Summary
Insulin resistance and muscle mass loss often coincide in individuals with type 2 diabetes. Most patients with type 2 diabetes are overweight, and it is well established that obesity and derangements in lipid metabolism play an important role in the development of insulin resistance in these individuals. Specifically, increased adipose tissue mass and dysfunctional adipose tissue lead to systemic lipid overflow and to low‐grade inflammation via altered secretion of adipokines and cytokines. Furthermore, an increased flux of fatty acids from the adipose tissue may contribute to increased fat storage in the liver and in skeletal muscle, resulting in an altered secretion of hepatokines, mitochondrial dysfunction, and impaired insulin signalling in skeletal muscle. Recent studies suggest that obesity and lipid derangements in adipose tissue can also lead to the development of muscle atrophy, which would make insulin resistance and muscle atrophy two sides of the same coin. Unfortunately, the exact relationship between lipid accumulation, type 2 diabetes, and muscle atrophy remains largely unexplored. The aim of this review is to discuss the relationship between type 2 diabetes and muscle loss and to discuss some of the joint pathways through which lipid accumulation in organs may affect peripheral insulin sensitivity and muscle mass.
Keywords: insulin resistance, interorgan crosstalk, muscle atrophy, obesity
Abbreviations
- 1RM
one repetition maximum
- DAG
diacylglycerol
- FGF‐19
fibroblasts growth factor 19
- FGF‐21
fibroblasts growth factor 21
- FXR
farnesoid X receptor
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- IL
interleukin
- IMCL
intramyocellular lipid droplets
- IκB
inhibitor of kappa B
- mTOR
mammalian target of rapamycin
- MuRF1
muscle RING finger 1
- NAFLD
nonalcoholic fatty liver disease
- NF‐κB
nuclear factor kappa B
- PA
phosphatidic acid
- PEDF
pigment epithelium‐derived factor
- PGC1α
peroxisome proliferator‐activated receptor Gamma Coactivator 1‐alpha
- PKC
protein kinase C
- PPARγ
peroxisome proliferator‐activated receptors gamma
- PXR
pregane X receptor
- RBP4
retinol binding protein 4
- ROS
reactive oxygen species
- T2D
type 2 diabetes
- TNFα
tumour necrosis factor α
1. INTRODUCTION
The global prevalence of diabetes is rising rapidly. The number of adults with diabetes in the world increased from 108 million in 1980 to 422 million in 2014,1 and by 2045, this number is expected to increase to 693 million.2 Type 2 diabetes (T2D) is the most common form of this disease and accounts for 85% to 95% of the cases.
Muscle insulin resistance is one of the key features of T2D. In recent years, however, it has been increasingly recognized that there is also a deterioration in muscle mass and muscle strength in patients with T2D,3, 4 and this is independent of the length of disease, metabolic control, vitamin D status, and the presence of microvascular complications and pain.5 In healthy individuals, muscle mass decreases at an annual rate of 1% to 2% after the age of 50.6 This means that an average male person of 80 kg with 35 kg of muscle mass would lose 350 to 700 g a year, which is the equivalent of 7 to 14 kg over 20 years. Individuals with T2D have an accelerated ageing process, which places them at greater risk for developing frailty at an earlier age. We, as well as others, have shown that the problem of muscle loss is most striking in individuals with T2D who are older; it is estimated that 30% to 50% of patients with T2D older than 65 suffer from moderate to severe muscle loss, which is fourfold to fivefold higher than the general population older than 65.4, 7, 8 Indeed, one of our previous studies showed that leg lean mass and appendicular skeletal muscle mass were 3% lower in patients with T2D compared with control subjects.4 Muscle loss in patients with T2D is often not without consequences and can result in poor physical performance and decreased quality of life. A study performed in greater than 6000 participants showed that diabetes was associated with a two to three times increased odds of disability related to lower‐extremity mobility, general physical activities, activities of daily living, instrumental activities of daily living, and leisure and social activities.9 Patients enter a vicious cycle in which increased incidence of falls and hospitalization lead to more muscle loss, a further deterioration in quality of life, and premature death (Figure 1).10, 11, 12 Given the steep rise in the number of patients with T2D, the number of people that are affected by muscle loss is expected to increase dramatically in the coming decades.
Figure 1.
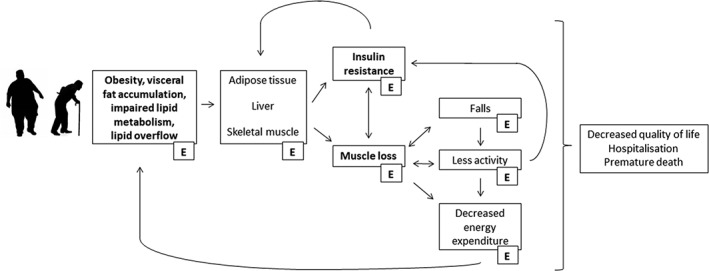
Individuals with obesity and older individuals experience an increase in lipid deposition in visceral and ectopic fat depots. This may affect metabolism in key organs including adipose tissue, liver, and skeletal muscle, which may result in the development of muscle insulin resistance and a decrease in muscle mass. Patients often enter a vicious cycle in which decreased activity levels and increased incidence of falls lead to more muscle loss, a deterioration in quality of life, and premature death. Exercise (E) is an effective strategy to reduce obesity and improve lipid metabolism. It will ameliorate muscle mass loss and the development of insulin resistance, and it will reduce the incidence of falls and improve quality of life
Studies estimated that approximately 80% of all individuals with T2D suffer from overweight or obesity.13 This means there is increased fat storage in subcutaneous and visceral fat depots,14, 15 as well as lipid accumulation in ectopic fat depots, including skeletal muscle and liver.15, 16, 17, 18 It is well established that obesity plays a crucial role in the development of insulin resistance.19 In the past two decades, an increasing number of papers also linked obesity to a reduced muscle mass, and this phenomenon has been named “sarcopenic obesity (Figure 1).”20 Sarcopenic obesity is now frequently observed and led to the suggestion that obesity not only causes insulin resistance but also plays a role in the development of muscle atrophy.21 If this is true, this would mean that insulin resistance and muscle atrophy are “two sides of the same coin” and it would explain the simultaneous occurrence of insulin resistance and muscle atrophy in many patients with T2D. Notably, the combination of increased adipose tissue mass and muscle atrophy may aggravate cardiometabolic complications.22, 23 In this review, we will discuss the relationship between T2D and muscle mass loss, and we will discuss some of the pathways through which obesity may affect insulin sensitivity and muscle mass. To date, data on the link between T2D and muscle loss is still in its infancies, and a significant body of research comes from studies performed in older individuals, who are also characterized by increased adiposity or ectopic fat accumulation, insulin resistance, and muscle loss. Therefore, when information in patients with T2D is missing, observations made in older individuals will be reported. To conclude, this review will also briefly discuss strategies as a way to counteract insulin resistance and muscle mass loss.
2. MUSCLE LOSS IN INDIVIDUALS WITH T2D: CAUSE OR CONSEQUENCE?
T2D and muscle atrophy develop hand in hand, and it has previously been speculated that muscle loss might be a cause as well as a consequence of T2D. Studies suggesting a causal role for muscle loss in the development of metabolic disturbances are relatively scarce though,24, 25 and to our knowledge, there is only one study that evaluated the association between low muscle mass and incidence of T2D in a longitudinal manner.24 Specifically, Son et al followed 6895 participants from Asian descent for a period of approximately 9 years and observed an inverse association between muscle mass index and the development of T2D. Main covariates included age, sex, urban or rural residence, family history of diabetes, hypertension, smoking status, education level, monthly income, physical activity, alcohol consumption, and diet. Also, additional adjustments for body fat mass, waist circumference, and body mass index (BMI) did not modify the relationship.24 The assumption that muscle loss may play a causal role in the development of T2D is partly based on the fact that skeletal muscle is a major determinant of total energy expenditure.26 Skeletal muscle accounts for approximately 80% of the variance in resting metabolic rate, whereas resting metabolic rate represents approximately 60% of total energy expenditure. Thus, if muscle mass decreases with age, total energy expenditure decreases accordingly, possibly leading to obesity, abdominal fat accumulation, and metabolic disturbances if energy intake is not properly reduced.27 However, it is important to keep in mind that T2D is a complex and multifactorial disease and it would be an oversimplification to reduce such a complex system of closely interrelated mechanisms to simple cause‐effect relationships. T2D is the resultant of insulin resistance, in combination with impaired beta‐cell function and decreased glucose effectiveness.28, 29 Therefore, disturbances on the level of skeletal muscle only are unlikely sufficient for T2D to manifest, and derangements on the level of multiple organs are required. In agreement with this, a study found that in a model of severe muscle wasting, mice did not exhibit differences in metabolism compared to wild‐type mouse.30 Furthermore, it was shown that activation of nuclear factor kappa B (NF‐κB) through muscle‐specific transgenic expression of activated inhibitor of kappa B (IκB) kinase beta caused profound muscle wasting that resembles clinical cachexia, but insulin sensitivity, glucose tolerance, and glucose uptake in the extensor digitorum longus were not different between mice.30 Also, a recent study in humans found that the prevalence of the metabolic syndrome was higher in individuals with low muscle mass, but this relationship was lost after controlling for fat mass.31 Thus, overall, there seems to be limited evidence that low muscle mass alone leads to the development of T2D. Nonetheless, of considerable interest are two studies investigating the interplay between high fat and low muscle mass on cardiometabolic risk factors. The major finding of these studies was that participants with both high percent body fat and low skeletal muscle mass index had higher HbA1c compared with those with high percent body fat or low skeletal muscle mass index alone.22, 25 Furthermore, low muscle mass attenuated the exercise response on changes in visceral adipose tissue, insulin resistance, and triglyceride concentration in individuals that were affected by obesity, but not in individuals that were lean.22 How exactly low muscle mass can act synergistically with increased adiposity to worsen metabolic control is an intriguing question and should be the topic of further investigation.
In contrast to the few studies that suggest a direct role for muscle loss in relation to the development of T2D, there are more studies that argue that muscle loss is a consequence of T2D.32, 33, 34, 35 A longitudinal study showed that the decline in muscle mass in subjects with T2D happens twice as fast compared with those without diabetes, with the greatest declines in lean mass in persons with undiagnosed diabetes.8 Also, in patients undergoing chronic dialysis who were followed over the course of a year, the presence of diabetes was the most significant independent predictor of muscle loss.35, 36 The reason behind the increased prevalence of muscle loss in individuals with T2D is still under debate. Some studies found that the decrease in muscle strength was related to the severity of neuropathy but not to degree of nephropathy or retinopathy, suggesting that the decrease in muscle strength may be, at least partly, due to neuropathy.32, 37, 38 It has also been suggested that insulin resistance may contribute to the development of muscle loss. Insulin plays an important role in the regulation of muscle protein metabolism by direct activation of the translation machinery and via vasodilatory actions; thus, it has been hypothesized that insulin resistance may lead to a decreased anabolic response, resulting in muscle loss. Several studies would support this hypothesis. For example, it was shown that chronic intake of sucrose induced insulin resistance and accelerated muscle loss in old rats.39 In a longitudinal study in 3000 older men without diabetes, it was shown that lean mass loss over the course of approximately 5 years was more pronounced, while fat mass gain was less pronounced in men that were insulin resistant as compared with men that were insulin sensitive.34 Treatment with metformin resulted in a significant improvement in insulin sensitivity in adults with newly diagnosed T2D, and this went along with an improvement in lean‐to‐fat ratio.40 Women with obesity showed a blunted protein anabolic response to hyperinsulinaemia.41 And it was also found that lipid‐induced insulin resistance was associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men.42 However, whether the changes in muscle mass are due to changes in insulin sensitivity per se is difficult to say. Animal research indicated that mice can develop insulin resistance without subsequently developing muscle atrophy.43 Furthermore, in the studies discussed above,34, 39, 40, 41, 42 there was a simultaneous change in adiposity or ectopic lipid accumulation. It is therefore also possible that the insulin resistance is merely a reflection of adiposity and that muscle mass changes due to increased lipid availability.
3. DETERMINANTS OF MUSCLE MASS
Muscle mass is regulated by the delicate balance between muscle protein synthesis and muscle protein breakdown. Food—and protein in particular—is an important stimulator of muscle protein synthesis. The postprandial muscle protein synthetic response to feeding is regulated on a number of levels, including dietary protein digestion and amino acid absorption, splanchnic amino acid retention, postprandial insulin release, skeletal muscle tissue perfusion, amino acid uptake by muscle, and intramyocellular signalling.44 Muscle contraction is a very potent anabolic stimulus that can further increase basal as well as postprandial muscle protein synthesis rates. Interestingly, the anabolic response to food and exercise is blunted in older individuals.45 This may be due to a delay in amino acid digestion or absorption, or to a decrease in physical activity levels. Alternatively, it could be caused by excess adiposity or circulating lipid levels. It is estimated that approximately 80% of all individuals with T2D are affected by overweight or obesity,13 which leads to increased lipid deposition in visceral14, 15 and ectopic fat depots, including skeletal muscle and liver.15, 16, 17, 18 In addition, many patients with T2D have decreased physical activity levels,46 which contributes to the accumulation of fat. While ectopic fat accumulation in individuals with T2D is usually the result of obesity, fat infiltration in organs in older individuals can happen independent of weight changes.47 Nevertheless, there are indications in both groups that expansion of adipose tissue, as well as lipid accumulation in other organs, plays an important role in the development of muscle atrophy. To test the hypothesis that the development of anabolic resistance can be caused by excess lipids, a study was performed in which young healthy volunteers received a 7‐hour saline or intralipid infusion on two randomized occasions.42 The authors observed that excess lipid availability per se induced insulin resistance of skeletal muscle glucose metabolism as well as anabolic resistance of amino acid metabolism, and given the randomized crossover design, it could be concluded that this was independently of any changes in amino acid handling or physical activity levels.42 It is therefore likely that an increase in lipid levels in older people and people with T2D contribute to the development of muscle atrophy. In the following paragraphs, several possible pathways will be discussed.
3.1. Expansion of the adipose tissue
Besides being a lipid buffering organ, adipose tissue is a major endocrine organ and is known to secrete hundreds of adipokines.48 Adipokines are important regulators of metabolism, and expansion of adipose tissue leads to a change in the secretion of adipokines, which have been linked to the development of insulin resistance in skeletal muscle through interorgan crosstalk (Figure 2).49, 50 Adipokines that are well‐known to modulate metabolism and insulin sensitivity include leptin,51 retinol binding protein 4 (RBP4),52 adiponectin,53 resistin,54 and pigment epithelium‐derived factor (PEDF).55 Macrophages are also believed to be an important contributor of insulin resistance.56, 57 Macrophages account for up to 40% of adipose cell content in individuals with obesity compared with only 10% in lean humans58 and secrete pro‐inflammatory cytokines such as interleukin (IL) 6, IL1β, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), and tumour necrosis factor α (TNFα).59 In addition, macrophage infiltration in adipose tissue leads to adipose tissue dysfunction, thereby contributing to the altered secretion pattern of adipokines. It is interesting to note that the secretion pattern of adipokines and cytokines not only changes with increased adiposity but also varies across fat depots. Specifically, the secretion products of the mesenteric and omental adipose tissue depots are more strongly associated with metabolic complications of obesity compared with the secretion products of the subcutaneous fat depots.60, 61, 62 This is consistent with the fact that individuals with T2D have increased visceral fat depots14, 15 and are characterized by increased inflammatory profile and increased insulin resistance. Several studies show that inflammatory cytokines and adipokines are also related to decreased muscle mass and strength (Figure 2).63 In humans, high levels of IL‐6 (greater than 5 pg/mL) was associated with a twofold to threefold greater risk of losing more than 40% of muscle strength.64 Furthermore, individuals that performed low on a physical activity test were characterized by smaller muscle volume and lower muscle strength and had higher levels of interleukin 1β, 6, 10, 12, 13, TNFα, IL‐6, and GM‐CSF compared with individuals that performed well on the physical activity test.65, 66, 67 Concentrations of the anti‐inflammatory adipokine adiponectin were found to be decreased in individuals with sarcopenia,66 while levels of the pro‐inflammatory adipokine leptin68 were increased. Although there seems to be a lot of evidence to suggest that adipokines and cytokines contribute to lower muscle mass and strength, the studies mentioned here are cross‐sectional studies, and therefore, it is difficult to differentiate between cause and consequence. Nevertheless, it has been shown in diet‐induced obese mice that administration of quercetin reduced levels of inflammatory cytokines and macrophage accumulation in the skeletal muscle, along with reduced transcript and protein levels of the specific atrophic factors, Atrogin‐1 and muscle RING finger 1 (MuRF1), and protected as such against the reduction of muscle mass and muscle fibre size.69 Furthermore, in 2015, Pellegrinelli et al demonstrated for the first time that the secretome of human obese adipocytes directly decreased the expression of contractile proteins in myotubes, consequently inducing atrophy,70 and this latter study provides evidence for a direct link between the adipose tissue secretome and the development of muscle loss. In support of this, also, O'Leary et al recently found that the secretome of human obese subcutaneous adipose tissue impaired the myogenesis of old myoblasts, an effect that was mediated via resistin‐induced activation of NFκB.71 Interestingly, the secretome of obese subcutaneous adipose tissue did not impair myogenesis of young myoblasts,71 suggesting that the effect of obesity on muscle mass may be particularly harmful in older individuals.
Figure 2.
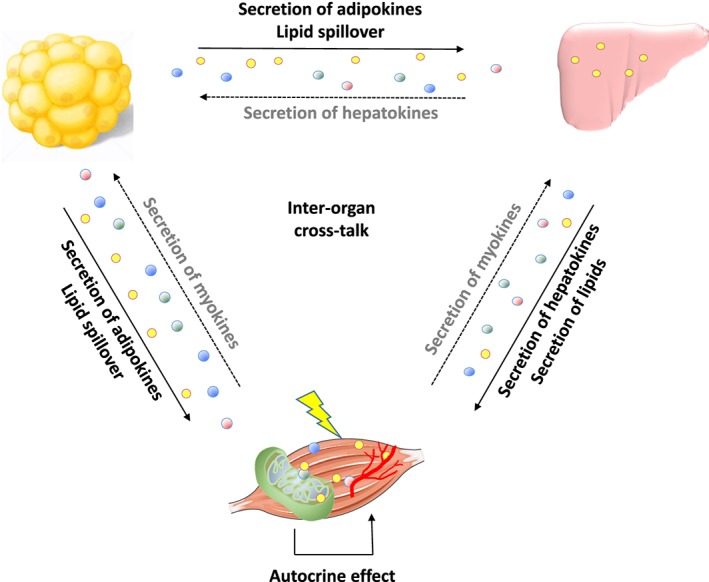
Schematic overview of interorgan crosstalk between adipose tissue, liver, and skeletal muscle, leading to muscle insulin resistance and decrease of muscle mass. Increased lipid deposition in visceral and ectopic fat depots include skeletal muscle and liver. Increased lipid levels may lead to a change in the secretion pattern of cytokines, which may lead to muscle insulin resistance and a decrease in muscle mass via interorgan crosstalk. Lipids and cytokines may also affect mitochondrial function and vascularization in skeletal muscle, which contributes to the problem. Full arrows indicate pathways discussed in this review. Broken arrows indicate pathways outside the scope of this review
3.2. Lipid accumulation in the liver
Liver steatosis is clinically defined as a hepatic triglyceride content that exceeds 5% of the total liver weight72 and is present in many individuals with obesity and in patients with T2D. Studies showed that approximately 60% of liver triglyceride content originates from lipolysis in adipose tissue. It is likely that in individuals with obesity, enlarged adipose tissue depots as well as insulin resistance of the adipose tissue contribute to fat disposition in the liver, thereby providing a link between obesity, insulin resistance, and liver steatosis. The liver on its turn also secretes a wide range of lipids, including high levels of triacylglycerols via the secretion of very‐low‐density lipoproteins.73 These lipids can accumulate in skeletal muscle and contribute to the development of insulin resistance (see following paragraph). In addition, lipids secreted by the liver can also play an important role in the development of atherosclerosis and increase the risk for cardiovascular problems and other complications such as nephropathy, retinopathy, and neuropathy. Neuropathy can result in deceased muscle strength, decreased physical activity, and muscle atrophy. Importantly, impaired vascular function also hampers glucose and insulin delivery to the muscle, which leads to a worsening of the hyperglycaemic state and a further deteriorating health.
In recent years, there has also been a growing interest regarding the role of liver‐secreted proteins (ie, hepatokines) in relation to the development of insulin resistance.74, 75 We showed that the protein secretory profile of hepatocytes is altered with steatosis and that this altered profile leads to inflammation and insulin resistance in muscle cells (Figure 2).76 We also identified fetuin B as a liver‐secreted protein that is increased in individuals with obesity and in patients with liver steatosis and that impairs glucose uptake in mice.76 Examples of other liver‐derived endocrine factors that have been linked to insulin resistance and impaired glucose metabolism include fetuin A,77 adropin,78 angiopoietin‐like protein 6,79 and selenoprotein P.80 In the last few years, liver‐secreted proteins have also been linked to muscle wasting (Figure 2). For example, individuals with high levels of α1‐antichymotrypsin were 40% less likely to experience loss of muscle strength and tended to have a smaller decline in muscle mass compared with those with low levels of α1‐antichymotrypsin.64 In addition, Cystatin C and Beta‐2‐macroglob ulin, two liver‐secreted proteins, were found to be positively associated with the development of severe muscle loss81, 82, 83; fetuin A was identified as a predictor of sarcopenic left ventricular dysfunction84; and tissue iron levels were elevated in muscle loss, which went along with an increase in transferrin, another liver‐secreted protein. Interestingly, secretion of Cystatin C and Beta‐2‐macroglobulin, fetuin A, and transferrin is increased from a fatty liver,76 suggesting a role for liver steatosis in the development of muscle loss. Unfortunately, research regarding the role of hepatokines is only in its infancies, and future studies are needed to identify the mechanisms through which certain hepatokines regulate insulin resistance and muscle loss.
3.3. Lipid accumulation in skeletal muscle
Intramyocellular lipid droplets (IMCLs) are lipid droplets present in skeletal muscle tissue. IMCLs are dynamic functional organelles, involved in lipid metabolism, vesicle trafficking, and cell signalling, and they are covered and surrounded by lipases and lipid droplet coating proteins that play an important role in controlling the balance between IMCL storage, mobilization, and oxidation (for extended reviews on this topic, please refer to previous works85, 86, 87, 88, 89). While IMCL is important for energy metabolism, lipid overflow from the expanded adipose tissue and the liver leads to increased fat deposition in skeletal muscle. In addition, also, resistin has been shown to promote IMCL storage in primary human myotubes.71 Importantly, increased IMCL storage results in the accumulation and dysregulation of detrimental lipid intermediates such as diacylglycerols (DAGs) and ceramides, and those intermediates lead to insulin resistance through activation of protein kinase C (PKC).90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100 High levels of DAG and ceramides have also been suggested to play a role in the development of muscle loss.16, 101, 102, 103 In C2C12 and L6 myotubes, treatment with the fatty acid palmitate induced ceramide accumulation, and this was associated with increased expression of pro‐atrophic genes such as atrogin‐1/MAFbx, increased levels of FoxO3, upregulated eIF2α phosphorylation, and decreased protein synthesis.16, 101, 104 Conversely, blocking ceramide synthesis prevented muscle atrophy, and this went along with improved mammalian target of rapamycin (mTOR) signalling, suppressed levels of Foxo3, and decreased atrogin‐1/MAFbx expression.103 Rivas and colleagues found that obese animals had significantly higher storage of ceramide and DAG compared with lean, and there was an attenuated insulin response in components of the mTOR anabolic signalling pathway.102 Interestingly, conversion of DAG to phosphatidic acid (PA) activated the mTOR signalling pathway and resulted in hypertrophy in isolated mouse extensor digitorum longus muscle.105 In skeletal muscle of older humans, there were no observable differences in total ceramide content; however, ceramide species C16:0 was increased with 156% and C20:0‐ceramide was up with 30%. In addition, there was a negative correlation between C16:0‐ceramide content with lower leg lean mass and an attenuated activation of anabolic signalling molecules such as Akt, FOXO1, and S6K1 after an acute bout of high‐intensity resistance exercise.106 When ageing and obesity in mice occurred in combination, ceramide accumulation was even more pronounced and negatively affected protein synthesis rate.16 Thus, there seems to be evidence that lipid intermediates in skeletal muscle such as DAG's and ceramides negatively affect insulin sensitivity as well as muscle mass. For completeness, however, it should be mentioned that there are also studies that report conflicting results regarding the role of lipid intermediates in relation to insulin resistance as well as muscle loss. For example, Turpin et al found that apoptosis in skeletal muscle myotubes was induced by ceramides104 but could not find any signs of apoptosis, autophagy, or proteolysis in high fat diet fed mice, in ob/ob mice, or in mice after an intralipid infusion.107 Moreover, a number of studies dissociated increased DAG levels from the development of insulin resistance,108, 109, 110 suggesting that this relationship is not quite as straightforward as previously thought. Possible explanations for these inconsistencies include not taking into account the importance of compartmentalization of the lipids, ignoring the structures, chain lengths, and the degree of saturation of the lipids, or bypassing any information on oxidation rates and fluxes. Either way, more research will be needed to establish the exact role of IMCL and lipid intermediates in the development of insulin resistance and muscle loss.
3.4. Mitochondrial dysfunction
Skeletal muscle mitochondrial dysfunction has previously been linked to insulin resistance.111, 112 Kelley et al observed mitochondrial abnormalities with respect to content, size, and morphology in patients with T2D,113 while Mootha et al showed a lower gene expression of peroxisome proliferator‐activated receptor gamma coactivator 1‐alpha (PGC1α), a key transcriptional cofactor in mitochondrial biogenesis, and a lower gene expression of its target genes encoding key enzymes in oxidative mitochondrial metabolism.114 Decreased mitochondrial function has also been reported in first degree relatives of patients with T2D,111, 115, 116 suggesting that mitochondrial dysfunction may actually be causally related to insulin resistance (Figure 2). It is interesting to note that also age‐related muscle loss has also been associated with mitochondrial dysfunction. Specifically, age‐related muscle loss has been linked to increased reactive oxygen species (ROS) production, increased mitochondrial apoptotic susceptibility, decline in mitochondrial respiratory chain function,117 reduced transcriptional drive for mitochondrial biogenesis,118 and morphological changes in mitochondria119; and also, most,120, 121, 122, 123, 124 but not all, studies125, 126 state that the capacity to mobilize and/or oxidize IMCL is substantially impaired in older individuals. In congruence with this, it has recently been suggested that accumulation of dysfunctional mitochondria initiate a signalling cascade leading to motor neuron and muscle fibre death and culminating in muscle loss (Figure 2).127 Not all studies point in the same direction though, and a number of articles raised doubts concerning mitochondrial dysfunction as a causal factor in the development of insulin resistance and muscle atrophy.128, 129, 130, 131, 132 A study reported normal in vivo mitochondrial function in Zucker diabetic rats throughout the pathogenesis of T2D130 and comparable in vivo mitochondrial function was found between subjects with normal glycaemia, individuals with impaired glucose tolerance, and individuals with T2D.131 In addition to that, we showed that although mitochondrial function was decreased in subjects with T2D, it could be completely restored towards the levels of healthy control subjects upon an exercise training programme, whereas insulin sensitivity could only be partially restored.133 We also recently showed that 5 days of muscle disuse was sufficient to induce substantial loss of both muscle mass and strength in young and older subjects but did not reduce the maximal activity of key mitochondrial enzymes.129 It seems fair to state that mitochondrial dysfunction is present in many individuals with T2D and may contribute to the development or progression of insulin sensitivity and muscle atrophy. However, considering the multifactorial disease that T2D is, mitochondrial dysfunction cannot be regarded as the main or sole causal factor in general.
4. STRATEGIES TO COUNTERACT INSULIN RESISTANCE AND MUSCLE LOSS
It may be clear that IMCL accumulation is strongly associated with the severity of insulin resistance; however, this is only true for individuals that are sedentary or untrained. Trained athletes are also characterized by elevated fat content in the muscle, but in contrast to their sedentary counterparts, they are highly insulin sensitive.134, 135, 136, 137 The precise mechanism by which trained persons are protected from the insulin desensitizing effects of IMCL is still incompletely understood, but here is substantial evidence that exercise enhances the regulation of lipid droplet degradation and synthesis and increases oxidative capacity, resulting in lower levels of detrimental lipid intermediates.138, 139 Furthermore, exercise leads to improved insulin sensitivity in adipose tissue, possibly resulting in lower levels of uncontrolled fatty acid release,140 and thus less fatty acid spillover to skeletal muscle and liver.141 Considering this, it is no wonder that exercise is fundamental in the treatment of diabetes.142 Exercise can delay and reduce the incidence of diabetes in persons at high risk by 58%143 and can improve (or even restore) abnormalities in oxidative capacity, substrate selection (ie, metabolic flexibility),133 mitochondrial function and density,133 capillary density, and lipid profile.144 Interesting from a therapeutic point of view is the evidence that exercise is at least as effective in older participants as it is in younger participants143 and that also patients on long‐standing insulin treatment can benefit from exercise training.145 For example, in a group of patients with long‐standing T2D on insulin therapy, 1 year of exercise training raised in vivo skeletal muscle ATP production capacity by 21%, increased expression levels of β‐oxidation, Krebs cycle, and oxidative phosphorylation system‐related genes, and tended to increase mitochondrial density and complex I activity,132 underscoring the importance of exercise in the treatment of T2D. Also, nutritional intervention programmes have been shown to be successful in preventing or delaying the onset insulin resistance. Especially weight reduction has been shown to lead to improved insulin sensitivity,146, 147, 148 suggesting that nutritional treatment should primarily focus on achieving weight loss. The underlying mechanism may differ from exercise though; while exercise training leads to an improved mitochondrial function but not to a decreased IMCL content, the opposite observation has been made after weight loss.149 Importantly, exercise and nutritional interventions are also key in the treatment of muscle loss. Muscle loss is mainly attributed to a decrease in type 2 muscle fibers size,150, 151, 152 and it has been shown that 6 months of strength training can increase type 2 fibers size up to 24%.150, 153 In addition to that, resistance exercise has been shown to prevent muscle atrophy and strength loss in subjects undergoing step reduction154 and bed rest,155 while their sedentary counterparts displayed a deterioration in muscle mass and strength. Also, in very old adults in institutions, exercise has shown to be effective156, 157; a 6‐month exercise training programme consisting of twice weekly resistance and balance exercises resulted in a significant increase in grip strength, which may transfer to reduce disability and muscle loss transition.156 Not only traditional high‐load strength exercise has been shown to be effective in the treatment of muscle loss. Low‐load, high‐volume exercise (30% one repetition maximum [1RM]) to volitional fatigue,158 low‐load blood flow restricted exercise, and walking154, 159 has also shown to stimulate muscle protein synthesis rate. Nutrition is often given in combination with exercise to counter muscle loss, and interestingly, it has been shown that they act synergistically to increase muscle protein synthesis.160 Importantly though, nutritional interventions should focus on obtaining fat mass loss as well as on ways to improve protein synthesis and gain lean mass. There are indications that older individuals and individuals with obesity have a blunted muscle protein synthesis response upon protein ingestion,161 and increasing both the quality and the dose of protein might be an effective strategy to maximally stimulate muscle protein synthesis.162, 163, 164 (For informative reviews regarding the role of exercise and nutrition in relation muscle loss, see other works165, 166, 167). It must be noted though that exercise as well as weight loss can improve insulin sensitivity in patients with T2D without increasing muscle mass.139, 168 This is not necessarily surprising as it is compatible with the multifactorial character of the disease. Apart from ectopic lipid disposition, insulin sensitivity is also determined by other parameters such as vascularization and oxidative capacity, and these parameters can improve without absolute changes in muscle mass. On a similar note, also, changes in muscle strength do not necessarily go hand in hand with changes in muscle mass; longitudinal studies previously showed that the decline in strength in patients with T2D is much more rapid than the concomitant loss of muscle mass.169 In fact, changes in lean mass can only explain a small part (5%) of the variability in strength decline, underscoring the importance of muscle quality and the central neural and neuromuscular components determining muscle strength and function.169, 170
Apart from exercise and nutrition, also, pharmacological interventions are applied to treat patients with T2D. The use of metformin is widespread among patients with T2D. Metformin ameliorates hyperglycaemia via suppression of hepatic glucose production and via improvements in peripheral glucose uptake, and although the exact mode of action is not entirely clear, metformin is known for its weight‐loss properties.171 Some mechanistic studies also demonstrated a positive effect on lipid accumulation in the liver. However, other studies show that the effect on the liver was no longer present after correction for weight loss or that it could be attributed to the use of suprapharmacological dosages of metformin.172 Also, multiple meta‐analysis studies suggested that metformin has no effect on histological responses in the liver.171, 173, 174 Nevertheless, due to its safe use and its weight‐losing and insulin‐sensitizing properties, the use of metformin remains the first‐line agent to treat insulin resistance in patients with T2D. Another well‐known class of medication that improves insulin sensitivity in individuals with T2D are the peroxisome proliferator‐activated receptors gamma (PPARγ) agonists thiazolidinediones (TZDs).175, 176 Interestingly enough, TZD use leads to an increase in extramyocellular lipid content and sometimes even weight gain.177, 178 On the other hand, TZDs ameliorate ectopic fat storage and improve lipid storage and metabolism in adipocytes,177 and this finding supports the notion that it is not the total amount of fat mass that is important, but rather where and how the fat is stored. Unfortunately, not all patients respond to TZDs, and its clinical use is also limited due to adverse events,176 and therefore, other pharmacological therapies should be explored. In the past few years, a lot of research has been directed towards identifying new molecular mechanisms to target for the treatment of nonalcoholic fatty liver disease (NAFLD). Potential drug targets include microRNAs, incretin analogues/antagonists, liver‐specific thyromimetics, AMP‐activated protein kinase activators, farnesoid X receptor (FXR) and pregane X receptor (PXR) agonists, fibroblasts growth factor 19 (FGF‐19) and fibroblasts growth factor 21 (FGF‐21) analogues, and antilipemic agents.179 There is evidence that some of these drugs improve liver steatosis and fibrosis, and ameliorate lipid metabolism and insulin sensitivity in cellular and animal models. However, (long‐term) efficiency has not yet been proven in humans, and some drugs come with severe side effects.179, 180, 181 Therefore, more basic research and more randomized controlled trials of adequate size and duration are needed in the search for novel treatments. Also, pharmacological targets to treat muscle loss are sparse. In 2014, a review summarized the impact of different therapeutic interventions in relation to body composition changes in men and females that were older and affected by obesity. The authors concluded that weight loss based on diet combined with exercise—and not drugs—represents the best strategy to treat phenotypic aspects of sarcopenic obesity.182 A very recent study investigated systematic reviews and meta‐analyses. The study included vitamin D, combined oestrogen‐progesterone, dehydroepiandrosterone, growth hormone, growth hormone‐releasing hormone, combined testosterone‐growth hormone, insulin‐like growth factor‐1, pioglitazone, testosterone, and angiotensin‐converting enzyme inhibitors. While the authors found that testosterone and vitamin D mildly increased muscle mass in older males and females respectively, this was only the case in individuals with low starting values.183 Up to now, there is insufficient evidence to recommend pharmacological interventions for the treatment of muscle loss, and more research is needed in this field.183
5. CONCLUSION
Many patients with T2D are characterized by peripheral insulin resistance and muscle atrophy. Derangements in lipid storage have been identified as a hallmark of the development and progression of insulin resistance, and an increasing amount of evidence suggest that it also plays an important role in the development of muscle atrophy. The exact mechanisms have not been established yet, but there is evidence that it may be via the accumulation of harmful lipid intermediates in skeletal muscle, decreased mitochondrial function, lipid accumulation in other organs, or a combination of those. Unfortunately, research is often hampered by the fact that many patients with T2D have decreased physical activity levels compared with the rest of the population. In many studies, it is therefore difficult to distinguish whether insulin resistance and muscle loss are caused by the mechanisms mentioned in this review and/or by a decrease in exercise per se. Furthermore, since insulin signalling plays a permissive role in skeletal muscle amino acids delivery and metabolism, it is possible that insulin resistance may aggravate skeletal muscle anabolic resistance. Future research will be needed to investigate the underlying mechanisms of muscle atrophy. This is important not only in the setting of T2D but also in other conditions of lipid‐induced insulin resistance, including ageing, disuse, and critical illness. With regard to drug therapy, there are currently no well‐established guideline recommendations to reduce lipid accumulation in the liver or other organs. Results of basic research over the past decade have been encouraging, and a number of new molecular mechanisms have been explored. However, most drugs pose long‐term safety issues, and so the biggest challenge lies in the translation into the clinical setting. Up till now, nutritional and exercise interventions are key in the treatment of T2D and muscle loss, and if possible, both should be applied to achieve optimal results.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this paper.
FINANCIAL SUPPORT
R.C.R.M. is supported by a “Marie Sklodowska Curie individual fellowship” by the European Commission (H2020‐MSCA‐IF).
Meex RCR, Blaak EE, van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obesity Reviews. 2019;20:1205–1217. 10.1111/obr.12862
REFERENCES
- 1. Collaboration NCDRF . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population‐based studies with 4.4 million participants. Lancet. 2016;387:1513‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271‐281. [DOI] [PubMed] [Google Scholar]
- 3. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55(6):1813‐1818. [DOI] [PubMed] [Google Scholar]
- 4. Leenders M, Verdijk LB, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14(8):585‐592. [DOI] [PubMed] [Google Scholar]
- 5. Guerrero N, Bunout D, Hirsch S, et al. Premature loss of muscle mass and function in type 2 diabetes. Diabetes Res Clin Pract. 2016;117:32‐38. [DOI] [PubMed] [Google Scholar]
- 6. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010;33(7):1497‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30(6):1507‐1512. [DOI] [PubMed] [Google Scholar]
- 9. Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and nutrition examination survey (NHANES), 1999‐2006. Diabetes Care. 2010;33(5):1055‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31(5):652‐658. [DOI] [PubMed] [Google Scholar]
- 11. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ulley J, Abdelhafiz AH. Frailty predicts adverse outcomes in older people with diabetes. Practitioner. 2017;261(1800):17‐20. [PubMed] [Google Scholar]
- 13. Lean ME. Obesity: burdens of illness and strategies for prevention or management. Drugs Today (Barc). 2000;36(11):773‐784. [DOI] [PubMed] [Google Scholar]
- 14. Hirose H, Takayama M, Iwao Y, Kawabe H. Effects of aging on visceral and subcutaneous fat areas and on homeostasis model assessment of insulin resistance and insulin secretion capacity in a comprehensive health checkup. J Atheroscler Thromb. 2016;23(2):207‐215. [DOI] [PubMed] [Google Scholar]
- 15. Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot‐specific mechanisms. Exp Gerontol. 2007;42(6):463‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tardif N, Salles J, Guillet C, et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2α activation. Aging Cell. 2014;13(6):1001‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zoico E, Rossi A, Di Francesco V, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65:295‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89(8):3864‐3871. [DOI] [PubMed] [Google Scholar]
- 19. Abbasi F, Brown BW Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40(5):937‐943. [DOI] [PubMed] [Google Scholar]
- 20. Heber D, Ingles S, Ashley JM, Maxwell MH, Lyons RF, Elashoff RM. Clinical detection of sarcopenic obesity by bioelectrical impedance analysis. Am J Clin Nutr. 1996;64(3):472S‐477S. [DOI] [PubMed] [Google Scholar]
- 21. Reinders I, Visser M, Schaap L. Body weight and body composition in old age and their relationship with frailty. Curr Opin Clin Nutr Metab Care. 2017;20(1):11‐15. [DOI] [PubMed] [Google Scholar]
- 22. Terada T, Boule NG, Forhan M, et al. Cardiometabolic risk factors in type 2 diabetes with high fat and low muscle mass: at baseline and in response to exercise. Obesity (Silver Spring). 2017;25(5):881‐891. [DOI] [PubMed] [Google Scholar]
- 23. Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116(7):1171‐1178. [DOI] [PubMed] [Google Scholar]
- 24. Son JW, Lee SS, Kim SR, et al. Low muscle mass and risk of type 2 diabetes in middle‐aged and older adults: findings from the KoGES. Diabetologia. 2017;60(5):865‐872. [DOI] [PubMed] [Google Scholar]
- 25. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity‐associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS ONE. 2010;5(5):e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24‐hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123‐148. [DOI] [PubMed] [Google Scholar]
- 28. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5(6):1003‐1015. [DOI] [PubMed] [Google Scholar]
- 29. Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19(9):1018‐1030. [DOI] [PubMed] [Google Scholar]
- 30. Cai D, Frantz JD, Tawa NE Jr, et al. IKKbeta/NF‐kappaB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285‐298. [DOI] [PubMed] [Google Scholar]
- 31. Koo HS, Kim MJ, Kim KM, Kim YS. Decreased muscle mass is not an independent risk factor for metabolic syndrome in Korean population aged 70 or older. Clin Endocrinol. 2015;82(4):509‐516. [DOI] [PubMed] [Google Scholar]
- 32. Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes. 2004;53(6):1543‐1548. [DOI] [PubMed] [Google Scholar]
- 33. Workeneh B, Bajaj M. The regulation of muscle protein turnover in diabetes. Int J Biochem Cell Biol. 2013;45(10):2239‐2244. [DOI] [PubMed] [Google Scholar]
- 34. Lee CG, Boyko EJ, Strotmeyer ES, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59(7):1217‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pupim LB, Heimburger O, Qureshi AR, Ikizler TA, Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005;68(5):2368‐2374. [DOI] [PubMed] [Google Scholar]
- 36. Pupim LB, Flakoll PJ, Majchrzak KM, Aftab Guy DL, Stenvinkel P, Ikizler TA. Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int. 2005;68(4):1857‐1865. [DOI] [PubMed] [Google Scholar]
- 37. Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles—a follow‐up study of long‐term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia. 2009;52(6):1182‐1191. [DOI] [PubMed] [Google Scholar]
- 39. Gatineau E, Savary‐Auzeloux I, Migne C, Polakof S, Dardevet D, Mosoni L. Chronic intake of sucrose accelerates sarcopenia in older male rats through alterations in insulin sensitivity and muscle protein synthesis. J Nutr. 2015;145(5):923‐930. [DOI] [PubMed] [Google Scholar]
- 40. Aghili R, Malek M, Valojerdi AE, Banazadeh Z, Najafi L, Khamseh ME. Body composition in adults with newly diagnosed type 2 diabetes: effects of metformin. J Diabetes Metab Disord. 2014;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. Whole‐body protein anabolic response is resistant to the action of insulin in obese women. Am J Clin Nutr. 2005;82:355‐365. [DOI] [PubMed] [Google Scholar]
- 42. Stephens FB, Chee C, Wall BT, et al. Lipid‐induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men. Diabetes. 2015;64(5):1615‐1620. [DOI] [PubMed] [Google Scholar]
- 43. Ostler JE, Maurya SK, Dials J, et al. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am J Physiol Endocrinol Metab. 2014;306(6):E592‐E605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gorissen SH, Remond D, van Loon LJ. The muscle protein synthetic response to food ingestion. Meat Sci. 2015;109:96‐100. [DOI] [PubMed] [Google Scholar]
- 45. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41:169‐173. [DOI] [PubMed] [Google Scholar]
- 46. Crespo CJ, Keteyian SJ, Heath GW, Sempos CT. Leisure‐time physical activity among US adults: results from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 1996;156:93‐98. [PubMed] [Google Scholar]
- 47. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lehr S, Hartwig S, Lamers D, et al. Identification and validation of novel adipokines released from primary human adipocytes. Mol Cell Proteomics. 2012;11(1):M111 010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ebert T, Roth I, Richter J, et al. Different associations of adipokines in lean and healthy adults. Horm Metab Res. 2014;46(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 50. Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36(7):461‐470. [DOI] [PubMed] [Google Scholar]
- 51. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763‐770. [DOI] [PubMed] [Google Scholar]
- 52. Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356‐362. [DOI] [PubMed] [Google Scholar]
- 53. Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty‐acid oxidation by activating AMP‐activated protein kinase. Nat Med. 2002;8(11):1288‐1295. [DOI] [PubMed] [Google Scholar]
- 54. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307‐312. [DOI] [PubMed] [Google Scholar]
- 55. Crowe S, Wu LE, Economou C, et al. Pigment epithelium‐derived factor contributes to insulin resistance in obesity. Cell Metab. 2009;10(1):40‐47. [DOI] [PubMed] [Google Scholar]
- 56. Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest. 2003;112(12):1821‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heilbronn LK, Liu B. Do adipose tissue macrophages promote insulin resistance or adipose tissue remodelling in humans? Horm Mol Biol Clin Invest. 2014;20:3‐13. [DOI] [PubMed] [Google Scholar]
- 60. Mazaki‐Tovi M, Bolin SR, Schenck PA. Differential secretion of adipokines from subcutaneous and visceral adipose tissue in healthy dogs: association with body condition and response to troglitazone. Vet J. 2016;216:136‐141. [DOI] [PubMed] [Google Scholar]
- 61. Kranendonk ME, van Herwaarden JA, Stupkova T, et al. Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis. 2015;239(2):419‐427. [DOI] [PubMed] [Google Scholar]
- 62. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin‐6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847‐850. [DOI] [PubMed] [Google Scholar]
- 63. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age‐associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200‐221. [DOI] [PubMed] [Google Scholar]
- 64. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526.e9‐526.e17. [DOI] [PubMed] [Google Scholar]
- 65. Calvani R, Marini F, Cesari M, et al. Systemic inflammation, body composition, and physical performance in old community‐dwellers. J Cachexia Sarcopenia Muscle. 2017;8(1):69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Can B, Kara O, Kizilarslanoglu MC, et al. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin Exp Res. 2017;29(4):745‐752. [DOI] [PubMed] [Google Scholar]
- 67. Toth MJ, Ades PA, Tischler MD, Tracy RP, LeWinter MM. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. Int J Cardiol. 2006;109:179‐187. [DOI] [PubMed] [Google Scholar]
- 68. Kohara K, Ochi M, Tabara Y, Nagai T, Igase M, Miki T. Leptin in sarcopenic visceral obesity: possible link between adipocytes and myocytes. PLoS ONE. 2011;6:e24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Le NH, Kim CS, Park T, et al. Quercetin protects against obesity‐induced skeletal muscle inflammation and atrophy. Mediat Inflamm. 2014;2014:834294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pellegrinelli V, Rouault C, Rodriguez‐Cuenca S, et al. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes. 2015;64(9):3121‐3134. [DOI] [PubMed] [Google Scholar]
- 71. O'Leary MF, Wallace GR, Davis ET, et al. Obese subcutaneous adipose tissue impairs human myogenesis, particularly in old skeletal muscle, via resistin‐mediated activation of NFκB. Sci Rep. 2018;8(1):15360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kleiner DE, Brunt EM, van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313‐1321. [DOI] [PubMed] [Google Scholar]
- 73. Peng KY, Watt MJ, Rensen S, et al. Mitochondrial dysfunction‐related lipid changes occur in nonalcoholic fatty liver disease progression. J Lipid Res. 2018;59(10):1977‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509‐520. [DOI] [PubMed] [Google Scholar]
- 75. Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9:144‐152. [DOI] [PubMed] [Google Scholar]
- 76. Meex RC, Hoy AJ, Morris A, et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab. 2015;22(6):1078‐1089. [DOI] [PubMed] [Google Scholar]
- 77. Pal D, Dasgupta S, Kundu R, et al. Fetuin‐A acts as an endogenous ligand of TLR4 to promote lipid‐induced insulin resistance. Nat Med. 2012;18(8):1279‐1285. [DOI] [PubMed] [Google Scholar]
- 78. Kumar KG, Trevaskis JL, Lam DD, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8(6):468‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oike Y, Akao M, Yasunaga K, et al. Angiopoietin‐related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11(4):400‐408. [DOI] [PubMed] [Google Scholar]
- 80. Misu H, Takamura T, Takayama H, et al. A liver‐derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12(5):483‐495. [DOI] [PubMed] [Google Scholar]
- 81. Kim H, Suzuki T, Kim M, et al. Incidence and predictors of sarcopenia onset in community‐dwelling elderly Japanese women: 4‐year follow‐up study. J Am Med Dir Assoc. 2015;16(1):85.e1‐85.e8. [DOI] [PubMed] [Google Scholar]
- 82. Kim JK, Choi SR, Choi MJ, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end‐stage renal disease. Clin Nutr. 2014;33(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 83. Lippi G, Sanchis‐Gomar F, Montagnana M. Biological markers in older people at risk of mobility limitations. Curr Pharm Des. 2014;20(19):3222‐3244. [DOI] [PubMed] [Google Scholar]
- 84. Chang WT, Tsai WC, Wu CH, et al. Fetuin‐A as a predicator of sarcopenic left ventricular dysfunction. Sci Rep. 2015;5(1):12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Badin PM, Langin D, Moro C. Dynamics of skeletal muscle lipid pools. Trends Endocrinol Metab. 2013;24(12):607‐615. [DOI] [PubMed] [Google Scholar]
- 86. Bosma M. Lipid droplet dynamics in skeletal muscle. Exp Cell Res. 2016;340:180‐186. [DOI] [PubMed] [Google Scholar]
- 87. Meex RC, Schrauwen P, Hesselink MK. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol. 2009;297:R913‐R924. [DOI] [PubMed] [Google Scholar]
- 88. MacPherson RE, Peters SJ. Piecing together the puzzle of perilipin proteins and skeletal muscle lipolysis. Appl Physiol Nutr Metab. 2015;40:641‐651. [DOI] [PubMed] [Google Scholar]
- 89. Stinkens R, Goossens GH, Jocken JW, Blaak EE. Targeting fatty acid metabolism to improve glucose metabolism. Obes Rev. 2015;16(9):715‐757. [DOI] [PubMed] [Google Scholar]
- 90. Conte M, Vasuri F, Bertaggia E, et al. Differential expression of perilipin 2 and 5 in human skeletal muscle during aging and their association with atrophy‐related genes. Biogerontology. 2015;16(3):329‐340. [DOI] [PubMed] [Google Scholar]
- 91. Aquilano K, Baldelli S, La Barbera L, Lettieri Barbato D, Tatulli G, Ciriolo MR. Adipose triglyceride lipase decrement affects skeletal muscle homeostasis during aging through FAs‐PPARα‐PGC‐1α antioxidant response. Oncotarget. 2016;7:23019‐23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cho KA, Kang PB. PLIN2 inhibits insulin‐induced glucose uptake in myoblasts through the activation of the NLRP3 inflammasome. Int J Mol Med. 2015;36:839‐844. [DOI] [PubMed] [Google Scholar]
- 93. Szendroedi J, Yoshimura T, Phielix E, et al. Role of diacylglycerol activation of PKCθ in lipid‐induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111(26):9597‐9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Boon J, Hoy AJ, Stark R, et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62(2):401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Badin PM, Louche K, Mairal A, et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes. 2011;60(6):1734‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Straczkowski M, Kowalska I, Baranowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50(11):2366‐2373. [DOI] [PubMed] [Google Scholar]
- 97. Thrush AB, Brindley DN, Chabowski A, Heigenhauser GJ, Dyck DJ. Skeletal muscle lipogenic protein expression is not different between lean and obese individuals: a potential factor in ceramide accumulation. J Clin Endocrinol Metab. 2009;94(12):5053‐5061. [DOI] [PubMed] [Google Scholar]
- 98. Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat‐induced insulin resistance. J Clin Invest. 2007;117:1679‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jocken JW, Goossens GH, Boon H, et al. Insulin‐mediated suppression of lipolysis in adipose tissue and skeletal muscle of obese type 2 diabetic men and men with normal glucose tolerance. Diabetologia. 2013;56(10):2255‐2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bryner RW, Woodworth‐Hobbs ME, Williamson DL, Alway SE. Docosahexaenoic acid protects muscle cells from palmitate‐induced atrophy. ISRN Obes. 2012;2012:647348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rivas DA, McDonald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R561‐R569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. de Larichaudy J, Zufferli A, Serra F, et al. TNF‐α‐ and tumor‐induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet Muscle. 2012;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab. 2006;291:E1341‐E1350. [DOI] [PubMed] [Google Scholar]
- 105. You JS, Lincoln HC, Kim CR, et al. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem. 2014;289(3):1551‐1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rivas DA, Morris EP, Haran PH, et al. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol (1985). 2012;113(11):1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Turpin SM, Ryall JG, Southgate R, et al. Examination of ‘lipotoxicity’ in skeletal muscle of high‐fat fed and ob/ob mice. J Physiol. 2009;587(7):1593‐1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Amati F. Revisiting the diacylglycerol‐induced insulin resistance hypothesis. Obes Rev. 2012;13(Suppl 2):40‐50. [DOI] [PubMed] [Google Scholar]
- 109. Amati F, Dube JJ, Alvarez‐Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance‐trained athletes? Diabetes. 2011;60(10):2588‐2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dube JJ, Amati F, Toledo FG, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54(5):1147‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Phielix E, Schrauwen‐Hinderling VB, Mensink M, et al. Lower intrinsic ADP‐stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57(11):2943‐2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mogensen M, Sahlin K, Fernstrom M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56(6):1592‐1599. [DOI] [PubMed] [Google Scholar]
- 113. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944‐2950. [DOI] [PubMed] [Google Scholar]
- 114. Mootha VK, Lindgren CM, Eriksson KF, et al. PGC‐1α‐responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267‐273. [DOI] [PubMed] [Google Scholar]
- 115. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin‐resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin‐resistant offspring of type 2 diabetic patients. Diabetes. 2007;56(5):1376‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992;113(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 118. Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7(1):2‐12. [DOI] [PubMed] [Google Scholar]
- 119. Leduc‐Gaudet JP, Picard M, St‐Jean Pelletier F, et al. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget. 2015;6(20):17923‐17937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Blaak EE, van Baak MA, Saris WH. Beta‐adrenergically stimulated fat oxidation is diminished in middle‐aged compared to young subjects. J Clin Endocrinol Metab. 1999;84(10):3764‐3769. [DOI] [PubMed] [Google Scholar]
- 121. Sial S, Coggan AR, Carroll R, Goodwin J, Klein S. Fat and carbohydrate metabolism during exercise in elderly and young subjects. Am J Phys. 1996;271:E983‐E989. [DOI] [PubMed] [Google Scholar]
- 122. Toth MJ, Tchernof A. Lipid metabolism in the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S121‐S125. [DOI] [PubMed] [Google Scholar]
- 123. Calles‐Escandon J, Arciero PJ, Gardner AW, Bauman C, Poehlman ET. Basal fat oxidation decreases with aging in women. J Appl Physiol (1985). 1995;78(1):266‐271. [DOI] [PubMed] [Google Scholar]
- 124. Calles‐Escandon J, Poehlman ET, Garcia‐Rubi E. Lipolysis in elderly postmenopausal women. Metabolism. 1997;46(11):1312‐1315. [DOI] [PubMed] [Google Scholar]
- 125. Melanson EL, Donahoo WT, Grunwald GK, Schwartz R. Changes in 24‐h substrate oxidation in older and younger men in response to exercise. J Appl Physiol (1985). 2007;103:1576‐1582. [DOI] [PubMed] [Google Scholar]
- 126. Bonadonna RC, Groop LC, Simonson DC, DeFronzo RA. Free fatty acid and glucose metabolism in human aging: evidence for operation of the Randle cycle. Am J Phys. 1994;266(3 Pt 1):E501‐E509. [DOI] [PubMed] [Google Scholar]
- 127. Alway SE, Mohamed JS, Myers MJ. Mitochondria initiate and regulate sarcopenia. Exerc Sport Sci Rev. 2017;45:58‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Barrientos A, Casademont J, Rotig A, et al. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem Biophys Res Commun. 1996;229(2):536‐539. [DOI] [PubMed] [Google Scholar]
- 129. Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr. 2014;144:1196‐1203. [DOI] [PubMed] [Google Scholar]
- 130. de Feyter HM, Lenaers E, Houten SM, et al. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. FASEB J. 2008;22(11):3947‐3955. [DOI] [PubMed] [Google Scholar]
- 131. de Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol. 2008;158:643‐653. [DOI] [PubMed] [Google Scholar]
- 132. van Tienen FH, Praet SF, de Feyter HM, et al. Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J Clin Endocrinol Metab. 2012;97(9):3261‐3269. [DOI] [PubMed] [Google Scholar]
- 133. Meex RC, Schrauwen‐Hinderling VB, Moonen‐Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2009;59:572‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance‐trained athletes. J Clin Endocrinol Metab. 2001;86:5755‐5761. [DOI] [PubMed] [Google Scholar]
- 135. Schrauwen‐Hinderling VB, Schrauwen P, Hesselink MK, et al. The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab. 2003;88(4):1610‐1616. [DOI] [PubMed] [Google Scholar]
- 136. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak‐Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271‐R1278. [DOI] [PubMed] [Google Scholar]
- 137. van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab. 2004;287:E558‐E565. [DOI] [PubMed] [Google Scholar]
- 138. Zacharewicz E, Hesselink MKC, Schrauwen P. Exercise counteracts lipotoxicity by improving lipid turnover and lipid droplet quality. J Intern Med. 2018;284:505‐518. [DOI] [PubMed] [Google Scholar]
- 139. Meex RC, Schrauwen‐Hinderling VB, Moonen‐Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59(3):572‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hickner RC, Racette SB, Binder EF, Fisher JS, Kohrt WM. Effects of 10 days of endurance exercise training on the suppression of whole body and regional lipolysis by insulin. J Clin Endocrinol Metab. 2000;85:1498‐1504. [DOI] [PubMed] [Google Scholar]
- 141. Brouwers B, Schrauwen‐Hinderling VB, Jelenik T, et al. Exercise training reduces intrahepatic lipid content in people with and people without nonalcoholic fatty liver. Am J Physiol Endocrinol Metab. 2018;314(2):E165‐E173. [DOI] [PubMed] [Google Scholar]
- 142. Albright A, Franz M, Hornsby G, et al. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2000;32(7):1345‐1360. [DOI] [PubMed] [Google Scholar]
- 143. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357‐369. [DOI] [PubMed] [Google Scholar]
- 145. Praet SF, Jonkers RA, Schep G, et al. Long‐standing, insulin‐treated type 2 diabetes patients with complications respond well to short‐term resistance and interval exercise training. Eur J Endocrinol. 2008;158(2):163‐172. [DOI] [PubMed] [Google Scholar]
- 146. Weyer C, Hanson K, Bogardus C, Pratley RE. Long‐term changes in insulin action and insulin secretion associated with gain, loss, regain and maintenance of body weight. Diabetologia. 2000;43:36‐46. [DOI] [PubMed] [Google Scholar]
- 147. Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587:4949‐4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Penn L, White M, Lindstrom J, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European diabetes prevention study RCT. PLoS ONE. 2013;8(2):e57143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57(4):987‐994. [DOI] [PubMed] [Google Scholar]
- 150. Nilwik R, Snijders T, Leenders M, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492‐498. [DOI] [PubMed] [Google Scholar]
- 151. Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22‐‐65 years. Acta Physiol Scand. 1978;103:31‐39. [DOI] [PubMed] [Google Scholar]
- 152. Lexell J, Henriksson‐Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588‐595. [DOI] [PubMed] [Google Scholar]
- 153. Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance‐type exercise training. J Gerontol A Biol Sci Med Sci. 2013;68:769‐779. [DOI] [PubMed] [Google Scholar]
- 154. Devries MC, Breen L, von Allmen M, et al. Low‐load resistance training during step‐reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep. 2015;3(8):e12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bamman MM, Clarke MS, Feeback DL, et al. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol (1985). 1998;84(1):157‐163. [DOI] [PubMed] [Google Scholar]
- 156. Hassan BH, Hewitt J, Keogh JW, Bermeo S, Duque G, Henwood TR. Impact of resistance training on sarcopenia in nursing care facilities: a pilot study. Geriatr Nurs. 2015. [DOI] [PubMed] [Google Scholar]
- 157. Stewart VH, Saunders DH, Greig CA. Responsiveness of muscle size and strength to physical training in very elderly people: a systematic review. Scand J Med Sci Sports. 2014;24:e1‐e10. [DOI] [PubMed] [Google Scholar]
- 158. Burd NA, West DW, Staples AW, et al. Low‐load high volume resistance exercise stimulates muscle protein synthesis more than high‐load low volume resistance exercise in young men. PLoS ONE. 2010;5(8):e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Breen L, Stokes KA, Churchward‐Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98(6):2604‐2612. [DOI] [PubMed] [Google Scholar]
- 160. Churchward‐Venne TA, Burd NA, Mitchell CJ, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590(11):2751‐2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Murphy J, Chevalier S, Gougeon R, Goulet ED, Morais JA. Effect of obesity and type 2 diabetes on protein anabolic response to insulin in elderly women. Exp Gerontol. 2015;69:20‐26. [DOI] [PubMed] [Google Scholar]
- 162. Pennings B, Groen B, de Lange A, et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302(8):E992‐E999. [DOI] [PubMed] [Google Scholar]
- 163. Wall BT, Hamer HM, de Lange A, et al. Leucine co‐ingestion improves post‐prandial muscle protein accretion in elderly men. Clin Nutr. 2013;32(3):412‐419. [DOI] [PubMed] [Google Scholar]
- 164. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997‐1005. [DOI] [PubMed] [Google Scholar]
- 165. Churchward‐Venne TA, Breen L, Phillips SM. Alterations in human muscle protein metabolism with aging: protein and exercise as countermeasures to offset sarcopenia. Biofactors. 2014;40:199‐205. [DOI] [PubMed] [Google Scholar]
- 166. Phillips SM. Nutritional supplements in support of resistance exercise to counter age‐related sarcopenia. Adv Nutr. 2015;6(4):452‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wall BT, Cermak NM, van Loon LJ. Dietary protein considerations to support active aging. Sports Med. 2014;44(Suppl 2):S185‐S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Roberts MD, Haun CT, Mobley CB, et al. Physiological differences between low versus high skeletal muscle hypertrophic responders to resistance exercise training: current perspectives and future research directions. Front Physiol. 2018;9:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985). 2001;90(6):2157‐2165. [DOI] [PubMed] [Google Scholar]
- 170. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059‐1064. [DOI] [PubMed] [Google Scholar]
- 171. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis of randomised trials. Diabetologia. 2012;55:885‐904. [DOI] [PubMed] [Google Scholar]
- 172. Erion DM, Park HJ, Lee HY. The role of lipids in the pathogenesis and treatment of type 2 diabetes and associated co‐morbidities. BMB Rep. 2016;49:139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Biomed Rep. 2013;1:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta‐analysis: insulin sensitizers for the treatment of non‐alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32:1211‐1221. [DOI] [PubMed] [Google Scholar]
- 175. Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188‐1193. [DOI] [PubMed] [Google Scholar]
- 176. Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23:205‐215. [DOI] [PubMed] [Google Scholar]
- 177. Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51(3):797‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kelly IE, Han TS, Walsh K, Lean ME. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care. 1999;22(2):288‐293. [DOI] [PubMed] [Google Scholar]
- 179. Musso G, Gambino R, Cassader M. Emerging molecular targets for the treatment of nonalcoholic fatty liver disease. Annu Rev Med. 2010;61:375‐392. [DOI] [PubMed] [Google Scholar]
- 180. Fiorucci S, Biagioli M, Distrutti E. Future trends in the treatment of non‐alcoholic steatohepatitis. Pharmacol Res. 2018;134:289‐298. [DOI] [PubMed] [Google Scholar]
- 181. Musso G, Gambino R, Cassader M, Pagano G. A meta‐analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79‐104. [DOI] [PubMed] [Google Scholar]
- 182. Poggiogalle E, Migliaccio S, Lenzi A, Donini LM. Treatment of body composition changes in obese and overweight older adults: insight into the phenotype of sarcopenic obesity. Endocrine. 2014;47:699‐716. [DOI] [PubMed] [Google Scholar]
- 183. de Spiegeleer A, Beckwee D, Bautmans I, Petrovic M. Sarcopenia guidelines development group of the Belgian Society of G, geriatrics. Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta‐analyses. Drugs Aging. 2018;35(8):719‐734. [DOI] [PubMed] [Google Scholar]


