Abstract
Pulmonary alveolar microlithiasis (PAM) is a rare autosomal recessive condition characterized by widespread alveolar deposition of calcium microliths. A mutation in the SLC34A2 gene in the alveolar Type II pneumocytes is responsible for decreased phosphate clearance and accumulation of calcium as spherules in the alveoli. The presence of this gene in other organs is responsible for the systemic phenotype of the disease. PAM is characterized by the lack of defining symptoms such as cough and progressive dyspnea until it reaches the stage of cor pulmonale and presents with features of respiratory and right ventricular (RV) failure. Radiologically, it is characterized by intense calcification in the lung parenchyma producing specific signs such as the “sandstorm appearance” in the early stages to the “white out lung” with “black pleura sign” in the later stages of the disease. While conventional therapy has not been successful at treatment, bilateral lung transplantation offers to be the only effective remedy. In this report, we present the case of a 54-year-old female who presented in the stage of respiratory and RV failure, with oxygen and noninvasive ventilation (NIV) dependence. She was treated with bilateral lung transplantation. Postoperatively, she was monitored closely for immunosuppression, prophylactic anti-infective measures, and bronchoscopies to evaluate for airway complications. The patient gradually improved and was discharged from the hospital without any need for oxygen or NIV.
KEY WORDS: Lung calcification, lung transplant, pulmonary alveolar microlithiasis, pulmonary alveolar microlithiasis, stone lung
INTRODUCTION
PAM is one of the rare lung diseases which often present late in disease course due to lack of symptoms. This autosomal recessive disease has a characteristic radiological appearance which often helps clinch the diagnosis. Lung biopsy is required to establish diagnosis. While no definite treatment is available lung transplant presents as a viable option for patients with severe disease.
CASE REPORT
A 54-year-old female patient presented with progressive dyspnea eventually becoming oxygen and NIV dependent. Her history is significant for hypothyroidism and systemic hypertension on regular medications. On physical examination, there was mild respiratory distress with peripheral cyanosis, marked clubbing, and bilateral fixed inspiratory crackles. On presentation, her arterial blood gas (ABG) was suggestive of severe hypoxemia with room air PO2 of 47.2 mmHg and SpO2 of 82.8%. Routine blood investigations did not reveal any significant abnormality.
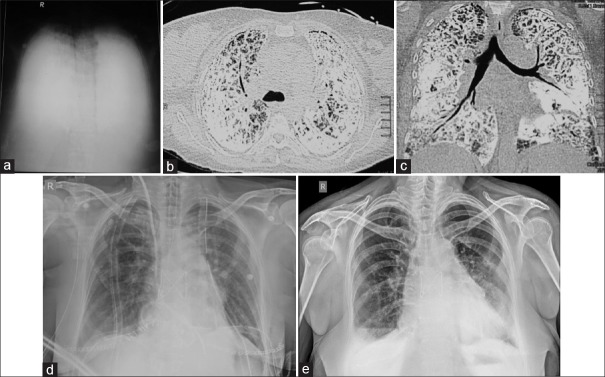
Chest X-ray [Figure 1a] showed that “white out lungs” with opacification of bilateral lung parenchyma also obscuring all mediastinal details [Figure 1b and c]. High-resolution computed tomography of the chest revealed diffuse dense calcification of lung parenchyma, including the subpleural, peribronchovascular, and centrilobular distribution. Interlobular septal thickening and parenchymal bands were visualized. Thus, a diagnosis of pulmonary alveolar microlithiasis (PAM) was established. Pulmonary function test (PFT) suggested restrictive lung defect. An echocardiogram demonstrated good biventricular systolic function and severe pulmonary hypertension (pulmonary arterial systolic pressure 80 mmHg). In view of diffuse lung involvement on CT, worsening dyspnea, increasing oxygen and NIV dependence and severe pulmonary hypertension (with preserved RV function), lung transplantation was offered to the patient as a solution for her disease. The patient was treated with a program for pulmonary rehabilitation and optimization of general condition and underwent bilateral lung transplantation with size and ABO-matched donor [Figure 1d].
Figure 1.
(a) Chest X-ray showing “white out lung,” (b and c) computed tomography showing dense calcific fibrosis and sub-pleural cysts (d). Immediate posttransplant Chest X-ray. (e) Chest X-ray 1-year posttransplant
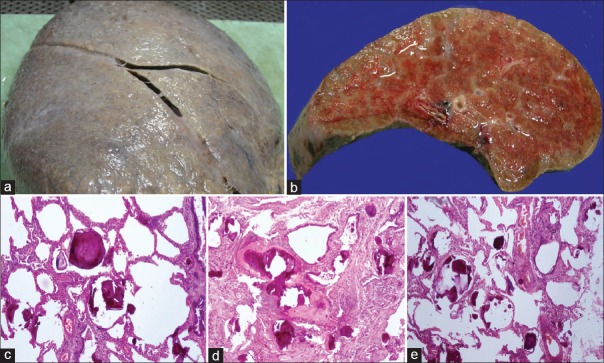
On pathological examination, explant lungs were enlarged and heavy. The right lung weight was 1450 g, and it measured 20 cm × 17 cm × 7 cm. The left lung weight was 1300 g and it measured 20 cm × 17 cm × 9 cm. External surface was firm and finely to coarsely granular, cut surface was firm-to-hard with granularity, and gritty sensation on cutting [Figure 2a and b]. On microscopic examination, Figure 2c–e sections demonstrated the alteration of pulmonary architecture with a diffuse filling of alveolar spaces with calcific deposits/microliths with lamellated appearance. There were foci with ossification and trabeculae formation, patchy interstitial fibrosis with lymphocytic inflammation, and few lymphoid aggregates. Vessels showed increased intimal fibrous and medial thickening with luminal narrowing. Few areas with Type 2 pneumocyte hyperplasia were noted. The final impression was PAM.
Figure 2.
(a) Lung explant with stony hard texture and rough external surface. (b) Gross gritty feeling on cut surface with granular appearance. (c-e) Microscopy sections showing intra-alveolar lamellar microliths in lung tissue with fibrosis
Postoperatively, she was maintained on immunosuppression and appropriate anti-infective prophylaxis. Regular microspirometry assessment surveillance bronchoscopy was done to monitor for any airway complications. She was discharged from the hospital with no oxygen/NIV requirement, no dyspnea, and improved exercise capacity. After >1-year posttransplant, the patient is under continuous follow-up, maintaining a forced expiratory volume in 1 s of 2.8 L. Chest X-ray [Figure 1e] showing no significant abnormality and full range of functions with a return to predisease/debilitation lifestyle.
DISCUSSION
PAM is a rare autosomal recessive disease characterized by an intra-alveolar accumulation of calcium calculi or microliths. It is caused by mutations in the SLC34A2 gene which encodes for a sodium-dependent phosphate cotransporter. SLC34A2 is expressed in alveolar epithelial cells Type II, and it is responsible for the uptake of phosphate released from phospholipids in metabolized surfactant.[1] The first case of PAM was reported by Harbitz in 1918, hence the name Harbitz' syndrome. Although PAM is reported worldwide, the majority of cases are reported from Asia.[2] The disease affects both sexes but has a slight predominance among the males, and most of the cases are found in the second and third decades of life.[2]
Generally, PAM presents with a paucity of symptoms. A cough with occasional microlith expectoration and dyspnea on exertion appears early. Other features of cor pulmonale and respiratory failure appear with disease progression. Expression of SLC34A2 gene in other tissues is responsible for the systemic phenotype, with calcifications in male genitalia being the most prevalent.[1] PFT, ABG, and diffusion capacity are normal in the initial stages of PAM. With disease progression, PFT typically shows a restrictive pattern and a reduction in diffusion capacity. Over time, patients start becoming hypoxic as evident in ABGs.
PAM is characterized by clinical-radiological dissociation. Generally, asymptomatic patients present with diffuse fine sand-like infiltrates on chest radiograph called “sandstorm lung.” Radiologically, it is divided into four phases: (i) early or precalcific phase with smaller number of microliths, (ii) sandy appearance with micronodules having a uniform size and distribution throughout the lung fields, (iii) number and volume of opacification increase often obscuring the mediastinal outlines, and (iv) fourth phase is characterized by intense calcific fibrosis-”white out lung,” “crazy paving pattern,” and “black pleura sign,” subpleural cysts delineated from the underlying fibrosed and calcified lung, these may occasionally lead to pneumothorax.
Bronchoscopic transbronchial lung biopsy and bronchoalveolar lavage help to establish definitive diagnosis of PAM. Histologically, the microliths are periodic acid-Schiff positive and consist of calcified spherules filling the alveoli. The microliths consist of calcareous concentric lamellae around a central nucleus with an amorphous or granular aspect. In autopsy specimen, the outer surfaces of lungs are granular and irregular due to the protrusion of microliths through the visceral pleura.[2]
No definite treatment other than lung transplantation has been proven to change the course of the disease. Systemic steroids and bronchoalveolar lavage are ineffective.[3] The disease may progress with chronic alveolar calcification and interstitial fibrosis leading to reduced lung volumes and the right heart failure. Lung transplantation has been beneficial in patients to increase RV ejection fraction and reduce oxygen requirements.[4] Bilateral lung transplant is preferred to unilateral because the replacement of only one lung might result in persistent shunting of blood through the native lung, filling of alveolar spaces, and the consequent creation of large areas of intrapulmonary shunts. Survival and long-term follow-up data of postlung transplant patients with recurrence are not available. The longest recorded survival without recurrence postlung transplantation is 15 years.[5]
CONCLUSION
PAM remains a challenging disease worldwide. The lack of clinical symptoms usually allows the disease to progress in silence, even though the characteristic radiological appearance helps to identify the condition easily. It is an “orphan” lung disease with no benefits from conventional therapy, and lung transplantation being the only effective treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–6. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellana G, Castellana G, Gentile M, Castellana R, Resta O. Pulmonary alveolar microlithiasis: Review of the 1022 cases reported worldwide. Eur Respir Rev. 2015;24:607–20. doi: 10.1183/16000617.0036-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tachibana T, Hagiwara K, Johkoh T. Pulmonary alveolar microlithiasis: Review and management. Curr Opin Pulm Med. 2009;15:486–90. doi: 10.1097/MCP.0b013e32832d03bb. [DOI] [PubMed] [Google Scholar]
- 4.Stamatis G, Zerkowski HR, Doetsch N, Greschuchna D, Konietzko N, Reidemeister JC, et al. Sequential bilateral lung transplantation for pulmonary alveolar microlithiasis. Ann Thorac Surg. 1993;56:972–5. doi: 10.1016/0003-4975(93)90370-w. [DOI] [PubMed] [Google Scholar]
- 5.Jackson KB, Modry DL, Halenar J, L'abbe J, Winton TL, Lien DC, et al. Single lung transplantation for pulmonary alveolar microlithiasis. J Heart Lung Transplant. 2001;20:226. doi: 10.1016/s1053-2498(00)00500-3. [DOI] [PubMed] [Google Scholar]