Abstract
Background:
Associations between smoking and health-related quality of life (HRQoL) in the general population remain unclear. The aim of the study was to quantify the independent associations between smoking and HRQoL.
Methods:
A cross-sectional population-based study was conducted on a total sample of 2197 participants obtained by multistage sampling to investigate the associations between smoking and HRQoL in the general population of southeast and southwest of Iran, aged 18–100 years in 2012–2013. Data were collected using a self-administrated of the 36-Item Short Form Survey (SF-36) questionnaire. Linear regression analyses were used to evaluate the associations between HRQoL and smoking while adjusting for various socioeconomic variables. In this study, P < 0.05 was considered a significant difference.
Results:
Out of the total of 2197 participants, current smokers and never smokers accounted for 13% and 87%, respectively. The mean HRQoL indices were for the current smokers 66.66 ± 17.86, and never smokers 71.35 ± 18.47 (P < 0.001). Independent associations between smoking and HRQoL were found, including negative associations (P < 0.001). The multivariate associations between smoking status and HRQoL, male smokers had a lower physical functioning, mental health, and total SF-36 score.
Conclusions:
Smoking was independently related to HRQoL, with large differences according to the gender. This study showed that there is a significant difference in the quality of life related to health in male smokers compared to male nonsmokers.
Keywords: Cigarette smoking, Iran, quality of life, sex
Introduction
Smoking is a significant health-economic problem and one of the most significant health threats to individuals. Smoking is the most preventable cause of mortality worldwide. Based on reported the World Health Organization (WHO) in 2008, tobacco-related deaths accounted for 5 million people, which will touch 8 million by 2030. In addition to high mortality, smoking imposes many costs on society.[1,2] Based on the WHO in 2010, tobacco was the second leading cause of death and the fourth-most significant cause of the incidence disease worldwide.[3] Based on recent estimates, approximately one-third of the world's population is smoking.[4] As in advanced countries, approximately 35% of men and 22% of women and in developing countries, approximately 50% of men and 9% of women are smoking.[5] Approximately 84% of the world's smokers live in the developing countries, which is around 1.3 billion people.[6] The prevalence of smoking in Iran has been studied in various studies and various aspects. In the meta-analysis study of Amin Esmaeili et al., the prevalence of cigarette smoking in different regions was reported between 9.2 and 26.8.[7] In the study of Meysami et al., the current and daily prevalence of smoking in Iran was 12.5% (23.4% men and 1.4% women, 6.1 million people) and 11.3% (21.4% men and 1.4% women, 5.6 million people).[6] A significant aspect of smoking is health-related quality of life (HRQoL). Smoking not only causes mortality but also affects people's quality of life. For example, in England, it is estimated that smoking accounts for 19% (27% men and 11% women) of all mortalities in 2002, and also it directly accounts for 12% of years lost due to disability.[8] Recently, only a few studies have examined the relationship between smoking and HRQoL in the general population. These studies also considerably vary in methods of measuring the HRQoL and smoking status.[1,9] Considering that there is insufficient knowledge approximately the association between smoking and HRQoL in the general population, and there has not been a comprehensive study in Iran so far, therefore, this study aimed to investigate the association between smoking and HRQoL in the general population.
Methods
A cross-sectional population-based study was conducted to investigate the associations between smoking and HRQoL in the general population of Shiraz and Zabol, Iran (Southeast and Southwest of Iran), during 2012–2013. The sample size using formula was determined at 1080. Since the cluster sampling was used in the second step of the sampling, the sample size was determined with considering of design effect = 2, in result sample size calculated 2160. Assuming the nonresponse or incomplete responses have final sample was increased to 2197. Using a multistage sampling method of clustering, a random sample of 2197 individuals aged 18 years and over who resided in Shiraz and Zabol was selected. In this survey, sampling was performed in four stages. First, districts were defined as strata and the sample size was determined proportionate to each stratum's population. Through random sampling in the next stage, each of the areas was divided into blocks. Afterward, the households were selected by the systematic sampling. If someone refused to take part in the research or was unable to answer the questions because of a language barrier, he/she was excluded from the study [Figure 1]. Face-to-face interviews were done to collect data.
Figure 1.
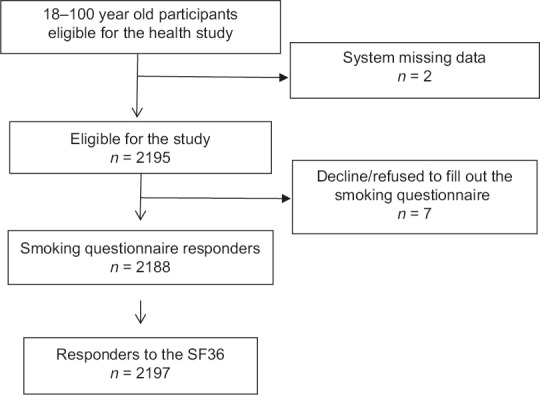
Flowchart of the study design
Two questionnaires were used to collect data. The first one included questions regarding basic demographic data such as age, gender, years of education, marital status, occupational status, income (per household unit per month), family size, insurance status, type of insurance, supplemental insurance, and cigarette smoking status. A cigarette smoker in this study was defined as a person who answered “yes” to the following question: “Are you currently a cigarette smoker?”
The second questionnaire was the 36-Item Short Form Survey (SF-36). The Persian version of the SF-36 questionnaire was used in this study, and this had previously been translated from English and validated.[10] The SF-36 is a well-recognized, self-administered quality of life scoring system. It consists of eight independent scales and two major dimensions. The eight multi-item scales include physical functioning (PF), role-physical, bodily pain, general health (GH), vitality, social functioning, role emotional, and mental health (MH). Each question is rated on an ordinal scale with 2–6 categories; the score of each dimension is the sum of the item scores of the related dimension further normalized to a score of 0–100, with higher values representing better perceived HRQoL. The first five scales are summarized into the physical health dimension and the last three scales into the MH dimension.[11]
Statistical analysis was done using the Statistics/Data Analysis software, STATA (Version 14, Stata Corporation, College Station, TX, USA). The results were expressed as mean values ± standard deviations and proportions, as appropriate. The Chi-square test was used to compare between smokers and nonsmokers relating to each variable of sociodemographic characteristics. The independent samples t-test was used to compare scores of HRQoL in smokers and nonsmokers.
The general strategy for disentangling the independent relationship of smoking with HRQoL from the confounding of sociodemographic consisted in the construction of regression models. Linear regression models were therefore constructed in two steps. First, models were constructed including only the smoking variable. Second, sociodemographic variables were also introduced to predictors identified as significant by the univariate analysis with smoking tested. Due to large differences in the associations between smoking and HRQoL according to gender, all analyses were stratified by gender. All tests assumed a two-sided alternative hypothesis, a 0.05 significance level.
Results
Overall 2197 individuals to be included in the study. Demographic and socioeconomic characteristics, marital status, and insurance are presented according to smoking status [Table 1]. Most females were never smokers, whereas in males were 18.46% current smokers. Individuals of younger age, less educated, technical workers, and worker occupation with lower income at the time of the survey were more often smokers.
Table 1.
Characteristics of 18-88-year-old individuals included in the study (n=2197), according to smoking status
| Total (n=2197), n* (%) | Current smoker (n=284), n (%) | Nonsmoker (n=1904), n (%) | P | |
|---|---|---|---|---|
| Gender | ||||
| Male | 1184 (53.94) | 218 (18.54) | 958 (81.46) | <0.001 |
| Female | 1011 (46.06) | 66 (6.53) | 945 (93.47) | |
| Age (years) | ||||
| 18-29 | 878 (40.18) | 94 (10.73) | 782 (89.27) | 0.001 |
| 30-39 | 441 (20.18) | 50 (11.42) | 388 (88.58) | |
| 40-49 | 374 (17.12) | 52 (13.98) | 320 (86.02) | |
| 50-59 | 278 (12.72) | 49 (17.63) | 229 (82.37) | |
| 60-65 | 103 (4.71) | 24 (23.30) | 79 (76.70) | |
| Upper 65 | 111 (5.08) | 12 (10.91) | 98 (89.09) | |
| Education (years) | ||||
| Lower than 5 | 179 (8.20) | 28 (15.82) | 149 (84.18) | 0.01 |
| 5 | 171 (7.83) | 22 (12.87) | 149 (87.13) | |
| 6-8 | 230 (10.53) | 36 (15.86) | 191 (84.14) | |
| 9-12 | 687 (31.46) | 104 (15.16) | 582 (84.84) | |
| Upper than 12 | 917 (41.99) | 93 (10.16) | 822 (89.84) | |
| Occupation | ||||
| Self-employed | 186 (8.56) | 30 (16.22) | 155 (83.78) | <0.001 |
| Staff | 454 (20.88) | 63 (13.97) | 388 (86.03) | |
| Technical workers | 65 (2.99) | 20 (30.77) | 45 (69.23) | |
| Worker | 106 (4.88) | 41 (39.05) | 64 (60.95) | |
| Housewife | 567 (26.08) | 27 (4.77) | 539 (95.23) | |
| Students | 429 (19.73) | 32 (7.48) | 396 (92.52) | |
| Unemployment or retired | 124 (5.70) | 22 (17.83) | 102 (82.26) | |
| Other | 243 (11.18) | 48 (19.83) | 194 (80.17) | |
| Marital status | ||||
| Married | 1386 (63.29) | 187 (13.56) | 1192 (86.44) | <0.001 |
| No spouse (divorce, separation, death) | 167 (7.63) | 20 (11.98) | 147 (88.02) | |
| Never married | 637 (29.09) | 76 (11.95) | 560 (88.05) | |
| Income (per household unit per month, “IRR”)** | ||||
| <500 | 782 (35.59) | 106 (13.57) | 675 (86.43) | 0.11 |
| 500-1000 | 950 (43.24) | 132 (13.94) | 815 (86.06) | |
| >1000 | 221 (10.06) | 26 (11.82) | 194 (88.18) | |
| Not mentioned | 244 (11.11) | 20 (8.33) | 220 (91.67) | |
| Family size | ||||
| ≤4 | 1264 (58.30) | 157 (12.44) | 1105 (87.56) | 0.52 |
| ≥5 | 904 (41.70) | 120 (13.36) | 778 (86.64) | |
| Insurance status | ||||
| Yes | 1876 (86.21) | 226 (12.10) | 1642 (87.90) | 0.003 |
| No | 300 (13.79) | 55 (18.33) | 245 (81.67) | |
| Type of insurance | ||||
| Social security insurance | 938 (49.58) | 124 (13.26) | 811 (86.74) | 0.07 |
| Health insurance | 587 (31.03) | 73 (12.50) | 511 (87.50) | |
| Armed forces of insurance | 149 (7.88) | 10 (6.76) | 138 (93.24) | |
| Other | 218 (11.52) | 20 (9.22) | 197 (90.78) | |
| Supplementary insurance | ||||
| Yes | 674 (33.68) | 93 (13.84) | 579 (86.16) | 0.73 |
| No | 1327 (66.32) | 176 (13.28) | 1149 (86.72) |
*There are missing data in some variables, **Amounts are in 10,000 Rials (1 US dollar=32,000 Islamic Republic of Iran’s Rials). IRR=Iranian Rial
The mean scores of SF-36 questionnaire are shown in Table 2. Table 2 indicates, there were statistically significant differences between current smoker and nonsmokers on all scales (P < 0.001). Table 3 shows the multivariate associations between smoking status and HRQoL, stratified by gender. The first striking result is that the number and the magnitude of the associations identified as significant in women were very substantially reduced by adjustments for socioeconomic variables (models B). However, male smokers had lower PF, MH and total SF-36 score. So that, on average PF in smokers 3.07, the MH 3.78 and total SF-36 score 3.58 has decreased (Model B, in men).
Table 2.
Comparison of Short Form Health Survey scores of the participants in terms of smoking
| Current smoke (n=284) | No smoker (n=1904) | P* | |
|---|---|---|---|
| Physical health (mean±SD) | 66.51±19.42 | 71.17±19.81 | <0.001 |
| Mental health (mean±SD) | 65.04±16.65 | 69.44±17.53 | <0.001 |
| Total SF-36 score (mean±SD) | 66.66±17.86 | 71.35±18.47 | <0.001 |
*Two sample independent t-test. SD=Standard deviation, SF-36=36- Item Short Form Survey
Table 3.
Multivariate associations between smoking status and health-related quality of life (Short Form Health Survey scales), stratified by gender
| Scale | Model | Smoking | Adjusted R2 for Model B | |
|---|---|---|---|---|
| Current smoke | No smoker | |||
| Men | ||||
| Physical health |
A | −5.32 (−8.10-−2.55) |
Reference | |
| B | −3.07 (−5.91-−0.22) |
0.187 | ||
| Mental health |
A | −5.09 (−7.65-−2.53) |
Reference | |
| B | −3.78 (−6.52-−1.03) |
0.128 | ||
| Total SF-36 score |
A | −5.31 (−7.93-−2.69) |
Reference | |
| B | −3.58 (−6.31-−0.84) |
0.163 | ||
| Women | ||||
| Physical health |
A | −10.90 (−15.89-−5.92) |
Reference | |
| B | −3.24 (−8.97-2.49) |
0.178 | ||
| Mental health | A | −8.06 (−12.32-−3.80) |
Reference | |
| B | −2.92 (−8.12-2.27) |
0.06 | ||
| Total SF-36 score |
A | −10.09 (−14.69-−5.50) |
Reference | |
| B | −2.93 (−8.31-2.46) |
0.137 | ||
Model A=Estimates (95% CI) unadjusted for the smoking variable, Model B=Estimates (95% CI) adjusted for socioeconomic variables (insurance status, marital status, occupation, education, income, age, and type of insurance). CI=Confidence interval, SF-36=36-Item Short Form Survey
Discussion
In this study, the association between smoking and HRQoL was studied in the general population of Shiraz and Zabol cities in the age group of 18–88 years. The results of this study showed that there is a statistically significant difference between the mean of mental and physical health and the overall quality of life among smokers and nonsmokers (P < 0.001). In men, physical and MH, and quality of life in smokers were lower than nonsmokers, and there was a statistically significant relationship between smoking and HRQoL. This statistic relationship was significant after adjusting the socioeconomic status. In women, there was a significant statistical relationship between smoking status and physical, MH and HRQoL before adjusting the socioeconomic status, while after adjusting the socioeconomic variable, this relationship was not statistically significant.
In this study, the prevalence of smoking was 18.5% in men and 6.5% in women. The prevalence of smoking in Iran has been studied in several studies, including the study of Aghamolaii and Zare in which the prevalence of smoking in men was 22.7% and in women 0.9%,[12] in the study of Fotouhi et al. in which the prevalence of smoking in men was 20.6 and in women 2.9%,[13] also the study of Salimzadeh et al. showed that the prevalence of smoking was 15.5% in men and 0.8% in women.[14] The prevalence of smoking was 23.4% in men and 1.4% in women,[6] based on the national estimates in Iran in 2011. Based on the WHO's latest report, the prevalence of smoking in Iranian adults is 22.4% for men and 1% for women.[15] Furthermore, the prevalence of smoking in adolescents aged 6–18 years in boys is 7.5% and 4.2% for girls.[16] The prevalence of smoking in this study was higher in males than in other studies, and the average is lower in the country but higher in women. The reasons for the difference in the prevalence of smoking in different studies can be the difference in the age groups under study as well as the sociocultural and family norms dominating on the different populations under the study.
The results of this study showed that the average mental and physical health and the general quality of life in smokers are lower than nonsmokers, and this difference is statistically significant,[17] which was consistent with the results of Tavafian et al. and Schmitz et al.[18,19] In the study of Laaksonen et al., MH, physical performance and overall health in smokers were lower compared to nonsmokers. In addition, in the study of Castro et al., the quality of life of smokers was lower than those who never smoked, and the average scales related to physical, mental aspects, and social relations in smokers were lower than nonsmokers.[20] In this regard, findings of the study conducted by Wilson et al. on smoking and quality of life showed that quality of life in smokers was lower compared to nonsmokers.[21] The higher prevalence of chronic diseases in smokers compared to nonsmokers can be one of the significant reasons for lower quality of life in smokers. One of these diseases is depression. The relationship between depression and smoking is well-known[22,23] and studies have shown that smoking is significantly higher in patients with mental disorders, especially in intense cases.[24,25,26,27] Hence, the higher prevalence of depression in smokers can be one of the reasons for the decline in their quality of life compared to nonsmokers. In addition, the results of various studies have shown that smokers have less physical activity than nonsmokers,[28,29] which can increase the chances of developing a variety of chronic diseases such as cardiovascular disease in smokers and consequently, results in the decline of physical and MH and a decline in HRQoL and life expectancy.[28] In addition, various epidemiological studies have shown the simultaneous consumption of alcohol and smoking.[30,31] Therefore, in addition to high rates of chronic illness in smokers, alcohol addiction in smokers can also be associated with a decline in quality of life.
Based on the findings of the present study, smoking in men is related to a decline in quality of life. Even after adjusting the socioeconomic status (insurance status, marital status, occupation, education level, income, age, and type of insurance), this relationship was statistically significant in men, which is consistent with the results of the study of Džubur et al.[32] Smoking and socioeconomic status in men accounted for 18.7% of changes in physical health, 12.8% of changes in MH and in general, and 16.3% of the quality of life. In the present study, there was a significant statistical relationship between smoking and physical and mental dimensions of quality of life in women, while there was no relationship after adjusting socioeconomic status. In this regard, the results of Coste et al.[9] and Heikkinen et al.[33] showed that the relationship between smoking and quality of life in women was weaker than men. Coste et al. showed that in women, there is no relationship between smoking and the physical aspects of quality of life. In the study of Samardzić and Marvinac, smokers in their different age groups assessed their GH better than nonsmokers.[34]
The lack of relationship between smoking and quality of life after adjusting socioeconomic status indicates the role of socioeconomic status as a confounding variable in the study of the relationship between smoking and quality of life in women. The observed difference in the quality of life related to health in men and women is particularly significant because it has been shown that women are more sensitive to the effects of smoking than men[35,36] and underlying diseases are more in women.[37] One of the possible reasons for the difference in the effect of smoking on the quality of life of men and women in the younger age of women participating in this study compared to men.
Conclusions
In this large representative study, smoking was found to be independently related to HRQoL. The results of this study showed that there is a significant difference in the quality of life related to health in male smokers compared to male nonsmokers. Although the results of the present study did not show a significant difference in the quality of life related to health between female smokers and female nonsmokers, this could indicate the significance and effect of socioeconomic factors on women's quality of life and health.
This study is a cross-sectional study, and thus, there is a fundamental limitation when making cause and effect arguments. In addition, the correlation between depression and alcohol addiction with smoking, in order to prevent confounding factors in the study of the relationship between smoking and HRQoL, it was better to control the alcohol addiction and depression in individuals participating in the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in english general population: Implications for economic evaluations. BMC Public Health. 2012;12:203. doi: 10.1186/1471-2458-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Brazil: World Health Organization; 2008. [Google Scholar]
- 3.Peykari NF, Tehrani FR, Afzali HM, Dovvon MR, Djalalinia SS. Smoking habits among Iranian general practitioners. J Egypt Public Health Assoc. 2010;85:97–112. [PubMed] [Google Scholar]
- 4.Slama K. Current challenges in tobacco control. Int J Tuberc Lung Dis. 2004;8:1160–72. [PubMed] [Google Scholar]
- 5.Meysamie A, Ghaletaki R, Zhand N, Abbasi M. Cigarette smoking in Iran. Iran J Public Health. 2012;41:1–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Meysamie A, Ghaletaki R, Haghazali M, Asgari F, Rashidi A, Khalilzadeh O, et al. Pattern of tobacco use among the Iranian adult population: Results of the National Survey of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007) Tob Control. 2010;19:125–8. doi: 10.1136/tc.2009.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin-Esmaeili M, Rahimi-Movaghar A, Sharifi V, Hajebi A, Radgoodarzi R, Mojtabai R, et al. Epidemiology of illicit drug use disorders in Iran: prevalence, correlates, comorbidity and service utilization results from the Iranian Mental Health Survey. Addiction. 2016;111:1836–47. doi: 10.1111/add.13453. [DOI] [PubMed] [Google Scholar]
- 8.Allender S, Balakrishnan R, Scarborough P, Webster P, Rayner M. The burden of smoking-related ill health in the UK. Tob Control. 2009;18:262–7. doi: 10.1136/tc.2008.026294. [DOI] [PubMed] [Google Scholar]
- 9.Coste J, Quinquis L, D’Almeida S, Audureau E. Smoking and health-related quality of life in the general population. Independent relationships and large differences according to patterns and quantity of smoking and to gender. PLoS One. 2014;9:e91562. doi: 10.1371/journal.pone.0091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B. The short form health survey (SF-36): Translation and validation study of the Iranian version. Qual Life Res. 2005;14:875–82. doi: 10.1007/s11136-004-1014-5. [DOI] [PubMed] [Google Scholar]
- 11.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Aghamolaii T, Zare S. Pattern of cigarette and water pipe use among people above 15 years in Bandarabas. Hormozgan Univ Med J. 2008;11:241–6. [Google Scholar]
- 13.Fotouhi A, Khabazkhoob M, Hashemi H, Mohammad K. The prevalence of cigarette smoking in residents of Tehran. Arch Iran Med. 2009;12:358–64. [PubMed] [Google Scholar]
- 14.Salimzadeh H, Najafipour H, Mirzaiepour F, Navadeh S, Shadkam-Farrokhi M, Mirzazadeh A, et al. Prevalence of active and passive smoking among adult population: Findings of a population-based survey in Kerman (KERCADRS), Iran. Addict Health. 2016;8:16–24. [PMC free article] [PubMed] [Google Scholar]
- 15.Boer K, Smit BJ, van Huis AM, Hogerzeil HV. Substance use in pregnancy: Do we care? Acta Paediatr Suppl. 1994;404:65–71. doi: 10.1111/j.1651-2227.1994.tb13386.x. [DOI] [PubMed] [Google Scholar]
- 16.Bowman J, Wiggers J, Colyvas K, Wye P, Walsh RA, Bartlem K, et al. Smoking cessation among Australian methadone clients: Prevalence, characteristics and a need for action. Drug Alcohol Rev. 2012;31:507–13. doi: 10.1111/j.1465-3362.2011.00408.x. [DOI] [PubMed] [Google Scholar]
- 17.Laaksonen M, Rahkonen O, Martikainen P, Karvonen S, Lahelma E. Smoking and SF-36 health functioning. Prev Med. 2006;42:206–9. doi: 10.1016/j.ypmed.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Tavafian SS, Aghamolaei T, Zare S. Water pipe smoking and health-related quality of life: A population-based study. Arch Iran Med. 2009;12:232–7. [PubMed] [Google Scholar]
- 19.Schmitz N, Kruse J, Kugler J. Disabilities, quality of life, and mental disorders associated with smoking and nicotine dependence. Am J Psychiatry. 2003;160:1670–6. doi: 10.1176/appi.ajp.160.9.1670. [DOI] [PubMed] [Google Scholar]
- 20.Castro MR, Matsuo T, Nunes SO. Clinical characteristics and quality of life of smokers at a referral center for smoking cessation. J Bras Pneumol. 2010;36:67–74. doi: 10.1590/s1806-37132010000100012. [DOI] [PubMed] [Google Scholar]
- 21.Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Prev Med. 1999;29:139–44. doi: 10.1006/pmed.1999.0523. [DOI] [PubMed] [Google Scholar]
- 22.Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking. A longitudinal investigation. Arch Gen Psychiatry. 1998;55:161–6. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- 23.Tsuang MT, Francis T, Minor K, Thomas A, Stone WS. Genetics of smoking and depression. Hum Genet. 2012;131:905–15. doi: 10.1007/s00439-012-1170-6. [DOI] [PubMed] [Google Scholar]
- 24.Waxmonsky JA, Thomas MR, Miklowitz DJ, Allen MH, Wisniewski SR, Zhang H, et al. Prevalence and correlates of tobacco use in bipolar disorder: Data from the first 2000 participants in the systematic treatment enhancement program. Gen Hosp Psychiatry. 2005;27:321–8. doi: 10.1016/j.genhosppsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Kotov R, Guey LT, Bromet EJ, Schwartz JE. Smoking in schizophrenia: Diagnostic specificity, symptom correlates, and illness severity. Schizophr Bull. 2010;36:173–81. doi: 10.1093/schbul/sbn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 28.de Miguel Díez J, Esteban y Peña MM, Puente Maestu L, Hernández Barrera V, Carrasco Garrido P, Alvarez-Sala Walther LA, et al. Relationship between tobacco consumption and health-related quality of life in adults living in a large metropolitan area. Lung. 2010;188:393–9. doi: 10.1007/s00408-010-9256-1. [DOI] [PubMed] [Google Scholar]
- 29.Strine TW, Okoro CA, Chapman DP, Balluz LS, Ford ES, Ajani UA, et al. Health-related quality of life and health risk behaviors among smokers. Am J Prev Med. 2005;28:182–7. doi: 10.1016/j.amepre.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health. 2000;24:201–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Falk DE, Yi HY, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the national epidemiologic survey on alcohol and related conditions. Alcohol Res Health. 2006;29:162–71. [PMC free article] [PubMed] [Google Scholar]
- 32.Džubur A, Mehić B, Džubur A, Filipovska-Mušanović M, Denjalić A, Hasanbegović I, et al. Quality of life for tobacco smokers in relation to their socioeconomic status. Med Glas (Zenica) 2014;11:210–5. [PubMed] [Google Scholar]
- 33.Heikkinen H, Jallinoja P, Saarni SI, Patja K. The impact of smoking on health-related and overall quality of life: A general population survey in Finland. Nicotine Tob Res. 2008;10:1199–207. doi: 10.1080/14622200802163142. [DOI] [PubMed] [Google Scholar]
- 34.Samardzić S, Marvinac GV. Health related quality of life of smokers in croatia. Coll Antropol. 2009;33(Suppl 1):107–14. [PubMed] [Google Scholar]
- 35.Legleye S, Khlat M, Beck F, Peretti-Watel P. Widening inequalities in smoking initiation and cessation patterns: A cohort and gender analysis in France. Drug Alcohol Depend. 2011;117:233–41. doi: 10.1016/j.drugalcdep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine Tob Res. 1999;1:301–15. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 37.Mucha L, Stephenson J, Morandi N, Dirani R. Meta-analysis of disease risk associated with smoking, by gender and intensity of smoking. Gend Med. 2006;3:279–91. doi: 10.1016/s1550-8579(06)80216-0. [DOI] [PubMed] [Google Scholar]


