Abstract
Aims and Objectives:
This study aimed to compare the vitamin D levels between chronic obstructive pulmonary disease (COPD) patients and healthy controls and to describe the correlation between vitamin D levels and lung functions.
Methods:
Fifty COPD patients (cases) and 30 healthy volunteers (controls) were recruited and their serum vitamin D level was measured together with lung function (forced vital capacity and forced expiratory volume in 1 s [FEV1]) by spirometry. vitamin D was categorized as ≤20 nmol/l: deficient, 21–50 nmol/l: inadequate, and ≥51 nmol/l as sufficient.
Results:
In this case–control cross-sectional study, lower vitamin D levels were associated with lower lung function in both cases as well as controls, the effect being more pronounced in cases. Mean FEV1 at vitamin D ≤20 nmol/l (0.98 ± 0.40 vs. controls 1.93 ± 0.24 with P = 0.006), mean FEV1 at vitamin D 21–50 nmol/l (1.55 ± 0.54 vs. 2.20 ± 0.31 with P = 0.000), and mean FEV1 at vitamin D ≥51 nmol/l (2.06 ± 0.54 vs. 2.20 ± 0.31 with P = 0.002). Moreover, the severity of predicted postbronchodilator FEV1% was also much lower among COPD cohort versus healthy volunteers (mean FEV1%: cases 47.88 ± 14.22 vs. controls 58.76 ± 15.05 with P = 0.002).
Conclusions:
Importantly, lung function in both the groups was affected by decreased vitamin D level; decrease in FEV1 was more pronounced among COPD patients compared to controls showing more expiratory airflow limitation. Vitamin D levels are associated with changes in lung function in cases of COPD as well as healthy controls. Larger studies to confirm the association in Indian context are required and routine assessment of vitamin D may be undertaken to obviate the effects of low vitmain D level on lung function.
KEY WORDS: Chronic obstructive pulmonary disease, lung function, vitamin D
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) remains the second and fourth most common cause of mortality in India and the world, respectively.[1,2,3,4] It has been estimated that the average prevalence of COPD is 6.2% in Asia and almost one-fifth of identified individuals are categorized as having severe COPD.[5] WHO estimates suggest that 90% of COPD-related deaths occur in low- and middle-income countries.[6,7,8] India and China constitute 33% of the total human population and account for 66% of the global COPD mortality.[9] As per the Global Burden of Disease report of India from 1990 to 2016 based on a study by Salvi et al.[10] the contribution of chronic respiratory diseases to the total disability-adjusted life years (DALYs) in India increased from 4·5% (95% UI: 4.0–4.9) in 1990 to 6.4% (5.8–7.0) in 2016.
Of the total global DALYs due to chronic respiratory diseases in 2016, 32.0% occurred in India. COPD and asthma were responsible for 75.6% and 20.0% of the chronic respiratory disease DALYs, respectively, in India in 2016. The number of cases of COPD in India increased from 28·1 million (27.0–29.2) in 1990 to 55.3 million (53.1–57.6) in 2016, an increase in prevalence from 3.3% (3.1–3.4) to 4.2% (4.0–4.4). The highest DALY rates for both COPD and asthma in 2016 were in the low ETL states of Rajasthan and Uttar Pradesh. The DALYs per case of COPD and asthma were 1·7 and 2·4 times higher in India than the global average in 2016, respectively; most states had higher rates compared with other locations worldwide at similar levels of sociodemographic index. Of the DALYs due to COPD in India in 2016, 53.7% (43.1–65.0) were attributable to air pollution, 25.4% (19.5–31.7) to tobacco use, and 16.5% (14.1–19.2) to occupational risks, making these the leading risk factors for COPD.[10]
Vitamin D deficiency, on the other hand, is turning out to be pandemic affecting 50% of the population worldwide[11] with significant extraskeletal effects.[11,12] The “adequacy of exposure to sunlight of an individual's bare skin” required to photosynthesize vitamin D is grossly ill understood. Darker skin has high melanin content which acts as a natural sunscreen. In fact, darker skin produces a significantly lesser amount of vitamin D when compared with the individuals with fairer skin, such as Caucasians.[13,14,15] Indian social and or religious norms related to public modesty dictate that most parts of an individual's body, irrespective of gender, should be covered. Vitamin D deficiency is pandemic, yet it is the most underdiagnosed and undertreated nutritional deficiency in the world.[16] Vitamin D deficiency prevails in epidemic proportions all over the Indian subcontinent, with a prevalence of 70%–100% in the general population.[17]
The connection between vitamin D status and COPD has attracted attention in the recent years. There are several factors that could account for vitamin D deficiency in COPD patients: poor diet, a reduced capacity of aging skin for vitamin D synthesis, reduced outdoor activity and therefore sun exposure, an increased catabolism by glucocorticoids, impaired activation because of renal dysfunction, and a lower storage capacity in muscles or fat due to wasting. In general, many steps of the vitamin D pathway (intake, synthesis, storage, and metabolism) can potentially be disturbed in COPD patients. Two small studies of adults and children with asthma have found positive associations between serum 25(OH) D concentrations and forced expiratory volume in 1 s (FEV1).[18] This was confirmed recently in a larger study of adult asthma from China.[19] In fact, a recent study observed a positive association between 25(OH) D and FEV1 in patients with chronic obstructive pulmonary disease (COPD).[20] In the Third National Health and Nutrition Examination Survey (NHANES III), strong positive relationship between serum 25(OH) D and FEV1 and forced vital capacity (FVC) was reported.[21,22,23]
It was seen that vitamin D supplementation was associated with reduced moderate-to-severe COPD exacerbation by 45% with low baseline vitamin D level.[24] Currently, some studies raised the issue of vitamin D supplementation in household COPD patients and during winter time among all in order to maintain the adequate level of vitamin D to enhance the quality of life in these patients.[25]
GOLD document 2019 was unable to conclude about routine supplementation with vitamin D in COPD patients but states further that COPD patients with vitamin D deficiency should be substituted with vitamin D.
MATERIALS AND METHODS
This was a case–control cross-sectional study design including 50 COPD patients (cases) either diagnosed previously or on spot diagnosis or on spot COPD diagnosis based on history, examination, risk factors, and spirometry criteria FEV1/FVC <0.7% as per GOLD 2016 guidelines and 30 healthy volunteers (controls) aged >40 years old with no history of obstructive airway diseases (asthma and COPD) and bronchodilator and/or inhaled corticosteroid use who were recruited after approval from the ethical committee of the IMS-BHU and their serum vitamin D level was measured. Inclusion criteria were as follows: participants must have all of these: >40 years old, an Indian, postbronchodilator FEV1/FVC <70%, and postbronchodilator reversibility <200 ml and <12%. Participants were excluded if any one of these was present: domiciliary oxygen therapy, hemodynamically unstable patients, liver diseases, renal diseases, long-term steroid use, alcoholics, pregnancy, and active smoker (ex-smokers were included). Participants were recruited to minimize confounders such as age, sex, race, BMI, and smoking status which may influence vitamin D and lung functions as seen from Table 1.
Table 1.
Baseline characteristics of the cases and controls
| Variables | Group I (cases) | Group II (controls) | P |
|---|---|---|---|
| Age | 60.78±11.25 | 59.76±11.67 | 0.702 |
| Sex (n) | |||
| Male | 64% (32) | 63.3% (19) | 0.952 |
| Female | 36% (18) | 36.7% (11) | 0.952 |
| BMI (n) | |||
| <18.5 | 18% (9) | 6.7% (2) | 0.468 |
| 18.5-24.9 | 44% (22) | 43.3% (13) | |
| 25-29.9 | 30% (15) | 36.7% (11) | |
| >30 | 8% (4) | 13.3% (4) | |
| BMI | 23.37±4.67 | 25.18±3.90 | 0.079 |
| Anemia | 40% (20) | 30% (9) | 0.368 |
| Male <14gm/dl | |||
| Female < 11 gm/dl | |||
| WBC: >10000 cells/cubic millimeter | 62% (31) | 30% (9) | 0.006 |
| Neutrophils (n) | |||
| <50% | 0% | 3.3% (1) | <0.001 |
| 50%-70% | 18% (9) | 56.7% (17) | |
| >70% | 82% (41) | 40% (12) | |
| Serum Calcium: unit: mg/dl | |||
| <8.5 | 30% (15) | 20% (6) | 0.492 |
| 8.5-10.5 | 64% (32) | 76.7% (23) | |
| >10.5 | 6% (3) | 3.3% (1) | |
| Smokers (cigarette + biomass) (n) | 36 (25+11) | 14 (10+4) | 0.076 |
| Vitamin D (nmol/l) | 33.76±18.33 | 46.26±14.88 | 0.283 |
| FVC (l) | 2.58±0.84 | 2.79±0.80 | 0.279 |
| FEV1 | 1.71±0.74 | 1.97±0.71 | 0.128 |
| FEV1% | 47.88±14.22 | 58.76±15.05 | 0.002 |
| FEV1/FVC | 64.34±11.54 | 69.83±11.71 | 0.044 |
FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s, BMI: Body mass index, WBC: White blood cells
Vitamin D was measured from random serum sample of participants using chemiluminescent immunoassay in the laboratory. Vitamin D was categorized as <70 nmol/l or >70 nmol/l for categorizing deficient versus adequate so to become alert and watchful and increasing the sensitivity of test in addition to most laboratory considering 70 nmol/l as the cutoff value. A conversion factor of 2.5 is used to convert nmol/l to ng/ml, i.e., divide nmol/l by 2.5 to get ng/ml. However, vitamin D was grouped into I, II, and III for the purpose of seeing the impact on lung function at ≤20 nmol/l: deficient (Group I, n = 19), 21–50 nmol/l: inadequate (Group II, n = 40), and ≥51 nmol/l as sufficient (Group III, n = 21) to specifically identify the level of vitamin D at which lung function is affected most based on several studies considering values lower than 50 nmol/l associated with effect on Lung function.
Spirometer – Spiroexcel model, Brand Medicaid's make – was used to measure lung function. The procedure was explained and demonstrated before performing the test by a trained pulmonary technician. A standard ATS/ERS Task Force (2005) Standardization of Lung Function Testing was followed for performing spirometry. Baseline spirometry was done after making sure that the patient did not take any SABA/SAMA up to minimum of 4 h prior and LABA >15 h prior to performing the procedure followed by inhalation of 400 mcg of SABA via pMDI without using spacer and 15 min after a repeat spirometry was done to document the FEV1/FVC <0.7. The best of three efforts was taken into consideration. The flow chart of the study can be seen from Figure 1.
Figure 1.
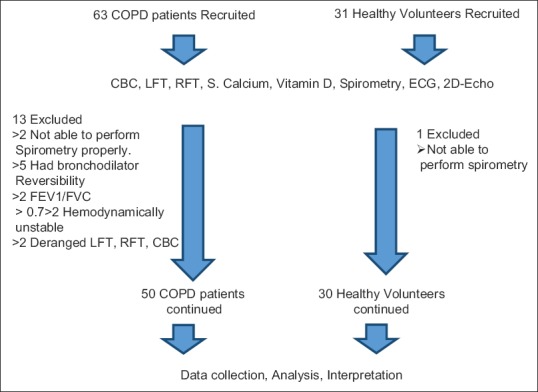
Schematic flow chart of the study
Sample size was calculated based on a pilot study of 15 subjects conducted prior to enrollment of participants to proper study by taking the mean of FEV1% predicted 50 among cases and the mean of FEV1% predicted 60 among controls with standard deviation of 14 in each group with level of confidence 5% at two-tailed test with 90% power and 1.5:1 ratio in cases and controls. Based on above variables, the sample size comes out to be 47 (cases) and 31 (controls). Data were analyzed using SPSS 20, (produced by SPSS Inc. Chicago, USA, acquired by IBM, New York, USA in 2009) in an age, sex, BMI, race, and smoking status matched.
RESULTS
The researcher found that as vitamin D level decreased, lung function in terms of FEV1 also decreased with significant P value as evident from Figure 2a–c:
Figure 2.
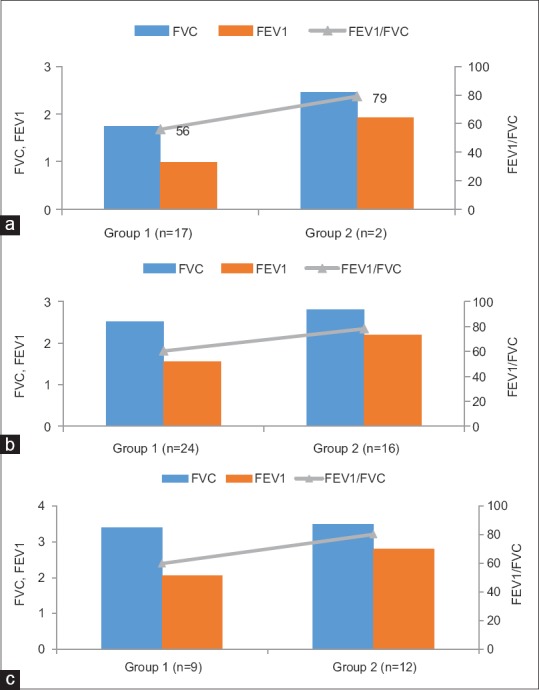
(a) Comparison of effect of vitamin D ≤20 nmol/l on lung function among groups. (b) Comparison of effect of vitamin D 21–50 (nmol/l) on lung function in groups. (c) Comparison of effect of vitamin D ≥51 (nmol/l) on lung function in groups. Group 1 = Vitamin D <21 nmol/l, Group 2 = Vitamin D >21–50 nmol/l, Group 3 = Vitamin D >50 nmol/l
It was seen that if vitamin D was ≤20 nmol/l, the mean FEV1 (in liters) among cases and controls was only 0.98 ± 0.40 and 1.93 ± 0.24, respectively, with P = 0.006 [Figure 2a]
It was seen that if vitamin D was 21–50 nmol/l, the mean FEV1 (in liters) among cases and controls was only 1.55 ± 0.54 and 2.20 ± 0.31, respectively, with P = 0.000 [Figure 2b]
It was seen that if vitamin D was ≥51 nmol/l, the mean FEV1 (in liters) among cases and controls was only 2.06 ± 0.54 and 2.80 ± 0.31, respectively, with P = 0.002 [Figure 2c].
Figure 2a–c shows the comparison of effect of various levels of vitamin D on lung function among COPD patients and controls.
Table 2 shows the comparative effect of various levels of vitamin D on lung function in terms of FEV1, FVC, FEV1/FVC as well as the post hoc analysis between groups. Groups were categorized based on their vitamin D level as Group 1 = Vitamin D <21, Group 2 = Vitamin D >21–50, and Group 3 = Vitamin D >50. The post hoc test showed a statistically significant difference in FVC and FEV1 between Groups I versus II, I versus III, and II versus III when each group compared to other group head to head without segregating cases from controls, i.e., it showed the linear effect of vitamin D on lung function; with decreased vitamin D level, FVC and FEV1 were also low and vice versa.
Table 2.
Comparison of effect of vitamin D on forced expiratory volume in 1 s, forced vital capacity, and forced expiratory volume in 1 s/forced vital capacity
| Group | FVC | FEV1 | FEV1/FVC |
|---|---|---|---|
| Group 1 (n=19) | 1.82±0.68 | 1.08±0.49 | 58.42±9.87 |
| Group 2 (n=40) | 2.64±0.62 | 1.81±0.56 | 67.52±10.61 |
| Group 3 (n=21) | 3.45±0.48 | 2.48±0.58 | 71.47±12.49 |
| F, P | 35.929, 0.000 | 32.124, 0.000 | 7.47, 0.001 |
| Post hoc test, P | |||
| I versus II | 0.000 | 0.000 | 0.004 |
| I versus III | 0.000 | 0.000 | 0.000 |
| II versus III | 0.000 | 0.000 | 0.186 |
FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s
Figure 3a and b shows the effect of vitamin D on FEV1 and FVC among COPD patients and controls. It is clear from these figures that decrease in FEV1 and FVC was more among COPD patients compared to controls.
Figure 3.
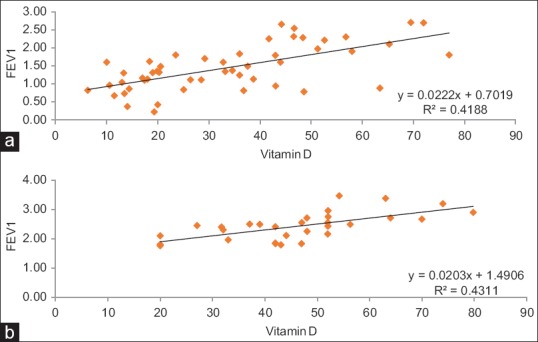
(a) Effect of vitamin D on forced expiratory volume in 1 s among chronic obstructive pulmonary disease patients. (b) Effect of vitamin D on forced expiratory volume in 1 s among healthy volunteers
Figure 4a and b shows a significant correlation between vitamin D and postbronchodilator FEV1% predicted among COPD patients and controls as the decline in FEV1% was more among COPD patients (47.88 ± 14.22) compared to controls (58.76 ± 15.05) with significant P = 0.002.
Figure 4.
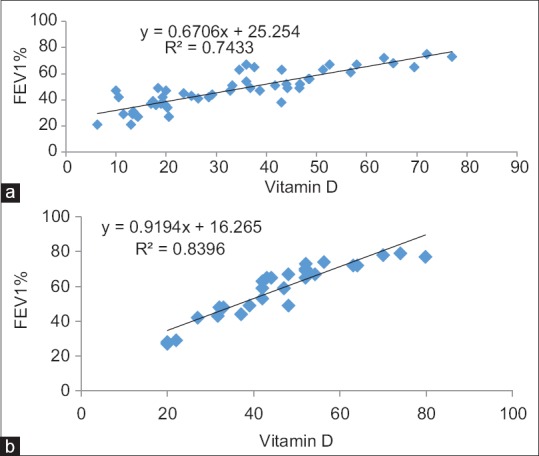
(a) Correlation of vitamin D with FEV1% among cases. (b) Correlation of vitamin D with FEV1% among controls
As evidenced from Tables 3–5 the various measure of lung functions included in this study like FEV1, FVC and FEV1/FVC ratios were lower at various vitamin D level as per study categorization among cases compared to controls.
Table 3.
Comparison of effect of vitamin D ≤20 nmol/l on lung function among groups
| Indicators (lung function) | Group 1 (n=17) Cases | Group 2 (n=2) Control | Remarks (t, P) |
|---|---|---|---|
| FVC | 1.74±0.68 | 2.45±0.39 | −1.405, 0.178 |
| FEV1 | 0.98±0.40 | 1.93±0.24 | −3.168, 0.006 |
| FEV1/FVC | 56.00±7.07 | 79.00±2.82 | −4.457, 0.000 |
FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s
Table 5.
Comparison of effect of vitamin D ≥51 (nmol/l) on lung function in groups
| Indicators (lung function) | Group 1 (n=9) | Group 2 (n=12) | Remarks (t, P) |
|---|---|---|---|
| FVC | 3.39±0.60 | 3.49±0.39 | −0.472, 0.642 |
| FEV1 | 2.06±0.54 | 2.80±0.39 | −3.638, 0.002 |
| FEV1/FVC | 59.88±9.14 | 80.16±5.54 | −6.318, 0.000 |
FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s
Table 4.
Comparison of effect of vitamin D 21-50 (nmol/l) on lung function in groups
| Indicators (lung function) | Group 1 (n=24) Cases | Group 2 (n=16) Controls | Remarks (t, P) |
|---|---|---|---|
| FVC | 2.52±0.73 | 2.81±0.37 | −1.440, 0.158 |
| FEV1 | 1.55±0.54 | 2.20±0.31 | −4.282, 0.000 |
| FEV1/FVC | 60.41±6.47 | 78.18±5.17 | −9.183, 0.000 |
FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s
Moreover, as seen from the Table 6; at Vit D cut off level of 70 nmol/l, more than >96% of COPD Cohort & 90% of control group were having vitamin D deficiency.
Table 6.
Frequency of vitamin D deficiency
| Vitamin D (nmol/l) | Group 1 (COPD cohort), n (%) | Group 2 (HV), n (%) | Total, n (%) |
|---|---|---|---|
| <70 | 48 (96.0) | 27 (90.0) | 75 (93.8) |
| ≥70 | 2 (4.0) | 3 (10.0) | 5 (6.2) |
| Total | 50 (100.0) | 30 (100.0) | 80 (100.0) |
COPD: Chronic obstructive pulmonary disease, HV: Healthy volunteer
DISCUSSION
Lot of interest had been given to the impact of vitamin D on extraskeletal system such as respiratory and cardiovascular in the past decade globally. Majority of the studies including randomized clinical trial and meta-analysis were able to show the significant impact of vitamin D in the prevention of exacerbation in COPD by decreasing infection, severity of COPD, improving quality of life, and FEV1.[24] To the contrary, few studies refuted such finding but agreed to the fact that replacement of vitamin D in a vitamin D-deficient COPD individual will definitely decrease exacerbation and improve FEV1.[24,26]
However, when analyzing data based on vitamin D categorization, statistically significant effect of vitamin D on lung function, i.e., FVC and FEV1 has been observed. The mean predicted postbronchodilator FEV1% among COPD group was 47.88 ± 14.22 versus 58.76 ± 15.05 among healthy volunteers, again showing a decrease in FEV1% among COPD patients. Furthermore, the researcher observed that the lower the vitamin D level, the lower was the predicted postbronchodilator FEV1% and vice versa. In this current hospital-based case–control cross-sectional study among Indian population, we demonstrated a positive linear relation between serum concentration of vitamin D and lung function (FVC and FEV1) after controlling for some confounder factors (age-, sex-, body mass index-, and smoking-matched participants) as evident in Table 1 and methodology and found strong evidence to suggest that individuals with lower serum concentrations of vitamin D were more likely to have expiratory airflow limitation. Our finding was similar to the finding by Black and Scragg who examined data from the NHANES III data set[24] (cross-sectional survey of 14091 adults in the US): After adjustment for potential confounders, a strong relationship between serum levels of vitamin D and lung function (FEV1 and FVC) was found. Zhu et al. concluded that serum deficiency of 25(OH) D (20 ng/mL) was significantly associated with COPD severity based on the meta-analyses between mild and moderate/severe COPD as well as moderate and severe COPD.[26] They further showed vitamin D supplementation prevented from worsening of COPD. However, Shaheen et al. did not confirm a positive association between blood 25(OH) D concentrations and adult lung function.[25]
The researcher also found that more than 90% of the participants were having Vitamin D deficiency which is similar to the studies conducted by Ritu et al.[17] who claimed vitamin D deficiency prevalence to be 70%–100% of the general Indian population. In addition, a report published in Times of India report by Janani Sampath, TNN, Updated: October 18, 2015, Chennai-based Metropolis Healthcare studied 1,496,683 samples over three years who underwent vitamin tests, and it was found that 81.28% of all samples tested were deficient in vitamin D. The current GOLD document 2019 says that vitamin D should not be supplemented routinely, but if a deficiency exists, it should be supplemented. Similarly, various studies recommend vitamin D supplementation, especially if vitamin D ≤20 nmol/l to prevent exacerbations as discussed in the introduction. In addition, we found that, the lower the vitamin D level the lower the FEV1. Hence, we believe measuring serum vitamin D level among COPD patients will have multiple benefits. Firstly, provides a scientific basis for supplementation and secondly prevent exacerbation of COPD and its ill-impact overall on expense, morbidity and mortality.
There are several factors that could account for vitamin D deficiency in COPD patients[12]: poor diet, a reduced capacity of aging skin for vitamin D synthesis, reduced outdoor activity and therefore sun exposure, an increased catabolism by glucocorticoids, impaired activation because of renal dysfunction, and a lower storage capacity in muscles or fat due to wasting. In general, many steps of the vitamin D pathway (intake, synthesis, storage, and metabolism) can potentially be disturbed in COPD patients.
The exact mechanism regarding the effect of vitamin D on lung function is unclear. However, several proposed biological mechanisms may explain the contribution of vitamin D deficiency to COPD in addition to calcemic effect of vitamin D on the skeletal system and impairing the lung function.
First, vitamin D acts as a potent inhibitor in either innate or adaptive immune response through the activation of vitamin D receptor (VDR). VDR is expressed in various types of inflammatory and structural cells. vitamin D deficiency fails to restrain the maturation of dendritic cell and macrophage by regulating the major histocompatibility complex class II molecules (77), decrease the production of pro-inflammatory cytokines and chemokines (78), promote monocyte and neutrophil recruitment depending on nuclear factor-κB-mediated pathway, and shift Th1 T-cell toward Th2 and regulatory T-cell. The dysregulated immune-inflammatory response leads to the development of chronic inflammation and lung structural destruction, which in turn, promotes the onset and progress of COPD. Second, vitamin D can upregulate the expression of antimicrobial peptides in response to infections.[27] vitamin D deficiency increases the susceptibility to respiratory infections, which in turn, contributes to airway colonization and chronic inflammation. Third, vitamin D deficiency has an effect on airway smooth muscle by regulating the expression of genes related to cell proliferation, glucocorticoid response, and smooth muscle contraction.[28] In addition, vitamin D deficiency contributes to the remodeling of airway smooth muscle and lung tissue by inducing fibroblast proliferation, promoting collagen synthesis, and increasing levels of matrix metalloproteinase.[29] Fourth, vitamin D is associated with the metabolism of bone and muscle. The association of vitamin D deficiency and reduced lung function could depend on the calcemic effects of vitamin D. The vital capacity and total lung capacity were found to decline with an increasing number of thoracic vertebral fractures as a direct consequence of vitamin D deficiency.[16] Nuti et al. observed 3030 ambulatory COPD patients and found a strong association between COPD severity and fractures.[16] Kyphosis related to osteoporosis caused limitation in rib mobility and inspiratory muscle function and correlated with a reduction in FEV1 and FVC.[5] The altered properties of the thoracic skeleton could result in failure of the respiratory muscles contributing to the pathophysiology of COPD. Vitamin D deficiency plays a role in the development of osteoporosis and skeletal muscle weakness, indicating the decline of lung function.[30,31]
Vitamin D deficiency is almost universal in Indian population perhaps because of inadequate sun exposure mostly, traditional clothing habits such as covering whole body parts, staying indoors, and inadequate dietary intake. Hence, an early morning sun exposure of approximately 20 min[32,33,34] to exposed body parts such as face, neck, arms, and legs at least could help prevent vitamin D deficiency and thence can prevent the adverse consequences of vitamin D deficiency leading to a better health with decreased economical burden to all stakeholders.
Limitations of the study
Firstly, the study design was a single-centered, cross-sectional hence causality link cannot be established, secondly, all co-morbidities were not taken into consideration, thirdly, limited sample size, fourthly, vitamin D was measured only once. The evidence just addressed the association between abnormal levels of vitamin D and COPD. However, it was still not clear whether abnormal level of vitamin D was a consequence of COPD or a contributor to COPD. Hence, further prospective, longitudinal, and well-designed cohort studies are needed. Second, potential confounders, such as sunlight exposure, seasonal variation, and diet intake, can affect vitamin D status and was not taken into consideration. However, insufficient information regarding these factors in included studies limited the adjustment of the results. Statistical heterogeneity was still assessed, even with stratified analyses based on latitude degree, assay method, and BMI (matched). Thus, the potential confounders may be the source of heterogeneity. Third, the limited number of eligible studies on the association between vitamin D and COPD severity as well as COPD exacerbations confined the analyses.
CONCLUSIONS
Vitamin D deficiency is surprisingly at peak and hence should be routinely looked for in COPD patients to reduce ill-effects on lung function and overall health. Vitamin D deficiency worsens lung function more among COPD patients compared to healthy adults. A routine evaluation of vitamin D in COPD patients seems to be logical to prevent further decline of lung function. A large prospective, randomized control trial must be carried out to ascertain these findings as normal healthy individuals with decrease vitamin D also had a decrease in lung function which further worsens lung function in COPD patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Agusti AG. GOLD 2018 Global Strategy for the Diagnosis, Management and Prevention of COPD. 2018 [Google Scholar]
- 2.Sharma S. Hindustan Times, The Top 10 Causes of Death in India. 2017 Sep 30; [Google Scholar]
- 3. [Last assessed on 2019 Sep 13]. Available from: h ttps://www.hindustantimes.com/health/the-top-10-causes-of-death-in-india/story-lFLxCFVHmF7svw2RKCl70K.html .
- 4. [Last assessed on 2019 Sep 13]. Available from: http://www.healthdata.org/india .
- 5.Lim S, Lam DC, Muttalif AR, Yunus F, Wongtim S, Lan le TT, et al. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: The EPIC Asia population-based survey. Asia Pac Fam Med. 2015;14:4. doi: 10.1186/s12930-015-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koul PA. Chronic obstructive pulmonary disease: Indian guidelines and the road ahead. Lung India. 2013;30:175–7. doi: 10.4103/0970-2113.116233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Chronic Obstructive Pulmonary Disease (COPD) Fact Sheet No 315. World Health Organization. 2011. [Last accessed on 2013 Jun 05]. Available from: http://www.who.int/mediacentre/factsheets/fs315/en/index.html .
- 8.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 9.Salvi SS, Manap R, Beasley R. Understanding the true burden of COPD: The epidemiological challenges. Prim Care Respir J. 2012;21:249–51. doi: 10.4104/pcrj.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvi S, Kumar GA, Dhaliwal RS, Paulson K, Agrawal A, Koul PA, Mahesh PA, et al. The burden of chronic respiratory diseases and their heterogeneity across the states of India: The global burden of disease study 1990-2016. Lancet Glob Health. 2018;6:e1363–74. doi: 10.1016/S2214-109X(18)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Burney P, Jithoo A, Kato B, Janson C, Mannino D, Nizankowska-Mogilnicka E, et al. Chronic obstructive pulmonary disease mortality and prevalence: The associations with smoking and poverty – A BOLD analysis. Thorax. 2014;69:465–73. doi: 10.1136/thoraxjnl-2013-204460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo CW, Paris PW, Holick MF. Indian and Pakistani immigrants have the same capacity as Caucasians to produce Vitamin D in response to ultraviolet irradiation. Am J Clin Nutr. 1986;44:683–5. doi: 10.1093/ajcn/44.5.683. [DOI] [PubMed] [Google Scholar]
- 14.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise Vitamin D3. Lancet. 1982;1:74–6. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of Vitamin D. Arch Dermatol. 1991;127:536–8. [PubMed] [Google Scholar]
- 16.Nuti R, Siviero P, Maggi S, Guglielmi G, Caffarelli C, Crepaldi G, Gonnelli S, et al. Vertebral fractures in patients with chronic obstructive pulmonary disease: The EOLO Study, Osteoporos Int. 2009;20:989–98. doi: 10.1007/s00198-008-0770-4. [DOI] [PubMed] [Google Scholar]
- 17.Ritu G, Gupta A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients. 2014;6:729–75. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, et al. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration. 2011;81:469–75. doi: 10.1159/000322008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the Vitamin D-binding gene. Thorax. 2010;65:215–20. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 21.Kunisaki KM, Niewoehner DE, Singh RJ, Connett JE. Vitamin D status and longitudinal lung function decline in the lung health study. Eur Respir J. 2011;37:238–43. doi: 10.1183/09031936.00146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, et al. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019;74:337–45. doi: 10.1136/thoraxjnl-2018-212092. [DOI] [PubMed] [Google Scholar]
- 23.Carson EL, Pourshahidi LK, Madigan SM, Baldrick FR, Kelly MG, Laird E, et al. Vitamin D status is associated with muscle strength and quality of life in patients with COPD: A seasonal prospective observation study. Int J Chron Obstruct Pulmon Dis. 2018;13:2613–22. doi: 10.2147/COPD.S166919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 25.Shaheen SO, Jameson KA, Robinson SM, Boucher BJ, Syddall HE, Sayer AA, et al. Relationship of Vitamin D status to adult lung function and COPD. Thorax. 2011;66:692–8. doi: 10.1136/thx.2010.155234. [DOI] [PubMed] [Google Scholar]
- 26.Zhu B, Zhu B, Xiao C, Zheng Z. Vitamin D deficiency is associated with the severity of COPD: A systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1907–16. doi: 10.2147/COPD.S89763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gombart AF. The Vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–65. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee A, Panettieri R., Jr Vitamin D modulates airway smooth muscle function in COPD. Curr Opin Pharmacol. 2012;12:266–74. doi: 10.1016/j.coph.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of Vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011;406:127–33. doi: 10.1016/j.bbrc.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of Vitamin D in skeletal muscle: Form, function, and metabolism. Endocr Rev. 2013;34:33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 31.Heulens N, Korf H, Janssens W. Innate immune modulation in chronic obstructive pulmonary disease: Moving closer toward Vitamin D therapy. J Pharmacol Exp Ther. 2015;353:360–8. doi: 10.1124/jpet.115.223032. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF. Sunlight and Vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes LE, Webb AR, Fraser HI, Kift R, Durkin MT, Allan D, et al. Recommended summer sunlight exposure levels can produce sufficient (> or=20 ng ml(-1)) but not the proposed optimal (> or=32 ng ml(-1)) 25(OH)D levels at UK latitudes. J Invest Dermatol. 2010;130:1411–8. doi: 10.1038/jid.2009.417. [DOI] [PubMed] [Google Scholar]
- 34.Cicarma E, Porojnicu AC, Lagunova Z, Dahlback A, Juzeniene A, Moan J, et al. Sun and sun beds: Inducers of Vitamin D and skin cancer. Anticancer Res. 2009;29:3495–500. [PubMed] [Google Scholar]


