Abstract
Aims
We evaluated risk factors for clinically relevant hypoglycaemia (blood glucose <3 mmol/L) in patients with type 2 diabetes during insulin glargine self‐titration. Data were from two clinical trials in which patients were able to improve glycaemic control by self‐titration of insulin glargine using a simple algorithm.
Materials and Methods
We performed post hoc analyses of pooled treatment groups from each of two Phase 3 studies comparing LY2963016 with LANTUS: ELEMENT‐2 (double‐blind) and ELEMENT‐5 (open label). Clinically relevant hypoglycaemia was analysed by category of HbA1c (<7%, 7%‐8.5%, >8.5%) at Week 12 (titration period) and at Week 24 (overall study), and by subgroups of age (<65, ≥65 years) and previous insulin use (naïve or not).
Results
In the ELEMENT‐2 study (N = 756), there were no overall differences in rate or incidence of hypoglycaemia among HbA1c categories. In the ELEMENT‐5 study (N = 493), patients with HbA1c greater than 8.5% had a lower rate and incidence of hypoglycaemia throughout the study compared to those in the lower HbA1c categories. In both studies, patients 65 years of age or older, compared to those less than 65 years, had a higher rate and incidence of hypoglycaemia during the titration phase, had lower baseline HbA1c, and experienced smaller increases in dose, with no differences in HbA1c post baseline. The rate and incidence of hypoglycaemia was similar between naïve patients and patients previously using basal insulin, across all levels of glycaemic control. With the exception of the older subgroup, hypoglycaemia rates were similar during titration and maintenance periods.
Conclusion
Our results support broader use of self‐titration algorithms for patients with type 2 diabetes.
Keywords: hypoglycaemia, insulin glargine, self‐titration
1. INTRODUCTION
Since its introduction to the market in 2000, insulin glargine (IGlar) 100 U/mL has been perceived as the gold standard basal insulin analogue1 and continues to be a benchmark for other therapies.2 Lessons from randomized clinical trials (RCTs) for IGlar, including effective use of titration algorithms (treat‐to‐target), have been adopted broadly in clinical practice for glargine and other basal insulins.3 Simplicity of treatment regimen and the relatively low risk of hypoglycaemia contribute to the growing popularity of adding basal insulin analogues to existing treatments as a way to introduce insulin in patients with type 2 diabetes (T2D) who are no longer controlled with oral glucose‐lowering medications.4
Despite the high efficacy of insulin treatment shown in RCTs, patients in the real world frequently fail to achieve the desired level of glycaemic control.5 There are multiple explanations, but one is lack of effective up‐titration, with patients continuing therapy at suboptimal doses of basal insulin. Reluctance to increase an insulin dose might be related to the fear of hypoglycaemia or of weight gain among patients or health care providers (HCPs). Also, the HC may lack expertise in dose adjustment, or may not have the resources or ability to monitor the titration process.6
Titration algorithms, which have been shown to be effective while maintaining a reasonable risk of hypoglycaemia, provide useful guidance to HCPs in titrating basal insulin.3, 7 Patient‐driven self‐titration algorithms may be used as an alternative to titration led by HCPs.8 Clinical trials comparing patient‐driven and physician‐driven basal insulin titration have provided reassuring results, showing similar or better glycaemic control with patient‐driven methods.9 However, HCPs may feel uncomfortable advising patients to implement self‐titration because of concerns about hypoglycaemia. Further research is needed to understand the factors that will identify patients who might require more cautious self‐titration monitoring and more frequent supervision because of the risk of hypoglycaemia.
We have undertaken a reanalysis of hypoglycaemia in two RCTs that involved glargine self‐titration, using the definition of clinically relevant hypoglycaemia (blood glucose <3 mmol/L [54 mg/dL]), which is in line with recent recommendations that glucose concentrations less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials.10 The ELEMENT‐2 and ELEMENT‐5 trials compared two IGlar U‐100 products, Lantus and LY2963016, in patients with T2D.11, 12 LY2963016 is an insulin glargine product developed by Eli Lilly and Company and Boehringer Ingelheim which is considered a follow‐on biologic to Lantus in the USA and a biosimilar product in the EU and some other countries. Treatment comprised a 3‐month titration period, during which doses were frequently adjusted based on self‐monitored blood glucose, and a 3‐month maintenance period.
This study sought to provide additional insight into the burden of hypoglycaemia in patients with T2D who were self‐titrating IGlar 100 U/mL by determining if glycaemic control, older age or previous insulin treatment affected the risk of clinically relevant hypoglycaemia (blood glucose <3 mmol/L) during IGlar self‐titration.
2. METHODS
2.1. Patients and study design
The ELEMENT‐2 and ELEMENT‐5 trials were Phase 3, randomized, multicentre, 24‐week studies in patients with type 2 diabetes, of which 60% and 45%, respectively, were insulin naive (HbA1c ≥7% and ≤11%) or were using IGlar previously (HbA1c ≤11%). Eligible patients were aged at least 18 years and had been receiving at least two oral antihyperglycaemia medications (OAMs) at stable doses for 12 weeks prior to screening, with or without IGlar. The ELEMENT‐2 trial (Czech Republic, France, Germany, Greece, Hungary, Italy, Republic of Korea, Mexico, Poland, Puerto Rico, Spain, Taiwan, USA) was double‐blind. The ELEMENT‐5 trial (India, Korea, Puerto Rico, Russia, Turkey, Taiwan, USA) was open label and also differed from the ELEMENT‐2 trial in enrolment of patients using pre‐study basal insulins other than IGlar. Both studies compared treatment with LY2963016 (LY IGlar) to treatment with LANTUS IGlar for 24 weeks; the titration period in each trial was Weeks 0 to 12, and the maintenance period was Weeks 13 to 24. Both treatments showed similar efficacy and safety.11, 12
The starting dose for all insulin‐naïve patients was 10 U/day; patient‐driven titration specified the addition of 1 U daily until fasting blood glucose (FBG) reached at least 5.6 mmol/L (100 mg/dL) or lower.13 Patients using LANTUS prior to study entry initiated the insulin to which they were randomized at their current dose, and increased their insulin dose by 1 U/day until the same FBG target was reached. Most of the titration was expected to be completed during the titration period, with any further adjustments after Week 12 made for safety concerns such as hypoglycaemia or unacceptable hyperglycaemia. Patients monitored their blood glucose with daily 4‐point self‐monitored blood glucose (SMBG) profiles, and were asked to perform three 7‐point SMBG profiles in the 2 weeks prior to each of four specified visits during the study.
In the ELEMENT‐2 trial, 621 patients (82.1%) were receiving two OAMs prior to randomization. The remaining patients were receiving three OAMs (15.9%), four OAMs (1.7%) or five OAMs (0.3%) prior to randomization. In the ELEMENT‐5 trial, 341 patients (69.2%) were receiving two OAMs prior to randomization, and the remaining patients were receiving three OAMs (26.4%), four OAMs (3.9%) or five OAMs (0.6%) prior to randomization. In both studies, the most commonly used combinations of two OAMs were sulfonylureas and metformin, and dipeptidyl peptidase‐4 inhibitors and metformin. The intent of the study was that patients not deviate from their OAM regimen except in an emergency.
2.2. Objectives
In these post hoc analyses, we aimed to estimate the incidence and rate of clinically relevant hypoglycaemia in patients who participated in the ELEMENT‐2 or ELEMENT‐5 clinical trials. Clinically relevant hypoglycaemia is a new category introduced for the purpose of these analyses. It includes both non‐severe and severe episodes of hypoglycaemia, confirmed with blood glucose less than 3 mmol/L (54 mg/dL), which were routinely recorded during the trials and reported, in line with the original protocol definitions.11, 12
With treatment groups pooled in each trial, we determined whether subgroups of age (<65 vs ≥65 years), previous insulin use (naïve vs previous use of insulin) or level of glycaemic control achieved during the trial and at the endpoint (HbA1c at 8, 12 and 24 weeks) affected the risk of hypoglycaemia. Hypoglycaemia was analysed for the titration period, the maintenance period and the overall study. We also determined whether any baseline factors would predict the risk of experiencing at least two episodes of clinically relevant hypoglycaemia during the study.
2.3. Statistical analyses
Given the similar efficacy and safety of LY IGlar and LANTUS,14, 15 treatment arms were pooled within each trial for this study. Because of differences in design and statistical analysis of the two trials, populations of patients from the two studies were pooled only for the regression analysis.
For baseline values of HbA1c, FBG and insulin dose, least square (LS) means were computed using an analysis of variance (ANOVA) model, with baseline of response variable as dependent variable and subgroup as independent variable (type III sums of squares).
For the ELEMENT‐2 trial, consistent with pre‐planned analyses, LS means of HbA1c, FBG and insulin dose at post‐baseline time‐points were computed using an analysis of covariance (ANCOVA) model, with the post‐baseline value of the response variable as dependent variable and the baseline value of the response variable, country, sulfonylurea use, time of basal insulin injection (daytime, evening/bedtime) and subgroup as independent variables (type III sums of squares). For endpoints at Week 24, last observation carried forward (LOCF) was used as an imputation mechanism.
For the ELEMENT‐5 trial, LS means estimates of HbA1c, FBG and insulin dose were computed for post‐baseline time‐points using a mixed model repeated measures (MMRM) model, with the response variable value as dependent variable and baseline value, pooled country, sulfonylurea use, subgroup, time and subgroup*time as independent variables (type III sum of squares and variance–covariance structure = unstructured). For endpoints in the ELEMENT‐5 trial, an ANCOVA model was implemented, with response variable value as dependent variable and baseline value, pooled country, sulfonylurea use, time of basal insulin injection and subgroup as independent variables (type III sums of squares). For endpoints at Week 24, LOCF was used as an imputation mechanism.
For ANCOVA and MMRM models of HbA1c at 12 weeks, the end of the titration phase, the baseline value of HbA1c was not entered in the model to avoid confounding with the subgroup categories; that is, higher baseline HbA1c values would have a higher probability of being present in the higher HbA1c categories (>8.5%) at 12 weeks.
For analysis of the incidence of hypoglycaemia, Fisher's exact test was used to compare analysed subgroups. To compare maintenance and titration periods within participants, a generalized estimating equations (GEE) logistic regression model was used to account for the paired nature of this test, with maintenance vs titration as covariate and country, sulfonylurea use at screening and HbA1c at baseline as fixed effects.
For analysis of the rate of hypoglycaemia per 100 person‐years, negative binomial (NB) models were used, with the number of hypoglycaemia events as outcome variable, subgroup as independent variable, and with log (exposure in days/365.25) as an offset variable to calculate the rate per 100 person‐years. For the ELEMENT‐2 trial, the following covariates were used in these models: baseline HbA1c, sulfonylurea use and time of basal insulin injection. For the ELEMENT‐5 trial, the following covariates were used in these models: baseline HbA1c, sulfonylurea use at screening, pooled country and basal insulin status at study entry. For comparison of titration and maintenance periods, GEE versions of the above NB models were used to accommodate for the paired nature of this test.
Assuming a similar effect of covariates on hypoglycaemia between the two studies, we implemented a multiple logistic regression model with stepwise selection on pooled ELEMENT‐2 and ELEMENT‐5 data. We aimed to assess the impact of possible baseline variables on the incidence of multiple (≥2) hypoglycaemia events within a period (maintenance, titration or overall). The variables analysed were: diabetes duration (arbitrary cut‐offs of >7.5, >10 or >12.5 years), basal insulin status at baseline (insulin naïve vs prior insulin use), gender, baseline HbA1c (≤8.5% or <7.0%), sulfonylurea use at screening, daytime basal insulin regimen, moderate to severe chronic kidney disease, and age (≥65 or ≥75 years). A separate analysis was performed for each period (maintenance, titration and overall). All independent variables were entered into a stepwise logistic regression model, predicting the occurrence of multiple hypoglycaemia (binary variable, occurrence of ≥2 hypoglycaemic events within the corresponding period). The stepwise selection process iteratively added variables to the model when the multivariable P value was <0.30 (“entry P value) and were allowed to stay in the model if the multivariable P value remained below P <0.10 (“stay P value”). Results of the final model for each period are presented as odds ratios (ORs), with 95% confidence intervals (CIs) for each retained variable, in a forest plot per period.
3. RESULTS
Demographic and baseline characteristics of the pooled treatment groups in each trial, by analysis subgroups, are given in Table 1. The overall (0‐24 weeks) rate and incidence of clinically relevant hypoglycaemia (blood glucose <3 mmol/L) by subgroup is given in Table 2. For each subgroup, mean HbA1c at baseline and at Week 24 and mean daily insulin dose at Week 24 are also shown.
Table 1.
Demographic and baseline characteristics
| Subgroups by age | Subgroups by prior insulin use | Subgroups by HbA1c at week 12 | |||||
|---|---|---|---|---|---|---|---|
| <65 y | ≥65 y | Naïve | Not naïve | <7% | 7% to 8.5% | >8.5% | |
| ELEMENT‐2 (N = 756) | |||||||
| N at baseline | n = 542 | n = 214 | n = 457 | n = 299 | n = 288 | n = 367 | n = 45 |
| Age, y | 54.3 ± 7.8 | 70.4 ± 4.4 | 58.1 ± 10.3 | 60.0 ± 9.6 | 59.3 ± 10.4 | 59.0 ± 9.2 | 56.6 ± 11.7 |
| Men, n (%) | 275 (50.7) | 103 (48.1) | 237 (51.9) | 141 (47.2) | 145 (50.3) | 176 (48.0) | 21 (46.7) |
| Race, n (%) | |||||||
| American Indian or Alaska native | 36 (6.6) | 2 (0.9) | 20 (4.4) | 18 (6.0) | 14 (4.9) | 17 (4.6) | 3 (6.7) |
| Asian | 50 (9.2) | 14 (6.5) | 36 (7.9) | 28 (9.4) | 21 (7.3) | 29 (7.9) | 5 (11.1) |
| Black or African American | 49 (9.0) | 9 (4.2) | 42 (9.2) | 16 (5.4) | 15 (5.2) | 30 (8.2) | 7 (15.6) |
| Multiple | 3 (0.6) | 0 (0.0) | 2 (0.4) | 1 (0.3) | 2 (0.7) | 1 (0.3) | 0 (0.0) |
| White | 404 (74.5) | 189 (88.3) | 357 (78.1) | 236 (78.9) | 236 (81.9) | 290 (79.0) | 30 (66.7) |
| Diabetes duration, y | 10.3 ± 6.2 | 14.4 ± 7.4 | 10.6 ± 6.5 | 12.7 ± 7.1 | 11.1 ± 6.6 | 11.5 ± 6.8 | 13.0 ± 7.5 |
| Prior insulin use (n, %) | 202 (37.3) | 97 (45.3) | N/A | N/A | 90 (31.3) | 162 (44.1) | 27 (60.0) |
| BMI ≥30 kg/m2, n (%) | 353 (65.1) | 112 (52.3) | 279 (61.1) | 186 (62.2) | 169 (58.7) | 238 (64.9) | 27 (60.0) |
| Baseline HbA1c, % | 8.43 ± 1.09 | 8.06 ± 0.99 | 8.45 ± 1.02 | 8.13 ± 1.13 | 7.80 ± 0.94 | 8.61 ± 0.99 | 9.48 ± 0.92 |
| Sulfonylurea use, n (%) | 447 (82.5) | 183 (85.5) | 380 (83.2) | 250 (83.6) | 229 (79.5) | 321 (87.5) | 38 (84.4) |
| ELEMENT‐5 (N = 493) | |||||||
| N at baseline | n = 388 | n = 105 | n = 223 | n = 270 | n = 149 | n = 256 | n = 56 |
| Age, y | 54.1 ± 7.3 | 69.8 ± 4.2 | 57.0 ± 9.4 | 57.8 ± 9.3 | 57.0 ± 9.2 | 57.9 ± 9.3 | 54.8 ± 8.4 |
| Men, n (%) | 203 (52.3) | 54 (51.4) | 117 (52.5) | 140 (51.9) | 82 (55.0) | 132 (51.6) | 30 (53.6) |
| Race, n (%) | |||||||
| American Indian or Alaska native | 1 (0.3) | 0(0.0) | 0 (0.0) | 1 (0.4) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Asian | 194 (50.0) | 40 (38.1) | 89 (39.9) | 145 (53.7) | 54 (36.2) | 130 (50.8) | 34 (60.7) |
| Black or African American | 22 (5.7) | 8 (7.6) | 15 (6.7) | 15 (5.6) | 9 (6.0) | 14 (5.5) | 4 (7.1) |
| Multiple | 2 (0.5) | 1 (1.0) | 2 (0.9) | 1 (0.4) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| White | 169 (43.6) | 56 (53.3) | 117 (52.5) | 108 (40.0) | 84 (56.4) | 112 (43.8) | 18 (32.1) |
| Diabetes duration, y | 11.0 ± 5.6 | 15.5 ± 6.7 | 10.2 ± 5.6 | 13.3 ± 6.7 | 11.7 ± 5.9 | 12.1 ± 6.6 | 12.0 ± 7.2 |
| Prior insulin use (n, %) | 206 (53.1) | 64 (61.0) | N/A | N/A | 68 (45.6) | 149 (58.2) | 41 (73.2) |
| BMI ≥30 kg/m2, n (%) | 156 (40.2) | 37 (35.2) | 87 (39.0) | 106 (39.3) | 58 (38.9) | 97 (37.9) | 27 (48.2) |
| Baseline HbA1c, % | 8.66 ± 1.06 | 8.42 ± 1.03 | 8.80 ± 0.98 | 8.45 ± 1.10 | 8.09 ± 1.02 | 8.70 ± 0.94 | 9.58 ± 0.81 |
| Sulfonylurea use, n (%) | 325 (83.8) | 89 (84.8) | 199 (89.2) | 215 (79.6) | 115 (77.2) | 219 (85.5) | 53 (94.6) |
Data are given as mean ± SD unless otherwise indicated.
Table 2.
HbA1c, insulin dose and hypoglycaemia summary (0‐24 weeks)
| Subgroups by age | Subgroups by prior insulin use | Subgroups by HbA1c at week 12 | |||||
|---|---|---|---|---|---|---|---|
| <65 y | ≥65 y | Naïve | Not naïve | <7% | 7% to 8.5% | >8.5% | |
| ELEMENT‐2 (N = 756) | |||||||
| N for subgroups | n = 542 | n = 214 | n = 457 | n = 299 | n = 288 | n = 367 | n = 45 |
| HbA1c, baseline, % | 8.43 ± 0.05 | 8.07 ± 0.07a | 8.46 ± 0.05 | 8.13 ± 0.06a | 7.81 ± 0.06 | 8.61 ± 0.05a | 9.48 ± 0.14a |
| HbA1c, week 24, % | 6.91 ± 0.06 | 7.00 ± 0.07 | 6.75 ± 0.06 | 7.23 ± 0.07b | 6.39 ± 0.05 | 7.22 ± 0.05b | 8.66 ± 0.11b |
| Insulin dose, week 24, U/kg/d | 0.556 ± 0.02 | 0.423 ± 0.03b | 0.592 ± 0.03 | 0.401 ± 0.03b | 0.442 ± 0.03 | 0.569 ± 0.03b | 0.629 ± 0.06b |
| Rate (events/p/y) | 2.73 ± 0.30 | 3.27 ± 0.50 | 2.94 ± 0.34 | 2.76 ± 0.38 | 3.03 ± 0.41 | 2.78 ± 0.36 | 2.92 ± 0.89 |
| RR (95% CI) | 1.19 (0.88, 1.62) | 0.94 (0.70, 1.25) | Reference | 0.92 (0.68, 1.25) | 0.96 (0.51, 1.82) | ||
| Incidence, n (%) | 226 (42.1) | 95 (44.8) | 199 (44.1) | 122 (40.9) | 141 (49.0) | 158 (43.1) | 15 (33.3) |
| Patients with ≥ two events, n (%) | 148 (27.6) | 70 (33.0) | 140 (31.0) | 78 (26.2) | 95 (33.0) | 111 (30.2) | 10 (22.2) |
| ELEMENT‐5 (N = 493) | |||||||
| N for subgroups | n = 388 | n = 105 | n = 223 | n = 270 | n = 149 | n = 256 | n = 56 |
| HbA1c, baseline, % | 8.65 ± 0.05 | 8.41 ± 0.10a | 8.82 ± 0.07 | 8.43 ± 0.06a | 8.09 ± 0.08 | 8.70 ± 0.06a | 9.58 ± 0.13a |
| HbA1c, week 24, % | 7.36 ± 0.05 | 7.43 ± 0.10 | 7.01 ± 0.07 | 7.67 ± 0.06c | 6.60 ± 0.07 | 7.52 ± 0.05c | 8.76 ± 0.11c |
| Insulin dose, week 24, U/kg/d | 0.605 ± 0.02 | 0.551 ± 0.04 | 0.697 ± 0.03 | 0.510 ± 0.02c | 0.572 ± 0.03 | 0.589 ± 0.02 | 0.705 ± 0.05 |
| Rate (events/p/y) | 0.94 ± 0.16 | 2.09 ± 0.89 | 0.89 ± 0.18 | 1.37 ± 0.34 | 1.58 ± 0.34 | 1.04 ± 0.25 | 0.35 ± 0.15b |
| RR (95% CI) | 2.24 (0.98, 5.10) | 1.53 (0.88, 2.69) | Reference | 0.66 (0.40, 1.07) | 0.22 (0.08, 0.60) | ||
| Incidence, n (%) | 113 (29.3) | 33 (32.0) | 58 (26.2) | 88 (32.8) | 62 (41.6) | 71 (27.7)d | 9 (16.1)d |
| Patients with ≥ two events, n (%) | 59 (15.3) | 24 (23.3) | 31 (14.0) | 52 (19.40) | 41 (27.5) | 34 (13.3)d | 5 (8.93)d |
Note: Data are given as least square means ± SE unless otherwise indicated.
Abbreviations: BG, blood glucose; CI, confidence interval; p, person; RR, relative rate.
P < 0.05 from ANOVA for differences between subgroups.
P < 0.05 from a negative binomial model for differences between subgroups.
P < 0.05 (from MMRM model) for differences between subgroups.
P < 0.05 (from Fisher's Exact test) for differences between subgroups.
Patients who were at least 65 years of age used a lower mean dose of insulin compared to patients less than 65 years of age throughout the studies (significant in ELEMENT‐2; not significant in ELEMENT‐5) (Table 2). The rate of clinically relevant hypoglycaemia for patients at least 65 years of age compared to those less than 65 years of age was higher during the titration period in both studies (Figure 1A), but this difference was not observed in the maintenance period of either study.
Figure 1.
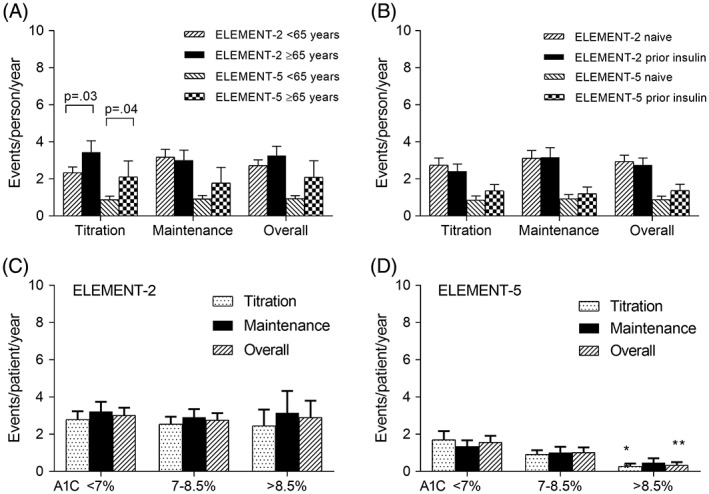
Rate of clinically relevant hypoglycaemia during titration, maintenance, and overall (weeks 0‐24) for each study by subgroup. A, Subgroups by age. B,Subgroups by prior insulin use. C, Subgroups by HbA1c category at week 12 for ELEMENT‐2. D, Subgroups by HbA1c category at week 12 for ELEMENT‐5. Data are presented as LS means ± SE. P values are given for differences between subgroups. **P < 0.01 compared to the corresponding HbA1c <7% category
Patients who were insulin naïve used a significantly higher dose of insulin throughout the studies, and their mean HbA1c values at the end of the titration period (Table 3) and at the 24‐week endpoint (Table 2) were lower compared to patients who previously used insulin. However, prior insulin use did not influence the overall rate or incidence of clinically relevant hypoglycaemia (Table 2). Rates of clinically relevant hypoglycaemia were also similar between prior insulin use and insulin naïve subgroups during the titration and maintenance periods in both studies (Figure 1B).
Table 3.
HbA1c, insulin dose and hypoglycaemia summary (0‐12 weeks)
| Subgroups by age | Subgroups by prior insulin use | Subgroups by HbA1c at week 12 | |||||
|---|---|---|---|---|---|---|---|
| <65 y | ≥65 y | Naïve | Not naïve | <7% | 7% to 8.5% | >8.5% | |
| ELEMENT‐2 (N = 756) | |||||||
| Number of patients | n = 542 | n = 214 | n = 457 | n = 299 | n = 288 | n = 367 | n = 45 |
| HbA1c, baseline, % | 8.43 ± 0.05 | 8.07 ± 0.07a | 8.46 ± 0.05 | 8.13 ± 0.06a | 7.81 ± 0.06 | 8.61 ± 0.05a | 9.48 ± 0.14a |
| HbA1c, week 12, % | 7.18 ± 0.05 | 7.23 ± 0.07 | 7.01 ± 0.05 | 7.48 ± 0.06b | 6.48 ± 0.03 | 7.54 ± 0.03b | 9.27 ± 0.07b |
| Insulin dose, week 12, U/kg/d | 0.506 ± 0.02 | 0.400 ± 0.02b | 0.548 ± 0.02 | 0.368 ± 0.02b | 0.429 ± 0.02 | 0.506 ± 0.02b | 0.542 ± 0.04b |
| Rate (events/p/y) | 2.34 ± 0.31 | 3.44 ± 0.61b | 2.75 ± 0.38 | 2.42 ± 0.39 | 2.79 ± 0.45 | 2.54 ± 0.40 | 2.46 ± 0.88 |
| RR (95% CI) | 1.47 (1.04, 2.09) | 0.88 (0.63, 1.23) | Reference | 0.91 (0.64, 1.30) | 0.88 (0.42, 1.83) | ||
| Incidence, n (%) | 154 (28.7) | 77 (36.3)d | 151 (33.5) | 80 (26.8) | 109 (37.8) | 106 (28.9) | 9 (20.0)d |
| Pts with ≥ two events n (%) | 86 (16.0) | 43 (20.3) | 86 (19.1) | 43 (14.4) | 57 (19.8) | 66 (18.0) | 5 (11.1) |
| ELEMENT‐5 (N = 493) | |||||||
| Number of patients | n = 388 | n = 105 | n = 223 | n = 270 | n = 149 | n = 256 | n = 56 |
| HbA1c, baseline, % | 8.65 ± 0.05 | 8.41 ± 0.10a | 8.82 ± 0.07 | 8.43 ± 0.06a | 8.09 ± 0.08 | 8.70 ± 0.06a | 9.58 ± 0.13a |
| HbA1c, week 12, % | 7.42 ± 0.04 | 7.59 ± 0.08 | 7.14 ± 0.06 | 7.71 ± 0.05c | 6.53 ± 0.04 | 7.64 ± 0.03c | 9.12 ± 0.06c |
| Insulin dose, week 12, U/kg/d | 0.550 ± 0.01 | 0.537 ± 0.03 | 0.639 ± 0.02 | 0.474 ± 0.02c | 0.514 ± 0.02 | 0.558 ± 0.02 | 0.618 ± 0.04c |
| Rate (events/p/y) | 0.88 ± 0.19 | 2.11 ± 0.86b | 0.85 ± 0.23 | 1.36 ± 0.34 | 1.70 ± 0.47 | 0.92 ± 0.23 | 0.28 ± 0.15b |
| RR (95% CI) | 2.38 (1.04, 5.46) | 1.60 (0.84, 3.06) | Reference | 0.54 (0.29, 1.01) | 0.16 (0.05, 0.56) | ||
| Incidence, n (%) | 79 (20.5) | 26 (25.2) | 38 (17.2) | 67 (25.0) | 47 (31.5) | 47 (18.4)d | 7 (12.5)d |
| Pts with ≥ two events, n (%) | 29 (7.5) | 12 (11.7) | 15 (6.79) | 26 (9.70) | 18 (12.08) | 18 (7.03) | 2 (3.57) |
Note: Data are given as least square means ± SE unless otherwise indicated.
Abbreviations: BG, blood glucose; CI, confidence interval; p, person; RR, relative rate.
P < 0.05 from ANOVA model for differences between subgroups.
P < 0.05 from a negative binomial model for differences between subgroups.
P < 0.05 (from Fisher's exact test) for differences between subgroups.
P < 0.05 (from MMRM model) for differences between subgroups.
The overall rate and incidence of hypoglycaemia according to the patient's HbA1c category at Week 12 of the study (end of titration period) is shown in Table 3. Results were similar when the patient's HbA1c category at Week 8 or Week 24 was used (not shown). The level of glycaemic control did not significantly influence the rate or incidence of clinically relevant hypoglycaemia in the ELEMENT‐2 trial (Table 2 and Figure 1C). However, in the ELEMENT‐5 trial, there was a significantly higher rate and incidence of clinically relevant hypoglycaemia in patients in the HbA1c less than 7% category compared to the greater than 8.5% category during the titration period and overall (Tables 2 and 3 and Figure 1D).
Risk factors that predicted patients who would experience multiple (at least two) hypoglycaemia events are shown in Figure 2. Results of the multiple logistic regression model predicted that, during the titration period, using sulfonylureas (SU), being 75 years of age or older, being female, having baseline HbA1c of at least 8.5%, or having a longer duration of diabetes significantly increased the risk. Only baseline HbA1c and diabetes duration were significant predictors during the maintenance period. All risk factors that were significant during the titration period, with the exception of being female, were also significant for the overall study period.
Figure 2.
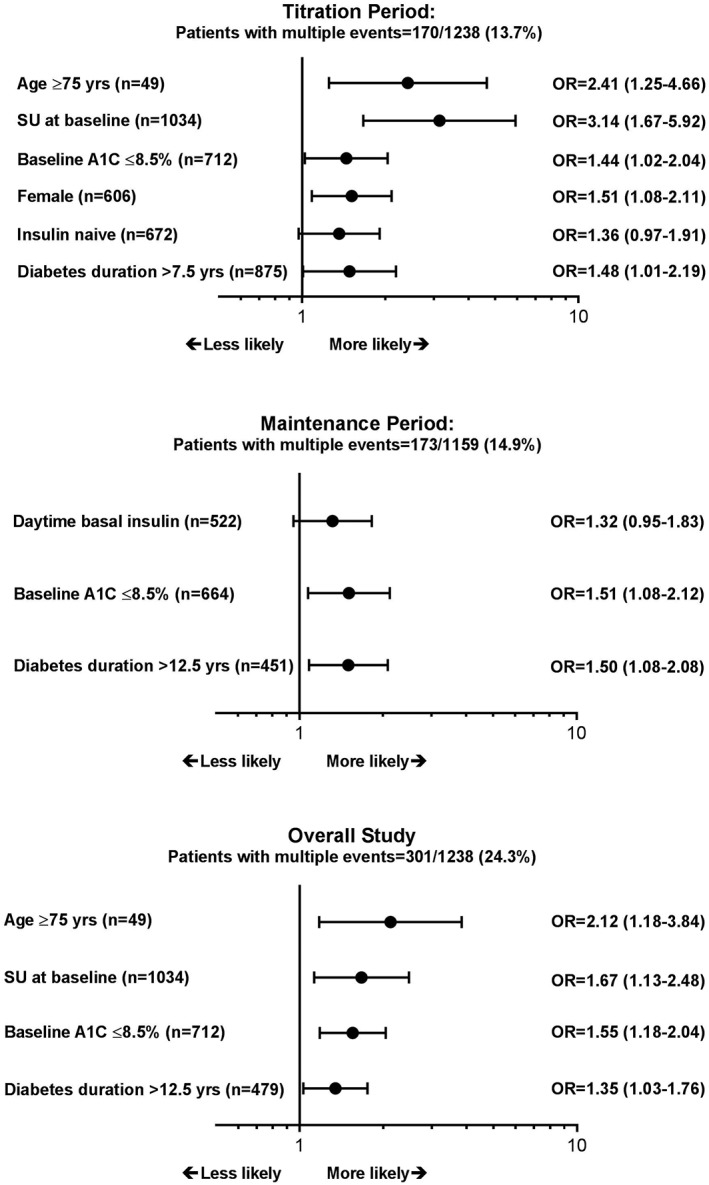
Adjusted odds ratios (OR) and 95% confidence intervals for factors that increased likelihood of experiencing multiple (≥2) hypoglycaemia events during each period of the study (P < 0.1); SU, sulfonylurea use
4. DISCUSSION
Self‐titration of IGlar has been shown to be simple and effective,16 but there is a concern that it may put some patients at increased risk of hypoglycaemia. Well‐known risk factors for hypoglycaemia among patients with T2D include older age, long duration of insulin treatment, intensity of treatment and glycaemic control.17, 18, 19, 20 We conducted this analysis to understand the extent to which these factors might affect the risk of hypoglycaemia in a population of patients with T2D who self‐titrated IGlar U‐100.
Patients in both the ELEMENT‐2 and ELEMENT‐5 trials self‐titrated IGlar doses effectively, resulting in a significant reduction in HbA1c and improvement of other parameters of glycaemia. Improvement was greater in the larger, double‐blind trial, ELEMENT‐2, with endpoint mean HbA1c values close to 7%, while in the ELEMENT‐5 trial, they remained close to 7.4%. IGlar doses were between 0.4 and 0.6 U/k/day throughout both studies, which is consistent with doses reported from other self‐titration studies with insulin analogues.9 As reported previously, the incidence of severe hypoglycaemia was very low (<1%) despite a high proportion of patients who continued pre‐study treatment with sulfonylureas.
In the ELEMENT‐2 and ELEMENT‐5 trials, up to 45% of patients experienced at least one episode of clinically relevant hypoglycaemia. In both trials, the risk of hypoglycaemia was similar, irrespective of previous insulin use. Level of glycaemic control, that attained at the end of the titration period, did not affect overall risk of hypoglycaemia in the ELEMENT‐2 rial, but was associated with a higher rate and incidence of hypoglycaemia in the ELEMENT‐5 trial.
Among older patients, those at least 65 years of age, in both trials, the risk of hypoglycaemia was higher during the insulin titration period, during intensive dose adjustments, compared to the risk during the maintenance period, when dose adjustments were completed. When data from the two trials were pooled and we looked at patients who had experienced two or more hypoglycaemia events, we found that patients at least 75 years of age (n = 49/1238) had a greater than 2‐fold higher risk during the titration period compared to younger patients. These results suggest that older patients might require a more cautious approach during the dose adjustment period. More frequent blood glucose monitoring, follow‐up contact, and slower up titration of glargine U‐100 might help to reduce the risk of hypoglycaemia in older patients until the doses of insulin become stable, after which a more routine approach may be sufficient.
When initiating insulin therapy in insulin naïve patients with T2D, concerns about hypoglycaemia may represent a barrier. Although the risk of hypoglycaemia among patients with T2D is typically low, it can increase significantly with duration and complexity of the treatment model, or can be exaggerated by other factors such as certain comorbidities and concomitant treatment with sulfonylureas. In our studies, the “previous insulin” or “insulin naïve” subgroups at randomization did not show a difference in hypoglycaemia during any period of either study. The overall rates of hypoglycaemia were low in both groups and comparable to rates reported in insulin naïve patients who initiated treatment with insulin degludec in the BEGIN ONCE‐LONG trial, which used a similar definition of hypoglycaemia (<3.1 mmol/L) and reported 1.5 to 1.9 events/person/year.21
It is well accepted that intensity of glucose‐lowering therapy is related to the risk of hypoglycaemia. In our study, we chose to use HbA1c at Week 12, the end of the titration period, to evaluate whether the level of glycaemic control achieved during titration correlated with the risk of hypoglycaemia. In the ELEMENT‐2 trial, there was no correlation of hypoglycaemia incidence or rate with HbA1c category at Week 12. In the ELEMENT‐5 trial, patients with HbA1c above 8.5% at Week 12 had a significantly lower incidence and rate of hypoglycaemia compared to the lower HbA1c categories, despite similar insulin doses.
Multiple episodes of hypoglycaemia are particularly concerning, and we pooled the two studies to identify patients who suffered from two or more hypoglycaemia events. In the pooled population, we found that risk of multiple episodes was greater for patients with baseline HbA1c of 8.5% or less compared to those with baseline HbA1c greater than 8.5% during all periods of the studies. Use of sulfonylureas, age of at least 75 years, and duration of diabetes longer than 12.5 years also increased the risk. These results are consistent with those of previous studies in suggesting that more caution is warranted when patients are within these categories.
In a patient‐level analysis of 12 studies by Riddle et al, baseline HbA1c was associated with the incidence, but not the rate, of symptomatic hypoglycaemia (blood glucose ≤3.9 mmol/L [70 mg/dL]) during titration of insulin in patients with T2D (adjusted OR for each 1% increase in baseline HbA1c: 0.84, 95% CI [0.76‐0.91]).20 In the current study, we analysed the incidence of multiple, at least two, hypoglycaemia events and implemented baseline HbA1c into a binary variable (≤8.5% vs >8.5%); this resulted in an adjusted OR (95% CI) of 1.6 (1.2‐2.0) for the overall study period. It should be noted that this reverses the direction of the effect; our study estimates the effect of a lower baseline HbA1c, while Riddle's results estimate the effect of a higher baseline HbA1c. Inverting Riddle's OR to achieve the same effect direction results in an OR of 1.2 per 1% decrease in baseline HbA1c. This is lower than our binary estimate (1.6), but can be explained by the fact that Riddle used baseline HbA1c as a continuous variable to predict incidence of any hypoglycaemia event, while our study used a dichotomized version (HbA1c ≤8.5% vs >8.5%) to predict incidence of multiple, at least two, hypoglycaemia events.
This study might be limited in its generalizability as the population, while broad, might not represent subgroups in which risk of hypoglycaemia is high (ie, the very elderly, the frail, those with renal failure or those with hypoglycaemia unawareness). Second, our “previous insulin” patients had used relatively low to moderate doses of insulin. The risk might have been higher if the population had included patients who had been treated with insulin for a very long time or had used high doses. Third, the size of the population in the two studies might be insufficient, and analysis of a larger pool of data and more trials might result in different findings. Finally, because of the exploratory nature of this post hoc analysis, no adjustments were made for multiple comparisons, and some possible confounding factors and/or interactions were not considered.
In conclusion, in this re‐analysis of two Phase 3 studies involving more than 1200 patients with T2D, self‐titration of IGlar did not increase the overall risk of clinically relevant hypoglycaemia. However, we found that older patients were at increased risk during the titration period, and all patients were at increased risk as their HbA1c approached the target value. Individualization of therapy, with particular attention to those above 65 years of age and those with better glycaemic control, will be important to avoid hypoglycaemia during titration.
CONFLICT OF INTEREST
Jacek Kiljanski, Erik Spaepen, and Cynthia Harris are employees and shareholders of Eli Lilly and Company. Priscilla Hollander serves on an advisory committee for Novo Nordisk.
AUTHOR CONTRIBUTIONS
J.K. and C.H. designed the analyses, interpreted the results, and drafted the manuscript. E.S. conducted the analyses, interpreted the analyses, and helped to draft the manuscript. P.H. interpreted the analyses and critically revised the manuscript. All authors approved the manuscript for publication.
ACKNOWLEDGMENTS
The data were presented, in part, at the European Association for the Study of Diabetes 54th Annual Meeting, October 1–5, 2018 in Berlin, Germany.
Caryl J. Antalis, PhD, Eli Lilly and Company, provided writing and editorial assistance.
Hollander PA, Kiljanski J, Spaepen E, Harris CJ. Risk of clinically relevant hypoglycaemia in patients with type 2 diabetes self‐titrating insulin glargine U‐100. Diabetes Obes Metab. 2019;21:2413–2421. 10.1111/dom.13822
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13822.
Funding information The study was sponsored by Eli Lilly and Company.
REFERENCES
- 1. Charbonnel PB. Basal insulin intensification in type 2 diabetes: a key role for GLP‐1 receptor agonists. Diabetes Metab. 2015;41:6S1‐6S2. [DOI] [PubMed] [Google Scholar]
- 2. Hilgenfeld R, Seipke G, Berchtold H, Owens DR. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs. 2014;74:911‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garber AJ. Treat‐to‐target trials: uses, interpretation and review of concepts. Diabetes Obes Metab. 2014;16:193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetologia. 2018;61:2461‐2498. [DOI] [PubMed] [Google Scholar]
- 5. Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19:1155‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polinski JM, Smith BF, Curtis BH, et al. Barriers to insulin progression among patients with type 2 diabetes: a systematic review. Diabetes Educ. 2013;39:53‐65. [DOI] [PubMed] [Google Scholar]
- 7. Arnolds S, Heise T, Flacke F, Sieber J. Common standards of basal insulin titration in type 2 diabetes. J Diabetes Sci Technol. 2013;7:771‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khunti K, Davies MJ, Kalra S. Self‐titration of insulin in the management of people with type 2 diabetes: a practical solution to improve management in primary care. Diabetes Obes Metab. 2013;15:690‐700. [DOI] [PubMed] [Google Scholar]
- 9. Blonde L, Merilainen M, Karwe V, Raskin P, TITRATE Study Group . Patient‐directed titration for achieving glycaemic goals using a once‐daily basal insulin analogue: an assessment of two different fasting plasma glucose targets ‐ the TITRATE study. Diabetes Obes Metab. 2009;11:623‐631. [DOI] [PubMed] [Google Scholar]
- 10. International Hypoglycaemia Study G . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care. 2017;40:155‐157. [DOI] [PubMed] [Google Scholar]
- 11. Pollom RK, Ilag LL, Lacaya LB, et al. Comparison of similar U‐100 insulin glargine products, LY2963016 and Lantus, in insulin‐naïve or basal‐insulin‐experienced adults with type 2 diabetes: a randomized, treat‐to‐target trial (ELEMENT 5). Diabetes Ther. 2018;9:827‐837. [Google Scholar]
- 12. Rosenstock J, Hollander P, Bhargava A, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus[R]) in patients with type 2 diabetes who were insulin‐naive or previously treated with insulin glargine: a randomized, double‐blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab. 2015;17:734‐741. [DOI] [PubMed] [Google Scholar]
- 13. Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose‐lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (implementing new strategies with insulin Glargine for Hyperglycaemia treatment) study. Diabet Med. 2006;23:736‐742. [DOI] [PubMed] [Google Scholar]
- 14. Blevins TC, Dahl D, Rosenstock J, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus[R]) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015;17:726‐733. [DOI] [PubMed] [Google Scholar]
- 15. Pollom RK, Lacaya LB, Ilag LL. Efficacy and safety between insulin Glargine products (LY2963016 and Lantus) in patients with T2DM: the ELEMENT 5 study. Diabetes. 2017;66:A249 963‐P. [Google Scholar]
- 16. Kennedy L, Herman WH, Strange P, Harris A, GOAL AIC Team . Impact of active versus usual algorithmic titration of basal insulin and point‐of‐care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the glycemic optimization with algorithms and labs at point of care (GOAL A1C) trial. Diabetes Care. 2006;29:1‐8. [DOI] [PubMed] [Google Scholar]
- 17. Dailey GE, Gao L, Aurand L, Garg SK. Impact of diabetes duration on hypoglycaemia in patients with type 2 diabetes treated with insulin glargine or NPH insulin. Diabetes Obes Metab. 2013;15:1085‐1092. [DOI] [PubMed] [Google Scholar]
- 18. Karl DM, Gill J, Zhou R, Riddle MC. Clinical predictors of risk of hypoglycaemia during addition and titration of insulin glargine for type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:622‐628. [DOI] [PubMed] [Google Scholar]
- 19. Kautzky‐Willer A, Kosi L, Lin J, Mihaljevic R. Gender‐based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient‐level pooled data of six randomized controlled trials. Diabetes Obes Metab. 2015;17:533‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riddle MC, Vlajnic A, Zhou R, Rosenstock J. Baseline HbA1c predicts attainment of 7.0% HbA1c target with structured titration of insulin glargine in type 2 diabetes: a patient‐level analysis of 12 studies. Diabetes Obes Metab. 2013;15:819‐825. [DOI] [PubMed] [Google Scholar]
- 21. Zinman B, Philis‐Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN once long). Diabetes Care. 2012;35:2464‐2471. [DOI] [PMC free article] [PubMed] [Google Scholar]


