sherrer ES et al. Self‐renewal of human embryonic stem cells requires insulin‐like growth factor‐1 receptor and ERBB2 receptor signaling. Blood 2007;110:4111–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakashima Y, Omasa T. What kind of signaling maintains pluripotency and viability in human‐induced pluripotent stem cells cultured on laminin‐511 with serum‐free medium? Biores Open Access 2016;5:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu C, Rosler E, Jiang J et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells 2005;23:315–323. [DOI] [PubMed] [Google Scholar]
- 6. Akopian V, Andrews PW, Beil S et al. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. Vitr Cell Dev Biol Anim 2010;46:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen G, Gulbranson DR, Hou Z et al. Chemically defined conditions for human iPS cell derivation and culture HHS public access. Nat Methods 2011;8:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skottman H, Hovatta O. Culture conditions for human embryonic stem cells. Reproduction 2006;132:691–698. [DOI] [PubMed] [Google Scholar]
- 9. Rinderknecht E, Humbel RE. The amino acid sequence of human insulin‐like growth factor I and its structural homology with proinsulin. J Biol Chem 1978;253:2169–2116. [PubMed] [Google Scholar]
- 10. Keyhanfar M, Booker GW, Whittaker J et al. Precise mapping of an IGF‐I‐binding site on the IGF‐1R. Biochem J 2007;401:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manning BD, Toker A. AKT/PKB signaling: Navigating the network. Cell 2017;169:381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Tian C, Zheng JC. FoxO3a contributes to the reprogramming process and the differentiation of induced pluripotent stem cells. Stem Cells Dev 2013;22:2954–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roche E, Jones J, Arribas MI et al. Role of small bioorganic molecules in stem cell differentiation to insulin‐producing cells. Bioorg Med Chem 2006;14:6466–6474. [DOI] [PubMed] [Google Scholar]
- 14. Teo AKK, Arnold SJ, Trotter MWB et al. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev 2011;25:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheung C, Bernardo AS, Pedersen RA et al. Directed differentiation of embryonic origin‐specific vascular smooth muscle subtypes from human pluripotent stem cells. Nat Protoc 2014;9:929–938. [DOI] [PubMed] [Google Scholar]
- 16. Ahmed M. Extracellular matrix regulation of stem cell behavior. Curr Stem Cell Rep 2016;2:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim Biophys Acta 2014;1840:2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L, Bennett SAL, Wang L. Role of E‐cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhe Migr 2012;6:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lane SW, Williams DA, Watt FM et al. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol 2014;32:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vitillo L, Kimber SJ. Integrin and FAK regulation of human pluripotent stem cells. Curr Stem Cell Rep 2017;3:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murrell M, Oakes PW, Lenz M et al. Forcing cells into shape: The mechanics of actomyosin contractility. Nat Rev Mol Cell Biol 2015;16:486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Canel M, Serrels A, Frame MC et al. E‐cadherin–Integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. [DOI] [PubMed] [Google Scholar]
- 23. Nagaoka M, Koshimizu U, Yuasa S et al. E‐cadherin‐coated plates maintain pluripotent ES cells without colony formation. PLoS One 2006;e15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giancotti FG, Ruoslahti E. Integrin signaling. Sci Compass 1999;285:1028–1033. [DOI] [PubMed] [Google Scholar]
- 25. Huttenlocher A, Horwitz AR. Integrins in cell migration. CSH Perspect 2011;3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol 2005;17:509–516. [DOI] [PubMed] [Google Scholar]
- 27. Müller EJ, Williamson L, Kolly C et al. Outside‐in signaling through integrins and cadherins: A central mechanism to control epidermal growth and differentiation? J Invest Dermatol 2008;128:501–516. [DOI] [PubMed] [Google Scholar]
- 28. Crossland H, Kazi AA, Lang CH et al. Focal adhesion kinase is required for IGF‐I‐mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1‐associated pathway. AJP Endocrinol Metab 2013;305:E183–E193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guilherme A, Torres K, Czech MP. Cross‐talk between insulin receptor and integrin α5β1 signaling pathways. J Biol Chem 1998;273:22899–22903. [DOI] [PubMed] [Google Scholar]
- 30. Shim SR, Kook S, Il KJ et al. Degradation of focal adhesion proteins paxillin and p130cas by caspases or calpains in apoptotic Rat‐1 and L929 cells. Biochem Biophys Res Commun 2001;286:601–608. [DOI] [PubMed] [Google Scholar]
- 31. Thomson JA, ItsKovitz‐Eldor J, Shapiro SS et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 32. Ludwig TE, Bergendahl V, Levenstein ME et al. Feeder‐independent culture of human embryonic stem cells. Nat Methods 2006;21:637. [DOI] [PubMed] [Google Scholar]
- 33. Ellerstrom C, Strehl R, Semb H. Facilitated expansion of human embryonic stem cells by single‐cell enzymatic dissociation. Stem Cells 2007;25:1690–1696. [DOI] [PubMed] [Google Scholar]
- 34. Pirone DM, Liu WF, Ruiz SA et al. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA‐ROCK signaling. J Cell Biol 2006;174:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beers J, Gulbranson DR, George N et al. Passaging and colony expansion of human pluripotent stem cells by enzyme‐free dissociation in chemically defined culture conditions. Nat Protoc 2012;7:2029–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen KG, Mallon BS, McKay RDG et al. Human pluripotent stem cell culture: Considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014;14:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen G, Hou Z, Gulbranson D et al. Actin‐myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 2010;7:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurosawa H. Application of Rho‐associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng 2012;114:577–581. [DOI] [PubMed] [Google Scholar]
- 39. Meng Y, Ren Z, Xu F et al. Nicotinamide promotes cell survival and differentiation as kinase inhibitor in human pluripotent stem cells. Stem Cell Rep 2018;11:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu J, Hu K, Smuga‐otto K et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009;324:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kohn AD, Takeuchi F, Roth RA. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem 1996;271:21920–21926. [DOI] [PubMed] [Google Scholar]
- 42. Carboni JM, Lee AV, Hadsell DL et al. Tumor development by transgenic expression of a constitutively active insulin‐like growth factor I receptor. Cancer Res 2005;9:3781–3788. [DOI] [PubMed] [Google Scholar]
- 43. Ben‐David U, Gan Q‐F, Golan‐Lev T et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high‐throughput screen. Cell Stem Cell 2013;12:167–179. [DOI] [PubMed] [Google Scholar]
- 44. Lin Y, Wang C, Yang S et al. Elimination of undifferentiated human embryonic stem cells by cardiac glycosides. Sci Rep 2017;65:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watanabe K, Ueno M, Kamiya D et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 2007;25:681. [DOI] [PubMed] [Google Scholar]
- 46. Fa R, Legate KR, Wickstro SA. Genetic and cell biological analysis of integrin outside‐in signaling. Genes Dev 2009;7:397–418. [DOI] [PubMed] [Google Scholar]
- 47. Fujita M, Ieguchi K, Cedano‐prieto DM et al. An integrin binding‐defective mutant of insulin‐like growth factor‐1 (R36E/R37E IGF1) acts as a dominant‐negative antagonist of the IGF1 receptor (IGF1R) and suppresses tumorigenesis but still binds to IGF1R. J Biol Chem 2013;288:19593–19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fujita M, Takada YK, Takada Y. Insulin‐like growth factor (IGF) signaling requires αvβ3‐IGF1‐IGF type 1 receptor (IGF1R) ternary complex formation in anchorage independence, and the complex formation does not require IGF1R and Src activation. J Biol Chem 2013;288:3059–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saegusa J, Yamaji S, Ieguchi K et al. The direct binding of insulin‐like growth factor‐1 (IGF‐1) to integrin αvβ3 is involved in IGF‐1 signaling. J Biol Chem 2009;284:24106–24114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vitillo L, Baxter M, Iskender B et al. Integrin‐associated focal adhesion kinase protects human embryonic stem cells from apoptosis, detachment, and differentiation loriana. Stem Cell Rep 2016;7:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (Related to Fig. 1). Insulin improves hESC survival.
(A) Plot showing the effects of E8 medium components on cell survival 24 hours after plating on Matrigel‐coated surface (n = 3 biological repeats). (B) Plots showing the survival of dissociated H9 and NL‐1 cells 24 hours after plating on Matrigel‐coated surface with or without insulin (n = 3 biological repeats). (C) Plots showing the survival of dissociated fibroblasts 24 hours after plating on Matrigel‐coated surface with or without insulin (n = 3 biological repeats). (D) Plot showing the dose effect of insulin on the cell survival on a matrigel‐coated surface 24 hours after plating (***p < .001, n = 3).
Figure S2 (Related to Fig. 2). Caspase inhibition by insulin.
(A) Quantification of the western blot shown in Figure 2D, normalized to GAPDH (*p < .05, n = 3 technical repeats). (B) Micrographs showing the phenotypes achieved with base medium, pan‐caspase inhibitor Z‐VAD‐FMK, insulin and Y‐27632 + Z‐VAD‐FMK on Matrigel after 24 hours of cell culture. Scale bar = 100 μm.
Figure S3 (Related to Fig. 3). Insulin promotes cell survival through IGF1R/PI3K pathway
(A) Plots showing the survival of dissociated H1 ES cells 24 hours after plating on Matrigel‐coated surface with or without neutralizing antibodies (**p < .01, n = 3 biological repeats). (B) Quantification of the western blot shown in Figure 3C. Levels of AKT and phospho‐AKT‐S473 were normalized to GAPDH and total AKT, respectively (*p < .05, n = 3 technical repeats). (C) Flow Cytometry analysis showing the cell cycle status with or without insulin and the PI3K inhibitor LY294002 after 24 hours treatment of undissociated H1 cells.
Figure S4 (Related to Fig. 4). Impact of insulin on cell attachment and adhesome formation
(A) Quantification of the western blot for Integrin β1 and phospho‐Integrin β1‐788‐789 shown in Figure 4B (*p < .05, n = 3 technical repeats). (B) Quantification of the western blot for IGF1R and phospho‐INSR/IGF1R‐Y1158‐1162‐1163 shown in Figure 4C (*p < .05, n = 3 technical repeats). (C) Quantification of the western blot for FAK, phospho‐FAK‐Y397, Paxillin and phospho‐Paxillin‐Y118 shown in Figure 4D (*p < .05, n = 3 technical repeats). (D) Phase contrast image showing hPSCs 2 hours after plating on Matrigel with or without Insulin. Scale bar = 100 μm. (E) Quantification of the immunostaining for Paxillin, FAK and phospho‐FAK‐Y397‐positive adhesomes per cell 2 hours after plating, corresponding to Figure 4E (**p < .01, *p < .05, n = 3 biological repeats). (F) Left panel: Immunostaining showing expression of Paxillin (green) in focal adhesions formed during 2 hours on Matrigel‐coated surface with or without insulin in AKT2 and IGF1R‐overexpression cell lines. Nuclei were stained with Hoechst (blue). Scale bar = 20 μm. Right panel: adhesomes/cell quantification of the image on the left (*p < .05, n = 3 biological repeats).
Figure S5 (Related to Fig. 5). Synergistic effect of insulin and ROCK inhibition on cell survival
(A) Quantification of the western blot for MLC2 phospho‐MLC2‐S19 shown in Figure 5B (*p < .05, n = 3 technical repeats). (B) Quantification of the western blot for MLC2 and phospho‐MLC2‐S19 shown in Figure 5D (*p < .05, n = 3 biological repeats). (C) Cell survival on matrigel in the presence of Insulin+Y27632 with Integrin β1 and α6 blocking antibodies, compared to Insulin+Y27632 + TNNT2 antibody (mock) (***p < .001, n = 3). (D) Quantification of the western blot for Integrin β1 and phospho‐Integrin β1‐788‐789 shown in Figure 5G (*p < .05, n = 3 technical repeats).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


