Abstract
Essentials.
TEG‐guided therapy has been shown to be valuable in a number of surgical settings.
This systematic review and analysis specifically evaluated the effects of TEG‐guided therapy.
TEG‐guided therapy can improve blood product utilization and enhance resource management.
Use of TEG improved key patient outcomes, including bleed rate, length of stay and mortality.
Background
Thromboelastography (TEG 5000 and 6s Thrombelastograph Hemostasis Analyzer; Haemonetics) is a point‐of‐care system designed to monitor and analyze the entire coagulation process in real time. TEG‐guided therapy has been shown to be valuable in a variety of surgical settings.
Objective
To conduct an analysis of published clinical trials to evaluate the effects of TEG‐guided transfusion for the management of perioperative bleeding on patient outcomes.
Patients/Methods
We searched MEDLINE (PubMed) and EMBASE for original articles reporting studies using TEG vs controls in a perioperative setting for inclusion in this systematic review. We identified nine eligible randomized controlled trials (RCTs) in two elective surgery settings (cardiac surgery and liver surgery), but only one RCT in the emergency setting.
Results
In the elective surgery study meta‐analysis, platelet (P = 0.004), plasma (P < 0.001) and red blood cell transfusion (P = 0.14), operating room length of stay (LoS) (P = 0.005), intensive care unit LoS (P = 0.04) and bleeding rate (P = 0.002) were reduced with TEG‐guided transfusion vs controls. Although blood product use was reduced, rates of mortality remained comparable between the TEG group and control group. In the emergency setting evaluation, the RCT reported lower mortality in the TEG group than in the control group (P = 0.049). In addition, there were significant reductions in platelet and plasma transfusion (P = 0.04 and P = 0.02, respectively), and the number of ventilator‐free days increased, in the TEG group as compared with the control group (P = 0.10).
Conclusions
This systematic review and analysis indicate that TEG‐guided hemostatic therapy can enhance blood product management and improve key patient outcomes, including LoS, bleeding rate, and mortality.
Keywords: blood coagulation, cardiovascular surgical procedures, elective surgical procedures, emergency treatment, thromboelastography
1. INTRODUCTION
Timely recognition and treatment of coagulopathy is crucial in preventing uncontrollable bleeding.1 However, standard coagulation tests cannot provide the required differential diagnosis of trauma‐induced coagulopathy or massive intraoperative blood loss.2 Because of the time required to conduct these assays in most trauma centers (~45 minutes), there are also limitations to their application in a trauma setting.3 Perioperative monitoring is essential for accurate understanding of the potential coagulopathic causes of hemorrhage and for prompt guidance of hemostatic therapies. Thromboelastography (TEG 5000 and 6s Thrombelastograph Hemostasis Analyzer; Haemonetics) is a method used for viscoelastic point‐of‐care detection, management, and monitoring of clot formation and hemostasis.1, 3, 4 TEG is designed to monitor and analyze blood coagulation in real time, and provides a graphical representation of the kinetics of clot formation.5 In contrast to those of standard coagulation tests, the results of viscoelastic testing are based on the entire coagulation process, and some parameters can be obtained more rapidly (within 10 minutes).3, 6
Viscoelastic testing is a rapid, reliable technique that can be used to guide hemostatic therapy in bleeding patients, enabling individualized therapy by targeting the actual deficiencies of each patient. Guided hemostatic therapy can be of critical value in situations of high blood loss, such as major surgery (eg, cardiac surgery and liver transplantation), or in emergency control of bleeding due to trauma or postpartum hemorrhage (PPH).1, 4, 7, 8, 9, 10, 11, 12, 13 Perioperative bleeding management guidelines recommend intervention algorithms based on viscoelastic coagulation monitoring, and highlight the predictive value of TEG results, particularly in an emergency setting.14, 15, 16, 17 However, there is no agreed, standardized algorithm for TEG‐guided hemostatic therapy.
In a systematic review, viscoelastic coagulation monitoring was found to be less expensive and more effective than standard coagulation tests in both cardiac surgery patients and trauma patients.18 In addition, the results from a systematic review with meta‐analysis and trial sequential analysis of TEG and rotational thromboelastometry (ROTEM) indicated that transfusion strategies guided by viscoelastic testing may reduce the utilization of blood product transfusion in patients with bleeding.19 However, there is a limited number of high‐quality randomized controlled trials (RCTs), and those available have used a variety of algorithms, making it difficult to assess the impact of TEG across these studies. Additionally, although there have been some published meta‐analyses regarding viscoelastic coagulation monitoring, few have specifically focused on the effects of TEG‐guided transfusion.16, 18, 20 Here, we report a systematic review and analysis evaluating the available data for TEG‐guided hemostatic therapy in patients undergoing surgery or massive transfusion, as compared with that guided by standard coagulation tests, with particular focus on allogeneic blood product transfusion and patient outcomes.
2. METHODS
This article adheres to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.21
2.1. Systematic literature review
A systematic literature search of MEDLINE (PubMed) and EMBASE up until January 2018 was conducted to identify original, English‐language articles that used TEG vs controls in a perioperative setting. The predefined search string utilized for MEDLINE was ((thromboelastography OR TEG OR viscoelastic) AND (surgery OR surgical OR perioperative) AND (transfusion OR haemosta* OR “blood management” OR hemorrhage)). The predefined search string utilized for EMBASE was ((thromboelastography OR TEG OR viscoelastic) AND (surgery OR surgical OR perioperative) AND (transfusion OR haemosta* OR “blood management” OR hemorrhage)) NOT MEDL(YES). The asterisk denotes a wildcard used to capture all words starting with ‘haemosta’.
The titles and abstracts of the identified articles were screened by the use of predefined inclusion and exclusion criteria. Inclusion criteria were as follows: RCTs, full original articles, English‐language articles, human subjects, and a perioperative setting. Exclusion criteria were as follows: review papers, conference abstracts or proceedings, opinion pieces, guidelines, meta‐analyses, systematic reviews, editorials, commentaries, case reports/cases series, articles not reporting original data, retracted papers, animal models, in vitro or ex vivo studies, healthy volunteers, and ROTEM or other devices. A second screen was undertaken with the same inclusion/exclusion criteria to review the full texts of the publications. Data were then extracted from identified RCTs that evaluated clinical outcomes of TEG‐guided hemostatic therapy as compared with that guided by standard coagulation tests. The study subjects, authors, methods and time periods for each article were examined in order to avoid inclusion of redundant data from multiple reports.
In addition, we used the systematic literature review to identify relevant prospective, observational, controlled studies. As these are not RCTs, they were not included in the main analysis, but were used to inform a narrative review of the available literature.
2.2. Data extraction and statistical analysis
Data were extracted from the identified studies for inclusion by two independent individuals, using a data extraction form developed for this purpose. In cases of disagreement, a third individual was consulted. Primary outcomes were blood transfusion requirements (units of red blood cells [RBCs], platelets, and plasma), operating room (OR) length of stay (LoS), intensive care unit (ICU) LoS, and rate of surgical reintervention. Secondary outcome measures were cryoprecipitate transfusion, bleeding rate (volume of blood lost over a defined period of time [12‐hour mediastinal tube drainage [MTD]; 24‐hour MTD; 12‐hour MTD and pleural drainage]), other complications beyond bleeding (eg, infections), mortality/survival, and total hospital LoS. In addition to the published data, information on further outcomes was obtained from the emergency setting study, including blood transfusion requirements among patients requiring transfusion of >2 units (RBCs, platelets, and plasma), transfusion of cryoprecipitate, ICU LoS, ICU LoS excluding patients who died within 48 hours, rates of complications (ventilator‐free days, rates of kidney dysfunction, thromboembolic complications, and infections [including sepsis]), and rates of these complications, excluding patients who died within 48 hours. Data unavailable from the selected publications were requested from the corresponding authors; if no data were forthcoming, additional data were obtained from selected meta‐analyses, where possible.18, 19 The risk of bias was assessed in included studies by use of the Cochrane Collaboration tool for assessment of risk of bias.22
Meta‐analysis methods were used to pool the results from the different studies in the elective surgery setting to give a single estimate of the differences in outcome between treatments. All analyses were performed with the DerSimonian‐Laird random‐effects method, regardless of the amount of heterogeneity between studies. The amount of heterogeneity between studies was assessed on the basis of the significance of the between‐study heterogeneity, and also on the size of the I 2 value (>50%).23 Treatment differences for continuous outcomes are given in the form of a standardized mean difference (SMD)/mean difference, and the relative risk (RR) is reported for binary outcomes. Corresponding confidence intervals (CIs) are reported with all treatment effects, along with P values indicating the significance of the difference between the TEG group and the control group. For evaluation of outcomes in the emergency setting study, differences in categorical outcomes between study arms were assessed with the χ2 test, and differences in continuous outcomes were assessed with the unpaired t test.
In order to accommodate the differences in how the results for continuous outcomes were reported between studies in the elective surgery setting, the analysis was performed by calculating the SMD between groups, rather than the raw difference. This provides the differences in outcome between groups in terms of the number of standard deviations (SDs) difference. The pooled outcomes for binary outcomes were expressed as an RR. Some studies presented continuous outcomes as only the median and interquartile range (IQR)/data range, whereas the data analysis required mean and SD values. When the summary statistics suggested that an underlying normal distribution was a reasonable assumption, these figures were converted to the mean and SD. In such instances, the mean value was assumed to be equivalent to the median, and the SD was assumed to be equal to either a quarter of the data range, or 0.74 times the width of the IQR. When the summary statistics suggested that an underlying normal distribution was not a reasonable assumption, no conversion to mean and SD was possible, and the data from those studies were excluded from the analysis. For the additional outcomes analyzed from the emergency setting study, only summary data were available. For the categorical outcomes, only the percentage of patients in whom the outcome occurred was reported, and these data were converted to patient numbers.
3. RESULTS
3.1. Results of the search
The search identified 1860 articles, with 35 passing the title and abstract screen (Figure 1). After a full‐text review, 11 met the predefined inclusion/exclusion criteria for the meta‐analysis. One of these studies was excluded from the analysis on the basis of the study design, owing to the sole use of TEG Platelet Mapping to guide treatment.24 Only one RCT conducted in an emergency setting was identified9; it was decided to include this in a separate emergency setting study evaluation. This resulted in nine studies being identified for inclusion in the elective surgery study meta‐analysis, and a single RCT for the emergency setting study evaluation. Thirty‐nine relevant prospective, observational, controlled studies were identified by the search to inform a narrative review.
Figure 1.
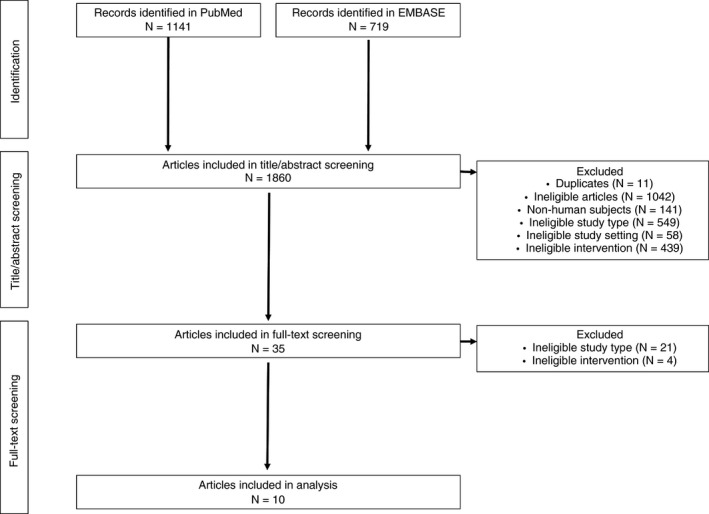
PRISMA flow diagram
3.2. Analysis of the RCTs
3.2.1. Included studies
A total of nine published RCTs4, 10, 11, 12, 25, 26, 27, 28, 29 conducted in an elective surgery setting were included in the meta‐analysis (Table 1). The population size varied from 28 patients to 224 patients, and there were only two types of surgery reported: cardiac surgery (7/9, 78%),10, 11, 12, 25, 26, 27, 29 and liver surgery (2/9, 22%).4, 28 The majority of studies (7/9, 78%)10, 11, 12, 25, 26, 28, 29 utilized both standard coagulation tests and clinical judgment as controls. The most common reason for study exclusion from outcome analyses was unavailability of the mean/SD. Among those studies providing details of the TEG‐based intervention transfusion algorithm employed, a variety of parameters were used to guide treatment (Table S1). One RCT conducted in an emergency setting was included in a separate analysis.
Table 1.
Details of studies included in the elective surgery meta‐analysis and emergency setting study evaluation
| No. of participants | Population | Study category | Study details | Intervention transfusion algorithm | Control group | |
|---|---|---|---|---|---|---|
| Elective surgery studies | ||||||
| Shore‐Lesserson et al 199912 | 105 | Cardiac surgery | Elective | High‐risk cardiac procedures (single or multiple valve replacement, combined artery bypass plus valvular procedure, cardiac reoperations, thoracic aortic replacement) | Fully TEG‐based | Standard coagulation tests |
| Clinical judgment | ||||||
| Nuttall et al 200110 | 92 | Cardiac surgery | Elective | Abnormal microvascular bleeding after CPB | Partly TEG‐based; POC standard coagulation tests and intraoperative algorithm also included | Standard coagulation tests |
| Clinical judgment | ||||||
| Royston et al 200111 | 60 | Cardiac surgery | Elective | High risk of requiring hemostatic products (heart transplantation, revascularization bypass, Ross procedure, multiple valve and revascularization surgery) | Fully TEG‐based | Standard coagulation tests |
| Clinical judgment | ||||||
| Avidan et al 200426 | 102 | Cardiac surgery | Elective | First‐time coronary artery bypass grafting with CPB | Partly TEG‐based; Hepcon and PFA‐100 platelet function analyzer also included | Standard coagulation tests |
| Clinical judgment | ||||||
| Ak et al 200925 | 224 | Cardiac surgery | Elective | First‐time coronary artery bypass grafting with CPB | Fully TEG‐based | Standard coagulation tests |
| Clinical judgment | ||||||
| Westbrook et al 200929 | 69 | Cardiac surgery | Elective | All procedure types except lung transplantation | Partly TEG‐based; Platelet Mapping also included | Standard coagulation tests |
| Clinical judgment | ||||||
| Cui et al 201027 | 31 | Cardiac surgery | Elective | Severely cyanotic pediatric patients with complex congenital heart disease undergoing arterial switch operation or double roots | Fully TEG‐based | Clinical judgment |
| Wang et al 201028 | 28 | Liver surgery | Elective | Orthotopic liver transplantation | Fully TEG‐based | Standard coagulation tests |
| Clinical judgment | ||||||
| De Pietri et al 20164 | 60 | Liver surgery | Elective | Patients with cirrhosis and significant coagulopathy before undergoing invasive procedures | Fully TEG‐based | Standard coagulation tests |
| Emergency setting studies | ||||||
| Gonzalez et al 20169 | 111 | Trauma | Emergency | Injured patients from an academic level 1 trauma center meeting the criteria for massive transfusion protocol activation | Fully TEG‐based | Standard coagulation tests |
| Clinical judgment | ||||||
Abbreviations: CPB, cardiopulmonary bypass; PFA, Platelet Function Analyzer; POC, point‐of‐care.
3.2.2. Risk of bias in the included studies
The overall methodologic quality of the studies was low to moderate (Figure 2). Among the nine elective surgery RCTs and the one RCT conducted in an emergency setting, an overall low risk of bias was identified for one study (9.1%).12 Random sequence generation was adequately reported in four studies (36.4%),4, 9, 10, 12 and allocation concealment was adequately reported in four studies (36.4%).10, 11, 12, 26 Blinding of group allocation was reported by four studies (36.4%),4, 12, 25, 29 complete outcome data were provided by eight studies (81.2%),9, 10, 11, 12, 25, 26, 28, 29 and non‐selective reporting was reported by nine studies.4, 9, 10, 11, 12, 25, 26, 28, 29 No other concerns about bias were identified for five studies (45.5%).4, 9, 12, 26, 28
Figure 2.
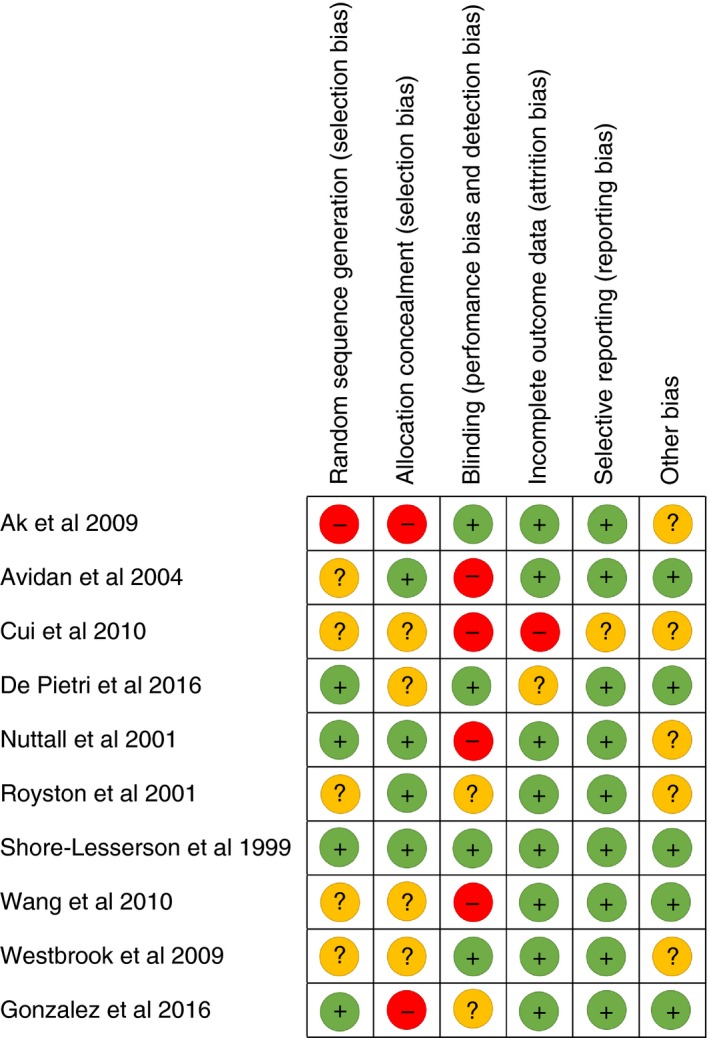
Risk of bias in included studies. Author‐judged risk of bias for each included study: low risk of bias (green “+” symbol), high risk of bias (red “−” symbol), and unclear risk of bias (yellow “?” symbol)
3.3. Elective surgery studies analysis
3.3.1. Effects of intervention on primary outcomes
As shown in Figure 3, the SMD (95% CI) showed statistically significant reductions in platelet transfusion (P = 0.004), plasma transfusion (P < 0.001), OR LoS (P = 0.005) and ICU LoS (P = 0.04) in the TEG group vs the control group. RBC transfusion was reduced in the TEG group vs the control group, but the difference did not reach statistical significance (P = 0.14). Surgical reintervention rates were not reduced with TEG‐guided transfusion (P = 0.83). There was relatively little heterogeneity between the different studies for all primary outcomes; all tests of heterogeneity gave non‐significant results, and the I 2 values were <50%.
Figure 3.
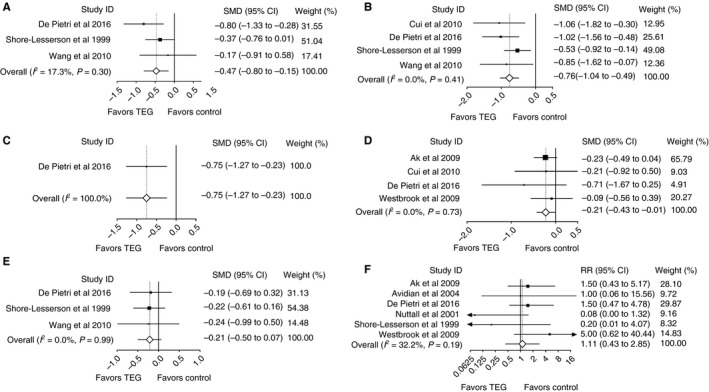
Primary outcomes from elective surgery studies: platelet transfusion (A), plasma transfusion (B), operating room length of stay (C), intensive care unit length of stay (D), red blood cell transfusion (E), and surgical reintervention (F). CI, confidence interval; SMD, standardized mean difference
3.3.2. Effects of intervention on secondary outcomes
A statistically significant effect of TEG‐guided transfusion vs control was shown for reduction in bleeding rate (P = 0.002) (Figure S1). Cryoprecipitate transfusion, total LoS and complications were numerically lower in the TEG group than in the control group. Rates of mortality were not reduced in the TEG group vs the control group (P = 0.85). There was little heterogeneity between studies for bleeding rate, complications, cryoprecipitate transfusion, and mortality outcomes. There was considerable heterogeneity for total LoS.
3.4. Emergency setting study evaluation
3.4.1. Effects of intervention on primary outcomes
Similar levels of RBC transfusion in the TEG group and in the control group were reported in the emergency setting study at all time points examined (Table 2). In the TEG group, significant reductions were shown for the volumes of plasma (P = 0.02) and platelets (P = 0.04) transfused at 2 hours and for the volume of plasma transfused at 4 hours (P = 0.044) vs the control group. There was also a significant increase in ICU‐free days (P = 0.09) for the TEG group vs the control group. This study did not provide data on OR LoS or surgical intervention.
Table 2.
Emergency setting study data (primary and secondary outcomes)
| TEG‐guided group | Control group | P value | |
|---|---|---|---|
| Primary outcomes | |||
| RBC transfusiona, median (range) | |||
| 0‐2 h | 4.5 (2‐8) | 5.0 (2‐11) | 0.317 |
| 0‐4 h | 6.0 (4‐13) | 8.0 (4‐14) | 0.434 |
| 0‐6 h | 8.0 (4‐14) | 8.0 (5‐15) | 0.716 |
| 0‐12 h | 9.5 (5‐16) | 10.5 (6‐15) | 0.496 |
| 0‐24 h | 9.5 (5‐16) | 11.0 (6‐16) | 0.413 |
| Platelet transfusiona, median (range) | |||
| 0‐2 h | 0.0 (0‐0) | 0.0 (0‐1) | 0.041 |
| 0‐4 h | 0.0 (0‐1) | 0.0 (0‐1) | 0.981 |
| 0‐6 h | 1.0 (0‐2) | 1.0 (0‐1) | 0.925 |
| 0‐12 h | 1.0 (0‐2) | 1.0 (0‐2) | 0.539 |
| 0‐24 h | 1.0 (0‐2) | 1.0 (0‐2) | 0.934 |
| Plasma transfusiona, median (range) | |||
| 0‐2 h | 0.0 (0‐3) | 2.0 (0‐4) | 0.022 |
| 0‐4 h | 2.0 (0‐5) | 4.0 (0‐6) | 0.044 |
| 0‐6 h | 4.0 (2‐6) | 5.0 (2‐8) | 0.350 |
| 0‐12 h | 5.0 (3‐8) | 6.0 (4‐8) | 0.533 |
| 0‐24 h | 5.0 (3‐9) | 6.0 (4‐9) | 0.509 |
| ICU‐free days | 16 (0‐22) | 8.5 (0‐19.5) | 0.091 |
| Secondary outcomes | |||
| Cryoprecipitate transfusiona, median (range) | |||
| 0‐2 h | 0.0 (0‐0) | 0.0 (0‐0) | 0.533 |
| 0‐4 h | 0.0 (0‐0) | 0.0 (0‐1) | 0.841 |
| 0‐6 h | 0.0 (0‐2) | 0.0 (0‐2) | 0.473 |
| 0‐12 h | 0.0 (0‐2) | 1.0 (0‐2) | 0.121 |
| 0‐24 h | 0.0 (0‐2) | 1.0 (0‐2) | 0.040 |
| Mortalityb | 11/56 (19.6) b | 20/55 (36.4) | 0.049 |
| RR (95% CI)c | |||
| Complications | 1.44 (0.98–2.10) | 0.06 | |
Abbreviations: CI, confidence interval; ICU, intensive care unit; RBC, red blood cells; RR, relative risk.
Data as reported in the published article.
n/N (%).
Calculated as values in the TEG group relative to those in the control group.
3.4.2. Effects of intervention on secondary outcomes
Lower rates of mortality within the first 6 hours from emergency department arrival (P = 0.032) and a greater probability of survival at 28 days (log‐rank, P = 0.032; and Wilcoxon, P = 0.027) in the TEG group than in the control group were reported.9 The volume of cryoprecipitate transfused was comparable between the groups. The results from our analysis showed that, whereas rates of mortality were reduced in the TEG group vs the control group (19.6% vs 36.4%; P = 0.049), rates of complications were higher in the TEG group than in the control group (P = 0.06) (Table 2), probably because of survival bias.
3.4.3. Analysis of the effects of intervention on additional outcomes
The results of the analysis for the additional emergency setting study outcomes are summarized in Table 3. A smaller proportion of patients received >2 units of plasma in the first 4 hours (P = 0.07) in the TEG group than in the control group. The proportion of patients receiving >2 units of RBCs during the 0‐2–hour period was also smaller in the TEG group than in the control group, but this difference did not reach statistical significance. The number of ventilator‐free days was higher in the TEG group than in the control group; however, the difference was not significant (P = 0.10). In an analysis excluding patients who died within 48 hours, which was performed to compensate for survival bias, rates of kidney dysfunction and infection were lower in the TEG group than in the control group (P = 0.16 and P = 0.14, respectively). Additional parameters were also compared between groups, with no statistically significant differences being found (Table 3).
Table 3.
Emergency setting study data analysis (additional outcomes)
| TEG group | Control group | Treatment effect | ||
|---|---|---|---|---|
| RR (95% CI)a | P value | |||
| Blood product outcomes, n/N (%) | ||||
| RBC transfusion >2 units | ||||
| 0‐2 h | 36/56 (64) | 40/55 (73) | 0.88 (0.69‐1.14) | 0.34 |
| 0‐4 h | 47/56 (84) | 49/55 (89) | 0.94 (0.81‐1.09) | 0.43 |
| 0‐6 h | 51/56 (91) | 50/55 (91) | 1.00 (0.89‐1.13) | 0.97 |
| 0‐12 h | 51/56 (91) | 51/55 (93) | 0.98 (0.88‐1.10) | 0.75 |
| 0‐24 h | 51/56 (91) | 51/55 (93) | 0.98 (0.88‐1.10) | 0.75 |
| Platelet transfusion >2 units | ||||
| 0‐6 h | 7/52 (14) | 6/44 (14) | 0.99 (0.36‐2.72) | 0.98 |
| 0‐12 h | 12/52 (23) | 8/44 (18) | 1.27 (0.57‐2.82) | 0.56 |
| 0‐24 h | 12/52 (23) | 11/43 (26) | 0.90 (0.44‐1.84) | 0.78 |
| Plasma transfusion >2 units | ||||
| 0‐2 h | 15/56 (27) | 18/52 (35) | 0.77 (0.44‐1.37) | 0.38 |
| 0‐4 h | 27/56 (48) | 34/52 (65) | 0.74 (0.53‐1.03) | 0.07 |
| 0‐6 h | 38/56 (68) | 38/51 (75) | 0.91 (0.72‐1.16) | 0.45 |
| 0‐12 h | 44/56 (79) | 42/51 (82) | 0.95 (0.79‐1.15) | 0.62 |
| 0‐24 h | 44/56 (79) | 43/51 (84) | 0.93 (0.78‐1.12) | 0.45 |
| Cryoprecipitate transfusion | ||||
| 0‐6 h | 19/55 (35) | 20/44 (45) | 0.76 (0.47‐1.24) | 0.27 |
| 0‐12 h | 24/55 (44) | 26/44 (59) | 0.74 (0.50‐1.09) | 0.13 |
| 0‐24 h | 24/55 (44) | 27/44 (61) | 0.71 (0.49‐1.04) | 0.08 |
| Complication and length of stay outcomes | ||||
| Total population, n/N (%) | ||||
| Kidney dysfunction | 12/56 (21) | 15/55 (27) | 0.79 (0.41‐1.52) | 0.47 |
| Thromboembolic complications | 9/56 (16) | 6/55 (11) | 1.47 (0.56‐3.86) | 0.43 |
| Infection | 13/56 (23) | 18/55 (33) | 0.71 (0.39‐1.30) | 0.26 |
| MD (95% CI)b | ||||
| Ventilator‐free days, (Mean ± SD) | 14.9 ± 11.0 | 11.4 ± 11.0 | 3.5 (−0.6 to 7.6) | 0.10 |
| ICU length of stay, (Mean ± SD) | 10.7 ± 7.3 | 10.0 ± 6.7 | 0.7 (−1.9 to 3.3) | 0.60 |
| Excluding patients who died within <48 h, n/N (%) | ||||
| Kidney dysfunction | 8/36 (22) | 10/26 (38) | 0.58 (0.26‐1.26) | 0.16 |
| Thromboembolic complications | 7/36 (19) | 4/24 (15) | 1.26 (0.41‐3.87) | 0.68 |
| Infection | 10/36 (28) | 12/26 (46) | 0.60 (0.31‐1.18) | 0.14 |
| MD (95% CI)b | ||||
| Ventilator‐free days, (Mean ± SD) | 10.8 ± 7.0 | 12.3 ± 6.3 | −1.5 (−5.0 to 2.0) | 0.39 |
| ICU length of stay, (Mean ± SD) | 19.7 ± 7.3 | 18.6 ± 6.5 | 1.1 (−2.5 to 4.7) | 0.54 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; MD, mean difference; RBC, red blood cell; RR, relative risk.
Calculated as values in the TEG group minus values in the control group.
Calculated as values in TEG group relative to those in the control group.
3.5. Prospective observational studies narrative
Thirty‐nine relevant prospective, observational, controlled studies were identified by the search. The available outcome data reported in these studies are summarized below. Overall, in the elective surgery setting, reduced transfusion of allogeneic blood products was found when TEG‐guided therapy was employed. In the emergency setting, a trend towards a reduction in the use of transfused blood products was also reported, alongside a decrease in blood loss.
Two identified prospective studies performed in a cardiovascular surgery setting reported that TEG‐guided coagulation management reduced the use of transfusion of allogeneic blood products.30 Both studies demonstrated a significant reduction in overall transfusion.30, 31 One of these studies showed a significant reduction in the use of platelet transfusion,30 and the other showed a significant reduction in the transfusion rates of RBCs, plasma, and any allogeneic blood product.31 Whereas Fassl et al 30 reported a significantly lower rate of postoperative chest tube drainage and significantly lower numbers of major bleeds, Aoki et al30 reported no difference in the rate of bleeding complications. One identified study performed in a liver surgery setting reported that TEG‐guided treatment reduced the use of transfusion of allogeneic blood products.32 The results demonstrated significant reductions in the number of units of RBCs and plasma transfused, but not in the mean total number of units of allogeneic blood products (TEG group, 67.9; control group, 71.4); a decrease in the total volume of blood products transfused was also noted.32
One identified study performed in a trauma setting showed that TEG‐guided therapy improved outcomes, and also reported a trend towards reduced use of allogeneic blood products.33 Mortality was also lower in the TEG group, but this was attributed, in part, to different baseline injury severity scores. In contrast, a separate study reported that none of the TEG variables was an independent predictor of massive transfusion or mortality.34 One study in a PPH setting was identified, and showed that TEG‐guided therapy reduced blood loss; blood loss (>2000 mL) and mean total blood loss occurred in a significantly lower percentage of patients.13 Further studies in a PPH setting are crucial, and, although recent guidelines do not recommend the use of viscoelastic hemostatic assays to predict PPH, they have highlighted their usefulness in major obstetric bleeding when used in conjunction with an agreed algorithm to guide blood transfusion.16, 18
4. DISCUSSION
This systematic review and analysis indicates that TEG‐guided hemostatic therapy reduces the transfusion of certain allogeneic blood products and improves many, but not all, patient outcomes (as detailed in the Results section). Reduced use of allogeneic blood products, resulting from more accurate guidance regarding patient requirements, may lead to reduced risks of complications that have been shown to be independently associated with transfusion, and also a reduction in the costs associated with blood product use.35, 36, 37 Although performed on a limited number of studies, our meta‐analysis in elective surgery and our evaluation of data from an emergency setting suggest that TEG‐guided transfusion reduced the use of allogeneic blood products.
Among the nine elective surgery studies analyzed, statistically significant reductions were demonstrated for the volumes of platelets and plasma transfused, and favorable trends were observed for RBC and cryoprecipitate transfusion. Typically, the primary outcome of elective surgery studies evaluating the use of TEG is a reduction in blood product use. In contrast, the primary outcome of emergency setting studies is typically reduction in mortality, which the single RCT in this setting identified.
In agreement with our results, three previous meta‐analyses of RCTs that included TEG showed that TEG‐guided transfusion significantly reduced the use of allogeneic blood products.18, 19, 38 They reported that viscoelastic‐guided transfusion led to a significant reduction in the proportion of patients transfused with platelets and/or RBCs,19 a significant reduction in transfusion of platelets, plasma, and RBCs,18 and a significant reduction in the proportion of patients receiving platelet and/or plasma transfusion,38 respectively. Among the prospective observational studies identified, significant reductions in transfusion of allogeneic blood products were reported in two studies performed in a cardiovascular surgery setting and one study performed in a liver surgery setting.30, 31, 32 Although the data available for emergency settings are limited, two studies also reported a trend towards reduced use of allogeneic blood products and a smaller proportion of patients requiring plasma and platelet transfusion.13, 33
Our analysis also demonstrated that TEG‐guided transfusion significantly improved other outcomes in an elective surgery setting. Statistically significant reductions were demonstrated for bleeding rate, and OR and ICU LoS, and positive trends were observed for total LoS and lower rates of complications. In agreement with our results, one previous meta‐analysis of RCTs showed that TEG‐guided transfusion reduced bleeding in patients requiring massive transfusion.38 Furthermore, three prospective observational cardiovascular surgery studies reported that TEG data were accurate in predicting bleeding,39, 40 including detection of abnormalities after protamine administration when conventional test results were normal.41 Two prospective observational studies performed in a trauma setting also showed an overall trend in favor of TEG‐guided treatment in predicting and diagnosing early trauma coagulopathy and the need for transfusion.42, 43 Although high‐quality prospective studies are limited, our results reporting a decrease in ICU LoS are supported by findings from a retrospective analysis among trauma patients. This article reported that employing TEG‐guided trauma resuscitation led to a significantly shorter ICU LoS, which corresponded to savings of $24 500 to $33 062.44 However, a previous meta‐analysis reported that, because of the limited data available, improvements in clinical outcomes such as hospital LoS were not supported.18 In addition, a separate meta‐analysis also showed no significant impact of employing a TEG/ROTEM‐based algorithm on total hospital LoS or ICU LoS.38 The authors highlighted that the majority of studies included were focused on reduction of blood loss or transfusion rather than hospital/ICU LoS. In contrast, our meta‐analysis is the first to identify a significant impact of TEG‐guided transfusion on OR LoS and ICU LoS in an elective surgery setting. Further studies will be needed to confirm these exploratory findings.
The only RCT in the emergency setting evaluation reported a reduction in mortality rates, and the recently published British Society for Haematology guidelines recommend that viscoelastic hemostatic assays, particularly TEG, may reduce mortality and overall transfusion exposure.16
When we analyzed other outcomes, that is, mortality and surgical reintervention, in an elective surgery setting, our results did not show that TEG‐guided transfusion led to improvements. These results are in agreement with three previous meta‐analyses of RCTs, which also did not identify improvements in rates of mortality and/or surgical reintervention.18, 19, 38 A separate meta‐analysis reported that the limited data available did not support improvements in mortality and surgical reintervention.18 These results are not surprising when we consider that rates of both mortality and surgical reintervention can be influenced by factors unrelated to TEG‐guided transfusion, such as the age and health of the subject.45, 46, 47 For this reason, we did not elect to include mortality or surgical reintervention as primary outcomes in the elective surgery assessment.
A more recent meta‐analysis did evaluate a reduction in all‐cause mortality as the primary outcome, using pooled results from three trials.48 In addition, a recent systematic review and meta‐analysis also used all‐cause mortality as a primary outcome for data extraction.49 In line with both our analysis and previous meta‐analyses, more recent analyses did not show that TEG‐guided transfusion affected mortality in an elective surgery setting.48, 49
In summary, this analysis demonstrates that the use of TEG to guide hemostatic therapy in the perioperative setting can reduce the requirement for blood product transfusion, enhancing resource management, and improving patient outcomes including bleeding rate, OR, ICU and total hospital LoS, and mortality. Because of the paucity of high‐quality studies, our analysis is based on cardiovascular surgery and liver surgery as key elective surgery settings, and trauma as an emergency setting. However, it is important to highlight that there are potential benefits of employing TEG in other settings, such as obstetrics, monitoring patients with congenital bleeding disorders, and assessing the coagulation status of stroke patients.50, 51 Our analysis has provided an up‐to‐date overview of the benefits of TEG‐guided transfusion in both an elective surgery setting and an emergency setting. However, to fully assess the benefit of TEG across studies, a standardized algorithm is required for use in high‐quality studies and RCTs, particularly in the emergency setting.
DISCLOSURE OF CONFLICT OF INTEREST
João D. Dias, Hardean E. Achneck and Jan Hartmann are, or were at the time of the research, employees of Haemonetics. The work of Angela Sauaia and Ernest E. Moore was supported in part by National Institute of General Medical Sciences P50 GM049222, National Heart, Lung, and Blood Institute UM 1HL 120877, and Department of Defense USAMRAA, W81XWH‐12‐2‐0028. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense. Ernest E. Moore received research support from Haemonetics, Instrumentation Laboratory, and Stago; Co‐founder of ThromboTherapeutics.
AUTHOR CONTRIBUTIONS
J. D. Dias, H. E. Achneck and J. Hartmann conceived and designed the study. A. Sauaia and E. E. Moore contributed to data collection and interpretation. All authors revised the manuscript for important intellectual content, and approved the final version for submission.
Supporting information
ACKNOWLEDGMENTS
The authors thank Meridian HealthComms, Plumley, UK, for providing medical writing support, which was funded by Haemonetics AS, Signy, Switzerland in accordance with Good Publication Practice (GPP3).
Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE. Thromboelastography‐guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: A systematic review and analysis. J Thromb Haemost. 2019;17:984–994. 10.1111/jth.14447
Manuscript handled by: James Douketis
Final decision: Frits Rosendaal, 28 March 2019
REFERENCES
- 1. National Institute for Clinical Excellence (NICE): Detecting, managing and monitoring haemostasis: viscoelastometric point‑of‑care testing (ROTEM, TEG and Sonoclot systems)2014 [accessed January 2019]. Available from: https://www.nice.org.uk/guidance/dg13.
- 2. Kozek‐Langenecker SA. Perioperative coagulation monitoring. Best Pract Res Clin Anaesthesiol. 2010;24:27–40. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point‐of‐care thromboelastography. Semin Thromb Hemost. 2010;36:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, et al. Thrombelastography‐guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63:566–73. [DOI] [PubMed] [Google Scholar]
- 5. Dias JD, Haney EI, Mathew BA, Lopez‐Espina CG, Orr AW, Popovsky MA. New‐generation thromboelastography: comprehensive evaluation of citrated and heparinized blood sample storage effect on clot‐forming variables. Arch Pathol Lab Med. 2017;141:569–77. [DOI] [PubMed] [Google Scholar]
- 6. Benes J, Zatloukal J, Kletecka J. Viscoelastic methods of blood clotting assessment: multidisciplinary review. Front Med. 2015;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth. 2012;109:851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124:3052–8. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal‐directed hemostatic resuscitation of trauma‐induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94:773–81. [DOI] [PubMed] [Google Scholar]
- 11. Royston D, von Kier S. Reduced haemostatic factor transfusion using heparinase‐modified thrombelastography during cardiopulmonary bypass. Br J Anaesth. 2001;86:575–8. [DOI] [PubMed] [Google Scholar]
- 12. Shore‐Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela‐Cantos F, Ergin MA. Thromboelastography‐guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–19. [DOI] [PubMed] [Google Scholar]
- 13. Barinov SV, Zhukovsky YG, Dolgikh VT, Medyannikova IV. Novel combined strategy of obstetric haemorrhage management during caesarean section using intrauterine balloon tamponade. J Mater Fetal Neonatal Med. 2017;30:29–33. [DOI] [PubMed] [Google Scholar]
- 14. Kozek‐Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017;34:332–95. [DOI] [PubMed] [Google Scholar]
- 15. American Society of Anesthesiologists (ASA) task force on perioperative blood management: practice guidelines for perioperative blood management: an updated report. Anesthesiology. 2015;122:241–75. [DOI] [PubMed] [Google Scholar]
- 16. Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Haematology Guideline. Br J Haematol. 2018;182:789–806. [DOI] [PubMed] [Google Scholar]
- 17. Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, et al. Rapid thrombelastography thresholds for goal‐directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82:114–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al. Viscoelastic point‐of‐care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost‐effectiveness analysis. Health Technol Assess. 2015;19:984–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016:CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NK. Effect of thromboelastography (TEG(R)) and rotational thromboelastometry (ROTEM(R)) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care. 2014;18:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al.; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]: The Cochrane Collaboration 2011. Available from http://www.handbook.cochrane.org [Google Scholar]
- 24. Agarwal S, Johnson RI, Shaw M. Preoperative point‐of‐care platelet function testing in cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29:333–41. [DOI] [PubMed] [Google Scholar]
- 25. Ak K, Isbir CS, Tetik S, Atalan N, Tekeli A, Aljodi M, et al. Thromboelastography‐based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg. 2009;24:404–10. [DOI] [PubMed] [Google Scholar]
- 26. Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, et al. Comparison of structured use of routine laboratory tests or near‐patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92:178–86. [DOI] [PubMed] [Google Scholar]
- 27. Cui Y, Hei F, Long C, Feng Z, Zhao J, Yan F, et al. Perioperative monitoring of thromboelastograph on blood protection and recovery for severely cyanotic patients undergoing complex cardiac surgery. Artif Organs. 2010;34:955–60. [DOI] [PubMed] [Google Scholar]
- 28. Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, et al. Thromboelastography‐guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42:2590–3. [DOI] [PubMed] [Google Scholar]
- 29. Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out‐performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. 2009;18:277–88. [DOI] [PubMed] [Google Scholar]
- 30. Aoki K, Sugimoto A, Nagasawa A, Saito M, Ohzeki H. Optimization of thromboelastography‐guided platelet transfusion in cardiovascular surgery. Gen Thorac Cardiovasc Surg. 2012;60:411–16. [DOI] [PubMed] [Google Scholar]
- 31. Fassl J, Matt P, Eckstein F, Filipovic M, Gregor M, Zenklusen U, et al. Transfusion of allogeneic blood products in proximal aortic surgery with hypothermic circulatory arrest: effect of thromboelastometry‐guided transfusion management. J Cardiothorac Vasc Anesth. 2013;27:1181–8. [DOI] [PubMed] [Google Scholar]
- 32. Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW Jr, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–96. [PMC free article] [PubMed] [Google Scholar]
- 33. Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, et al. Initial experiences with point‐of‐care rapid thrombelastography for management of life‐threatening postinjury coagulopathy. Transfusion. 2012;52:23–33. [DOI] [PubMed] [Google Scholar]
- 34. Johansson PI, Sorensen AM, Larsen CF, Windelov NA, Stensballe J, Perner A, et al. Low hemorrhage‐related mortality in trauma patients in a Level I trauma center employing transfusion packages and early thromboelastography‐directed hemostatic resuscitation with plasma and platelets. Transfusion. 2013;53:3088–99. [DOI] [PubMed] [Google Scholar]
- 35. Marcucci C, Madjdpour C, Spahn DR. Allogeneic blood transfusions: benefit, risks and clinical indications in countries with a low or high human development index. Br Med Bull. 2004;70:15–28. [DOI] [PubMed] [Google Scholar]
- 36. Brown CV, Foulkrod KH, Sadler HT, Richards EK, Biggan DP, Czysz C, et al. Autologous blood transfusion during emergency trauma operations. Arch Surg. 2010;145:690–4. [DOI] [PubMed] [Google Scholar]
- 37. Weber CF, Gorlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point‐of‐care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–47. [DOI] [PubMed] [Google Scholar]
- 38. Wikkelsø AJ, Afshari A, Wetterslev J, Brok J, Moeller AM. Monitoring patients at risk of massive transfusion with Thrombelastography or Thromboelastometry: a systematic review. Acta Anaesthesiol Scand. 2011;55:1174–89. [DOI] [PubMed] [Google Scholar]
- 39. Essell JH, Martin TJ, Salinas J, Thompson JM, Smith VC. Comparison of thromboelastography to bleeding time and standard coagulation tests in patients after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1993;7:410–15. [DOI] [PubMed] [Google Scholar]
- 40. Welsby IJ, Jiao K, Ortel TL, Brudney CS, Roche AM, Bennett‐Guerrero E, et al. The kaolin‐activated Thrombelastograph predicts bleeding after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:531–5. [DOI] [PubMed] [Google Scholar]
- 41. Tuman KJ, Spiess BD, McCarthy RJ, Ivankovich AD. Comparison of viscoelastic measures of coagulation after cardiopulmonary bypass. Anesth Analg. 1989;69:69–75. [PubMed] [Google Scholar]
- 42. Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997;42:716–20. [DOI] [PubMed] [Google Scholar]
- 43. Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, et al. Rapid thrombelastography delivers real‐time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71:407–14. [DOI] [PubMed] [Google Scholar]
- 44. Mohamed M, Majeske K, Sachwani GR, Kennedy K, Salib M, McCann M. The impact of early thromboelastography directed therapy in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2017;25:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karimi A, Ahmadi H, Davoodi S, Movahedi N, Marzban M, Abbasi K, et al. Factors affecting postoperative morbidity and mortality in isolated coronary artery bypass graft surgery. Surg Today. 2008;38:890–8. [DOI] [PubMed] [Google Scholar]
- 46. Yin YC, Peng SK, Huang JH, Hsieh CH, Tsai TC, Shih YR, et al. Risk factors affecting adverse outcomes of cardiac surgery in patients aged 70 years and older. Acta Anaesthesiol Taiwan. 2007;45:197–204. [PubMed] [Google Scholar]
- 47. Tort J, Rozenberg P, Traoré M, Fournier P, Dumont A. Factors associated with postpartum hemorrhage maternal death in referral hospitals in Senegal and Mali: a cross‐sectional epidemiological survey. BMC Pregnancy Childbirth. 2015;15:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Franchini M, Mengoli C, Cruciani M, Marietta M, Marano G, Vaglio S, et al. The use of viscoelastic haemostatic assays in non‐cardiac surgical settings: a systematic review and meta‐analysis. Blood Transfus. 2018;16:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal‐directing treatment with allogeneic blood products—a systematic review and meta‐analysis. Scand J Trauma Resusc Emerg Med. 2017;25:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramiz S, Hartmann J, Young G, Escobar MA, Chitlur M. Clinical utility of viscoelastic testing (TEG and ROTEM analyzers) in the management of old and new therapies for hemophilia. Am J Hematol. 2018;94:249–56. [DOI] [PubMed] [Google Scholar]
- 51. Hartmann J, Mason D, Achneck H. Thromboelastography (TEG) point‐of‐care diagnostic for hemostasis management. Point of Care. 2018;17:15–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


