Summary
This year marks the 50th anniversary of the discovery of σ70 as a protein factor that was needed for bacterial RNA polymerase to accurately transcribe a promoter in vitro. It was 25 years later that the Group IV alternative σs were described as a distinct family of proteins related to σ70. In the intervening time, there has been an ever‐growing list of Group IV σs, numbers of genes they transcribe, insight into the diverse suite of processes they control, and appreciation for their impact on bacterial lifestyles. This work summarizes knowledge of the Rhodobacter sphaeroides σE‐ChrR pair, a member of the ECF11 subfamily of Group IV alternative σs, in protecting cells from the reactive oxygen species, singlet oxygen. It describes lessons learned from analyzing ChrR, a zinc‐dependent anti‐σ factor, that are generally applicable to Group IV σs and relevant to the response to single oxygen. This MicroReview also illustrates insights into stress responses in this and other bacteria that have been acquired by analyzing or modeling the activity of the σE‐ChrR across the bacterial phylogeny.
Activation of a cascade of alternative σ factors by the reactive oxygen species 1O2. The master regulator of this response, the Group IV ECF11 protein Rb. sphaeroides σE, directly activates transcription of ~genes, one (rpoHII) encodes an alternative σ factor in the heat shock family. RpoHII directly activates ~145 genes; some 45 of which are also transcribed by RpoHI, the master regulator of the Rb. sphaeroides heat shock response.
Introduction
The study of Group IV or extracytoplasmic function (ECF) σ factor function has provided many new insights into the cell biology, stress responses and signaling pathways across the bacterial phylogeny (Staron et al., 2009; Feklístov et al., 2014), and provided strategies to allow for targeted control of gene expression in native and heterologous hosts (Rhodius et al., 2013; Pinto et al., 2019). This contribution will review what is known about the ECF11 sub‐family of Group IV σs (Staron et al., 2009). It will focus on the founding member of the ECF11 sub‐family, the Rhodobacter sphaeroides σE protein, its cognate cytoplasmic anti‐σ ChrR (Newman et al., 1999; 2001), and their role in a stress response to singlet oxygen, a reactive oxygen species (ROS) encountered by a variety of cells (Anthony et al., 2005; Ziegelhoffer and Donohue, 2009). It will summarize lessons learned about Group IV σ factor function by studying this system, and highlight unanswered questions about this response.
Singlet oxygen (1O2) is a ROS
Prior to the introduction of molecular oxygen (O2), organisms had a limited metabolic and regulatory repertoire. However, when photosynthetic cells acquired the ability to produce O2, they altered the Earth's atmosphere and influenced the forms of life that inhabited the planet (Raymond et al., 2003; Kerr, 2005). In particular, the accumulation of atmospheric O2 allowed evolution of pathways like aerobic respiration that couple the four‐electron reduction of O2 to formation of a proton gradient (Kerr, 2005). One advantage to aerobic respiration is the large amount of energy that is conserved as O2 is reduced to water (Gennis, 1986; Brzezinski and Gennis, 2008; Borisov et al., 2011; Bueno et al., 2012; Soo et al., 2019). However, there are other, potentially deleterious, consequences to life in the presence of O2. One trade off to accumulation of atmospheric O2, or its use as a terminal electron acceptor, is formation of different ROS (Rosner and Storz, 1997; Schulz et al., 2000; Mittler et al., 2004; Frick et al., 2015; Taverne et al., 2018).
When one electron is sequentially transferred to O2 (Fig. 1), Type I ROS (superoxide, hydrogen peroxide, or hydroxyl radicals) are formed (Rosner and Storz, 1997). Each Type I ROS can damage biomolecules, kill cells or trigger the onset of debilitating diseases (Schulz et al., 2000; Taverne et al., 2018). Consequently, considerable effort has been invested into determining the stress response(s) to these ROS (Rosner and Storz, 1997; Schulz et al., 2000; Zheng and Storz, 2000; Kiley and Storz, 2004; Taverne et al., 2018).
Figure 1.
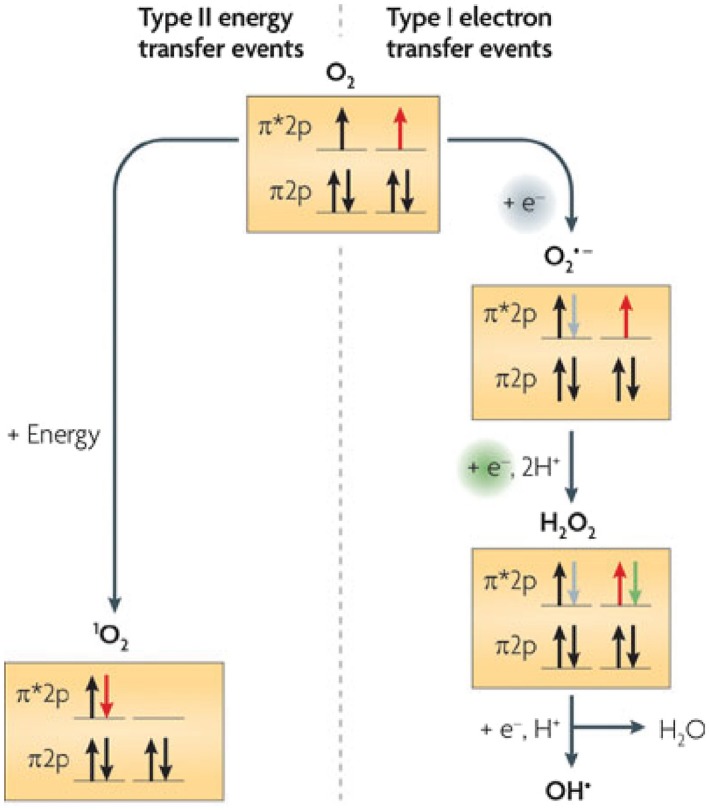
Formation and biological consequences of ROS generation. The right panel shows production of the ROS superoxide (O2 •−), hydrogen peroxide (H2O2) or hydroxyl radicals (OH•) by one‐electron transfer reactions. The left panel shows formation of singlet oxygen (1O2) by Type II energy transfer, typically from an excited, triplet state donor, to O2. The diagrams show the spin of electrons in shells of the outer p orbital of each compound. Note that 1O2 is formed by movement of an electron between outer p orbital shells (red arrow). Figure modified from (Ziegelhoffer and Donohue, 2009).
In contrast, less is known about how cells respond to the Type II ROS 1O2 (Ragàs et al., 2013; Dogra et al., 2018). 1O2 is formed when energy transfer from an excited, triplet state donor, to O2 alters the distribution of electrons in its outer orbital (Fig. 1). Enzymes which detoxify superoxide or H2O2 are ineffective against 1O2 due to differences in outer orbital electron organization between these Type 1 and Type II ROS (Ziegelhoffer and Donohue, 2009). Indeed, there are no known enzyme‐catalyzed systems for 1O2 detoxification (Davies, 2004).
The outer orbital electron organization of 1O2 makes it a strong oxidant (~900mV energy difference between 1O2 and O2). 1O2 is known or predicted to peroxidize and eventually cleave unsaturated bonds in olefins, oxidize amino acid side chains or nucleic acid bases and cleave peptide or phosphodiester bonds (Nymann and Hynninen, 2004; Godley et al., 2005). Thus, it is not surprising that 1O2 can also inhibit growth or kill cells (Anthony et al., 2005; Ziegelhoffer and Donohue, 2009; Lemke et al., 2014).
Biological formation of 1O2
Major cellular sources of 1O2 include the enzymes NADH oxidase, myloperoxidase or chloroperoxidase (Kochevar, 2004; Davies, 2004; Godley et al., 2005). Light energy capture by photosynthetic pigments is another significant source of 1O2 (Fig. 1). In the light reactions of photosynthesis, photons excite chlorophyll pigments to a high‐energy state (Cogdell, 2000; Frank and Brudvig, 2004; Kochevar, 2004; Triantaphylides and Havaux, 2009). Normally, these excited (triplet state) pigments transfer energy to a reaction center (in bacteria) or photosystem (in cyanobacteria, algae and plants) resulting in light‐driven oxidation of this membrane enzyme. However, at a significant frequency, energy transfer from light‐excited photopigments to O2 generates 1O2 (Cogdell, 2000; Frank and Brudvig, 2004; Kochevar, 2004; Uchoa et al., 2008; Triantaphylides and Havaux, 2009).
1O2 has a high reactivity, so it is predicted to have a short cellular half‐life (~100 ns), not travel far its site of synthesis, and produce localized damage (Kochevar, 2004). In phototrophs, 1O2 formation initiates a process called photo‐oxidative stress (Triantaphylides and Havaux, 2009; Ziegelhoffer and Donohue, 2009) that can inactivate integral photosynthetic membrane enzymes (Cogdell, 2000; Fryer et al., 2002; Frank and Brudvig, 2004; Kochevar, 2004; Szabó et al., 2005), peroxidize or cleave nearby olefins (carotenoids or unsaturated fatty acids), destroy bilayer integrity and function (Girotti and Kriska, 2004; Ramel et al., 2012; Lemke et al., 2014), signal changes in nuclear gene expression from the organelle (chloroplasts) where it is generated in eukaryotic phototrophs or trigger apoptosis (Danon et al., 2005; Foyer and Noctor, 2005).
1O2 promotes a bacterial transcriptional response
We uncovered a role for an ECF11 Group IV σ factor in a 1O2 stress response by studying the photosynthetic bacterium Rb. sphaeroides (Anthony et al., 2005; Dufour et al., 2008; Ziegelhoffer and Donohue, 2009; Nam et al., 2013). In the laboratory, photosynthetic growth of Rb. sphaeroides is often achieved by incubating cells anaerobically in the light (Tavano and Donohue, 2006; Donohue and Kiley, 2011), so 1O2 is not formed under these conditions. However, we discovered that Rb. sphaeroides mounts a transcriptional response to 1O2 either when pigmented cells are exposed to light and O2 or when non‐pigmented cells are exposed to the photosensitizer methylene blue, light and O2 (Anthony et al., 2005), two conditions that are well known to produce this ROS (Fig. 2). The master regulator of this transcriptional response to 1O2 in Rb. sphaeroides is the Group IV σ factor, σE (Anthony et al., 2005; Campbell et al., 2007; Greenwell et al., 2011).
Figure 2.
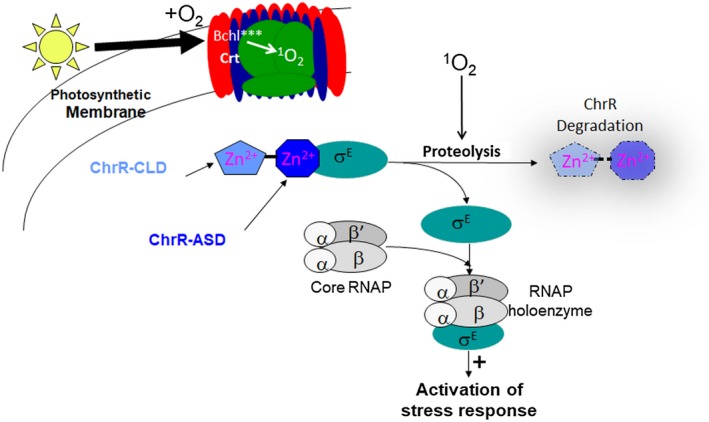
Model for activation of the Rb. sphaeroides σE‐dependent 1O2 stress response. Top Depicts formation of 1O2 during light‐driven energy transfer from excited pigments (Bchl***) of the photosynthetic membrane to O2. Middle Depicts the ability of 1O2 to somehow (signal unknown) promote ChrR degradation, releasing the Group IV sigma factor σE, so it binds RNA polymerase (RNAP) and directly activates transcription of genes in the resulting stress response. ChrR is color‐coded to denote interactions between its N‐terminal ASD domain with σE and the C‐terminal ChrR‐CLD (see text).
In many cells, the ability of carotenoids to quench 1O2 is generally accepted to be a major route of detoxification of this ROS (Cogdell, 2000; Frank and Brudvig, 2004; Kochevar, 2004). However, quenching by carotenoids must not provide complete protection against 1O2 since this ROS can inactivate proteins, and oxidize membrane fatty acids and other olefins (Rinalducci et al., 2004; Kochevar, 2004; Nishiyama et al., 2004; Estevam et al., 2004; Ramel et al., 2012; Lemke et al., 2014). In addition, 1O2 formation kills Rb. sphaeroides ΔσE cells, demonstrating the essential role of the σE‐dependent transcriptional response to this ROS (Anthony et al., 2005).
The Rb. sphaeroides σE‐dependent pathway is not activated by superoxide, H2O2 or hydroxyl radicals (Anthony et al., 2005; Greenwell et al., 2011). However, we and others found that the organohydroperoxide tert‐butylhydroperoxide (t‐BOOH) increases Rb. sphaeroides σE activity (Lourenco and Gomes, 2009; Greenwell et al., 2011; Nam et al., 2013). Based on studies with model compounds, 1O2 oxidization of biomolecules could form organohydroperoxides in the membrane (Stief, 2003; Davies, 2004; Kochevar, 2004; Watabe et al., 2007; Triantaphylides and Havaux, 2009). Thus, activation of the Rb. sphaeroides σE pathway by either 1O2 or t‐BOOH could reflect signal correlation, an evolutionary adaptation that allows cells to mount a response to two membrane‐localized signals (1O2 and organohydroperoxides) that are often found together in nature (Dufour et al., 2010; 2012; Dufour and Donohue, 2012). Several candidate enzymes that could detoxify t‐BOOH or its oxidation products are part of the Rb. sphaeroides σE‐dependent stress response (Dufour et al., 2012; Dufour and Donohue, 2012).
ChrR is a negative regulator of Rb. sphaeroides σE activity
Group IV σs typically bind to a cognate an anti‐σ factor that is co‐transcribed with the σ factor structural gene (Staron et al., 2009). Rb. sphaeroides σE follows this paradigm since ChrR, its cognate anti‐σ, which forms a complex with σE that prevents it transcribing target genes (Newman, 2001; Newman et al., 2001; Anthony et al., 2004; Campbell et al., 2007), is encoded by the rpoEchrR operon (Newman et al., 1999). However, unlike many other Group IV anti‐σ factors, which are integral membrane proteins (Staron et al., 2009), Rb. sphaeroides ChrR is a cytoplasmic protein (Newman et al., 2001; Anthony et al., 2003; 2004; Campbell et al., 2007). Indeed, Rb. sphaeroides ChrR was the founding member of the ECF11 family of Group IV anti‐σs (Staron et al., 2009).
How ChrR blocks σE activity
Key insights into the ECF11 family came from solving the three‐dimensional structure of the Rb. Sphaeroides σE‐ChrR complex (in collaboration with the Darst lab) (Campbell et al., 2007). In the σE‐ChrR complex, the Rb. sphaeroides σE fold is similar to that of other σs, including Escherichia coli σ70 and σE (Campbell et al., 2002; 2003), consisting of two α‐helical domains (σ regions 2 and 4) connected by a short domain 2‐4 linker (Fig. 3).
Figure 3.
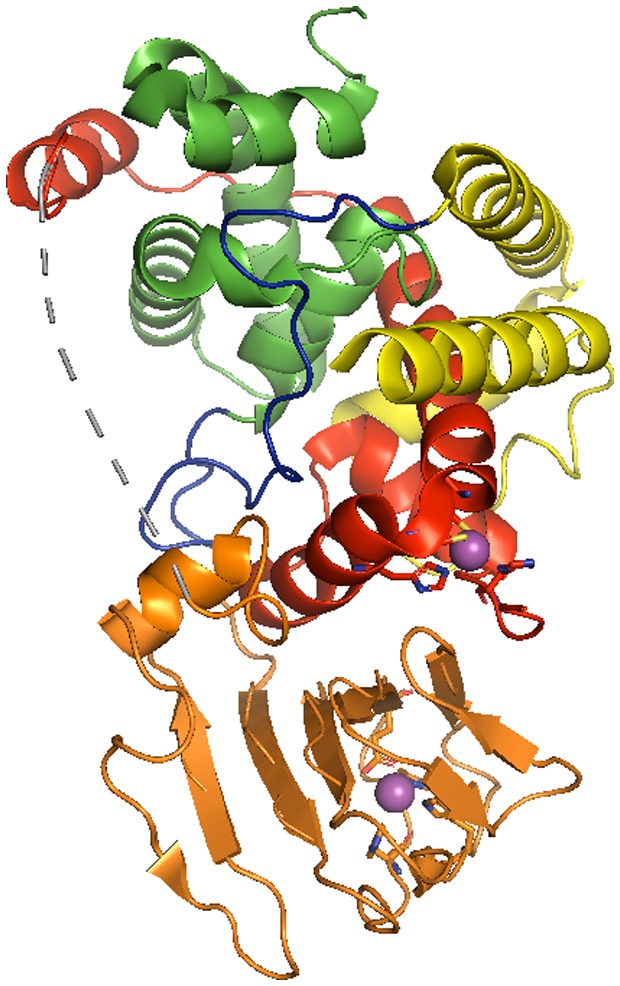
Structure of the Rb. sphaeroides σE‐ChrR complex. The Rb. sphaeroides σE protein is colored to reflect the major functional domains of sigma factors (green‐region 2; blue‐region 2‐4 linker; yellow‐region 4). ChrR is colored to indicate its two major structural domains (red‐the 4 helical bundle that contains the N‐terminal anti‐sigma domain, ASD; orange‐the C‐terminal cupin‐like domain, CLD). Note the extensive interactions of the ChrR‐ASD with σE regions 2 and 4. The 2 zinc atoms associated with ChrR are shown in magenta, along with the side chains of the amino acids that ligate these metals (6His, 31His, 35Cys & 38Cys in the ChrR‐ASD and 141His, 143His, 147Glu and 177His in the ChrR‐CLD respectively).
In this structure, ChrR contains two major structural elements that were connected by a flexible linker. The ChrR N‐terminal domain makes extensive contacts with those σE regions predicted to bind RNA polymerase and promoter DNA (Fig. 3), so this part of ChrR was called the anti‐sigma domain (ChrR‐ASD) to denote how it could block σ factor function.
Zinc binding to the ChrR‐ASD is needed to inhibit σE activity
Rb. sphaeroides ChrR and Streptomyces coelicolor RsrA were founding members of the ZAS anti‐σ proteins, a family of zinc‐dependent anti‐sigma factors that each bind a zinc metal in their N‐terminal ASDs (Paget et al., 2001a; Paget and Buttner, 2003; Bae et al., 2004; Zdanowski et al., 2006). It was known that zinc binding was required for ChrR to inhibit σE activity (Newman et al., 2001). In the σE:ChrR complex, zinc is tetrahedrally coordinated to amino acid side chains in the ChrR‐ASD (Fig. 3) that were predicted to be involved in zinc binding based on studying mutant ChrR proteins containing single alanine substitutions at these positions (Newman et al., 2001).
Conservation of structure and function among different Group IV anti‐σs
The structure of the σE‐ChrR complex contributed to developing an early model for how many anti‐sigma factors could inhibit function of Group IV σs (Campbell et al., 2002). This model was based on the unexpected finding that the ChrR‐ASD and the N‐terminal domain of the E. coli anti‐sigma factor RseA (RseA‐ASD) both contain similar α‐helical bundles despite the lack of significant primary amino acid sequence similarity between these proteins (Campbell et al., 2007). Each ASD contains one structurally conserved helix, helix IV, which interacts with region 2.1 of its cognate Group IV σ in their respective complexes (Fig. 4, right). Based on this, we proposed that many other ECF anti‐sigma factors will use a region structurally related to ASD helix IV to bind region 2.1 of their cognate ECF σs and block RNA polymerase binding (Campbell et al., 2007).
Figure 4.
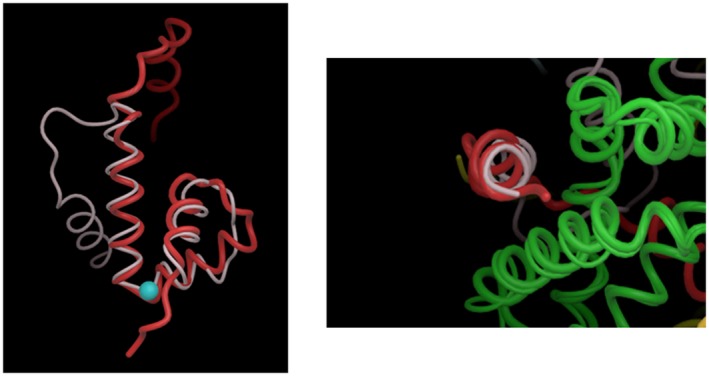
Structural similarity between the Rb. sphaeroides ChrR‐ & E. coli RseA‐ASD. The left panel shows the structural similarity between helices I‐III and the displacement of helix IV of the ASD of Rb. sphaeroides ChrR (red) and E. coli RseA (white). The blue sphere is the Zn2+ atom in the ChrR‐ASD. The right panel shows that, despite this displacement of helix IV in the ASD of ChrR (red) and RseA (white), it interacts with a structurally conserved part of region 2 in the cognate Group IV sigma factors (region 2 of the Rb. sphaeroides and E. coli σE proteins are both shown in green). Figures modified from (Campbell et al., 2007).
We also made the unexpected finding that the region of structural similarity between the ChrR‐ and RseA‐ASDs includes the ChrR zinc binding site (Fig. 4, left). The ability of both anti‐σs to adopt a similar fold shows that either zinc–protein interactions (in ChrR) or protein–protein interactions (in RseA) can stabilize the ASD helical bundle (Campbell et al., 2007).
The other structurally conserved helices in the ASD of each anti‐sigma factor (helices I‐III) interact with different regions of their cognate sigma factors, providing a way for each anti‐σ factor to recognize specific partner proteins (Campbell et al., 2007). When these observations were combined with comparative genomics, it predicted that the ASDs of ChrR homologs, as well as many other Group IV anti‐σ factors that have lower degrees of amino acid identity and thus fall into other ECF subfamilies (Staron et al., 2009), could adopt a similar fold when bound to their cognate σ factor (Campbell et al., 2007). Subsequent structural analysis of additional complexes has revealed that, while the ASD conformation has been highly conserved among anti‐sigma factors, the mechanism of inhibition of σ factor activity is unique for each cognate pair examined (Sineva et al., 2017).
The C‐terminal domain of ChrR is needed to release σE in the presence of 1O2
The ChrR C‐terminal domain binds a 2nd zinc atom within a structural element that adopts an overall fold similar to proteins in the cupin superfamily (Khuri et al., 2001), so we called this the ChrR cupin‐like domain (ChrR‐CLD, Fig. 3). In structurally characterized proteins that contain a CLD, it can have enzyme activity (isomerases) or bind a ligand (Khuri et al., 2001). The overall CLD fold and the residues known to bind zinc in the σE‐ChrR structure are predicted to exist in many other ChrR homologs (Campbell et al., 2007) and a variety of ZAS that are members of other ECF sub‐families (Rajasekar et al., 2016).
The ChrR‐CLD had little contact with σE, leading us to propose that this region was unnecessary for formation of the σE‐ChrR complex. As predicted, a truncated ChrR protein lacking the CLD (ChrR85) inhibited σE activity, but cells containing ChrR85 did not mount a transcriptional response to 1O2 (Campbell et al., 2007), predicting that the ChrR‐CLD is needed to activate the response. When we analyzed function of ChrR variants containing amino acid substitutions in the CLD zinc ligands, we identified side chains that are (147Glu and 177His) and are not (187Cys and 189Cys) needed for 1O2 or the organoperoxide like t‐BOOH (see below) to increase σE activity (Greenwell et al., 2011).
1O2 stimulates ChrR turnover
Some Group IV anti‐σ factors, including others that bind zinc, are reversibly modified by an inducing signal (Paget and Buttner, 2003; Antelmann and Helmann, 2011). However, there is no known mechanism for reversible protein modification by 1O2 (Davies, 2004). Instead, we found that 1O2 promotes ChrR proteolysis (Nam et al., 2013), releasing σE so it can bind RNA polymerase (Anthony et al., 2004) and directly activate transcription (Ziegelhoffer and Donohue, 2009; Dufour et al., 2010; 2012).
There is precedent for regulated proteolysis of a Group IV anti‐σ factor during a stress response, since cleavage of E. coli RseA is initiated by a protease cascade (including DegS and YaeL) that responds to envelope stress (Alba et al., 2002). After DegS and YaeL cleavage of RseA in its membrane spanning region, housekeeping proteases complete degradation of this anti‐σ, releasing the E. coli σE so it can activate transcription (Chaba et al., 2007). Homologs of extra‐cytoplasmic proteases that cleave RseA have been reported to promote ChrR turnover in vivo (Nuss et al., 2013). However, direct proteolytic cleavage of ChrR has yet to be reported and it is not clear how membrane‐ or periplasmic‐localized proteases promote direct or indirect degradation of this cytoplasmic anti‐σ. Indeed, it is possible that 1O2 initiates a conformational change in ChrR that can makes it protease‐susceptible since this ROS can remove zinc from a synthetic peptide that contains the metal ligands and mimics the fold found in the ChrR‐ASD (Chabert et al., 2019). There is precedent for zinc release playing such a regulatory role in the bacterial chaperone Hsp33 and the ZAS anti‐σ factor, RsrA, that each respond to oxidative stress signals (Jakob et al., 2000; Kim et al., 2001; Raman et al., 2001; Paget et al., 2001a; 2001b; Paget and Buttner, 2003; Zdanowski et al., 2006; Rajasekar et al., 2016), so the precise role(s) of the ChrR‐ASD zinc in the response to 1O2 is unknown.
Other proteins have been shown to be needed for ChrR turnover in the presence of 1O2 (Nam et al., 2013; Nuss et al., 2013). However, these proteins lack significant amino acid sequence similarity to proteases but some catalyze synthesis of an unusual furan‐containing fatty acid (Lemke et al., 2014). 1O2 formation promotes destruction of furan‐containing fatty acids, so it has been proposed that peroxidation of membrane bound olefins can act as a second messenger to stimulate the activity of one or more proteases that initiates degradation of ChrR in response to this ROS (Lemke et al., 2014).
Organohydroperoxides and 1O2 promote ChrR turnover by different mechanisms
While the presence of either 1O2 or an organoperoxide like t‐BOOH increases σE activity (Glaeser et al., 2011; Nam et al., 2013), they appear to inactivate ChrR by different mechanisms. By comparing the ability of either 1O2 or t‐BOOH to increase σE activity in cells containing wild‐type ChrR, a truncated ChrR85 protein, variant ChrR proteins with single amino acid changes (Greenwell et al., 2011), and host mutants with defects in furan fatty acid synthesis (Nam et al., 2013), one can genetically separate the effects of 1O2 and t‐BOOH on σE activation. To explain this observation, it has been proposed that ChrR inactivation in the presence of 1O2 (requires a ChrR‐CLD) or t‐BOOH (occurs in cells lacking an intact ChrR‐CLD) do not occur by identical mechanisms (Greenwell et al., 2011; Nam et al., 2013).
The biological response to 1O2
Identifying the members of a transcriptional regulon is often instrumental to understanding functions needed during a stress response (Guisbert et al., 2008). By combining computational (phylogenetic clustering), in vitro (transcription assays) and in vivo (gene fusions, global gene expression or chromatin immunoprecipitation) analyses, we found that 1O2 activated ~160 genes (Fig. 5), with the majority of them the indirect result of this ROS activating a σ factor cascade that includes RpoHII, one of two Rb. sphaeroides σ32 homologs (Green and Donohue, 2006; Dufour et al., 2008; 2012; Ziegelhoffer and Donohue, 2009). Indeed, a combination of in vitro and in vivo studies showed that < 10% of the 1O2 activated genes (~13/160 genes) were directly transcribed by σE‐containing RNA polymerase (Anthony et al., 2005; Green and Donohue, 2006; Dufour et al., 2008; 2012; Dufour and Donohue, 2012). Others predict that a larger number of genes are part of the σE‐dependent response to 1O2 (Glaeser et al., 2007; Nuss et al., 2009; Berghoff et al., 2009), but many studies often do not distinguish direct and indirect effects of 1O2 on downstream gene expression. The direct targets of Rb. sphaeroides σE encode proteins that could prevent or remove damage from lipid peroxidation, enzymes that can repair mutations, electron transport metalloproteins, another alternative sigma factor RpoHII and proteins of unknown function (Anthony et al., 2005; Green and Donohue, 2006; Watabe et al., 2007; Dufour et al., 2008; 2012; Ziegelhoffer and Donohue, 2009). The finding that rpoHII transcription is absolutely dependent on σE (Fig. 5) predicted that 1O2 activated a transcriptional cascade and that both σE and RpoHII have a role in this stress response (Anthony et al., 2005; Green and Donohue, 2006; Watabe et al., 2007; Dufour et al., 2008; 2012). As predicted, 1O2 is bacteriocidal to cells lacking σE or RpoHII (Anthony et al., 2005; Green and Donohue, 2006; Nuss et al., 2010). Additional direct σE target genes are needed for rapid ChrR proteolysis, while others encode proteins that could potentially reduce products of olefin oxidation, prevent oxidation of unsaturated fatty acids, serve as electron carriers or repair damaged macromolecules (Dufour et al., 2008; Ziegelhoffer and Donohue, 2009; Lemke et al., 2014).
Figure 5.

1O2 activates a transcriptional cascade. Shown is the transcriptional cascade that is activated by the presence of 1O2 in Rb. sphaeroides. The master regulator, σE, directly activates transcription of 13 genes, one of which (rpoHII) encodes one of two Rb. sphaeroides alternative σ factors in the heat shock family. RpoHII directly activates ~145 genes; some 45 of which are also transcribed by RpoHI, the master regulator of the Rb. sphaeroides heat shock response. Data summarized from (Dufour et al., 2012).
The ~145 direct RpoHII target genes (Fig. 5) encode bioenergetic enzymes that contain oxidant‐sensitive metal centers (NADH dehydrogenase, etc.), metalloenzymes that synthesize cofactors for bioenergetic enzymes (tetrapyrroles, quinone, etc.) and glutathione‐dependent enzymes that can repair oxidized macromolecules (Dufour et al., 2012; Dufour and Donohue, 2012). Numerous σE and RpoHII targets have no known function (Dufour et al., 2008; 2012; Dufour and Donohue, 2012), illustrating how little is known about the cellular and biological response to 1O2 and organoperoxide stress.
Unlike E. coli, which contains a single heat shock σ factor (Guisbert et al., 2008), Rb. sphaeroides contains two homologs, RpoHI and RpoHII (Karls et al., 1998; Green and Donohue, 2006). 1O2 is not bacteriocidal to ΔRpoHI cells, and RpoHI activity is increased during heat shock (Karls et al., 1998; Green and Donohue, 2006; Dufour et al., 2012). Indeed, many of the ~130 genes that are directly transcribed by RpoHI encode homologs of typical heat shock proteins (Green and Donohue, 2006; Dufour and Donohue, 2012), so it appears that its primary role is in thermal adaptation, similar to that of E. coli σ32 (Guisbert et al., 2008). However, many of the 45 genes which are directly transcribed by both RpoHII and RpoHI (Fig. 5) encode proteins that could act in both 1O2 and heat stress (Green and Donohue, 2006; Dufour et al., 2012; Dufour and Donohue, 2012).
Often, members of a stress regulon are part of a homeostatic loop that is needed to activate the response (Ades et al., 2003; Guisbert et al., 2008). This appears to be true for 1O2 stress, since mutants lacking σE target genes that produce furan‐containing fatty acids are defective in increasing activity of this σ factor when they are exposed to this ROS (Nam et al., 2013). However, cells lacking other ECF11 regulon members have normal activation of σE activity and rates of ChrR proteolysis in the presence of 1O2 (Hendrischk et al., 2007; Nam et al., 2013; Nuss et al., 2013).
Conservation of the ECF11 system across the bacterial phylogeny
Selective pressures experienced by cells in nature can dictate a relationship between signals and regulated genes, so the function of a given regulon may have evolved to accommodate variance in environmental conditions across cells with different lifestyles or habitats. For example, a comparative analysis of the E. coli σE regulon in nine γ‐proteobacteria revealed the existence of a ‘core regulon’ that encodes functions involved in envelope stress, plus an ‘extended regulon’ that includes functions related to pathogenesis or symbiosis, and led to the proposal that host‐microbe interactions also activate this stress response (Rhodius et al., 2006).
A similar phylogenetic analysis of σE‐ChrR homologs across bacterial divisions also suggested that this system evolved prior to the divergence of the α‐ and γ‐proteobacteria, and shows that it includes species which have both photosynthetic and non‐photosynthetic lifestyles (Dufour et al., 2008). By analyzing these genomes for genes orthologous to those transcribed by σE and promoters that contain the motif recognized by this Group IV alternative σ, it was found that many of the direct Rb. sphaeroides σE‐ChrR regulon members were present and predicted to contain a σE promoter in these diverse species. The σE targets that were most conserved across species, which comprise a so‐called ‘core σE‐ChrR regulon’ of ~8 genes, include the rpoEchrR operon and genes involved in synthesis of furan fatty acids that are required for ChrR turnover in the presence of 1O2 (Dufour et al., 2008). Therefore, it is possible that the photosynthetic and non‐photosynthetic species which contain σE‐ChrR homologs both encounter 1O2 in nature (Dufour et al., 2008). The observation that proteins required for ChrR turnover in the presence of 1O2 are conserved members of the core σE regulon gene suggests that a similar homeostatic feedback loop activates this stress response in other bacteria (Dufour et al., 2008; Nam et al., 2013; Lemke et al., 2014). As predicted by these phylogenetic analyses, the Caulobacter crescentus σE‐ChrR system was rapidly activated by 1O2 and organic hydroperoxides and exhibited a slower response to other inducers (Lourenco and Gomes, 2009), suggesting other signals or pathways activate the ECF11 regulon in this and other species.
Comparative genomics also identified another group of genes directly transcribed by σE that are not highly conserved among bacterial species and constitute an ‘extended σE‐ChrR regulon’ (Dufour et al., 2008). This extended σE‐ChrR regulon contains numerous genes of unknown function, illustrating the potential to reveal new biology by elucidating their function. There are also gene sets which are only part of the extended σE‐ChrR regulon in selected bacteria, suggesting they encode functions associated with the lifestyle or ecological niche of these organisms (Dufour et al., 2008).
Future directions
Like other optogenetic circuits (Zhao et al., 2018), one advantage of studying Rb. sphaeroides σE‐ChrR is the ease of controlling production of the stimulating signal, 1O2, by the presence or absence of light (Anthony et al., 2005). In addition, biochemical, genetic, genomic and computational methods were combined to reveal control principles of this system, define processes that are impacted by 1O2 formation, and predicted the properties of σE‐ChrR networks in other bacteria that contain ECF11 proteins (Newman et al., 1999; Anthony et al., 2003; Campbell et al., 2007; Dufour et al., 2008; 2012; Greenwell et al., 2011; Dufour and Donohue, 2012; Nam et al., 2013; Lemke et al., 2014).
Despite the knowledge accumulated by studying Rb. sphaeroides σE‐ChrR, major gaps remain in our understanding on important aspects of its function. For example, to understand how Rb. sphaeroides σE activity is increased, information is needed on the events and proteins that regulate ChrR turnover in the presence of 1O2. Other needs include insight into a direct interaction of 1O2, peroxidation products of fatty acids, or other biomolecules with the ChrR‐ASD and ChrR‐CLD, the protease(s) that degrade ChrR, and the signal transduction pathway used to promote turnover of a cytoplasmic anti‐σ factor by a membrane ROS. In addition, identifying the function of genes that are directly transcribed by σE‐containing RNA polymerase but only found in selected species (extended members of the σE regulon) can provide needed insight into stress response functions associated with lifestyles or ecological niches of these bacteria.
It is crucial to point out that many of the above questions illustrate knowledge gaps for other Group IV alternative σs, so it is likely that answers obtained by analyzing ECF11 proteins will have broad applicability to other regulatory networks. In this way, analysis of the Group IV alternative σs will continue to illuminate new features of biological processes across the bacterial phylogeny.
Accession numbers
The atomic coordinates for proteins discussed are deposited in the Protein Data Bank (http://wwpdb.org/) under ID codes 2Q1Z, 2Z2S, and 1OR7. The gene expression and chromatin immunoprecipitation data sets discussed can be found in the Gene Expression Omnibus through series accession number GSE39806 (https://www.ncbi.nlm.nih.gov/geo/).
Acknowledgements
Preparation of this MicroReview and our ongoing studies on furan fatty acid synthesis is funded by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE‐FC02‐07ER64494 and DE‐SC0018409). I thank the students, trainees and staff in the Donohue lab who contributed to work on this topic, reviewers of past papers who made important suggestions, and colleagues from around the globe for sharing ideas or collaborating on structural, genomic, and other analyses summarized in this review.
References
- Ades, S.E. , Grigorova, I.L. and Gross, C.A. (2003) Regulation of the alternative sigma factor σE during initiation, adaptation, and shutoff of the extracytoplasmic heat shock response in Escherichia coli . Journal of Bacteriology, 185, 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba, B.M. , Leeds, J.A. , Onufryk, C. , Lu, C.Z. and Gross, C.A. (2002) DegS and YaeL participate sequentially in the cleavage of RseA to activate the σE‐dependent extracytoplasmic stress response. Genes & Development, 16, 2156–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelmann, H. and Helmann, J.D. (2011) Thiol‐based redox switches and gene regulation. Antioxidants & Redox Signaling, 14, 1049–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, J.R. , Green, H.A. and Donohue, T.J. (2003) Purification of Rhodobacter sphaeroides RNA polymerase and its sigma factors. Methods in Enzymology, 370, 54–65. [DOI] [PubMed] [Google Scholar]
- Anthony, J.R. , Newman, J.D. and Donohue, T.J. (2004) Interactions between the Rhodobacter sphaeroides ECF sigma factor, σE, and its anti‐sigma factor. ChrR. J. Mol. Biol., 341, 345–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, J.R. , Warczak, K.L. and Donohue, T.J. (2005) A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proceedings of the National Academy of Sciences, 102, 6502–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, J.‐B. , Park, J.‐H. , Hahn, M.‐Y. , Kim, M.‐S. and Roe, J.‐H. (2004) Redox‐dependent changes in RsrA, an anti‐sigma factor in Streptomyces coelicolor: zinc release and disulfide bond formation. Journal of Molecular Biology, 335(2), 425–435. [DOI] [PubMed] [Google Scholar]
- Berghoff, B.A. , Glaeser, J. , Sharma, C.M. , Vogel, J. and Klug, G. (2009) Photooxidative stress‐induced and abundant small RNAs in Rhodobacter sphaeroides . Molecular Microbiology, 74, 1497–1512. [DOI] [PubMed] [Google Scholar]
- Borisov, V.B. , Gennis, R.B. , Hemp, J. and Verkhovsky, M.I. (2011) The cytochrome bd respiratory oxygen reductases. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics, 1807, 1398–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski, P. and Gennis, R.B. (2008) Cytochrome c oxidase: exciting progress and remaining mysteries. Journal of Bioenergetics and Biomembranes, 40, 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno, E. , Mesa, S. , Bedmar, E.J. , Richardson, D.J. and Delgado, M.J. (2012) Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox control. Antioxidants & redox signaling, 16, 819–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, E.A. , Muzzin, O. , Chlenov, M. , Sun, J.L. , Olson, C.A. , Weinman, O. , et al. (2002) Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Molecular Cell, 9, 527–539. [DOI] [PubMed] [Google Scholar]
- Campbell, E. , Tupy, J. , Gruber, T. , Wang, S. , Sharp, M. , Gross, C. and Darst, S. (2003) Crystal structure of Escherichia coli σE with the cytoplasmic domain of its anti‐sigma RseA. Molecular Cell, 11, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Campbell, E.A. , Greenwell, R. , Anthony, J.R. , Wang, S. , Lim, L. , Das, K. , et al. (2007) A conserved structural module regulates transcriptional responses to diverse stress signals in bacteria. Molecular Cell, 27, 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaba, R. , Grigorova, I.L. , Flynn, J.M. , Baker, T.A. and Gross, C.A. (2007) Design principles of the proteolytic cascade governing the σE‐mediated envelope stress response in Escherichia coli: keys to graded, buffered, and rapid signal transduction. Genes & Development, 21, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabert, V. , Lebrun, V. , Lebrun, C. , Latour, J.‐M. and Sénèque, O. (2019) Model peptide for anti‐sigma factor domain HHCC zinc fingers: high reactivity toward 1O2 leads to domain unfolding. Chemical Science, 12, 3608–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell, R.J. (2000) How carotenoids protect bacterial photosynthesis. Phil. Trans. R. Soc. Lond. B, 355, 1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon, A. , Miersch, O. , Felix, G. , Camp, R.G.L. and Apel, K. (2005) Concurrent activation of cell death‐regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. The Plant Journal, 41, 68–80. [DOI] [PubMed] [Google Scholar]
- Davies, M. (2004) Reactive species formed on proteins exposed to singlet oxygen. Photochemical & Photobiological Sciences, 3, 17–25. [DOI] [PubMed] [Google Scholar]
- Dogra, V. , Rochaix, J.D. and Kim, C. (2018) Singlet oxygen‐triggered chloroplast‐to‐nucleus retrograde signalling pathways: an emerging perspective. Plant, Cell & Environment, 41, 1727–1738. [DOI] [PubMed] [Google Scholar]
- Donohue, T.J. and Kiley, P.J. (2011) Bacterial responses to O2 limitation In: Storz G. and Hengge R. (Eds.) Bacterial Stress Responses. Washington, DC: American Society for Microbiology, pp. 175–189. [Google Scholar]
- Dufour, Y. and Donohue, T.J. (2012) Signal correlations in ecological niches can shape the organization and evolution of bacterial gene regulatory networks In: Poole R.K. (Ed.) Advances in Microbial Physiology. London: Elsevier, pp. 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour, Y.S. , Landick, R. and Donohue, T.J. (2008) Organization and evolution of the biological response to singlet oxygen stress. Journal of Molecular Biology, 383, 713–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour, Y. , Kiley, P.J. and Donohue, T.J. (2010) Reconstruction of the core and extended regulons of global transcription factors. PLoS Genetics, 6, e1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour, Y.S. , Imam, S. , Koo, B.‐M. , Green, H.A. and Donohue, T.J. (2012) Convergence of the transcriptional responses to heat shock and singlet oxygen stresses. PLoS Genetics, 8, e1002929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevam, M.L. , Nascimento, O.R. , Baptista, M.S. , Di Mascio, P. , Prado, F.M. , Faljoni‐Alario, A. , et al. (2004) Changes in the spin state and reactivity of cytochrome c induced by photochemically generated singlet oxygen and free radicals. Journal of Biological Chemistry, 279, 39214–39222. [DOI] [PubMed] [Google Scholar]
- Feklístov, A. , Sharon, B.D. , Darst, S.A. and Gross, C.A. (2014) Bacterial sigma factors: a historical, structural, and genomic perspective. Annual Review of Microbiology, 68, 357–376. [DOI] [PubMed] [Google Scholar]
- Foyer, C.H. and Noctor, G. (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell, 17, 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, H.A. and Brudvig, G.W. (2004) Redox functions of carotenoids in photosynthesis. Biochemistry, 43, 8605–8615. [DOI] [PubMed] [Google Scholar]
- Frick, K. , Schulte, M. and Friedrich, T. (2015) Reactive oxygen species production by Escherichia coli respiratory complex I. Biochemistry, 54, 2799–2801. [DOI] [PubMed] [Google Scholar]
- Fryer, M.J. , Oxborough, K. , Mullineaux, P.M. and Baker, N.R. (2002) Imaging of photo‐oxidative stress responses in leaves. Journal of Experimental Botany, 53, 1249–1254. [PubMed] [Google Scholar]
- Gennis, R.B.a.V.S. (1986) Respiration In: Neidhardt F.C. (Ed.). Escherichia coli and Salmonella Cellular and Molecular Biology. Washington, DC: ASM Press, pp. 217–261. [Google Scholar]
- Girotti, A.W. and Kriska, T. (2004) Role of lipid peroxides in photo‐oxidative stress signalling. Antioxidants & Redox Signalling, 6, 301–310. [DOI] [PubMed] [Google Scholar]
- Glaeser, J. , Zobawa, M. , Lottspeich, F. and Klug, G. (2007) Protein synthesis patterns reveal a complex regulatory response to singlet oxygen in Rhodobacter . Journal of Proteome Research, 6, 2460–2471. [DOI] [PubMed] [Google Scholar]
- Glaeser, J. , Nuss, A.M. , Berghoff, B.A. and Klug, G. (2011) Singlet oxygen stress in microorganisms In: Robert K.P. (Ed.) Advances in Microbial Physiology. Oxford, UK: Academic Press, pp. 141–173. [DOI] [PubMed] [Google Scholar]
- Godley, B.F. , Shamsi, F.A. , Liang, F.‐Q. , Jarrett, S.G. , Davies, S. and Boulton, M. (2005) Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. Journal of Biological Chemistry, 280, 21061–21066. [DOI] [PubMed] [Google Scholar]
- Green, H.A. and Donohue, T.J. (2006) Activity of Rhodobacter sphaeroides RpoHII, a second member of the heat shock sigma factor family. Journal of Bacteriology, 188, 5712–5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell, R.S. , Nam, T.W. and Donohue, T.J. (2011) Aspects of the zinc metalloprotein ChrR required for dissociation of σE/ChrR complexes. Journal of Molecular Biology, 407, 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisbert, E. , Yura, T. , Rhodius, V.A. and Gross, C.A. (2008) Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiology and Molecular Biology Reviews, 72, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrischk, A.‐K. , Braatsch, S. , Glaeser, J. and Klug, G. (2007) The phrA gene of Rhodobacter sphaeroides encodes a photolyase and is regulated by singlet oxygen and peroxide in a σE‐dependent manner. Microbiology, 153, 1842–1851. [DOI] [PubMed] [Google Scholar]
- Jakob, U. , Eser, M. and Bardwell, J.C. (2000) Redox switch of Hsp33 has a novel zinc‐binding motif. Journal of Biological Chemistry, 275, 38302–38310. [DOI] [PubMed] [Google Scholar]
- Karls, R.K. , Brooks, J. , Rossmeissl, P. , Luedke, J. and Donohue, T.J. (1998) Metabolic roles of a Rhodobacter sphaeroides member of the σ32 family. Journal of Bacteriology, 180, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, R.A. (2005) Earth science: the story of O2 . Science, 308, 1730–1732. [DOI] [PubMed] [Google Scholar]
- Khuri, S. , Bakker, F.T. and Dunwell, J.M. (2001) Phylogeny, function, and evolution of the cupins, a structurally conserved, functionally diverse superfamily of proteins. Molecular Biology and Evolution, 18(4), 593–605. [DOI] [PubMed] [Google Scholar]
- Kiley, P.J. and Storz, G. (2004) Exploiting thiol modifications. PLoS Biology, 2, 1714–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.J. , Jeong, D.G. , Chi, S.W. , Lee, J.S. and Ryu, S.E. (2001) Crystal structure of proteolytic fragments of the redox‐sensitive Hsp33 with constitutive chaperone activity. Natural Structural Biology, 8, 459–466. [DOI] [PubMed] [Google Scholar]
- Kochevar, I. (2004) Singlet oxygen signaling: from intimate to global. Science Signaling, 2004(221), pe7. [DOI] [PubMed] [Google Scholar]
- Lemke, R.A.S. , Peterson, A.C. , Ziegelhoffer, E.C. , Westphall, M.S. , Tjellström, H. , Coon, J.J. and Donohue, T.J. (2014) Synthesis and scavenging role of furan fatty acids. Proceedings of the National Academy of Sciences, 111, E3450–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco, R.F. and Gomes, S.L. (2009) The transcriptional response to cadmium, organic hydroperoxide, singlet oxygen and UV‐A mediated by the σE‐ChrR system in Caulobacter crescentus . Molecular Microbiology, 72, 1159–1170. [DOI] [PubMed] [Google Scholar]
- Mittler, R. , Vanderauwera, S. , Gollery, M. and Van Breusegem, F. (2004) Reactive oxygen gene network of plants. Trends in Plant Science, 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Nam, T.W. , Ziegelhoffer, E.C. , Lemke, R.A.S. and Donohue, T.J. (2013) Proteins needed to activate a transcriptional response to the reactive oxygen species singlet oxygen. MBio, 4, e00541‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, J. (2001) The sigma factor/anti‐sigma factor pair, σE/ChrR, in Rhodobacter sphaeroides. PhD Thesis, UW‐Madison. [Google Scholar]
- Newman, J.D. , Falkowski, M.J. , Schilke, B.A. , Anthony, L.C. and Donohue, T.J. (1999) The Rhodobacter sphaeroides ECF sigma factor, σE, and the target promoters cycA P3 and rpoE P1. Journal of Molecular Biology, 294, 307–320. [DOI] [PubMed] [Google Scholar]
- Newman, J.D. , Anthony, J.R. and Donohue, T.J. (2001) The importance of zinc‐binding to the function of Rhodobacter sphaeroides ChrR as an anti‐sigma factor. Journal of Molecular Biology, 313, 485–499. [DOI] [PubMed] [Google Scholar]
- Nishiyama, Y. , Allakhverdiev, S.I. , Yamamoto, H. , Hayashi, H. and Murata, N. (2004) Singlet oxygen inhibits the repair of Photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry, 43, 11321–11330. [DOI] [PubMed] [Google Scholar]
- Nuss, A.M. , Glaeser, J. and Klug, G. (2009) RpoHII activates oxidative‐stress defense systems and is controlled by RpoE in the singlet oxygen‐dependent response in Rhodobacter sphaeroides . Journal of Bacteriology, 191, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss, A.M. , Glaeser, J. , Berghoff, B.A. and Klug, G. (2010) Overlapping alternative sigma factor regulons in the response to singlet oxygen in Rhodobacter sphaeroides . Journal of Bacteriology, 192, 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss, A.M. , Adnan, F. , Weber, L. , Berghoff, B.A. , Glaeser, J. and Klug, G. (2013) DegS and RseP homologous proteases are involved in singlet oxygen dependent activation of RpoE in Rhodobacter sphaeroides . PLoS ONE, 8, e79520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymann, E.S. and Hynninen, P.H. (2004) Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. Journal of Photochemistry and Photobiology B: Biology, 73, 1–28. [DOI] [PubMed] [Google Scholar]
- Paget, M.S.B. and Buttner, M.J. (2003) Thiol‐based regulatory switches. Annual Review of Genetics, 37, 91–121. [DOI] [PubMed] [Google Scholar]
- Paget, M.S. , Bae, J.B. , Hahn, M.Y. , Li, W. , Kleanthous, C. , Roe, J.H. and Buttner, M.J. (2001a) Mutational analysis of RsrA, a zinc‐binding anti‐sigma factor with a thiol‐disulphide redox switch. Molecular Microbiology, 39, 1036–1047. [DOI] [PubMed] [Google Scholar]
- Paget, M.S. , Molle, V. , Cohen, G. , Aharonowitz, Y. and Buttner, M.J. (2001b) Defining the disulphide stress response in Streptomyces coelicolor A3(2): identification of the σR regulon. Molecular Microbiology, 42, 1007–1020. [DOI] [PubMed] [Google Scholar]
- Pinto, D. , Dürr, F. , Froriep, F. , Araújo, D. , Liu, Q. and Mascher, T. (2019) Extracytoplasmic function σ factors can be implemented as robust heterologous genetic switches in Bacillus subtilis . iScience, 13, 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragàs, X. , He, X. , Agut, M. , Roxo‐Rosa, M. , Gonsalves, A. , Serra, A. and Nonell, S. (2013) Singlet oxygen in antimicrobial photodynamic therapy: photosensitizer‐dependent production and decay in E. coli . Molecules, 18, 2712–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar, K.V. , Zdanowski, K. , Yan, J. , Hopper, J.T.S. , Francis, M.‐L.R. , Seepersad, C. , et al. (2016) The anti‐sigma factor RsrA responds to oxidative stress by reburying its hydrophobic core. Nature Communications, 7, 12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, B. , Siva Kumar, L.V. , Ramakrishna, T. and Mohan Rao, C. (2001) Redox‐regulated chaperone function and conformational changes of Escherichia coli Hsp33. FEBS Letters, 489, 19–24. [DOI] [PubMed] [Google Scholar]
- Ramel, F. , Birtic, S. , Ginies, C. , Soubigou‐Taconnat, L. , Triantaphylidès, C. and Havaux, M. (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proceedings of the National Academy of Sciences, 109, 5535–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, J. , Zhaxybayeva, O. , Gogarten, J.P. and Blankenship, R.E. (2003) Evolution of photosynthetic prokaryotes: a maximum‐likelihood mapping approach. Philosophical Transactions: Biological Sciences, 358, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodius, V.A. , Suh, W.C. , Nonaka, G. , West, J. and Gross, C.A. (2006) Conserved and variable functions of the σE stress response in related genomes. PLoS Biology, 4, 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodius, V.A. , Segall‐Shapiro, T.H. , Sharon, B.D. , Ghodasara, A. , Orlova, E. , Tabakh, H. , et al. (2013) Design of orthogonal genetic switches based on a crosstalk map of σ's, anti‐σ's, and promoters. Molecular Systems Biology, 9, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinalducci, S. , Pedersen, J.Z. and Zolla, L. (2004) Formation of radicals from singlet oxygen produced during photoinhibition of isolated light‐harvesting proteins of photosystem II. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics, 1608, 63–73. [DOI] [PubMed] [Google Scholar]
- Rosner, J.L. and Storz, G. (1997) Regulation of bacterial responses to oxidative stress. Current Topics in Cellular Regulation, 35, 163–177. [DOI] [PubMed] [Google Scholar]
- Schulz, J.B. , Lindenau, J. , Seyfried, J. and Dichgans, J. (2000) Glutathione, oxidative stress and neurodegeneration. European Journal of Biochemistry, 267, 4904–4911. [DOI] [PubMed] [Google Scholar]
- Sineva, E. , Savkina, M. and Ades, S.E. (2017) Themes and variations in gene regulation by extracytoplasmic function (ECF) sigma factors. Current Opinion in Microbiology, 36, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo, R.M. , Hemp, J. and Hugenholtz, P. (2019) Evolution of photosynthesis and aerobic respiration in the cyanobacteria. Free Radical Biology and Medicine, in press. 10.1016/j.freeradbiomed.2019.03.029 [DOI] [PubMed] [Google Scholar]
- Staron, A. , Sofia, H.J. , Dietrich, S. , Ulrich, L.E. , Liesegang, H. and Mascher, T. (2009) The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Molecular Microbiology, 74, 557–581. [DOI] [PubMed] [Google Scholar]
- Stief, T.W. (2003) The physiology and pharmacology of singlet oxygen. Medical Hypotheses, 60(4), 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó, I. , Bergantino, E. and Giacometti, G.M. (2005) Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo‐oxidation. EMBO Reports, 6, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano, C.L. and Donohue, T.J. (2006) Development of the bacterial photosynthetic apparatus. Current Opinion in Microbiology, 9, 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne, Y.J. , Merkus, D. , Bogers, A.J. , Halliwell, B. , Duncker, D.J. and Lyons, T.W. (2018) Reactive oxygen species: radical factors in the evolution of animal life. BioEssays, 40, 1700158. [DOI] [PubMed] [Google Scholar]
- Triantaphylides, C. and Havaux, M. (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends in Plant Science, 14, 219–228. [DOI] [PubMed] [Google Scholar]
- Uchoa, A.F. , Knox, P.P. , Turchielle, R. , Seifullina, N.K. and Baptista, M.S. (2008) Singlet oxygen generation in the reaction centers of Rhodobacter sphaeroides . European Biophysics Journal, 37, 843–850. [DOI] [PubMed] [Google Scholar]
- Watabe, N. , Ishida, Y. , Ochiai, A. , Tokuoka, Y. and Kawashima, N. (2007) Oxidation decomposition of unsaturated fatty acids by singlet oxygen in phospholipid bilayer membranes. Journal of Oleo Science, 56, 73–80. [DOI] [PubMed] [Google Scholar]
- Zdanowski, K. , Doughty, P. , Jakimowicz, P. , O'Hara, L. , Buttner, M.J. , Paget, M.S.B. and Kleanthous, C. (2006) Assignment of the zinc ligands in RsrA, a redox‐sensing ZAS protein from Streptomyces coelicolor . Biochemistry, 45, 8294–8300. [DOI] [PubMed] [Google Scholar]
- Zhao, E.M. , Zhang, Y. , Mehl, J. , Park, H. , Lalwani, M.A. , Toettcher, J.E. and Avalos, J.L. (2018) Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature, 555, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M. and Storz, G. (2000) Redox sensing by prokaryotic transcription factors. Biochemical Pharmacology, 59(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Ziegelhoffer, E.C. and Donohue, T.J. (2009) Bacterial responses to photo‐oxidative stress. Nature Reviews Microbiology, 7, 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]


