Abstract
The biodiversity of food webs is composed of horizontal (i.e. within trophic levels) and vertical diversity (i.e. the number of trophic levels). Understanding their joint effect on stability is a key challenge. Theory mostly considers their individual effects and focuses on small perturbations near equilibrium in hypothetical food webs. Here, we study the joint effects of horizontal and vertical diversity on the stability of hypothetical (modelled) and empirical food webs. In modelled food webs, horizontal and vertical diversity increased and decreased stability, respectively, with a stronger positive effect of producer diversity on stability at higher consumer diversity. Experiments with an empirical plankton food web, where we manipulated horizontal and vertical diversity and measured stability from species interactions and from resilience against large perturbations, confirmed these predictions. Taken together, our findings highlight the need to conserve horizontal biodiversity at different trophic levels to ensure stability.
Keywords: Equilibrium, horizontal diversity, large perturbations, small perturbations, stability, vertical diversity
Introduction
Diversity (i.e. species richness) within food webs is important for sustaining ecosystem functions such as biomass production, energy flow and nutrient uptake (Otto et al. 2007; Rooney & McCann 2012; Soliveres et al. 2016; Barnes et al. 2018; Wang & Brose 2018). Diversity can be characterised in two dimensions (Duffy et al. 2007; Srivastava & Bell 2009; Wang & Brose 2018): the number of species within trophic levels (i.e. horizontal diversity) and the number of trophic levels (i.e. vertical diversity). Horizontal and vertical diversity both affect the functioning and stability of food webs, via different mechanisms (Duffy et al. 2007). Effects of horizontal diversity are driven by competitive interactions, while effects of vertical diversity are mediated by predation. Horizontal and vertical diversity may interact with each other (Duffy et al. 2007). For instance, producer coexistence can be indirectly mediated by consumer diversity (Brose 2008).
Until now, the effects of horizontal and vertical diversity on food web stability (i.e. via local stability analysis) have been mostly treated separately (Pimm & Lawton 1977; Duffy et al. 2007), and mainly using small trophic modules (Pimm & Lawton 1977; McCann et al. 1998; Thébault & Loreau 2005). No information is available on their joint effect in multitrophic food webs. Horizontal diversity of consumers is expected to increase stability (McCann et al. 1998), because a higher number of consumer species decrease the per capita energy flux in consumer–resource interactions by decreasing the per capita consumption rate (Crowder et al. 1997; Perna et al. 2004; Finke & Denno 2005), hence stabilising the consumer–resource links (Rip & Mccann 2011; Gilbert et al. 2014). Producer diversity can increase stability (McCann 2000) by increasing the potential for niche differentiation among consumers (Novotny et al. 2006; Jetz et al. 2009; Poisot et al. 2013) or again weaken consumer–resource interactions (Berlow 1999; Hillebrand & Cardinale 2004; Edwards et al. 2010; Moore & de Ruiter 2012). In contrast, vertical diversity is expected to decrease stability in simple food chains via increasing recovery times (Pimm & Lawton 1977; Morin & Lawler 1995; Post 2002). This negative vertical diversity effect has been evoked as an explanation for the limited number of trophic levels in natural food webs (Pimm & Lawton 1977; Morin & Lawler 1995; McHugh et al. 2010; Sabo et al. 2010).
In natural systems, horizontal and vertical diversity will vary jointly. For example, the decrease in vertical diversity (e.g. the extinction of top predators) could cause cascades that lead to species extinction, lowering horizontal diversity (Crooks & Soulé 1999; Borrvall & Ebenman 2006; Srivastava & Bell 2009). In addition, ecosystem succession and degradation often change both horizontal and vertical diversity (Ferris & Matute 2003; Maharning et al. 2009; Yang et al. 2018). Hence, it is critical to understand how horizontal (both producer and consumer) and vertical diversity interact and shape food web stability.
The individual effects of horizontal and vertical diversity on local stability are often examined by analysing the Jacobian matrix (hereafter ‘Jacobian’). This approach assumes that systems are near equilibrium and exposed to small perturbations (May 2001; Allesina & Tang 2012, 2015). However, ecosystems are often far away from equilibrium (Allesina & Tang 2015) and face large perturbations (De Laender et al. 2016). This makes it uncertain whether stability analyses based on the Jacobian provide useful information for real‐world perturbations (May 2001). Alternative stability measures have therefore been proposed (Grimm & Wissel 1997; Arnoldi et al. 2016; Donohue et al. 2016). Examples include population recovery and resistance following severe perturbations (Isbell et al. 2015; Baert et al. 2016; Hillebrand et al. 2018) and the coefficient of temporal variation of population dynamics (McCann 2000; Pennekamp et al. 2018). Recent work indicates that these alternative stability measures may correlate poorly (Ives & Carpenter 2007; Montoya et al. 2013; Hillebrand et al. 2018; Radchuk et al. 2019). For example, temporal stability is positively associated with diversity, while the latter is negatively correlated with resistance (Pennekamp et al. 2018).
In this paper, we combine models and experiments to examine the joint effect of horizontal and vertical diversity on food web stability. We define stability using two kinds of metric: either based on the assumption of small near equilibrium perturbations or based on biomass and compositional recovery following large perturbations away from equilibrium. To this end, we first analysed the joint effect of horizontal (the number of producer/consumer species) and vertical diversity (the number of trophic levels) on the Jacobian‐based stability of randomly created food webs. Second, we manipulated horizontal and vertical diversity in an experiment with a planktonic food web and quantified their joint effect on stability, measured using empirically established Jacobian matrices. Finally, we quantified the effect of horizontal and vertical diversity on the stability of the same food web, but now measured as resilience following large perturbations caused by two types of chemicals.
Overall, our results show for the first time that the positive effect of producer diversity on stability increases with consumer diversity, regardless of vertical diversity. In contrast, vertical diversity always decreased stability. This trend emerged from all analyses and suggests that conserving diversity within multiple trophic levels is key to promote food web stability.
Materials and Methods
Model and simulations
We conducted a full factorial design with 24 food web configurations: four levels of horizontal diversity at the first trophic level (producer diversity equalled 6, 7, 8 or 9), three levels of horizontal diversity at the second trophic level (consumer diversity equalled 3, 4 or 5) and two levels of vertical diversity (2 or 3 trophic levels). This design reflects the empirically observed triangularity of food webs (Woodward et al. 2005; Turney & Buddle 2016). We deliberately omitted omnivores (species consuming at multiple trophic levels), because omnivores have already been proven to stabilise food webs by creating weak predator–prey interactions (Neutel et al. 2002, 2007). Food web connectance (i.e. the number of links divided by the square of the number of species) was set to 0.10 (Williams et al. 2002; Dunne et al. 2002a,2002b). The links were randomly distributed between adjacent trophic levels.
We described community dynamics with generalised Lotka–Volterra equations (eqn 1) (Emmerson & Yearsley 2004; Gibbs et al. 2018; Maynard et al. 2018):
| (1) |
where N i and N j are the population density of species and , respectively; is the intrinsic per capita growth rate of species . The is positive for producers, where it represents the density‐independent growth rate, while is negative for consumers and predators, where it represents a death rate. The is the per capita effect of species on the growth rate of species .
The growth rate for all producers was equal to 1, which guaranteed that emergent food web patterns were a direct effect of horizontal/vertical diversity, rather than fitness differences among species (Maynard et al. 2018). For consumers and predators, we randomly drew from a uniform distribution U(−0.001, 0) while for predators was generated from U(−0.0001,0) (Eklöf & Ebenman 2006). We ensured that the of predators was less negative than the of consumers, because species at higher trophic levels often have larger body sizes and therefore lower mortality rates (Borrvall et al. 2000). We ensured that intraspecific competition (i = j) was stronger for primary producers (−1) than for consumers and predators (−0.1) (Berg et al. 2011; Kadoya et al. 2018). Interspecific competitions (i ≠ j) among producers were sampled from U(−0.5, 0) and set symmetrically to avoid cycling or chaos (Eklöf & Ebenman 2006; Maynard et al. 2018). Consumers competed indirectly by sharing producers, and direct interspecific interactions among consumers were thus set to zero (Eklöf & Ebenman 2006).
Finally, the (i ≠ j), the per capita effect of consumers (or predators) species j on the per capita growth rate of producers (or prey) species i, was sampled from U(−0.5, 0) when a consumer (or predator) only consumed one producer (or prey) (Eklöf & Ebenman 2006). Considering that interaction strengths in natural system communities often have skewed distributions with mostly weak and only few strong interactions (Borrvall et al. 2000), one strong was sampled from U(−0.4, 0) and assigned randomly (Eklöf & Ebenman 2006), if the number of producers (or prey) was larger than one. The weak was sampled from U(−0.1,0) divided by the number of prey species minus one (Borrvall et al. 2000; Borrvall & Ebenman 2006). Hence, the total effect of a consumer (or predator) on all its producers (or prey), , always varied between −0.5 and 0, but the average per capita effect of a consumer (or predator) on its producers (or prey) decreased with the number of producers (or prey) (McCann et al. 1998; Borrvall et al. 2000). A rationale for this approach and more details can be found in the Supplementary Information S1. The effect of producers (or prey) on consumers (or predators) is given by , which is positive: , with representing the efficiency of the resources being converted into consumers, which was set at 0.2 (Borrvall & Ebenman 2006; Eklöf & Ebenman 2006).
Per food web configuration, we created 10 000 food webs, yielding 240 000 food webs. For each food web, we calculated stability as follows. First, we calculated equilibrium population density (directly solving the equations on eqn 1) and verified if all equilibrium densities were positive. If this was the case, we retained the particular food web; otherwise, we discarded it. For each food web configuration, more than 95% of the generated food webs were feasible with positive equilibrium densities (Table S1). Next, we used these equilibria to compute the Jacobian for this food web. Finally, we quantify stability using the recovery time, defined as the negative reciprocal of the real part of the dominant eigenvalue of the Jacobian, that is ()) (Pimm & Lawton 1977; Emmerson & Yearsley 2004; Moore & de Ruiter 2012). A larger recovery time indicates a lower stability. Finally, we conducted two sensitivity analyses to inspect how our results changed with the selected parameter ranges (Figs. S1–S3).
Experiments: general conditions
We experimentally tested the effect of horizontal and vertical diversity on the stability of a freshwater plankton food web representative of Dutch ditches. These two experiments, each lasted for 21 days, were performed in 900‐mL glass jars, filled with 500 mL WC medium (Guillard & Lorenzen 1972; Frenken et al. 2018) and contained in a water bath at constant temperature (19.9 ºC ± 0.8 ºC) and a light regime of 12 h : 12 h (light : dark). The light intensity at the surface (measured with a LI‐COR LI‐250A, LI‐COR Biosciences, Lincoln, USA) was 120 μmol m−2 s−1 and was created using Ceramalux® Philips 430 Watt High Pressure Sodium Non‐Cycling Lamps. We worked with field‐collected organisms (details are in the Supplementary Information S2). The total initial biovolume of producers (algae) and consumers (invertebrate grazers) was always 25 mm3 and 0.2 mm3, respectively, regardless of producer and consumer diversity (richness). For the systems with three trophic levels, we added one individual of predator Chaoborus to each system. The predators used in the experiments had mean individual body length 11.21 ± 0.04 mm. In both experiments, we worked with four replicates.
Experiment 1: empirical Jacobian matrices
The aim of the experiment was to examine how stability, based on empirically constructed Jacobian matrices, varied with horizontal and vertical diversity. We manipulated horizontal diversity, at the first (producers; 1 or 5 species) and second trophic level (consumers; 1 or 4 species), and vertical diversity (2 or 3 trophic levels) in a full factorial design (Table S2). At all combinations, we estimated interactions (within and between trophic levels) to characterise the Jacobian on day 21 after the start of the experiment. The off‐diagonal elements of this matrix are per capita interactions, which we estimated as the per capita material fluxes between consumers (or predators) and producers (or consumers) (de Ruiter et al. 1995; Neutel et al. 2007; Schwarz et al. 2017). The effect of consumers (or predators) on producers (or consumers) is given by , and the effect of producers (or consumers) on consumers (or predators) is given by , where is the energy flux from to (e.g. from producers to consumers), is the assimilation efficiency of , and and (g m−2) are the biomass of and , respectively (Schwarz et al. 2017). The diagonal elements of the Jacobian are , where is the metabolism of trophic level , and s is a free parameter between 0 and 1 (Schwarz et al. 2017). Because cannot be determined empirically in complex food webs, we determined the smallest leading to all eigenvalues of the Jacobian having negative real parts. The value of represents the stability of the community against small perturbations, assessed based on estimated interactions (Schwarz et al. 2017). It is therefore conceptually similar to recovery time (smaller values indicate more stable food webs) obtained with the model and is referred to as the degree of self‐damping. Details on the calculation of , and are provided in the Supplementary Information S3.
Experiment 2: large perturbations
The objective of this experiment was to examine how horizontal and vertical diversity affected the stability against large perturbations. Here, we applied functional and compositional resilience as stability metrics. We manipulated the same experimental factors as in experiment 1 and added one additional factor: pesticide exposure (absent or present). We performed this experiment twice, once using the insecticide chlorpyrifos (1 μg L−1) and once using the herbicide linuron (100 μg L−1), selectively targeting consumers and producers, respectively (Wijngaarden et al. 1996; Daam et al. 2009). Experimental procedures were identical to the experiment 1. Information on chemical administration is provided in Supplementary Information S4. We measured community biomass, community composition (using the same methods as for experiment 1 and on days 6 and 21) and stability. To measure stability, we first measured functional resilience (the recovery rate of total biomass) as (Isbell et al. 2015; Baert et al. 2016):
| (2) |
where B control,6, B control,21, B stress,6 and B stress,21 represent the total biomass in the control (no pesticide) and exposure (pesticide present) on days 6 and 21. Functional resilience is > 1 if biomass differences between the control and stress treatment decrease between day 6 and day 21, and < 1 otherwise. Larger values mean faster recovery.
Next, we measured compositional resilience (compositional recovery) (Baert et al. 2016; Hillebrand et al. 2018):
| (3) |
Compositional resilience can be considered an abundance‐based change of Bray–Curtis similarity between day 6 (BC6) and day 21 (BC21) (Baert et al. 2016; Hillebrand et al. 2018), where is abundance of species i. Positive values reflect that compositions of the control and disturbed communities converge between day 6 and day 21, while negative values imply compositional divergence. Again, larger values mean faster recovery.
Analysis of simulated and empirical data
To the simulated data, we applied linear regression to estimate the effect of producer, consumer and vertical diversity, and their pairwise interactions, on the recovery time. To interpret potential effects on recovery time, we also tested for diversity effects on average interaction strengths, defined as the square root of the average of all the off‐diagonal elements in the interaction matrix Jij (i ≠ j) with total species T, that is, (May 2001; Moore & de Ruiter 2012), again using linear regression.
To the data from experiment 1, we applied linear mixed models to test for the effect of producer, consumer and vertical diversity, and their pairwise interactions, on the degree of self‐damping, as calculated from the estimated interactions. We used species identity as a random effect to exclude the potential confounding effect of species identity.
To understand possible effects of diversity on the degree of self‐damping, we examined diversity effects on three variables underlying the degree of self‐damping: consumer biomass, the energy flux into consumers and interaction strengths. We did so by first applied the mixed model to test for the effect of producer, consumer and vertical diversity, and their pairwise interactions (again with species identity as a random effect) on these three variables. Next, we constructed linear regression models to examine the relationship between (1) consumer biomass and energy flux into consumers, (2) energy flux into consumers and the absolute value of interaction strength of consumers to producers and finally (3) the absolute value of interaction strength of consumers to producers and degree of self‐damping (minimum s). Again, we used mixed models with species identity as a random effect and included interactions between horizontal and vertical diversity. We adopted the same approach for predator biomass, energy flux into predator and absolute value of interaction strength of predator to consumer. However, note that by definition, vertical diversity here was always three, so we could only analyse the effects of horizontal diversity.
To the data from experiment 2, we again used linear mixed‐effects models (species identity was again a random effect) to test for the effect of producer, consumer and vertical diversity, and their pairwise interactions on the two measures of recovery (eqns 2 and 3). Because these measures depend on how total biomass changed with time, we also included sampling time and chemical concentrations into the analysis of total biomass. All models were fitted with the lme4 package in R (Bates et al. 2014).
Results
Model simulations
Producer and consumer diversity both promoted stability, that is, decreased recovery time (Fig. 1). The positive effect of producer diversity on stability increased with increasing consumer diversity, and this trend was not qualitatively changed by vertical diversity. Vertical diversity on itself always decreased stability. Stability was highest at high horizontal (producer and consumer) diversity and low vertical diversity, and lowest at low horizontal diversity and high vertical diversity (Fig. 1a,b), indicating that high horizontal diversity can compensate the stability loss caused by vertical diversity. These results were robust to changing all parameters simultaneously from their reference value by −20% and +20% (Fig. S1). Outside of this range, the model results were sensitive to the conversion efficiency (Fig. S2), where larger destabilised the food webs and switched the diversity–stability relationship, as expected (Rip & Mccann 2011; Barbier & Loreau 2019). When fixing the conversion efficiency to its reference value, the model results were robust to changes of up to − 60% and + 60% of all parameters except (Fig. S3).
Figure 1.
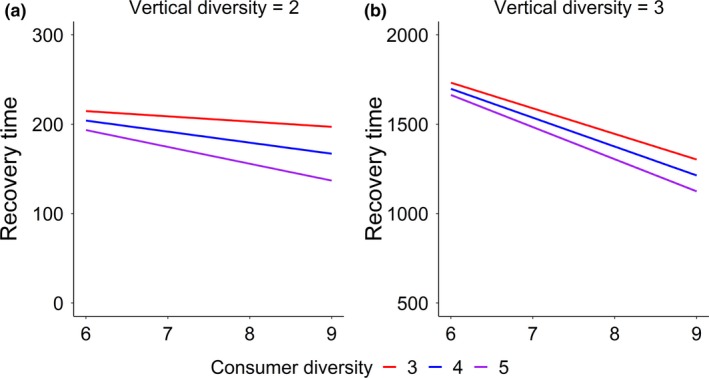
Model simulations illustrating the interactive effects of horizontal (producer and consumer) and vertical diversity on recovery time (a lower recovery time indicates a greater stability)
Experiment 1: empirical Jacobian matrices
Producer, consumer and vertical diversity all affected food web stability. In line with the model predictions, both producer and consumer diversity increased food web stability (i.e. decreasing the degree of self‐damping) and the impact of producer diversity on stability increased with increasing consumer diversity. Also in line with the model results, vertical diversity on itself decreased stability (Fig. 2a,b). Stability was highest at high horizontal (both producer and consumer) diversity and low vertical diversity, and was lowest at low horizontal diversity (producer and consumer) and high vertical diversity (Fig. 2a,b).
Figure 2.

The interactive effects of horizontal (producer and consumer) and vertical diversity on stability (the degree of self‐damping) (a, b), on consumer biomass (c, d), on energy flux from producers to consumers (e, f) and on the absolute value of interaction strength of consumers to producers (g, h). Plotted are sample mean ± 1 SD. Detailed statistical results are listed in Table S4.
The effects of horizontal and vertical diversity on stability were associated with effects on consumer biomass, energy fluxes and interaction strengths between trophic levels. Consumer biomass increased with producer and consumer diversity but decreased with vertical diversity (Fig. 2c,d). Diversity did not affect predator biomass (Table S3).
Interactions of producer, consumer and vertical diversity affected the energy flux into consumers (Fig. 2e,f). At high vertical diversity (i.e. 3), horizontal diversity of either producers or consumers increased the energy flux into consumers (Fig. 2f). This higher energy flux was associated with higher consumer biomass (Fig. 3a). Under low vertical diversity (i.e. 2), however, horizontal diversity decreased the energy flux (Fig. 2e), while increasing consumer biomass (Fig. 3a). We found no effect of diversity on the energy flux into predators (Table S3).
Figure 3.
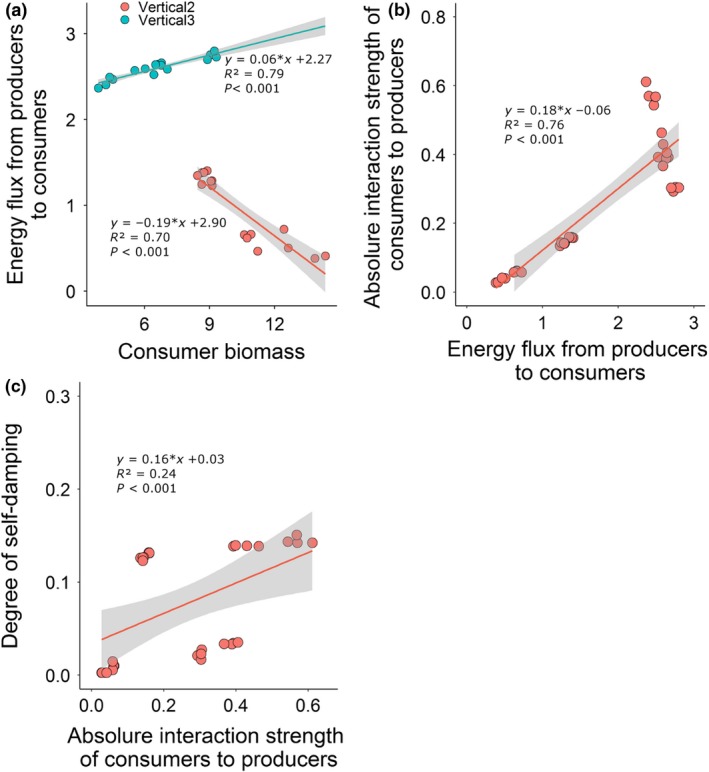
Relationships between consumer biomass (g m−2) and energy flux from producers to consumers (g c m−2 h−1) (a), between the energy flux from producers to consumers (g c m−2 h−1) and the absolute value of interaction strength of consumers to producers (b), and between the absolute value of interaction strength of consumers to producers and the degree of self‐damping (c)
The interaction strength of consumers to producers was influenced by interactions of producer, consumer and vertical diversity. Horizontal diversity decreased the interaction strength, whereas vertical diversity increased it (Fig. 2g,h). The interaction strength was lowest at high horizontal and low vertical diversity, but highest at low horizontal and high vertical diversity (Fig. 2g,h), where the interaction strength was positively correlated with the energy flux into consumers (Fig. 3b). No significant diversity effects were detected on the interaction strength of predators to consumers (Table S3). Finally, the interaction strength of consumers to producers was positively correlated with the degree of self‐damping (Fig. 3c), indicating that strong interactions decreased food web stability.
Experiment 2: large perturbations
In line with the results obtained with the Jacobian method for simulated and empirical food web data, producer and consumer diversity both increased stability (i.e. functional resilience) against severe perturbations and the positive effect of producer diversity was stronger when consumer diversity was high (Fig. 4a–d). Again, vertical diversity decreased stability (Fig. 4a–d). Therefore, functional resilience was highest at high horizontal diversity and low vertical diversity, and it was lowest when horizontal diversity was low and vertical diversity was high (Fig. 4a–d). We found qualitatively identical results for stability measured by the compositional resilience (Fig. 5a–d), even though the interactive effect of producer and consumer diversity was weaker for the case of herbicide exposure.
Figure 4.
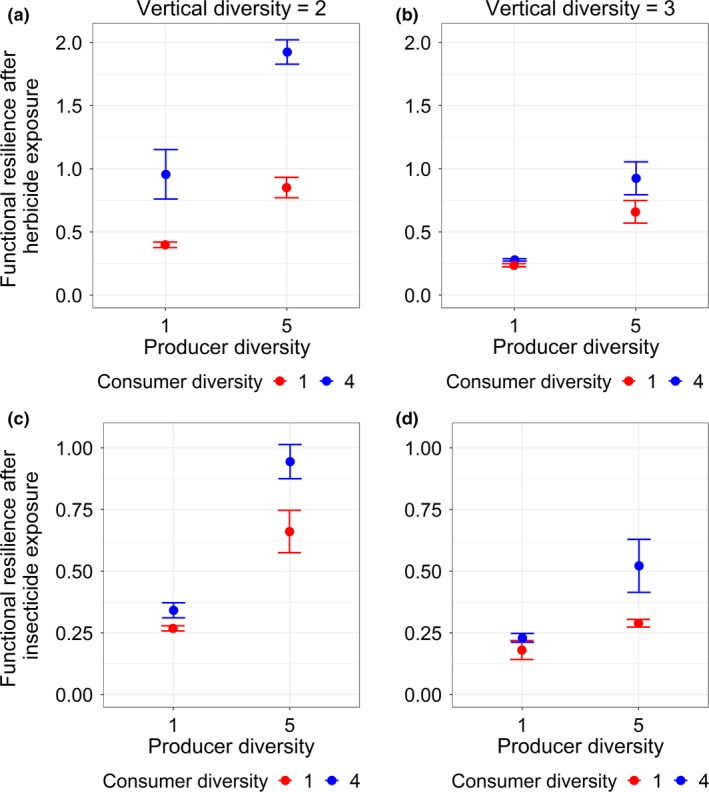
The interactive effects of horizontal (producer and consumer) and vertical diversity on the functional resilience after herbicide (a, b) and insecticide (c, d) exposure. Plotted are sample mean ± 1 SD. Detailed statistical results are listed in Table S5.
Figure 5.
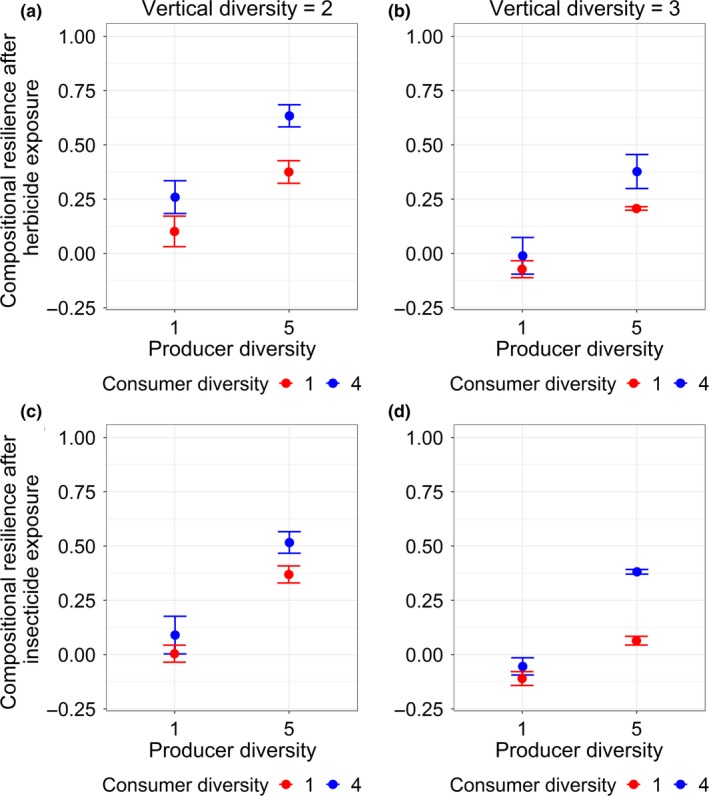
The interactive effects of horizontal (producer and consumer) and vertical diversity on the compositional resilience after herbicide (a, b) and insecticide (c, d) exposure. Plotted are sample mean ± 1 SD. Detailed statistical results are listed in Table S5.
The effects of horizontal and vertical diversity on the functional and compositional resilience were associated with effects on total biomass (sum across all trophic levels) and composition, respectively. Total biomass showed signs of recovery after exposure to the herbicide and insecticide, but horizontal diversity increased the biomass recovery rate while vertical diversity decreased it. This result can be understood from the smaller effect the pesticides had on the horizontally more diverse communities (Fig. S4a–d and Fig. S5a–d). Indeed, this smaller effect translates to the numerator and especially denominator of eqn 2 being smaller at higher horizontal diversity, making their ratio (i.e. functional resilience) inevitably larger. The opposite occurred for vertical diversity, which increased biomass differences (Fig. S4e,f and Fig. S5e,f) and therefore decreased the recovery rate.
On average, the composition of the exposed and control communities was more similar on day 21 than on day 6, indicating compositional recovery. Horizontal and vertical diversity had also opposite effects on compositional recovery. Because producer abundance accounted for more than 97% of the whole community, the effects of horizontal and vertical diversity on compositional recovery can be understood by focusing on the producer community.
The herbicide directly decreased the abundance of sensitive producers (Desmodesmus pannonicum, Chlorella vulgaris and Selenastrum capricornutum, Fig. S6a) on day 6, but did not change consumer composition (Fig. S6c,d). A greater producer diversity caused an insurance effect as tolerant producers (e.g. Scenedesmus obliquus in Fig. S6a) became dominant, which caused compositional differences between the control and the herbicide‐treated systems. This difference translates to the last term of eqn 3 () being smaller at higher producer diversity (no composition changes on day 21), making the difference between and (i.e. compositional resilience) inevitably greater. We also found that the magnitude of this insurance effect was increased by consumer diversity, but decreased by vertical diversity, which respectively increased and decreased compositional recovery (Fig. S6a–d).
The insecticide directly decreased the abundance of sensitive consumers (i.e. Daphnia pulex and Moina macrocopa in Fig. S7a), and tolerant species (e.g. Daphnia lumholtzi in Fig. S7a) became dominant. The dominance of tolerant species had indirect, top‐down, effects on its preferred algae (Scendesmus acutus, C. vulgaris and S. capricornutum), which increased the abundance of non‐preferred algae (D. pannonicum), compensating the loss of the preferred algae (Fig. S7c). Again, this represents an insurance effect, but this time driven by consumer diversity. This mechanism caused composition to be more different between control and insecticide‐exposed systems on day 6 (no composition discrepancy on day 21), which again translated to the last term of eqn 3 () being smaller at higher consumer diversity, making the difference between and (i.e. compositional resilience) inevitably greater. This insurance effect was again increased by producer diversity, but decreased by vertical diversity, which increase and decrease compositional recovery, respectively (Fig. S7a–d).
Discussion
Our model and empirical results show for the first time that horizontal diversity and vertical diversity jointly affect stability. Specifically, the effect of producer diversity was stronger when consumer diversity was higher, regardless of vertical diversity. Vertical diversity consistently decreased stability. Taken together, these results suggest that food webs that are horizontally diverse at various trophic levels, but contain relatively few trophic levels will be more stable. These conclusions are broadly supported. First, both model simulations and two independent experiments with natural food webs yield consistent results. Second, we applied both Jacobian‐based stability assessments that assume small perturbations and population equilibrium, but also alternative stability measures following large perturbations.
The results from the simulations and empirical food webs (Experiment 1) indicate that, under the assumption of small perturbations and population at equilibrium, horizontal and vertical diversity affect food web stability by changing (average) interaction strength. The individual and joint effects of producer and consumer diversity as well as the effect of vertical diversity, as found through modelling, can be understood from changing average interaction strengths (Fig. S8). The results from experiment 1 can be explained by biomass changes and energy flows between trophic levels, which finally change interaction strengths between trophic levels. We show that the well‐known positive (and negative) effects of horizontal (and vertical) diversity on consumer biomass (Duffy 2002; Cardinale et al. 2003) underpin these proposed effects. The positive interactive effects of producer and consumer diversity on consumer biomass reflect a greater niche differentiation among producers and consumers, optimising consumer biomass (Cardinale et al. 2006; Tilman et al. 2014; Barnes et al. 2018). The negative effect of vertical diversity on biomass reflects predation on consumers. It should be noted that, in this study, we only added a single predator individual. Given that natural systems are controlled by predator populations (Cardinale et al. 2003; Snyder et al. 2008; Griffin et al. 2013), biomass depression by vertical diversity can be higher than reported here.
Increasing the biomass of a focal trophic group generally increases the energy flux into this group (Otto et al. 2007; Ehnes et al. 2011; Barnes et al. 2014). At high vertical diversity (i.e. 3), we found a positive interactive effect of producer and consumer diversity on consumer biomass, which was indeed positively associated with energy fluxes into consumers. However, the positive association between biomass and energy flux can be overruled by other factors such as body size structure (Barnes et al. 2014, 2018). Under low vertical diversity (i.e. 2), we detected that high consumer biomass was negatively correlated with the energy fluxes to consumers. We found some support that individual body mass distributions could explain this result (Fig. S9). The treatments with high consumer biomass had a higher proportion of large individuals, which have slower metabolic rates, and thus generate lower energy fluxes, than small organisms.
High energy flux between trophic levels can increase interaction strength (McCann 2000; Rip & Mccann 2011; Schwarz et al. 2017; Kadoya et al. 2018), which in turn decreases food web stability (McCann 2000; Rip & Mccann 2011; Ushio et al. 2018). We found that the large energy flux into consumers indeed increased the interaction strength between consumers and producers, which led to lower stability. More specifically, producer and consumer diversity positively interacted to decrease interaction strength, which increased food web stability. Vertical diversity increased the interaction strength and decreased stability.
Taken together, interactive effects of producer and consumer diversity can change consumer biomass and the energy flux into consumers, leading to weak interactions and increased stability. Vertical diversity, in contrast, makes for strong links which will decrease stability.
Pesticide effects on community biomass were a direct result of effects on community composition and were buffered by horizontal diversity. This buffering effect has been shown before for competitive systems (Gonzalez & Loreau 2009; Isbell et al. 2015; Baert et al. 2016). Our findings suggest that this effect also holds for food webs. Importantly, we found that – in our system where producers were the largest community – this effect occurs both when the pesticide directly affects producers and when it affects producers indirectly by depressing consumers.
We are cognizant of our study’s limitations. First, in our experiments, we only considered two levels per horizontal and vertical diversity treatment. Previous studies have shown that food webs with higher horizontal (producer or consumer) diversity have larger niche differentiation and lower consumption rate (Duffy et al. 2007; Edwards et al. 2010). We therefore expect the positive effect of producer diversity on stability to be stronger than reported here. Second, natural systems often vary not only in species richness but also in how species biomasses are distributed. Our results may therefore change when considering alternative diversity indices (e.g. Shannon’s index in Kato et al. 2018). However, a combination of Shannon’s index and species richness may provide a deeper insight into future work. Third, our model assumed pairwise interactions and neglected potential higher‐order interactions, that is pairwise interactions being modulated by a third species, which have been found to stabilise communities (Bairey et al. 2016; Grilli et al. 2017; Mayfield & Stouffer 2017; Letten & Stouffer 2019). We expect that adding high‐order interactions will reinforce the positive effect of horizontal diversity we found here, but weaken the negative effect of vertical diversity on stability. Finally, our results cannot be extrapolated to food webs that include omnivores. Previous studies indeed showed that complex food webs with omnivores potentially hold many stabilising weak links (Neutel et al. 2002, 2007), making the destabilising effect of vertical diversity we report here possibly weaker. Recent studies demonstrated that the presence of omnivores can alter the relationship between vertical diversity and primary productivity in complex food webs (Wang et al. 2019).
Our results show that different aspects of biodiversity may affect stability in different ways, through effects on biomass, energy fluxes and eventually interaction strengths. How our results scale up to more complex food webs is an outstanding question, but our findings suggest that the benefits of horizontal diversity can in theory overcompensate the negative effects of vertical diversity. Our results show that conserving horizontal diversity across trophic levels (multiple horizontal biodiversity) can offer a solution to maintain both functioning and stability of natural ecosystems with high vertical diversity.
Authorship
QHZ, FDL, CC, PRS, CX, YXGW and SPW conceived and developed the models; PJVDB and QHZ designed the experiments; SV, FG and MV assisted the experiments; QHZ and YXGW analysed all data; QHZ and FDL drafted the manuscript; and all authors contributed substantially to revisions.
Supporting information
Acknowledgements
We acknowledge feedback and advice from the editor, Tim Coulson and three anonymous referees. We thank Carlos Melian and Jürg W. Spaak for valuable suggestions and comments. QHZ is supported by the China Scholarship Council (No. 201606190229).
Data accessibility statement
The data supporting the results are archived at Dryad digital repository (https://doi.org/10.5061/dryad.t46kr73). The model code is archived at GitHub (https://github.com/qhz2019/stability).
References
- Allesina, S. & Tang, S. (2012). Stability criteria for complex ecosystems. Nature, 483, 205–208. [DOI] [PubMed] [Google Scholar]
- Allesina, S. & Tang, S. (2015). The stability–complexity relationship at age 40: a random matrix perspective. Popul. Ecol., 57, 63–75. [Google Scholar]
- Arnoldi, J. , Loreau, M. & Haegeman, B. (2016). Resilience, reactivity and variability: a mathematical comparison of ecological stability measures. J. Theor. Biol., 389, 47–59. [DOI] [PubMed] [Google Scholar]
- Baert, J.M. , De Laender, F. , Sabbe, K. & Janssen, C.R. (2016). Biodiversity increases functional and compositional resistance, but decreases resilience in phytoplankton communities. Ecology, 97, 3433–3440. [DOI] [PubMed] [Google Scholar]
- Bairey, E. , Kelsic, E.D. & Kishony, R. (2016). High‐order species interactions shape ecosystem diversity. Nat. Commun., 7, 12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier, M. & Loreau, M. (2019). Pyramids and cascades: a synthesis of food chain functioning and stability. Ecol. Lett., 22, 405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, A.D. , Jochum, M. , Mumme, S. , Haneda, N.F. , Farajallah, A. , Widarto, T.H. , et al. (2014). Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat. Commun., 5, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, A.D. , Jochum, M. , Lefcheck, J.S. , Eisenhauer, N. , Scherber, C. , O’Connor, M.I. , et al. (2018). Energy flux: The link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol., 33, 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. & Walker, S. (2014). Fitting linear mixed‐effects models using lme4. J. Stat. Softw., 67, 1–48. [Google Scholar]
- Berg, S. , Christianou, M. , Jonsson, T. & Ebenman, B. (2011). Using sensitivity analysis to identify keystone species and keystone links in size‐based food webs. Oikos, 120, 510–519. [Google Scholar]
- Berlow, E.L. (1999). Strong effects of weak interactions in ecological communities. Nature, 398, 330. [Google Scholar]
- Borrvall, C. & Ebenman, B. (2006). Early onset of secondary extinctions in ecological communities following the loss of top predators. Ecol. Lett., 9, 435–442. [DOI] [PubMed] [Google Scholar]
- Borrvall, C. , Ebenman, B. & Jonsson, T. (2000). Biodiversity lessens the risk of cascading extinctions in model food webs. Ecol. Lett., 3, 131–136. [Google Scholar]
- Brose, U. (2008). Complex food webs prevent competitive exclusion among producer species. Proc. R. Soc. B Biol. Sci., 275, 2507–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale, B.J. , Harvey, C.T. , Gross, K. & Ives, A.R. (2003). Biodiversity and biocontrol: Emergent impacts of a multi‐enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett., 6, 857–865. [Google Scholar]
- Cardinale, B.J. , Srivastava, D.S. , Duffy, J.E. , Wright, J.P. , Downing, A.L. , Sankaran, M. , et al. (2006). Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature, 443, 989–992. [DOI] [PubMed] [Google Scholar]
- Crooks, K.R. & Soulé, M.E. (1999). Mesopredator release and avifaunal extinctions in a fragmented system. Nature, 400, 563–566. [Google Scholar]
- Crowder, L.B. , Squires, D.D. & Rice, J.A. (1997). Nonadditive effects of terrestrial and aquatic predators on juvenile estuarine fish. Ecology, 78, 1796–1804. [Google Scholar]
- Daam, M.A. , Van den Brink, P.J. & Nogueira, A.J.A. (2009). Comparison of fate and ecological effects of the herbicide linuron in freshwater model ecosystems between tropical and temperate regions. Ecotoxicol. Environ. Saf., 72, 424–433. [DOI] [PubMed] [Google Scholar]
- Donohue, I. , Hillebrand, H. , Montoya, J.M. , Petchey, O.L. , Pimm, S.L. , Fowler, M.S. , et al. (2016). Navigating the complexity of ecological stability. Ecol. Lett., 19, 1172–1185. [DOI] [PubMed] [Google Scholar]
- Duffy, J.E. (2002). Biodiversity and ecosystem function: the consumer connection. Oikos, 99, 201–219. [Google Scholar]
- Duffy, J.E. , Cardinale, B.J. , France, K.E. , McIntyre, P.B. , Thébault, E. & Loreau, M. (2007). The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett., 10, 522–538. [DOI] [PubMed] [Google Scholar]
- Dunne, J.A. , Williams, R.J. & Martinez, N.D. (2002a). Food‐web structure and network theory: the role of connectance and size. Proc. Natl Acad. Sci., 99, 12917–12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne, J.A. , Williams, R.J. & Martinez, N.D. (2002b). Network structure and biodiversity loss in food webs: robustness increase with connectance. Ecol. Lett., 5, 558–567. [Google Scholar]
- Edwards, K.F. , Aquilino, K.M. , Best, R.J. , Sellheim, K.L. & Stachowicz, J.J. (2010). Prey diversity is associated with weaker consumer effects in a meta‐analysis of benthic marine experiments. Ecol. Lett., 13, 194–201. [DOI] [PubMed] [Google Scholar]
- Eklöf, A. & Ebenman, B. (2006). Species loss and secondary extinctions in simple and complex model communities. J. Anim. Ecol., 75, 239–246. [DOI] [PubMed] [Google Scholar]
- Emmerson, M. & Yearsley, J.M. (2004). Weak interactions, omnivory and emergent food‐web properties. Proc. R. Soc. B Biol. Sci., 271, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, H. & Matute, M.M. (2003). Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol., 23, 93–110. [Google Scholar]
- Finke, D.L. & Denno, R.F. (2005). Predator diversity and the functioning of ecosystems: the role of intraguild predation in dampening trophic cascades. Ecol. Lett., 8, 1299–1306. [Google Scholar]
- Frenken, T. , Wierenga, J. , van Donk, E. , Declerck, S.A.J. , de Senerpont Domis, L.N. , Rohrlack, T. , et al. (2018). Fungal parasites of a toxic inedible cyanobacterium provide food to zooplankton. Limnol. Oceanogr., 63, 2384–2393. [Google Scholar]
- Gibbs, T. , Grilli, J. , Rogers, T. & Allesina, S. (2018). Effect of population abundances on the stability of large random ecosystems. Phys. Rev. E, 98, 1–16. [DOI] [PubMed] [Google Scholar]
- Gilbert, B. , Tunney, T.D. , Mccann, K.S. , Delong, J.P. , Vasseur, D.A. , Savage, V. , et al. (2014). A bioenergetic framework for the temperature dependence of trophic interactions. Ecol. Lett., 17, 902–914. [DOI] [PubMed] [Google Scholar]
- Gonzalez, A. & Loreau, M. (2009). The causes and consequences of compensatory dynamics in ecological communities. Annu. Rev. Ecol. Evol. Syst., 40, 393–414. [Google Scholar]
- Griffin, J.N. , Byrnes, J.E.K. & Cardinale, B.J. (2013). Effects of predator richness on prey suppression: A meta‐analysis. Ecology, 94, 2180–2187. [DOI] [PubMed] [Google Scholar]
- Grilli, J. , Barabás, G. , Michalska‐Smith, M.J. & Allesina, S. (2017). Higher‐order interactions stabilize dynamics in competitive network models. Nature, 548, 210–213. [DOI] [PubMed] [Google Scholar]
- Grimm, V. & Wissel, C. (1997). Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia, 109, 323–334. [DOI] [PubMed] [Google Scholar]
- Guillard, R.R. & Lorenzen, C.J. (1972). Yellow‐green algae with chlorophyllide C12. J. Phycol., 8, 10–14. [Google Scholar]
- Hillebrand, H. & Cardinale, B.J. (2004). Consumer effects decline with prey diversity. Ecol. Lett., 7, 192–201. [Google Scholar]
- Hillebrand, H. , Langenheder, S. , Lebret, K. , Lindström, E. , Östman, Ö. & Striebel, M. (2018). Decomposing multiple dimensions of stability in global change experiments. Ecol. Lett., 21, 21–30. [DOI] [PubMed] [Google Scholar]
- Isbell, F. , Craven, D. , Connolly, J. , Loreau, M. , Schmid, B. , Beierkuhnlein, C. , et al. (2015). Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature, 526, 574–577. [DOI] [PubMed] [Google Scholar]
- Ives, A.R. & Carpenter, S.R. (2007). Stability and diversity of ecosystems concepts of stability. Science, 80(317), 58–62. [DOI] [PubMed] [Google Scholar]
- Jetz, W. , Kreft, H. , Ceballos, G. & Mutke, J. (2009). Global associations between terrestrial producer and vertebrate consumer diversity. Proc. R. Soc. London B Biol. Sci., 276, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya, T. , Gellner, G. & McCann, K.S. (2018). Potential oscillators and keystone modules in food webs. Ecol. Lett., 21, 1330–1340. [DOI] [PubMed] [Google Scholar]
- Kato, Y. , Kondoh, M. , Ishikawa, N.F. , Togashi, H. , Kohmatsu, Y. , Yoshimura, M. , et al. (2018). Using food network unfolding to evaluate food–web complexity in terms of biodiversity: theory and applications. Ecol. Lett., 21, 1065–1074. [DOI] [PubMed] [Google Scholar]
- De Laender, F. , Rohr, J.R. , Ashauer, R. , Baird, D.J. , Berger, U. , Eisenhauer, N. , et al. (2016). Reintroducing environmental change drivers in biodiversity‐ecosystem functioning research. Trends Ecol. Evol., 31, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letten, A.D. & Stouffer, D.B. (2019). The mechanistic basis for higher‐order interactions and non‐additivity in competitive communities. Ecol. Lett., 22, 423–436. [DOI] [PubMed] [Google Scholar]
- Maharning, A.R. , Mills, A.A.S. & Adl, S.M. (2009). Soil community changes during secondary succession to naturalized grasslands. Appl. Soil Ecol., 41, 137–147. [Google Scholar]
- May, R.M. (2001). Stability and Complexity in Model Ecosystems, vol. 6. Princeton University Press, Princeton, NJ. [Google Scholar]
- Mayfield, M.M. & Stouffer, D.B. (2017). Higher‐order interactions capture unexplained complexity in diverse communities. Nat. Ecol. Evol., 1, 0062. [DOI] [PubMed] [Google Scholar]
- Maynard, D.S. , Serván, C.A. & Allesina, S. (2018). Network spandrels reflect ecological assembly. Ecol. Lett., 21, 324–334. [DOI] [PubMed] [Google Scholar]
- McCann, K.S. (2000). The diversity–stability debate. Nature, 405, 228–233. [DOI] [PubMed] [Google Scholar]
- McCann, K. , Hastings, A. & Huxel, G.R. (1998). Weak trophic interactions and the balance of nature. Nature, 395, 794–798. [Google Scholar]
- McHugh, P.A. , McIntosh, A.R. & Jellyman, P.G. (2010). Dual influences of ecosystem size and disturbance on food chain length in streams. Ecol. Lett., 13, 881–890. [DOI] [PubMed] [Google Scholar]
- Montoya, M. , Jackson, A.L. , Viana, M. & Connor, N.E.O. (2013). On the dimensionality of ecological stability. Ecol. Lett., 16, 421–429. [DOI] [PubMed] [Google Scholar]
- Moore, J.C. & de Ruiter, P.C. (2012). Energetic Food Webs: An Analysis of Real and Model Ecosystems, 1st edn Oxford University Press, Oxford, UK. [Google Scholar]
- Morin, P.J. & Lawler, S.P. (1995). Food web architecture and population dynamics: theory and empirical evidence. Annu. Rev. Ecol. Syst., 26, 505–529. [Google Scholar]
- Neutel, A.M. , Heesterbeek, J.A.P. & de Ruiter, P.C. (2002). Stability in real food webs: weak links in long loops. Science, 80(296), 1120–1124. [DOI] [PubMed] [Google Scholar]
- Neutel, A.M. , Heesterbeek, J.A.P. , Van De Koppel, J. , Hoenderboom, G. , Vos, A. , Kaldeway, C. , et al. (2007). Reconciling complexity with stability in naturally assembling food webs. Nature, 449, 599–602. [DOI] [PubMed] [Google Scholar]
- Novotny, V. , Drozd, P. , Miller, S.E. & Kulfan, M. (2006). Why are there so many species of herbivorous insects in tropical rainforests? Science, 80(738), 1115–1118. [DOI] [PubMed] [Google Scholar]
- Otto, S.B. , Rall, B.C. & Brose, U. (2007). Allometric degree distributions facilitate food‐web stability. Nature, 450, 1226–1229. [DOI] [PubMed] [Google Scholar]
- Pennekamp, F. , Pontarp, M. , Tabi, A. , Altermatt, F. , Alther, R. , Choffat, Y. , et al. (2018). Biodiversity increases and decreases ecosystem stability. Nature, 563, 109–112. [DOI] [PubMed] [Google Scholar]
- Perna, M. , Pinto, M.Di & Roualec, J.M. (2004). Predator diversity dampens trophic cascades. Nature, 429, 407–410. [DOI] [PubMed] [Google Scholar]
- Pimm, S.L. & Lawton, J.H. (1977). Number of trophic levels in ecological communities. Nature, 268, 329–330. [Google Scholar]
- Poisot, T. , Mouquet, N. & Gravel, D. (2013). Trophic complementarity drives the biodiversity – ecosystem functioning relationship in food webs. Ecol. Lett., 16, 853–861. [DOI] [PubMed] [Google Scholar]
- Post, D.M. (2002). The long and short of food chain length. Trends Ecol. Evol., 17, 269–277. [Google Scholar]
- Radchuk, V. , De Laender, F. , Sarmento Cabral, J. , Boulangeat, I. , Crawford, M. , Bohn, F.J. , et al. (2019). The dimensionality of stability depends on disturbance type. Ecol. Lett., 22, 674–684. [DOI] [PubMed] [Google Scholar]
- Rip, J.M.K. & Mccann, K.S. (2011). Cross‐ecosystem differences in stability and the principle of energy flux. Ecol. Lett., 14, 733–740. [DOI] [PubMed] [Google Scholar]
- Rooney, N. & McCann, K.S. (2012). Integrating food web diversity, structure and stability. Trends Ecol. Evol., 27, 40–45. [DOI] [PubMed] [Google Scholar]
- de Ruiter, P.C. , Neutel, A.‐M. & Moore, J.C. (1995). Energetics, patterns of interaction strengths, and stability in real ecosystems. Science, 80(269), 1257–1260. [DOI] [PubMed] [Google Scholar]
- Sabo, J.L. , Finlay, J.C. , Kennedy, T. & Post, D.M. (2010). The role of discharge variation in scaling of drainage area and food chain length in rivers. Science, 330, 965–7. [DOI] [PubMed] [Google Scholar]
- Schwarz, B. , Barnes, A.D. , Thakur, M.P. , Brose, U. , Ciobanu, M. , Reich, P.B. , et al. (2017). Warming alters energetic structure and function but not resilience of soil food webs. Nat. Clim. Chang., 7, 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, G.B. , Finke, D.L. & Snyder, W.E. (2008). Predator biodiversity strengthens aphid suppression across single‐ and multiple‐species prey communities. Biol. Control, 44, 52–60. [Google Scholar]
- Soliveres, S. , Van Der Plas, F. , Manning, P. , Prati, D. , Gossner, M.M. , Renner, S.C. , et al. (2016). Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature, 536, 456–459. [DOI] [PubMed] [Google Scholar]
- Srivastava, D.S. & Bell, T. (2009). Reducing horizontal and vertical diversity in a foodweb triggers extinctions and impacts functions. Ecol. Lett., 12, 1016–1028. [DOI] [PubMed] [Google Scholar]
- Thébault, E. & Loreau, M. (2005). Trophic interactions and the relationship between species. Am. Nat., 166, E95–E114. [DOI] [PubMed] [Google Scholar]
- Tilman, D. , Isbell, F. & Cowles, J.M. (2014). Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst., 45, 471–493. [Google Scholar]
- Turney, S. & Buddle, C.M. (2016). Pyramids of species richness: the determinants and distribution of species diversity across trophic levels. Oikos, 125, 1224–1232. [Google Scholar]
- Ushio, M. , Hsieh, C.H. , Masuda, R. , Deyle, E.R. , Ye, H. , Chang, C.W. , et al. (2018). Fluctuating interaction network and time-varying stability of a natural fish community. Nature, 554, 360–363. [DOI] [PubMed] [Google Scholar]
- Wang, S. & Brose, U. (2018). Biodiversity and ecosystem functioning in food webs: the vertical diversity hypothesis. Ecol. Lett., 21, 9–20. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Brose, U. & Gravel, D. (2019). Intraguild predation enhances biodiversity and functioning in complex food webs. Ecology, 10, e02616. [DOI] [PubMed] [Google Scholar]
- van Wijngaarden, R.P.A. , van den Brink, P.J. , Crum, S.J.H. , Peter, T.C.M.B. , Voshaar, L. & Voshaar, O.J.H. (1996). Effects of the insecticide Dursban(R) 4E (active ingredient chlorpyrifos) in outdoor experimental ditches. 1. Comparison of short‐term toxicity between the laboratory and the field. Environ. Toxicol. Chem., 15, 1133–1142. [Google Scholar]
- Williams, R.J. , Berlow, E.L. , Dunne, J.A. , Barabasi, A.‐L. & Martinez, N.D. (2002). Two degrees of separation in complex food webs. Proc. Nat. Acad. Sci., 99, 12913–12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, G. , Ebenman, B. , Emmerson, M. , Montoya, J.M. , Olesen, J.M. , Valido, A. , et al. (2005). Body size in ecological networks. Trends Ecol. Evol., 20, 402–409. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Yan, C. , Zhao, Q. , Holyoak, M. & Fortuna, M.A. (2018). Forest Ecology and Management Ecological succession drives the structural change of seed‐rodent interaction networks in fragmented forests. For. Ecol. Manage., 419, 42–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the results are archived at Dryad digital repository (https://doi.org/10.5061/dryad.t46kr73). The model code is archived at GitHub (https://github.com/qhz2019/stability).


