Abstract
Background
Activated eosinophils cause major pathology in stable and exacerbating asthma; however, they can also display protective properties like an extracellular antiviral activity. Initial murine studies led us to further explore a potential intracellular antiviral activity by eosinophils.
Methods
To follow eosinophil‐virus interaction, respiratory syncytial virus (RSV) and influenza virus were labeled with a fluorescent lipophilic dye (DiD). Interactions with eosinophils were visualized by confocal microscopy, electron microscopy, and flow cytometry. Eosinophil activation was assessed by both flow cytometry and ELISA. In a separate study, eosinophils were depleted in asthma patients using anti‐IL‐5 (mepolizumab), followed by a challenge with rhinovirus‐16 (RV16).
Results
DiD‐RSV and DiD‐influenza rapidly adhered to human eosinophils and were internalized and inactivated (95% in ≤ 2 hours) as reflected by a reduced replication in epithelial cells. The capacity of eosinophils to capture virus was reduced up to 75% with increasing severity of asthma. Eosinophils were activated by virus in vitro and in vivo. In vivo this correlated with virus‐induced loss of asthma control.
Conclusions
This previously unrecognized and in asthma attenuated antiviral property provides a new perspective to eosinophils in asthma. This is indicative of an imbalance between protective and cytotoxic properties by eosinophils that may underlie asthma exacerbations.
Keywords: CD69, exacerbation, influenza, rhinovirus_16, RSV
1,19 – dioctadecyl ‐ 3, 3, 39, 39 – tetramethylindocarbocyanine (DiD)‐respiratory syncytial virus and DiD‐influenza adhered to human eosinophils. Both viruses were internalized and inactivated by eosinophils. The capacity of eosinophils to capture virus was reduced with increasing severity of asthma. IL‐5 Tg: IL‐5 transgenic; RSV: Respiratory syncytial virus; WT: Wild‐type

1. INTRODUCTION
Asthma is a chronic but heterogeneous inflammatory airway disease characterized by episodes of variable symptoms like wheezing, coughing, chest tightness, and shortness of breath.1 Patients can experience periods of acute worsening of their asthma symptoms (exacerbations), which are predominantly triggered by respiratory viral infections, and to a lesser extent by allergens and air pollution.2, 3 Exacerbations are paralleled by increased airway inflammation. These symptoms severely impact the patient's quality of life and even are life‐threatening in severe asthma. Corticosteroids, the cornerstone in anti‐inflammatory treatment of asthma, are not always effective during exacerbations and in severe asthma, despite enhanced doses. Therefore, there is a need for specific interventions tailored to the underlying inflammatory process. This was illustrated by the recent finding that eosinophil depletion by monoclonal anti‐IL‐5 antibody (eg, mepolizumab) treatment in moderate to severe eosinophilic asthma attenuated corticosteroid use and reduced exacerbations,4, 5 the latter of which was also shown for treatment with anti‐IL‐5 receptor antibody.6
Airway eosinophilia is a hallmark of allergic asthma and of severe nonatopic late‐onset asthma. It has been associated with both allergen‐ and respiratory virus‐induced asthma exacerbations, airway hyperresponsiveness, and remodeling.7 In asthma, eosinophils are considered predominantly pro‐inflammatory and contributing to tissue damage by the release of specific cytotoxic granular constituents and reactive oxygen species.8, 9 However, earlier and recent studies have provided further support to earlier claims that eosinophils can also display protective regulatory functions in asthma.13, 14 Eosinophils have been found to accumulate in airways of asthma patients during virus‐induced exacerbations suggesting that eosinophils may also be actively involved in the antiviral response. Extracellular eosinophil‐derived neurotoxin (EDN) displays RNAse activity and can enter viral capsids to degrade RNA from respiratory syncytial virus (RSV).13, 16, 17 In vivo relevance of this activity was shown in eotaxin2/IL‐5 double transgenic (tg) mice that were protected against a lethal pneumonia virus infection.16 Apparently in line with these findings, we recently found that anti‐IL‐5‐mediated depletion of eosinophils resulted in enhanced influenza X31 viral loads in house dust mite‐sensitized mice, whereas the enhanced morbidity was reduced.20
These initial findings urged us to further study the antiviral activity of eosinophils. Here, we show that mouse and human eosinophils efficiently capture and rapidly reduce infectivity of respiratory viruses. In line herewith, we21 showed that depletion of eosinophils by anti‐IL‐5 treatment significantly enhanced viral loads in airways from asthma patients challenged with rhinovirus 16 (RV16). Intriguingly, we here found that DiD‐labeled virus interacted less with eosinophils from asthma patients compared to those from healthy controls, which may lead to a less effective virus inactivation. These findings support a newly recognized antiviral activity of eosinophils and place the role of eosinophils during virus‐induced asthma exacerbations in a new perspective.
2. RESULTS
2.1. Murine lung eosinophils capture influenza virus and are activated in vivo
In a mouse model of eosinophilia (IL‐5 transgenic (tg) mice), recovery after infection with a nonlethal dose of influenza (PR8) was faster as compared to controls (WT), as was apparent from the reduced weight loss, a surrogate marker of inflammation (Figure 1A). In concordance, the pulmonary viral load at day 8, but not yet at the viral peak at day 6, was significantly lower than in WT mice (Figure 1B). IL‐5 tg mice have elevated blood and bronchoalveolar lavage (BAL) eosinophils at homeostasis, and upon infection, the eosinophils were recruited to the airways (Figure S1A). The BAL inflammatory profile upon influenza infection did not differ with respect to the recruitment of other immune cells (Figure S1B). By using DiD‐labeled PR8,22 purified by density gradient centrifugation, we traced virus not only to alveolar macrophages (CD11chigh CD11blow SiglF+) as was expected, but also to eosinophils (F4/80− CD11c− CD11b+ SiglF+ cells) in BAL and draining mediastinal lymph nodes (mLN) of IL‐5 tg mice (Figure 1C). The differentiation between alveolar macrophages and eosinophils is shown in Figure S1D. Within 4 hours after infection, more than 60% of the BAL eosinophils were associated with DiD‐labeled PR8 (Figure 1D), as was confirmed by confocal microscopy (Figure 1E). Part of these PR8+ eosinophils migrated to the draining mLNs (Figure 1D). Both BALF and mLN PR8+ eosinophils showed elevated expression of CD86, CD80 (only mLN), MHCII, and CCR7 compared to their PR8− counterpart (Figure S1C). So besides their potential extracellular antiviral activity, these data indicate that murine pulmonary eosinophils can also capture virus in vivo and become activated.
Figure 1.
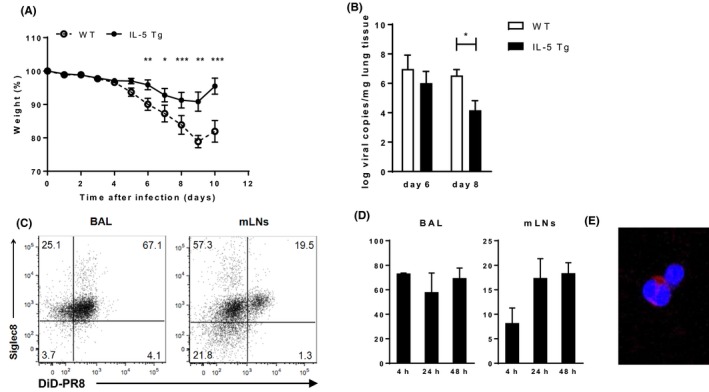
Reduced virus‐induced pathology in mice with eosinophilia. A, Weight loss of IL‐5 tg mice and controls after a PR8 infection. B, Viral load in the lungs at day 8 after PR8 infection. C, Representative flow cytometry plots of DiD‐labeled PR8 uptake by BALF and mLN eosinophils from IL‐5 tg mice. D, Quantification of DiD‐labeled PR8 in BALF (left panel) and mLNs (right panel). E, Representative confocal microscopy image of DiD‐labeled PR8 virus with BALF eosinophils. Data are representative of 2 experiments with 3 (C‐E) mice each or combined from 2 experiments with 2‐12 mice (A,B) and expressed as mean ± SEM.: 2‐way ANOVA (for A) or normal t test (B); *P < 0.05; **P < 0.01; ***P < 0.001
2.2. Rapid capture and inactivation of respiratory viruses by human eosinophils
Next, we extended these findings to human cells by exposing blood eosinophils from healthy donors to DiD‐labeled respiratory syncytial virus (RSV) and influenza (PR8) in vitro. After 2 hours coculture, for both viruses, three distinct staining patterns were observed by confocal microscopy: (a) those showing separate viral particles spread across the eosinophil membrane (diffuse staining), (b) those with predominantly clustered virus (clustered staining), and (c) few cells without virus (Figure 2A,B). Similar staining patterns were found for BAL eosinophils (Figure 2C). DiD labeling of virus itself did not lead to aggregation of the virus as shown by confocal microscopy, indicating that the viral clusters on the eosinophils result upon interaction with virus.
Figure 2.
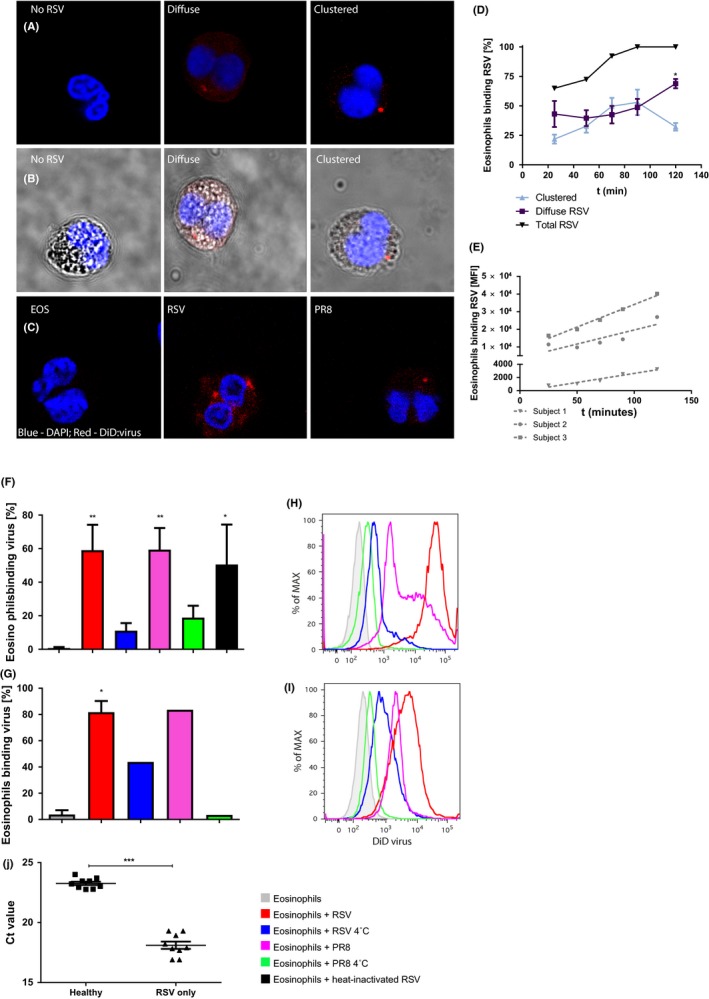
Rapid capture and inactivation of virus by human eosinophils. A, Representative confocal microscopy images of peripheral blood eosinophils incubated with DiD‐labeled RSV (red) for 2 h. B, Bright‐field images corresponding to (A). C, BALF eosinophils co‐incubated with either DiD‐labeled RSV or PR8 for 2 h. D, Patterns of virus associated with eosinophils over time, assessed by confocal microscopy (n = 4). E, RSV+ eosinophils over time, assessed by flow cytometry (G,H: n = 3). RSV (red), influenza (pink), heat‐inactivated RSV (black) binding at 37°C and at 4°C (blue) to both peripheral blood (F,H: n = 6) and BALF eosinophils (g and i: n = 2). J, RSV load in epithelial cells 24 h after exposure to lysates from eosinophils exposed for 2 h to RSV (Healthy). RSV only is RSV added directly to the epithelial cells (n = 9). Ct value of 27.89 (5000 c/PCR) refers to a virus concentration of 6250 c/mL. Data are expressed as mean ± SEM. Paired t test: *P < 0.01; **P < 0.001; ***P < 0.0001
After 25 minutes, about 70% of blood eosinophils were DiD‐positive, predominantly diffuse staining (Figure 2D). After 90 minutes, nearly all eosinophils were DiD‐positive, either diffusely or clustered, to an equal extent. At 120 minutes, most eosinophils were DiD‐positive with the majority showing diffuse staining. The number of viral particles (DiD label) associated per eosinophil over time increased as shown by increased mean fluorescence intensity, although this varies per donor (Figure 2E).
To exclude that association of DiD‐labeled virus by eosinophils is part of productive infection, eosinophils were incubated with heat‐inactivated DiD‐labeled RSV (HI:RSV) or GFP‐tagged RSV, which expresses GFP upon viral replication.23 Eosinophils and HI:RSV adhered (Figure 2F) to a similar extent as viable RSV, indicative of uptake instead of infection. Also, RSV‐GFP, which infects and replicates in epithelial cells, did not replicate in eosinophils (Figure S1). Furthermore, the capacity of RSV to infect epithelial cells was reduced by 95% when RSV was incubated with eosinophils for 2 hours (Figure 2J). Together this indicates that eosinophils rapidly capture viral particles and effectively reduce their infectivity.
Imaging of RSV‐exposed eosinophils by electron microscopy revealed viral particles with the typical RSV structure and the approximate size (120‐300 nm) within eosinophils, but only in the cytoplasm and not enclosed by a membrane (Figure 3). Combined these findings confirm uptake of viral particles by eosinophils.
Figure 3.
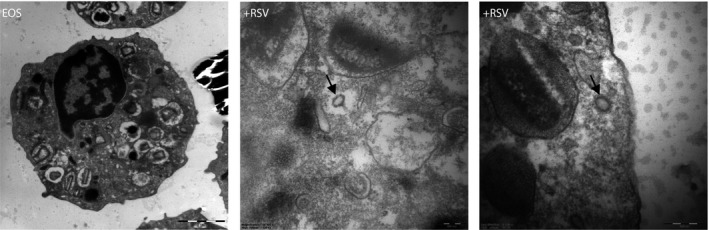
Representative EM images of eosinophils incubated with RSV (MOI 10). Intact RSV particles were found free in the cytoplasm but never surrounded by a plasma membrane (left panel)
2.3. Eosinophils from asthma patients capture less DiD‐labeled virus
As respiratory viral infections in asthma patients trigger acute worsening of asthma symptoms, we set out to determine how the antiviral properties of eosinophils from asthma patients compared to those from healthy controls. Strikingly, blood eosinophils from mild to severe asthma patients (Table S1) displayed reduced levels of DiD‐labeled RSV. Whereas more than 85% of eosinophils from healthy donors bound RSV, eosinophils from mild to moderate asthmatics could be divided into two subgroups: either binding identical to those from controls or up to a 40% reduced binding (Figure 4A). Interestingly, eosinophils showing binding similar to those from controls were all derived from patients using inhaled corticosteroids. In severe asthmatics, all on either high doses of inhaled corticosteroids or oral corticosteroids, a further reduced capacity by eosinophils to bind virus was observed. In some donors, only 20% of eosinophils bound RSV while others showed a moderate reduced binding capacity compared to that of healthy controls. To clarify whether eosinophils from asthma patients also display an aberrant capacity to reduce infectivity of virus, epithelial cells were exposed to a cell lysate of eosinophils from severe asthmatics and healthy controls exposed for 2 hours to RSV. Eosinophils from healthy controls reduced RSV infectivity (Figure 4B; P < 0.0001), and possibly slightly less by eosinophils from patients, as compared to epithelial cells directly infected with RSV (RSV only). In our analysis, we also considered that some severe asthma patients used oral corticosteroids (n = 4) (n = 3 for virus infectivity) or only inhaled corticosteroids (n = 5) (n = 4 for virus infectivity). We stratified the severe group by OCS use, and we examined a significant difference between severe asthmatics without OCS (−OCS) and healthy individuals (Figure S5A), but we do not see this in severe asthmatics on OCS (+OCS) and healthy subjects. There is also no difference between severe asthmatics (total group) and healthy subjects. Virus binding, however, is independent of OCS use (Figure S5B).
Figure 4.
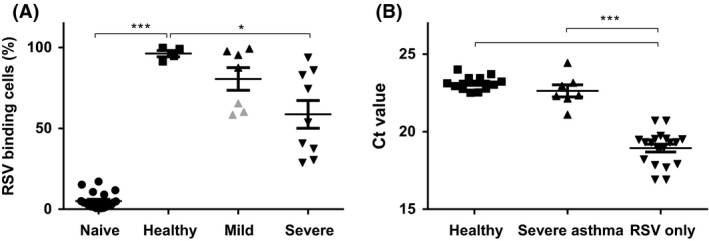
Eosinophils from asthma patients have a reduced capacity to bind and inactivate virus, but still inactivate virus in vivo (A). DiD‐labeled RSV determined by flow cytometry. Eosinophils without exposure to DiD‐labeled RSV (Naive), and after exposure to DiD‐labeled virus for eosinophils from 4 healthy (Healthy), 8 mild to moderate asthmatics (Mild asthma) and 9 severe asthmatics (Severe asthma) for 16 h. Eosinophils from mild asthma patients not on ICS are depicted in gray. B, Infectious RSV (depicted by Ct‐values) derived from lysates from RSV co‐incubated with eosinophils, reflected by 24‐h replication on epithelial cells. RSV only is RSV added directly to the epithelial cells (n = 16). Healthy refers to lysates from eosinophils from healthy donors (n = 4) and Severe asthma to those from severe asthma patients (n = 7). Ct value of 27.89 (5000 c/PCR) refers to a virus concentration of 6250 c/mL. Data are expressed as mean ± SEM. Paired or unpaired t test *P < 0.02, ***P ≤ 0.0001
Together this indicates that eosinophils from asthma patients bind less RSV and may have a reduced capacity to inactivate viruses as compared to eosinophils from healthy individuals.
2.4. Eosinophils are activated by virus interaction
Exposure of human blood eosinophils for 2 hours to RSV as well as to PR8 significantly enhanced expression of CD69 and trend wise upregulated CD11b expression. CD66b and CD62L expression were unaffected by viral exposure (Figure 5A). Eosinophils can synthesize mediators de novo as well as release preformed mediators selectively.24 Within 2 hours, RSV and PR8 increased IL‐8 secretion by eosinophils (Figure 5B), whereas IL‐6 secretion was increased by PR8, but not by RSV. Neither virus induced the release of eosinophil cationic protein (ECP) (Figure 5B). Together this suggests that eosinophils exposed to virus can drive subsequent inflammatory responses in a virus‐specific manner.
Figure 5.
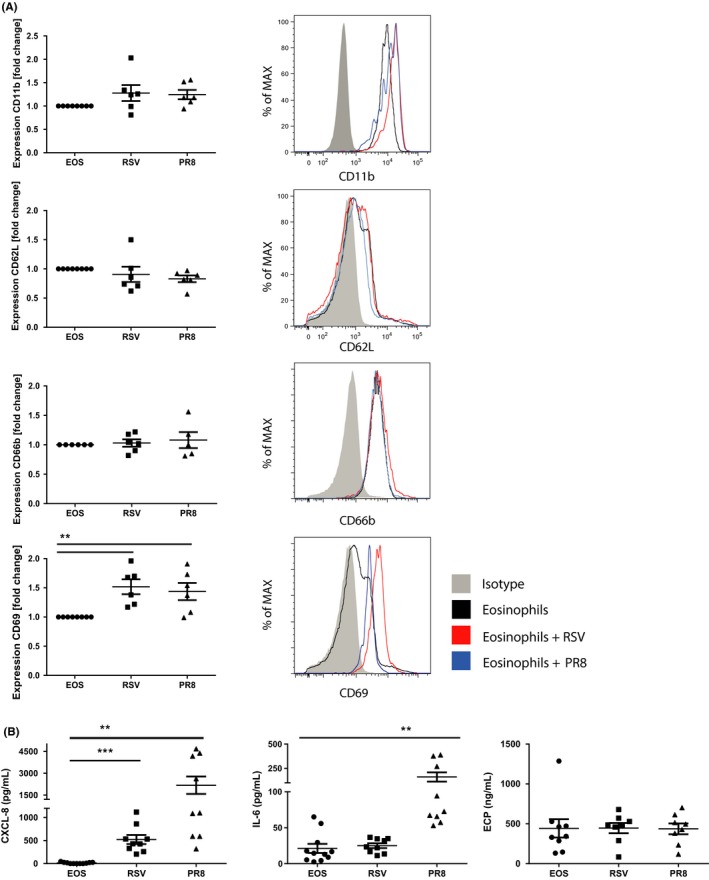
Eosinophils are activated upon exposure to virus in vitro (A). CD11b, CD62L, CD66b, and CD69 expression on blood eosinophils from healthy subjects after 2‐h incubation with DiD‐labeled RSV or influenza; gray, isotype‐matched antibodies (n = 6). B, IL‐8, IL‐6, and ECP secretion of blood eosinophils from healthy subjects after 2‐h incubation with RSV (MOI 10) or PR8 (MOI 2) (n = 9). Data are expressed as mean ± SEM. Paired t test: **P < 0.01; ***P < 0.001
2.5. CD69 correlates to FEV1 in RV‐16‐induced loss of asthma control
To extend these in vitro findings, in a separate study, mild to moderate asthmatics (patient characteristics in Table S2) receiving eosinophil‐depleting mepolizumab (anti‐IL‐5) or placebo were challenged with low‐dose (10TCID50) RV16.21 In this experimental exacerbation model, loss of asthma control as monitored by forced expiratory volume in 1 second (FEV1) started at 4‐5 days after RV16 exposure, peaked around day 7, and was paralleled by increased airway inflammation.25 CD69 expression by eosinophils recovered from BALF of patients receiving placebo or mepolizumab at day 7 after RV16 exposure strongly correlated inversely with FEV1 %pred (relative to predicted FEV1; Figure 6A right panel). This relationship was not found at day 0 (before RV16 was given, Figure 6A left panel), indicating that CD69 expression by eosinophils in RV16‐challenged asthma patients relates to the activation of eosinophils by RV16. Interestingly, eosinophils from patients treated with mepolizumab showed no significant increase in CD69 expression after RV16 exposure (Figure 6B).
Figure 6.
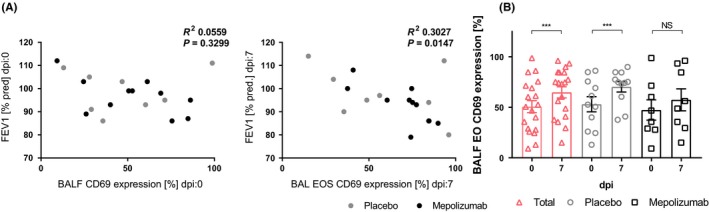
Eosinophils are activated upon exposure to virus in vivo (A). FEV1 of asthma patients before RV16 challenge does not correlate (left) with CD69 expression on BALF eosinophils, whereas they correlate after RV16 challenge (right) (placebo‐ and mepolizumab‐treated groups (Pearson r: −0.55; P = 0.01) and for placebo‐treated group alone (Pearson r: −0.74; P < 0.01)). (b) After RV16 exposure in vivo, eosinophils become more activated (all mild asthmatics (pink; n = 19), placebo (gray; n = 11) and mepolizumab (black; n = 8)). Data are expressed as mean ± SEM. Paired t test or Pearson correlation: ***P < 0.001
3. DISCUSSION
Eosinophils exert prominent cytotoxic properties that can harm the respiratory mucosa and attenuate lung function in stable and exacerbating asthma.13, 26 In the present study, however, we provide ex vivo and in vivo evidence that murine and human eosinophils can rapidly capture various respiratory viruses and significantly reduce infectivity of these viruses. Eosinophils that bound virus became activated, as reflected by altered expression of cell surface molecules like CD69 and the release of pro‐inflammatory cytokines such as IL‐6 and IL‐8. In line herewith, RV16‐induced loss of asthma control in patients revealed a strong correlation with CD69 expression by eosinophils. Intriguingly, eosinophils from asthma patients displayed a reduced capacity to bind virus.
Together this indicates that human eosinophils may be important scavengers of virus at the respiratory mucosa in vivo, preventing further viral propagation. With the murine allergic house dust mite model for severe asthma exacerbations, we showed that depletion of eosinophils using the anti‐IL‐5 antibody TRFK5 resulted in enhanced viral titers.20 Similarly, in our human study, depletion of eosinophils followed by a RV16 challenge resulted in enhanced viral titers.21 These studies underline the in vivo antiviral activity of eosinophils and are in accordance with earlier murine studies showing that the number of eosinophils influenced killing of pneumonia virus of mice (PVM), although the mechanisms proposed were distinct from that shown here.16, 18, 19, 27 Electron microscopy of eosinophils incubated with virus showed intact viral particles in the cytoplasm, but we failed to detect viral particles enclosed by a membrane, which would have supported the concept of intracellular inactivation of viral particles. During the infectious cycle, RSV fuses with the plasma membrane,28, 29 and thus, the presence of intact viral particles is indicative of viral uptake. Elucidation of the precise mechanisms by which viruses are inactivated by eosinophils, however, awaits further studies. Like eosinophils, macrophages also serve as a sink for virus; however, contrary to our findings for eosinophils, this involves an infectious cycle.30, 31
Recently, in a murine study, CD101‐low eosinophils residing in lung tissue with a regulatory signature were distinguished from inflammatory CD101‐high eosinophils, which typically are recruited into the lung.15 There are some indications that both eosinophil types exist in humans. In the present study, we did not distinguish between either types, although our findings with human eosinophils relate to circulating and recruited eosinophils and thus likely inflammatory eosinophils. In another study, we reported that CD101+ eosinophil influx in HDM‐sensitized mice was boosted in response to house dust mite (HDM) and further increased by influenza infection.32 Interestingly, blocking IL‐33 signaling, which drives influenza‐induced exacerbation in HDM‐sensitized mice, resulted in a significant reduction of CD86+ MHC‐II+ CD101− eosinophils, but did not affect the inflammatory (CD101+) eosinophil population.32 As blocking of IL‐33 signaling was paralleled by an increased clearance of virus, we consider it unlikely that resident eosinophils contributed to this. Although we did not distinguish between resident and inflammatory eosinophils in our anti‐IL‐5 studies,20, 31 the increase in viral load upon anti‐IL‐5 treatment is again suggestive of an antiviral role for inflammatory eosinophils and not resident cells, as the presence of the latter appears independent of IL‐5.15 Further studies, particularly in humans, however, are needed to clarify the role of resident eosinophils in the antiviral response.
As a consequence of viral binding in vitro, eosinophils become activated as reflected by increased expression of various activation markers and a fast release of mediators like IL‐6 and IL‐8, but not that of ECP. It is important to realize here that the DiD‐labeled virus was purified on a density gradient, and thus, the activation of eosinophils is unlikely to be due to pro‐inflammatory mediators released by cells to produce the virus. Thus, eosinophils activated by virus can exert virus‐specific immunomodulatory roles locally.33 The IL‐8 release can in part explain the influx of neutrophils during a viral infection. During a RSV infection in infants, neutrophilic inflammation is considered the most important cause of acute bronchiolitis.34 At early time points of a RSV infection, particularly eosinophilic degranulation products are found,35 which may emphasize the important role of eosinophils in RSV infections.
Also, the strong inverse correlation between CD69 expression by eosinophils and lung function (FEV1) in asthma patients 7 days after a challenge with RV16 is in line with virus‐induced activation of eosinophils and the cytotoxic properties of eosinophils in asthma (6‐8). Although in our in vitro studies eosinophils failed to release ECP upon activation by viruses, it is possible that in an in vivo setting ECP is released. In our RV16 challenge study in asthma patients,21 we found an increase in ECP although it did not reach significance. Along these lines, it is possible that in more severe patients and with a more virulent virus, the release of ECP is more prominent. This is important as ECP has ribonuclease activity, which may inactivate viruses, but on the other hand, ECP also exerts cytotoxic, neurotoxic, fibrosis‐promoting, airway‐inflammatory, and immune‐regulatory functions.36, 37 Besides the activation of eosinophils, the murine studies indicate that after viral exposure, eosinophils migrated to the draining lymph nodes and increased expression of MHCII and co‐stimulatory molecules. This points to an additional role for eosinophils in antigen presentation, as has been proposed earlier.40
It is known that eosinophils in asthma patients are primed, for example, by IL‐5.41 This could lead to a more pronounced virus‐induced activation of eosinophils in asthma patients and to the release of ECP and other granular proteins. In line herewith, CD69 expression on eosinophils did not increase in patients treated with mepolizumab. Remarkably, eosinophils from asthma patients displayed a reduced capacity to bind and inactivate virus, which was particularly evident in patients with severe asthma. So, on the one hand, eosinophils in asthma patients may be less capable of clearing virus, whereas on the other hand, virus may more profoundly activate eosinophils, because of their primed state. Therefore, both the number of eosinophils and their activation state control their antiviral response. As eosinophils from asthma patients are already activated in the circulation, this could explain their reduced capacity to bind viruses. Interestingly, mild to moderate asthma patients on corticosteroids, which attenuates eosinophil activation, had a higher antiviral activity, in support of the previous.42 The effect of corticosteroids may relate to inhibition of mediators that activate eosinophils but also to direct effects on eosinophils, such as by stabilizing the eosinophil's cell membrane.43 In the reported studies, we used predominantly PR8 and RSV to show the direct interaction between virus and eosinophils, whereas we used RV16 in the human challenge model. Although the apparent uniform interaction of RSV and PR8 with eosinophils may indicate a general mechanism applicable also to RV16, we have not been able to test that. RV16 is known to rapidly inactivate after thawing, which is incompatible with DiD labeling of RV16. Alternatively, we could not use another virus than RV16 in our human challenge model and so there is uncertainty whether RV16 is actually captured by eosinophils. However, exposure to RV16 resulted in eosinophil activation and, upon depletion of eosinophils, in enhanced viral titers,21 all in line with findings for RSV and PR8.
In summary, our data show that eosinophils are capable of rapid capture and inactivation of virus, which has been so far an unrecognized property of eosinophils. In addition, we found that eosinophils particularly from severe asthma patients are defective in these properties, which may lead to enhanced viral loads. These reduced antiviral and enhanced cytotoxic properties of eosinophils from asthma patients likely underlie, at least in part, the pathogenesis of virus‐induced asthma exacerbations. These findings also lead to a new perspective of therapeutic benefits in reducing eosinophils in the airway. Novel therapeutics like anti‐IL‐5 and IL‐5R are currently aimed at reducing eosinophils and eosinophil cytotoxic compounds, however, consequently also reduce the potential protective properties of eosinophils. Whether the current dosing (100 mg s.c.), which differs from what we used here (750 mg i.v.), and whether long‐term treatment with anti‐IL5 or anti‐IL‐5R has additional effects are currently unknown. Based on the apparent maintenance of the antiviral properties of eosinophils from mild to moderate asthma patients by corticosteroids, we speculate that tapering anti‐IL‐5 and IL‐5R treatments to limit activation of eosinophils, rather than eradicate eosinophils, may lead to even better clinical results.
4. METHODS
4.1. General methods
4.1.1. Virus and cells
Influenza strain A/PR/8/34, RSV‐A2 (a kind gift from AIMM therapeutics), and GFP‐RSV (a kind gift from Dr Mark Peepels (Nationwide Children's Hospital, Columbus, Ohio, USA) and Dr Peter Collins (NIH, USA) were used. RSV was propagated in HE‐P2 cells in IMDM (Lonza) culture medium supplemented with 1% FCS and influenza in NCI‐H292 cells in RPMI‐1640 with 1% FCS. At day 1‐3 postinfection, when cytopathic effects were observed, the supernatant was harvested. Cell debris was removed by centrifugation at 3000 g for 10 minutes, and the supernatant was snap frozen and stored at −80°C (Figure S4).
4.1.2. DiD labeling of virus
1,19 – dioctadecyl ‐ 3, 3, 39, 39 – tetramethylindocarbocyanine (DiD) (Molecular Probes, Invitrogen) was dissolved in DMSO at a concentration of 20 mg/mL and used to label influenza A/PR/8/34 or RSV‐A2. Influenza and RSV were incubated at room temperature for 30 minutes with 10 μL or 2 μL DiD, respectively, followed by density gradient centrifugation to obtain purified labeled virus, essentially as described elsewhere.22 DiD labeling varied between preparations, and so for comparisons, a single batch of DiD‐labeled virus was used. Direct DiD staining of eosinophils yielded homogeneous staining of the cell membrane, distinct from the staining seen upon exposure to DiD‐labeled virus.
4.1.3. Confocal microscopy
Coverslips were precoated with 50 μg/mL poly‐l‐lysine (Life technologies) for 2 hours at 37°C. Eosinophils (2.5 × 104) were seeded onto the precoated coverslips and fixated for 5 minutes in 3.7% paraformaldehyde. These were either immediately mounted with ProLong Gold (life technologies) anti‐fade reagent with DAPI (nuclear staining). Immunofluorescence images were made using the Confocal Microscope SP‐8 X SMD (Leica Microsystems) with a 63x/1.30 Oil CS2 objective.
4.1.4. Electron microscopy
Eosinophils were fixed in 1% glutaraldehyde and 4% PFA in 0.1 mol/L sodium cacodylate buffer (McDowell fixative) and postfixed with 1% osmium tetroxide (OsO4, Electron microscopy sciences) in cacodylate buffer. Samples were dehydrated in serial dilutions of alcohol and embedded into Epon (LX‐112 resin Ladd research). Ultrathin Epon (50‐85 nm) sections were collected on formvar‐coated grids, counterstained with uranyl acetate and lead citrate, and visualized with transmission electron microscope (FEI Tecnai 12). For negative staining of RSV (Figure S3), the particles were allowed to adhere to carbon‐coated grids, washed with PBS, and postfixed with 1% glutaraldehyde in PBS. Then, grids were washed with several drops of dH2O and counterstained with uranyl acetate.
4.2. Murine studies
4.2.1. Animals
IL‐5 transgenic mice (NJ.1638) were obtained from Drs. James and Nancy Lee (Mayo Clinic).44 All animals (male; C57BL/6J; NJ.1638) were housed in standardized specific pathogen‐free conditions.
4.2.2. Influenza infection
IL‐5 transgenic mice or negative littermates were infected in with 10 TCID50 influenza A/PR/8/34 and killed after 2, 4, 6, 8, or 10 days. For visualization of virus, in separate experiments (Figure 1C‐G), animals were infected with DiD‐labeled influenza as previously described45 and killed after 4, 24, or 48 hours.
All animal data are derived from at least two independent experiments with group sizes of 6‐8 animals, except the infection with DiD‐labeled influenza, which was performed twice with 3 animals per group.
4.3. Human studies
4.3.1. Rhinovirus 16 challenge in asthma patients under mepolizumab or placebo
This study was intended to clarify whether the attenuation of eosinophil numbers in asthma patients impacted the inflammatory response to a viral infection. To facilitate this, we aimed to not affect asthma pathophysiology otherwise than attenuating eosinophil numbers, as has been reported in detail in Ref.31 In a double‐blind placebo‐controlled two‐armed trial, mild to moderate, steroid‐naive asthma patients, characteristics shown in Table S2, received one infusion containing 750 mg mepolizumab or placebo, which was known to attenuate eosinophil numbers for up to 4 weeks. Two weeks after infusion, patients were intranasally inoculated with 100 TCID50 of rhinovirus type 16 to induce loss of asthma control (NCT01520051). Bronchoalveolar lavages (8 × 20 mL) were performed 0 days before and 7 days after RV16 infection with comparable recoveries. As the initial procedure for immunophenotyping of BALF cells was not satisfactory and we did not always obtain paired samples (before vs after rhinovirus challenge), we lost samples for analyses. For the specific analyses reported here, we only had complete data sets from eight patients on mepolizumab and 11 on placebo. This, however, did not result in a selection bias as there were no statistical significant differences between the placebo(P)‐ and mepolizumab(M)‐treated groups as is shown in the Table S2, like there were no significant differences in the P‐ vs M‐treated groups in table 1 in Ref.21
4.3.2. DiD‐labeled virus capture assay
Eosinophils used for this assay were isolated from peripheral blood from healthy, mild (different from those participating in the RV16 challenge study) to moderate and severe asthma patients, characteristics shown in Table S1 (described in more detail in Supplementary methods). Eosinophils were incubated with either A/PR/8/34 or RSV‐A2 at a MOI of 2 and 10, respectively. Different conditions were used; the cells maintained in PBS supplemented with 1% BSA and were either incubated at 4ºC or 37ºC for 2 hours at 95% humidity and 5% CO2.
4.3.3. Virus degradation assay
Eosinophils were incubated with RSV‐A2 at a MOI of 0.5, and the cells were incubated at 37ºC for 2 hours at 95% humidity and 5% CO2. Eosinophils were subsequently lysed by a three times freeze‐thaw cycle and spun down for 5 minutes at 400 g. H292 cells were incubated with eosinophil lysates for 1 hour, and RNA is collected after 24 hours (see also Figure S4 for explanation of the study set‐up). As a control, H292 cells were exposed directly to RSV‐A2 at a MOI of 0.5. Virus titer was determined as described elsewhere.46
4.4. Statistics
Data from the RV16 challenge study for cell populations, cell activation markers, and also the asthma‐related parameters were normally distributed and were expressed as mean ± SEM and analyzed using GraphPad Prism 6.0 software. The relevant statistical analyses for other analyses are described in legends to the figures. P < 0.05 was considered significant.
4.5. Study approval
The Animal Care and Use Committee of the University of Amsterdam approved all experiments. The human study protocol was approved by the local ethics committee, and the participants provided written informed consent.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
YSSP and SMB designed the study, did experiments, analyzed the data, and wrote the manuscript. AD, BSD, TD, EPH, LR, and DP did experiments and analyzed the data. CJM provided blood from patients and together with PIB performed the bronchoscopies. NNW analyzed data. LK and PJS contributed to the design of the study and wrote the manuscript. RL designed the study, analyzed the data, and wrote the manuscript.
Supporting information
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical assistance of Dr. E. Yasuda (AIMM Therapeutics, Amsterdam, the Netherlands) for virus propagation and Dr. Mark Peepels (Nationwide Children's Hospital, Columbus, Ohio, USA) and Dr. Peter Collins (NIH, USA) for providing recombinant green fluorescent protein‐expressing respiratory syncytial virus and Dr. S. R. Jacobino (UMCU, Utrecht, the Netherlands) for assistance on the use of GFP‐RSV. Drs. James and Nancy Lee (Mayo Clinic, Scottsdale, AZ, USA) are acknowledged for providing the IL‐5 transgenic mice. Dr. K. van der Sluijs is acknowledged for initiating the RV16 study. The authors are very grateful to all patients and healthy individuals for their participation in the present studies; without their commitment and suggestions, this study would not have been possible. Prof. T. Geijtenbeek and Dr. L.S. van Rijt are thanked for their comments to the manuscript. These studies were supported by the Netherlands Asthma Foundation (project: 3.2.10.069; currently Lung Foundation), GSK (CRT 114696), and internal funds.
Sabogal Piñeros YS, Bal SM, Dijkhuis A, et al. Eosinophils capture viruses, a capacity that is defective in asthma. Allergy. 2019;74:1898–1909. 10.1111/all.13802
Lead author: René Lutter, PhD, Amsterdam University Medical Centers, University of Amsterdam, room K0‐150, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. Email: r.lutter@amc.uva.nl. Phone: +31‐205668753.
Sabogal Piñeros, Bal, Ravanetti and Lutter equally contributed to this study.
REFERENCES
- 1. Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143‐178. [DOI] [PubMed] [Google Scholar]
- 2. Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804‐813. [DOI] [PubMed] [Google Scholar]
- 3. O'Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet. 2006;368(9537):794‐803. [DOI] [PubMed] [Google Scholar]
- 4. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549‐556. [DOI] [PubMed] [Google Scholar]
- 5. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189‐1197. [DOI] [PubMed] [Google Scholar]
- 6. Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti‐interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose‐ranging study. Lancet Respir Med. 2014;2(11):879‐890. [DOI] [PubMed] [Google Scholar]
- 7. Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120(19):3882‐3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Min A, Lee YA, Kim KA, El‐Benna J, Shin MH. NOX2‐derived ROS‐mediated surface translocation of BLT1 is essential for exocytosis in human eosinophils induced by LTB4. Int Arch Allergy Immunol. 2014;165(1):40‐51. [DOI] [PubMed] [Google Scholar]
- 9. Jaquet V, Bedard K. Editorial: genetic mapping—the path of discovery for novel functions of the NOX NADPH oxidases. J Leuko Biol. 2009;86(3):461‐463. [DOI] [PubMed] [Google Scholar]
- 10. Yuk CM, Kwon B‐I, Lee S‐H. Effect of NADPH oxidase 2 (Nox2) deficiency on the function of Th2 cells (HYP4P.415). J Immunol. 2014;192(1 supplement):55.2. [Google Scholar]
- 11. Weller PF, Spencer LA. Functions of tissue‐resident eosinophils. Nat Rev Immunol. 2017;17:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carr TF, Berdnikovs S, Simon H‐U, Bochner BS, Rosenwasser LJ. Eosinophilic bioactivities in severe asthma. World Allergy Organ J. 2016;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70(5):691‐698. [PubMed] [Google Scholar]
- 14. Tanizaki Y, Sudo M, Kitani H, et al. Eosinophilic leucocytes and arylsulfatase activity in bronchoalveolar lavage fluid of patients with bronchial asthma. Acta Med Okayama. 1988;42(4):227‐230. [DOI] [PubMed] [Google Scholar]
- 15. Mesnil C, Raulier S, Paulissen G, et al. Lung‐resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279‐3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Percopo CM, Dyer KD, Ochkur SI, et al. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123(5):743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samarasinghe AE, Melo RC, Duan S, et al. Eosinophils promote antiviral immunity in mice infected with influenza A virus. J Immunol. 2017;198(8):3214‐3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drake MG, Bivins‐Smith ER, Proskocil BJ, et al. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am J Respir Cell Mol Biol. 2016;55(3):387‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lacy P. 28 days later: eosinophils stop viruses. Blood. 2014;123(5):609‐611. [DOI] [PubMed] [Google Scholar]
- 20. Ravanetti L, Dijkhuis A, Sabogal Pineros YS, et al. An early innate response underlies severe influenza‐induced exacerbations of asthma in a novel steroid‐insensitive and anti‐IL‐5‐responsive mouse model. Allergy. 2016;72:737‐753. [DOI] [PubMed] [Google Scholar]
- 21. Piñeros Y, Bal SM, van de Pol MA et al. Anti‐IL5 in mild asthma alters rhinovirus‐induced macrophage, B cell and neutrophil responses (MATERIAL): a placebo‐controlled, double‐blind study. Am J Respir Crit Care Med. 2019;199:508‐517. [DOI] [PubMed] [Google Scholar]
- 22. Ho A, Prabhu N, Betts RJ, et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J Immunol. 2011;187(11):10. [DOI] [PubMed] [Google Scholar]
- 23. Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol. 2000;74(22):10508‐10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melo RC, Weller PF. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol Histopathol. 2010;25(10):1341‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Sluijs KF, van de Pol MA, Kulik W, et al. Systemic tryptophan and kynurenine catabolite levels relate to severity of rhinovirus‐induced asthma exacerbation: a prospective study with a parallel‐group design. Thorax. 2013;68(12):1122‐1130. [DOI] [PubMed] [Google Scholar]
- 26. Gleich GJ, Flavahan NA, Fujisawa T, Vanhoutte PM. The eosinophil as a mediator of damage to respiratory epithelium: a model for bronchial hyperreactivity. J Allergy Clin Immunol. 1988;81(5 Pt 1):776‐781. [DOI] [PubMed] [Google Scholar]
- 27. Su Y‐C, Townsend D, Herrero LJ, et al. Dual proinflammatory and antiviral properties of pulmonary eosinophils in respiratory syncytial virus vaccine‐enhanced disease. J Virol. 2015;89(3):1564‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghildyal R, Ho A, Jans DA. Central role of the respiratory syncytial virus matrix protein in infection. FEMS Microbiol Rev. 2006;30(5):692‐705. [DOI] [PubMed] [Google Scholar]
- 29. Zheng LL, Yang XX, Liu Y, et al. In situ labelling chemistry of respiratory syncytial viruses by employing the biotinylated host‐cell membrane protein for tracking the early stage of virus entry. Chem Commun (Camb). 2014;50(99):15776‐15779. [DOI] [PubMed] [Google Scholar]
- 30. Schneider C, Nobs SP, Heer AK, et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLOS Pathog. 2014;10(4):e1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84(15):7569‐7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ravanetti L, Dijkhuis A, Dekker T, et al. IL‐33 drives influenza‐induced asthma exacerbations by halting innate and adaptive anti‐viral immunity. J Allergy Clin Immunol. 2019;143:1355‐1370. [DOI] [PubMed] [Google Scholar]
- 33. Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil‐derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stokes Kl, Currier Mg, Sakamoto K, et al. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol. 2013;87(18):10070‐10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim CK, Callaway Z, Koh YY, Kim S‐H, Fujisawa T. Airway IFN‐γ production during RSV bronchiolitis is associated with eosinophilic inflammation. Lung. 2011;190(2):183‐188. [DOI] [PubMed] [Google Scholar]
- 36. Tomassini M, Magrini L, De Petrillo G, et al. Serum levels of eosinophil cationic protein in allergic diseases and natural allergen exposure. J Allergy Clin Immunol. 1996;97(6):1350‐1355. [DOI] [PubMed] [Google Scholar]
- 37. Bystrom J, Amin K, Bishop‐Bailey D. Analysing the eosinophil cationic protein ‐ a clue to the function of the eosinophil granulocyte. Respir Res. 2011;12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moneret‐Vautrin DA. Le dosage de la protéine cationique des éosinophiles est‐il un marqueur utile pour l'interniste? La Revue de Médecine Interne. 2006;27(9):679‐683. [DOI] [PubMed] [Google Scholar]
- 39. Venge P. Monitoring the allergic inflammation. Allergy. 2004;59(1):26‐32. [DOI] [PubMed] [Google Scholar]
- 40. Kambayashi T, Laufer TM. Atypical MHC class II‐expressing antigen‐presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14(11):719‐730. [DOI] [PubMed] [Google Scholar]
- 41. Schweizer R, Welmers B, Raaijmakers J, Zanen P, Lammers J, Koenderman L. RANTES‐ and interleukin‐8‐induced responses in normal human eosinophils: effects of priming with interleukin‐5. Blood. 1994;83(12):3697‐3704. [PubMed] [Google Scholar]
- 42. Grutters J, Brinkman L, Aslander M, van den Bosch J, Koenderman L, Lammers J. Asthma therapy modulates priming‐associated blood eosinophil responsiveness in allergic asthmatics. Eur Respir J. 1999;14(4):915‐922. [DOI] [PubMed] [Google Scholar]
- 43. Lamche HR, Silberstein PT, Knabe AC, Thomas DD, Jacob HS, Hammerschmidt DE. Steroids decrease granulocyte membrane fluidity, while phorbol ester increases membrane fluidity. Studies using electron paramagnetic resonance. Inflammation 1990;14(1):61‐70. [DOI] [PubMed] [Google Scholar]
- 44. Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL‐5 in thymocytes T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158(3):1332‐1344. [PubMed] [Google Scholar]
- 45. Ge MQ, Ho A, Tang YF, et al. NK cells regulate CD8(+) T cell priming and dendritic cell migration during influenza A infection by IFN‐gamma and perforin‐dependent mechanisms. J Immunol. 2012;189(5):2099‐2109. [DOI] [PubMed] [Google Scholar]
- 46. Jansen RR, Schinkel J, Koekkoek S, et al. Development and evaluation of a four‐tube real time multiplex PCR assay covering fourteen respiratory viruses, and comparison to its corresponding single target counterparts. J Clin Virol. 2011;51(3):179‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


