Abstract
Since the advent of soap, personal hygiene practices have revolved around removal, sterilization, and disinfection—both of visible soil and microscopic organisms—for a myriad of cultural, aesthetic, or health‐related reasons. Cleaning methods and products vary widely in their recommended use, effectiveness, risk to users or building occupants, environmental sustainability, and ecological impact. Advancements in science and technology have facilitated in‐depth analyses of the indoor microbiome, and studies in this field suggest that the traditional “scorched‐earth cleaning” mentality—that surfaces must be completely sterilized and prevent microbial establishment—may contribute to long‐term human health consequences. Moreover, the materials, products, activities, and microbial communities indoors all contribute to, or remove, chemical species to the indoor environment. This review examines the effects of cleaning with respect to the interaction of chemistry, indoor microbiology, and human health.
Keywords: antimicrobial resistance genes, chemical intervention, indoor microbiology, occupant health, surface microbiology, urface chemistry
Practical Implications.
Simple interventions, such as hand washing, can dramatically improve health and reduce infectious disease.
Chemical intervention, while effective, may encourage the development of microbial resistance over time if not implemented properly.
Microbial communities adapt, reassemble, and persist, and recent theory in microbial ecology suggests that curating microbial communities may be more sustainable than perpetually attempting to remove them.
1. WHY WE CLEAN AND WHAT WE LEAVE BEHIND
The idea of cleanliness has shaped our social and political landscape for millennia and has been as nuanced as the ways in which we accomplish it. The earliest recorded use of soap dates to 2800 BC, but historically, different cultures either prioritized hygiene and the removal of visible soil as well as recommended against it.1 Cleaning was often used for rituals or hospitality, or to remove odor, more than it was used to remove human or animal waste as a potential source of infection.1 Yet, urbanization over the last millennium fueled rapid human social and habitational development which altered our interactions and our environment, contributing to record‐setting, multinational epidemics, and intensifying the issues of waste management, sanitation practices, and public health.2, 3, 4
Over time, our reasons for cleaning evolved to incorporate management or prevention of infectious disease3; however, early infection control was frequently based on fear and anecdotal evidence.1 Town‐scale quarantine was used to prevent the spread of seemingly uncontainable epidemics beginning in the second century, and by the fifth century, hospitals became more common, though patients often languished in decrepit and unsanitary conditions—these places were regarded as areas in which to die rather than to heal.3 Not until the initial discovery of microorganisms in the seventeenth century did scientists, medical professionals, and the general public begin to understand the mechanism of cleaning for infection control, but new ideas and implementations of public health initiatives were sometimes met with resistance.2, 3
By the twentieth century, increasingly rapid developments in microbiology and chemistry yielded multitudinous consumer products with which to design, manipulate, and clean the built environment, most of which attempt to imbue or associate their product with pleasing odors. Our intricate sensory perceptions drive our desire for multifaceted indoor spaces which gratify visual, auditory, tactile, and olfactory senses. Whether driven by chemistry or microbiology, our “obsession with clean” continues to shape our world view and is intertwined with cultural beliefs, practices, and social, political, and economic dynamics.1, 5, 6 Yet, it also affects our health; we spend up to 90% of their time indoors,7 during which we are exposed to chemicals generated by building materials, as well as products for cleaning, personal care, and hygiene (Figure 1). Cleaning imbues the air we breathe and coats the surfaces we touch with a myriad of chemicals, the health or environmental effects of many of which have not been established.8, 9, 10, 11 Indoor chemistry is superbly complex,11 due to the diversity of chemical compounds present, the ability of materials to emit or adsorb, the propensity for gases to become trapped indoors, the proximity to occupants, and local environmental conditions—for example, ultraviolet (UV) light, temperature, humidity, ozone—which can alter chemical reactivity. The unintended consequence of cleaning is that occupants can be exposed to a myriad of chemicals and their byproducts.
Figure 1.
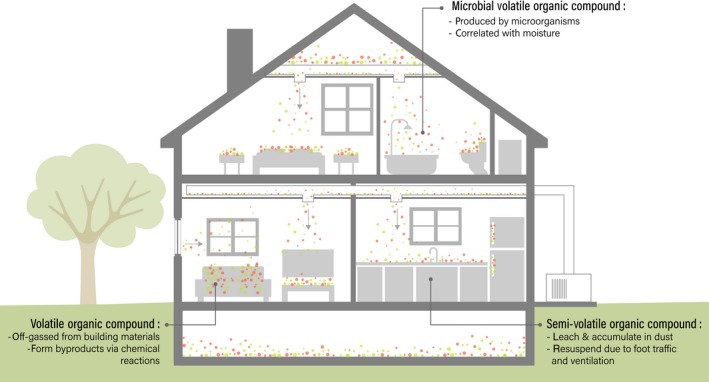
Chemical species indoors are sourced from building materials, material goods, cleaning and hygiene products, human or other biological occupant activities, microbial activities, and a variety of chemical reactions. The interaction between microorganisms, chemicals, and human occupants is complex and often affected by architectural or environmental factors. Airflow through a building (indicated by arrows) can affect dispersal and mixing of chemical and microbial species indoors
Of particular interest are volatile organic chemicals (VOCs), which have a high reactivity, are frequently off‐gassed by materials in the built environment, and are likely to become entrapped indoors (Figure 1). The majority of well‐described VOCs, and those in focus here, create displeasing odors, irritate mucous membranes, cause corrosion damage to cells, exacerbate respiratory problems, and can produce secondary or tertiary chemical reactions.12, 13, 14 Recent consumer demands have led to the development of “green products”—so named because they are marketed as less hazardous and more environmentally sustainable, as compared to synthetic cleaners. Yet “green” is an inexact term and regulation has not always kept pace with marketing; “green” products thus far do not inherently exhibit lower emission rates of all, or even some, classified hazardous VOCs, despite consumer perceptions.10, 13, 15 Semi‐volatile organic compounds (SVOCs), a subcategory of VOCs with a higher molecular weight, are commonly incorporated into materials in the built environment for a variety of purposes and can also be found in detergents (Figure 1); but these, too, may leach and accumulate in dust on surfaces.16, 17 Once leached into and incorporated into dust, chemicals can be resuspended into indoor air by occupant traffic, airflow from ventilation, and routine cleaning activities, allowing these chemical species to be inhaled, ingested, or otherwise come into contact with human skin.18, 19, 20, 21 Collectively, the chemicals present in cleaning solutions, as well as the reactions caused by solutions interacting with surfaces, can cause negative health effects in building occupants, especially those with close or frequent contact to the products.14, 21, 22, 23
Similarly, microorganisms can either process VOCs to create microbial volatile organic compounds (MVOCs) or independently produce MVOCs (Figure 1). Microorganisms can subsist off organic material found in dust, especially sloughed human cells,24, 25 and their production of ammonia and volatile fatty acids can be sufficient to spur odor complaints.25 However, low MVOC production, the plurality of microbial and chemical sources of chemicals indoors, and instrument detection limits can make it difficult to accurately classify a chemical as an MVOC.26, 27 As such, drawing conclusions from in situ MVOC studies has been difficult.
Typically, MVOC production is associated with damp buildings, and with fungal growth, especially molds.27, 28 Surface properties of different materials can contribute to localized areas of high moisture which, along with the chemicals absorbed there, can support microbial growth and MVOC production.29 There is a clear association between health and the dampness of buildings, especially with visible mold,30, 31, 32 yet a connection between MVOCs and health has yet to be firmly established beyond a mouse model.33 Some MVOC studies report a correlation between MVOCs and self‐reported adverse mucosal/respiratory symptoms (ie, stuffiness, sneezing, coughing) in occupants.26, 34 However, these correlations are always not statistically significant,34 suggesting a more intricate relationship between individual MVOCs and health, or a limitation of using self‐reported symptoms. This may also reflect the diversity of MVOCs and other microbial metabolites, very little of which is typically represented in sampling efforts.35 MVOCs produced by fungi, in particular, may be poorly characterized, and it has been hypothesized that many of these MVOCs could also be classified as mycotoxins.27 Collectively, MVOCs, microbial metabolites, and building‐sourced VOCs apparently affect indoor chemistry, human health, and well‐being,25, 34, 36 and the generalization of poor indoor air quality and resulting negative effects on occupants are known as sick building syndrome.8, 34, 37
The mechanisms of interaction between indoor microbes and chemicals are multifaceted. In addition to producing chemicals indoors, some microorganisms have the capacity to degrade chemical contaminants, like phthalates that are emitted from home vinyl flooring.38 However, a high relative humidity is required to achieve this in dust.38 Microbial activity indoors is strongly correlated with moisture availability,24, 39, 40 which is generally limited in most circumstances. However, a number of cleaning activities, for example mopping, involve adding water to an indoor environment, which may have a large amount of microbial biomass suspended in an inactive state. Additionally, many chemical compounds found in cleaning products can be metabolized by microorganisms.41 Thus, cleaning may aid in sustaining indoor microbial communities which are genetically dispositioned to react to intermittent growth conditions and resources to survive.42 And, while cleaning surfaces often removes microbial biomass, the microbial community can reestablish within a matter of days.43, 44 This dispersal is aided by various occupant activities, as well as by certain chemical species, such as ozone, which may deteriorate or kill microorganisms, as well as chemically react with VOCs to generate particulate matter that can carry microorganisms.45 Relatively little work has been done to understand how whole communities of microorganisms—bacteria, fungi, viruses, protozoa, and the oxygen‐tolerant archaea in dust react to chemicals from cleaning or consumer products or from building materials.46, 47 Further research is needed to determine whether community structure, microbial activity, and biochemical capabilities are altered and how these might impact human health.
Moreover, cleaning chemistry may have intensified a microscopic arms race: Faster evolution occurs in harsh environments.48, 49 Microorganisms may express phenotypic resistance or resilience to our attempts to remove or destroy them, as well as acquire additional genetic resistance through mobile gene elements, thus enhancing their pathogenicity and virulence.49, 50, 51 Despite their prodigious use in cleaning and hygiene products in the late twentieth and early twenty‐first century, antimicrobial products do not always reduce infections compared to traditional cleaning products.52, 53, 54, 55 Antimicrobial compounds may persist in built or natural environments, potentially driving microbial evolution as well as environmental exposure to humans, causing alteration of our resident microbiome and the functional benefits it provides.51, 56, 57, 58
Chemical disinfection or sterilization of surfaces varies widely by the active ingredients present in products, the target materials and types of microbes, and mechanisms of action.59, 60 A non‐exhaustive summary is provided in Table 1. However, the presence of certain organic materials can increase microbial tolerance or resistance to cleaning, for example, to sodium hypochlorite.61 Therefore, effective cleaning in settings containing significant amounts of carbohydrates, fats, proteins, or other organic materials may benefit from antimicrobial activity coupled with removal or disambiguation of organic materials, that is, “soil.” Similarly, cleaning solutions are less effective when microbial attachment and biofilm formation preclude the ability to access cells. In the same way that agriculture is embracing a “many little hammers” approach 62 to prevent herbicide resistance in weeds by using a combination of control methods, so, too, antimicrobial resistance can be delayed or thwarted by combining multiple strategies for cleaning, disinfection, and sterilization.
Table 1.
Summary of cleaning strategies presented
| Classification, examples | Benefits | Limitations/risks |
|---|---|---|
| Osmolarity disruption | ||
| Acids; acetic acid, chlorine, citric acid |
|
|
| Bases; ammonia, sodium bicarbonate | ||
| Alcohols; isopropanol, ethanol | ||
| Quaternary ammonia; Alkyldimethylbenzylammonium chloride, benzalkonium chloride, benzethonium chloride, dialkyldimethylammonium chloride |
|
|
| Oxidation | ||
| Halogens; iodine, chlorine, fluorine |
|
|
| Hydrogen peroxide | ||
| Sodium hypochlorite | ||
| Ozone |
|
|
| Coagulation | ||
| Alcohols and phenols |
|
|
| Aldehydes | ||
| Ammonia compounds | ||
| Halophenol; chloroxylenol |
|
|
| Detergents and surfactants | ||
| Detergents; sodium laureth sulfate, ammonium lauryl sulfate, amphoteric sodium deoxycholate, bile salts, Tween, and Triton |
|
|
| Enzyme targeting | ||
| Triclosan |
|
|
| Triclocarban |
|
|
| Microbial‐based products | ||
| Containing microbial byproducts (enzymes) |
|
|
| Containing microorganisms |
|
|
| Metals | ||
| Copper |
|
|
| Titanium dioxide |
|
|
2. ANTIMICROBIAL CLEANING
Many cleaning products can be considered antibiological more than specifically antimicrobial, as they are intended to remove, inactivate, or decay biological material. However, in the scope of cleaning, we aim to deactivate, destroy, or remove microbial communities on our surfaces; typically, bacteria, fungi, and viruses. As such, most cleaning aims to kill cells or inhibit their activity by targeting cell mechanisms that promote reproduction, proliferation, and survival (Table 1). These include targeting cell wall structure or ability to control transmembrane transport, stifling of target DNA replication, RNA translation, or inhibition of other sites for protein production, with variable efficacy or specificity of cell‐type targeting (Figure 2). While several antimicrobials have broad action against multiple taxa or domains of microorganisms, a number have specific action (Figure 3), include those listed above. Antibiotics refer to the subtype with action against bacteria; as such, antivirals target viruses, and antifungals target fungi (Figure 3). This distinction seems superfluous, but is a critical component of cleaning product selection by the lay user and the resulting effectiveness at controlling infectious disease.
Figure 2.
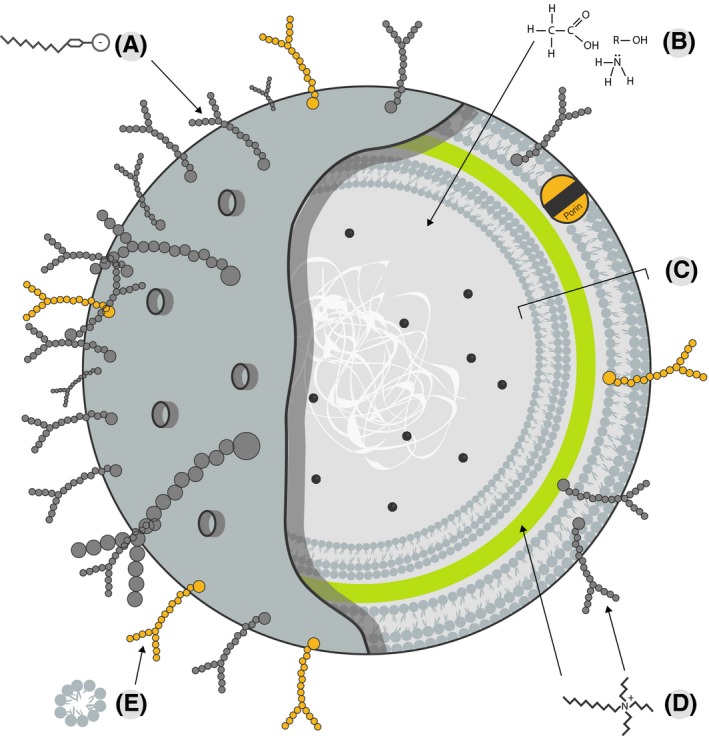
Chemical‐based cleaning acts on various cellular components which may be specific to a particular domain or cell type or generalizable across many types. (A) Anionic detergents disrupt lipopolysaccharides in the cell membrane of Gram‐negative bacteria. (B) Vinegars, ammonia, and alcohols disrupt the osmolarity of a cell. (c) The cell wall of a Gram‐negative bacterium is complex, including outer cell membrane, periplasmic space, peptidoglycan, and inner cell membrane. (D) Cationic detergents disrupt the normal activities of peptidoglycan in cell walls and lipopolysaccharides in cell membranes. (E) Detergents (eg, sodium laureth sulfate) disrupt the attachment of cells to surfaces and disrupt lipid membranes through hydrophobic interactions with glycopolysaccharides
Figure 3.
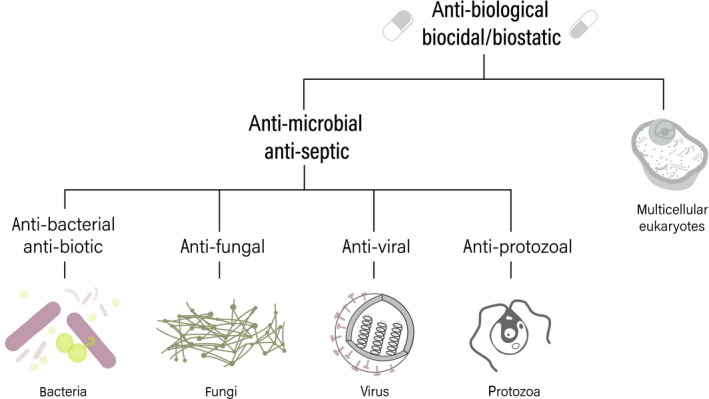
The action of a chemical against a particular domain of life or cell type informs its effectiveness at killing or preventing the growth and division of target microorganisms
2.1. Osmolarity disruption
Simple solutions can be used to lyse and destroy cells by disrupting osmolarity around the cell or chemical disruption of the cell membrane. Simple acid cleaners, such as dilute (10%) solutions of acetic acid (vinegar), can be very effective against cellular debris and non‐hazardous to building occupants.63 The same principle applies to chemicals such as ammonia or alcohols, like isopropanol or ethanol, which are widely reported as effective at minimum (ie, ≤10%) concentrations.64 The benefits to these cleaners include a lack of toxicity toward humans at working concentrations, provided they are not mixed with other chemicals, as well as the relative ease at which they are neutralized, thus reducing environmental contamination. Due to the reactive nature of acids, bases, chlorines, and alcohols, these cleaning products may not remain stable over time, and evaporation or dissociation may change the formulation rendering working batches ineffective. Microorganisms can be tolerant to acidic, basic, or alcoholic conditions and may be able to metabolize these cleaning products, particularly if the microorganism was growing in environments containing similar chemicals at tolerable concentrations.15, 65, 66, 67, 68
2.2. Oxidation
The choice of oxidizing or non‐oxidizing coagulating cleaners is often down to the target microorganisms,60 as cell wall composition, growth phase, and ability to form spores (sexual structures of fungi), endospores (dormant and resilient structures of some bacteria), or cysts (for some free‐living protozoans such as amoeba). Oxidizing agents, such as hydrogen peroxide, sodium hypochlorite (bleach), and halogens (iodine, chlorine, and fluorine), create reactive‐oxygen species which degrade DNA, RNA, proteins, and fatty acids by disrupting chemical bonds.60, 63, 69, 70, 71 Chlorine products are often only useful at specific pH ranges and can react with organic materials leaving behind toxic byproducts.72 Similarly, oxidizing agents can form harmful secondary compounds when reacting with fragrances and other VOCs.73
Oxidative sterilization is also achieved using gaseous or aqueous ozone, which is effective against a variety of microorganisms, including known pathogens, relative to the concentration of microbial cells present.74, 75 Ozone is highly reactive and is not only immediately effective but reacts or dissipates swiftly, thus reducing application and “turn‐around time” during cleaning.74 Due to its reactivity, however, it cannot be used with building occupants present, and it readily reacts with chemicals in air or on surfaces indoors, causing physical damage or creating chemical byproducts which are more toxic to building occupants.76, 77
2.3. Coagulation
Similar to oxidizing agents, chemicals causing coagulation damage DNA, RNA, proteins, and other cellular components; however, this is mainly achieved using alteration of chemical bonds that create cross‐linkage and a disfigurement and coagulation of cell components. Non‐oxidizing agents or coagulating agents include alcohols and phenols, aldehydes, ammonia compounds, and other chemical mixtures.60 Alcohol products work better in aqueous solutions, as water acts as a reaction catalyst and it allows proper penetration of cells that would not be otherwise possible in 100% alcohol solutions which have protein coagulase properties.60 Alcohols are commonly metabolized by microorganisms, allowing them to adapt to the use of alcohol‐based cleaners.67
2.4. Detergents and surfactants
Detergents or surfactants are not considered disinfectants, as their primary mode of action is to envelope or disrupt attachment of dirt and grease, ultimately removing them from their attached surface or skin. However, their action against lipids can cause them to lyse cells. This is done by using their polar and nonpolar regions to interact with other nonpolar molecules.78, 79 Active ingredients of detergents and surfactants are soluble in water, but contain a hydrophobic portion which makes them ideally suited to disrupting lipids. Common detergents include sodium laureth sulfate (sodium dodecyl sulfate), ammonium lauryl sulfate, amphoteric sodium deoxycholate, bile salts, Tween, and Triton. The effectiveness of detergents is based on their active ingredients and ionic charge—cationic, anionic, or ampholytic—as well as the type of microorganism being treated. Cationic detergents are more effective and work on Gram‐positive or Gram‐negative bacteria.80 Anionic detergents, however, may only act against Gram‐positive bacteria.80 Not only can detergents lyse cells, but they can decrease their metabolism as well, effectively inactivating them.80, 81 However, the disinfecting capacity of detergents can be diminished at temperatures below 27℃82 and insufficient detergent activation can actually stimulate bacterial metabolism.80, 81 Furthermore, detergents have been associated with irritation and health risks in humans, particularly in endocrine (hormone) disruption.14, 78, 83 Recently, detergents have been implicated in altering the human gut microbial community, both directly and indirectly through the alteration of host physiology.84, 85
2.5. Enzyme‐targeting
Lately, a number of new antimicrobial chemicals, which were originally thought to only target specific microbial enzymes, have become popular in cleaning products used in homes, hospitals, and other built environments. As previously stated, “antimicrobial” is a broad term which encompasses the action of many different cleaning strategies. However, it has become the archetype term for a group of relatively new chemical compounds with specific action against microorganisms and which were originally thought to have no effect on other domains of life. Primarily, these have included triclosan (TCS) and triclocarban (TCC). The use of TCS and TCC in consumer products has been remarkably widespread and includes personal products such as toothpaste, soaps, lotions, shaving cream, and deodorant.86 Additionally, TCS and TCC are used as a material preservative and thus found in consumer products such as adhesives, fabrics, plastics, textiles, and exposed interior surface coatings.87 They may also be used as disinfectants which can be sprayed on any surface and wiped down.
Triclosan prevents the formation of membranes in bacteria and causes mitochondrial dysfunction in eukaryotes, but is toxic to a number of mammalian cell types and has adverse effects in many biological organisms, including humans.88 Not only is TCS a known endocrine disrupter and carcinogen, but it also suppresses immune cell response in immunological disorders such as asthma, allergies, and atopy.83 TCC is particularly effective against Gram‐positive bacteria, but its specific mechanism of action is not well understood. TCC is a suspected endocrine disruptor, a nanomolar inhibitor of soluble epoxide hydrolase (sEH) enzyme, and exhibits high environmental persistence.89
In response to the information regarding the effect of triclosan and triclocarban on human health, these chemicals have recently been banned in hand soaps and a few other consumer products in the United States, yet are still present in many other products globally. In reaction, benzalkonium chloride has been substituted in as an antimicrobial additive, yet similar concerns over its safety are being voiced.90 Antimicrobials, like these compounds, are effective at deactivating microorganisms on non‐porous surfaces under laboratory conditions. However, the use of these products has no statistical difference in effectiveness for reducing disease transmission when compared to traditional plain soap.52, 54, 55, 91, 92, 93 Promoting basic hand washing hygiene and community education on hygiene, even without the use of antimicrobial additives, reliably reduces incidences of certain transmissible diseases.91 Thus, their risks may outweigh their benefits to the extent that they are not worth including in products.
2.6. Resistance and sustainability
Microorganisms have an indefatigable ability to develop survival strategies to adverse conditions, including cleaning solutions and sterilization methods. The advent of modern‐day antimicrobial chemicals was seen as the end of unwanted microbial growth, but this optimism was replaced with dismay that these too, were selecting for genetic resistance and fostering the evolution of “super‐bugs.”48, 49, 50, 51 However, the potential for and development of microbial resistance is nuanced. A year‐long study reported that using products containing TCS did not significantly increase antimicrobial‐resistant organisms on the skin,92 but it has been argued that evolution is a product of time and a significantly longer study would be needed to make definitive claims about the ecological impact of antimicrobials. However, the bacterial community found on skin may not be a dependable system to test this, as it is largely comprised of Gram‐positive species, which are often able to scavenge fatty acids from host epithelial cells rendering TCS ineffective.94 Thus, resistance might not increase because the community is likely to be inherently resistant.
Further, it has also been suggested that there are other factors that contribute to antimicrobial drug resistance in organisms.92 For example, antimicrobial resistance may simply be facilitated in ecosystems with higher microbial biomass and activity than the skin, as well as access to nutrients and moisture, such as the digestive tract or waste water systems.95, 96 Resistance may also be facilitated in areas with more consistent presence of antimicrobials such as building products,46, 47 which may be imbued with chemicals during manufacturing or may accrue chemicals from cleaning or hygiene produce residues. Mammalian host tissues readily absorb TCS, but sequester it in areas of the body apart from the host's microbial communities.
Antimicrobial compounds may persist in built or natural environments, driving microbial evolution as well as environmental exposure to humans, causing alteration of our resident microbiome and the functional benefits it provides.51, 56, 57, 58 Recent studies have shown that TCS can accumulate in indoor dust and potentially affect the microbial communities within, for example, by favoring organisms carrying antibiotic resistance genes47 or changes in cellular morphology and functions which can confer for antimicrobial resistance.46, 96
The concern over development of microbial resistance to chemical cleaners extends beyond the location and scope of their use; leftover residues and environmental contamination from chemical cleaners can also contribute. Some surface disinfectants are able to dissociate into non‐hazardous components, or may be used at low concentrations for extended periods without long‐term health effects,97 and are thus considered environmentally friendly and safer to use, including hydrogen peroxide, bleach, alcohol, and isopropanol. Others, such as halogens, are considered environmentally friendly at low concentrations. But large‐scale use, particularly the use of chlorine in water and food decontamination, has led to concerns about volatilization, as well as the possibility of selecting for microbial resistance.70, 72, 98, 99 More complex compounds require disposal at specific facilities to prevent contamination of water systems, making them more suitable for use in industrial settings where disposal regulations are enforced.100
As discussed, there are several different mechanisms by which antimicrobial cleaning works to destroy cells or disrupt their cellular functions. While some compounds may be effective at their proposed activity, they have not been proven to be significantly more effective at doing so than plain soap. Use of these antimicrobial products is likely propagating antimicrobial resistance among microbial communities. Although there are many products and methods that remove microbes from the system without using harsh chemicals, the byproducts produced can be just as or more harmful than the initial intervention.
3. PRO‐MICROBIAL CLEANING
Cleaning products which mimic or utilize microbial products, namely enzymes, have long been implemented as a method for degrading residues of synthetic or organic chemicals, including antimicrobial compounds, and inactivating or killing pathogens. Biofilms on medical equipment can be difficult to remove and contribute to the spread of infectious disease between healthcare patients.101 Certain solutions of enzymes mixed with biofilm removal cleaning solutions showed promise by removing biofilms more effectively than control solutions.101 Enzyme‐based cleaning formulations were even viable and effective after 24 weeks of storage at room temperatures.101 However, enzymatic cleaning solutions are limited by enzyme specificity, only functioning at specific temperatures with limited activity duration, and many enzymes only inhibit growth for certain strains of bacteria.
Similarly, incorporating microorganisms into systems to degrade materials has been effectively used in water systems, including aquariums, wastewater treatment plants, bioswales, green infrastructure, and more.102 More recently, alternatives to traditional household cleaners and disinfectants have become commercially available that include viable bacterial species chosen for their competitive abilities. Currently, most commercially available formulations are comprised of one or several bacterial species of Bacillus, with a few incorporating fungal species. Probiotic cleaning solutions aim to kill pathogenic bacteria while leaving a residual coating of non‐threatening probiotic bacteria to form a protective biofilm. The probiotic microorganisms on the surface produce enzymes which are capable of removing dirt and grime from the surface, or antimicrobial compounds to prevent further microbial accumulation. Probiotic cleaners embrace the theory that microbial competition can be harnessed to effectively control microbial populations in the built environment, by removing or selectively altering biomass and antimicrobial resistance gene abundance.103
Bacilli are well‐known for their ability to survive adverse environmental conditions, form endospores, and produce antimicrobial compounds 104, 105 and have also been demonstrated to antagonize a handful of bacterial pathogens,106 even on non‐porous surfaces.107 Moreover, Bacillus subtilis poses little threat to individuals who are not immunocompromised and is commonly used as a dietary probiotic.108 Bacilli are capable of degrading organic materials, ammonia, sulfides, and more,109 and some are capable of producing surfactants which can disrupt a variety of biological and synthetic oils.110 Yet, bacilli are commonly found in all environments and may compete with each other, possibly precluding the ability of the probiotic cleaner species to form a biofilm.106, 111, 112 They may also compete with other common environmental bacteria and fail to persist on a surface,113 potentially requiring continuous or frequent application. Further, environmental context can alter microbial communities: Exposure to outdoor dust enhanced the biofilm formation of a number of bacterial pathogens as well as their ability to proliferate in co‐culture with human cells.114 While plenty of information about commonly used probiotic species is available on their capacity in the laboratory, information justifying the selection of these as probiotic species is less abundant. For example, data stemming from studies involving in vitro experimentation using dust communities or in situ experimentation in complex, occupied built environments is lacking.
Studies have claimed that daily cleaning with the Probiotic Cleaning Hygiene System (PCHS), comprised of equal parts Bacillus subtilis, Bacillus pumilus and Bacillus megaterium, over sustained periods of time can lead to a reduction of viable bacterial pathogen colony forming units (CFU) present on hospital surfaces by >89%.115, 116 However, current literature lacks experimental design detail or rigor; one study did not include any experimental or technical replication and was not able to provide statistical support for reduction claims.116 This same combination of bacilli showed a significant reduction in CFU/m2 as well as fewer reported hospital acquired infections during the treatment phase.117, 118 However, compliance to hospital cleaning procedures is a persistent issue, and it is unclear from the reported study designs whether the probiotic treatment reduced pathogen biomass and hospital acquired infections, or simply the retraining of cleaning staff, though minimal, for use of the probiotic cleaning which caused a re‐adherence to cleaning protocols was sufficient to decrease bacterial load.54, 119, 120, 121 The previous study, which did use replication, also utilized randomized cleaning product treatments to which hospital cleaning staff were blind, and a return to conventional cleaning methods after a probiotic treatment,115 providing demonstrable promise to the use of PCHS in field trials. Yet it is also important to note that many hospital‐acquired infections follow a seasonal trend in infection rates affected by humidity or other factors122, 123; thus, a short‐term cleaning trial implemented during a downward trend in HAIs may yield false positives.
Another study showed that mixing probiotic cleaning solutions with phages in vitro yielded higher rates of destruction of multidrug‐resistant pathogens which can be extremely helpful in hospitals and schools where surfaces can hold larger amounts of pathogens due to the constant movement and transfer of people and microorganisms.124 The study showed that this mixture can be effective at low dosages against microorganisms on fomites typical of a hospital or workplace, over a duration of time (up to 13 days), and the mixture is sustainable for long‐term use meaning the probiotics or phages remain viable in solution longer than they would alone. While phages are often host‐specific, a number of bacteriophages may also affect humans, and if ingested, inhaled, or contacted via skin, the phage present in cleaning solutions could potentially remodel the host microbiome.125, 126 However, phages die quickly after being dispersed into the environment; thus, the potential for exposure to most building inhabitants is low. Nonetheless, newly cleaned surfaces could be dangerous to the public immediately after phage application, and cleaning personnel are subject to deleterious effects of phage exposure due to their continual spatial proximity to the cleaning solutions.
Although many products already exist on the market, microbial‐based cleaners are still a nascent technology that lack rigorous laboratory and field testing on long‐term microbial community and human health effects. As food additives, microorganisms or products may be categorized by the United States’ Food and Drug Administration (FDA) as “generally recognized to be safe” (GRAS), often based on their ubiquity in products and lack of evidence of harm. As a component of consumer cleaning products, there are no regulations in place for evaluating safety or efficacy.109 Fortunately, the vast majority of bacilli have not been identified as causative agents of disease in humans.
Another important consideration is eradication versus inactivation of transposable microbial elements, such as antimicrobial resistance genes. Microorganisms are capable of horizontal gene transfer (HGT) or moving genetic material between cells. HGT is accomplished with specialized cell components that trade DNA between cells, acquire relic DNA from the dead cells in the environment, and transfer viruses between cells. Bacilli‐based cleaners did not completely eradicate all antibiotic‐resistant strains of bacteria from surfaces. Rather, they appeared to kill or outcompete the other bacterial cells to ingest the antimicrobial resistance (AMR) genes.118, 127 Although several studies found correlations between the use of probiotic cleaning solutions and reductions in hospital‐acquired infections, a vital part of their reasoning is missing: longevity. The persistence of a non‐pathogenic biofilm that ultimately inhibits the growth of multidrug‐resistant strains of bacteria has not been shown to survive longer than a few days.
One of the major limitations that comes with the growing interest of these biologically based cleaning solutions comes with quality control and quality assurance. As of recently, there has been little regulation by most government organizations, and this has been reflected in inconsistent cell counts in products, effectiveness of use, and formulation.128 Moreover, the wider implications toward health have not been considered,128 as these formulations may come into contact with skin, whereby they might be accidentally ingested, as well inhaled, all of which can modulate the infectious potential of strains. To date, the microbial species known to be included in these products fall in the category of GRAS, but the potential for infection, allergy, immune sensitization, or the production of undesirable MVOCs nevertheless remains.128
4. PHYSICAL REMOVAL OR ADHESION PREVENTION
The physical removal of microorganisms in air or water systems can be achieved using mechanical filtration. For stationary surfaces, physical removal is achieved via cell destruction, or detachment from surfaces using abrasion or suction. Depending on the microorganism(s) requiring removal, the surface or environment (eg, pipes, air ducts), and the accessibility of the microbial community, many methods of physical removal have limited applicability. However, the complete removal of all microorganisms is not always the goal of surface cleaning, nor is it an easily achievable one. Physical removal may not equate to destruction of the cells, and these methods do not rely on chemical intervention, although incorporating both physical and chemical methods may render cleaning more effective and discourage the development of antimicrobial resistance.
Physical removal with light abrasion and water, even without the addition of a soap emulsifier or disinfectant, can remove some microbial biomass,53, 71 as well as inactivate some viruses.129 For example, wet mopping and moist mopping are the most effective ways to reduce the presence of organic material from floor surfaces.130 Even though moisture can foster microbial activity, it also improves the ability of microorganisms to be removed from surfaces to which they are adhered.131 However, the physical abrasion employed by common cleaning methods does not completely remove all microorganisms.43, 71, 132 While the act of cleaning and repetitive use of cleaning materials (eg, using the same rag to clean multiple surface) can spread unwanted organisms,132 the goal of physical abrasion during cleaning is to reduce microbial biomass and organic material on a surface. When microbial cells accrue, the density of the community may trigger phenotypic changes to cause the formation of a biofilm, the strength of which is often determined by oxygen or nutrient content, community structure, and surface characteristics. In the built environment, microbial biofilms commonly form in water systems or places of excess moisture. During their early stages of formation, biofilms can be removed using abrasive methods such as pressurized air or water, but once established, simple abrasion often fails to remove all or any of the microorganisms.132 Once cells begin to adhere to the surface, chemical sterilization remains the most effective at removing biofilms.133, 134
Physical destruction of cells may involve radiation, desiccation, heat, and/or pressurization (eg, autoclaving). Visible spectra of light, as well as ultraviolet light, triggers damage to cellular components by changing their biochemistry, reduces microbial biomass in dust,135 and inactivates some pathogens.136 It is well‐understood to be biocidal for many microorganisms, via disruption of chemical bonds in proteins and nucleic acids which cause lethal physical complications. Methods like non‐ionizing UV‐C and pulsed xenon UV systems have been shown to be effective at killing multidrug‐resistant strains, and review of their efficacy showed they were both equally effective at destroying these strains on non‐porous surfaces.137, 138, 139 In the case of UV‐C, it is assumed that microorganisms are unable to form resistance because UV‐C does not normally reach Earth's surface due to atmospheric spectral filtering. However, laboratory trials of UV‐A and UV‐B exposure highlight the ability of microbial communities to enhance their radiation resistance over time due to insufficient exposure140 and would suggest that UV‐C resistance is possible if effective protocols are not established and adhered to. Further, UV light can cause increased emission and chemical changes to VOCs and physical degradation to building finishes and furnishing.141
Photocatalytic chemicals, typically used in conjunction with UV light treatment, can imbue biocidal properties to surfaces. Titanium dioxide (TiO2) has biocidal properties against bacterial and eukaryotic (including human) cells, but when used in conjunction with UV light treatment will act against microorganisms.142, 143 When coated or imprinted onto surfaces, TiO2 reduced adhesion of the pathogen Streptococcus mutans, and a synergistic effect between chemical concentration, type of application onto surface, and use of UV light treatment was observed.144
A more proactive solution to physical removal is to prevent the accrual of microorganisms and their ability to adhere to surfaces,145 by utilizing the surface properties of different materials. Chemical composition and micro‐topography affect the cleanability of indoor surfaces; as a result, cleaning product efficacy varies between surface materials, and engineered self‐cleaning surfaces are designed to lessen the necessity for regular cleaning intervention. Surface composition may be biostatic or biocidal to microorganisms, or their composition or physical structure may inhibit microbial attachment—“self‐cleaning surfaces.”
A number of metals are inherently antimicrobial through chemical toxicity, and most notable among them is copper for its low risk of toxicity to humans and its ability to kill microorganisms in as little as one minute.146, 147, 148 While the mechanism is not known, it is hypothesized that once a microorganism contacts the copper surface, it is subject to cell membrane damage, allowing excess copper influx that results in lethal oxidative damage to DNA.146 Copper can reduce pathogenic species on surfaces more effectively than traditional stainless steel appliances, which are common in hospitals.148, 149 However, dry copper surfaces are significantly more effective at contact‐killing pathogens than wet copper surfaces, indicating a potential limitation to its application in the presence of oxygen and suggesting a mechanism for resistance to copper oxides which is not effective against copper ions.147 Like other methods of disinfection, microorganisms have also displayed mutations and modulation of cellular mechanisms which promote tolerance or resistance to toxic metals, including copper.150 Few studies have been conducted that show the effects of combined cleaning with copper surfaces, but those few indicate that it may actually be more beneficial to leave the surfaces alone rather than adding supplementary cleaning, as it can deteriorate the copper and render it less effective.149, 151
Hydrophobic surfaces are the most abundant and fundamental products in the self‐cleaning market.152 These surfaces were originally designed as a biomimicry of the lotus leaf, which effectively removes debris by maintaining water droplet surface tension down a funnel gradient.153 This type of design is typically seen with stain‐resistant fabric in which the substance applied to it beads and rolls off the fabric.154 Generally considered as hydrophobic surfaces, Teflon, PEHD, and PVC have been found to increase cell adhesion in certain strains of bacteria such as Bacillus subtilis, Bacillus cereus, and Escherichia coli.145 Interactions between the surface chemistry of the cell wall and the chemistry of the surface itself affect the cell's ability to adhere to the surface as previously stated. A second type of self‐cleaning surface involves a hydrophilic response of the surface and the substance applied to it: Hydrophilic surfaces tend to use metal oxides to “sheet” the water which removes dirt from the surface.154 These types of coatings are typically produced using nanostructured material which is water repellent, resistant to corrosion, and stable under ultraviolet radiation, and have several antimicrobial properties like preventing cell adhesion to surfaces.154, 155
While much of the research using surface micropattern and material for microbial control is promising and could potentially reduce the need for vigorous cleaning or chemicals, there are several limitations to the field itself. Mimicking nature using mathematical models to produce these self‐cleaning surfaces has proven technically challenging. There are a number of additional considerations for developing these self‐cleaning products, such as surface roughness, contact time, synthesis or replacement of the material, and intended use. One of the main benefits to using a copper surface is that the age of the copper itself is not a detrimental factor to the effectiveness of it; one study showed that having 6‐month‐old copper features in hospital settings did not affect the efficacy of microbial reduction in the environment.156 Further, the appealing aspect of self‐sterilizing or self‐cleaning surfaces is not only their reduced need for cleaning, but also their presumed lack of toxicity toward humans, but this is often related to dose. The toxicity of metals toward microorganisms is increased when incorporated with nanoparticles,157 and the effects on humans are largely unknown. However, the chemistry of these surfaces may change over time, surfaces wear,152 and a lack of moisture 153 or accrual of debris can all render the surface ineffective, further complicating our knowledge of chemical leaching or reactions.
5. COMPLEX SOLUTIONS FOR COMPLEX CHEMISTRY
In recent years, the primary motive for cleaning has been infection prevention, yet despite the advances in chemistry and microbiology in the last several centuries, evidence‐based infection control remains elusive: Proving the effectiveness of particular cleaning interventions in reducing infectious organisms or disease symptoms varies widely by type of intervention or is often absent altogether. This is true for studies that pertain to infection control on non‐human surfaces. For one, the definition of “clean” is vague and relies on personal or cultural definitions, as well as temporal context.1, 158 How often do surfaces need to be cleaned to maintain them free of organic matter and organisms?159 How long do surfaces need to be free from organic matter? From all living microorganisms or just certain ones? On that point, the methodology for measuring the presence of microorganisms, cellular debris, or general soil varies160 and is often measured indirectly through observed infection rates.119, 161
This disparity may have a number of root causes, including experimental design challenges, unexpected in situ conditions, resilience of the microbial community, and fluctuating adherence to cleaning product instructions and institutional protocols. Many cleaning studies in occupied buildings lack randomization or comparable control groups, implement multiple interventions simultaneously, or conflate treatment effects with seasonal trends in infections. Studies may also generate false positives when introducing a new product; making staff aware that samples are being collected (ie, The Hawthorne Effect 162) can cause cleaning personnel to re‐adhere to stringent cleaning practices, which have a tendency to lapse over time.54 It is possible that by maintaining compliance to enacted protocols, regular staff trainings, and effective staff communication to prevent certain hospital equipment from being overlooked in cleaning protocols,120, 121, 163, 164, 165 cleaning can be effective regardless of the products used. For example, basic hand hygiene is nearly uniformly effective at reducing disease transmission regardless of the cleaning products used,4, 6, 52, 166 while environmental cleaning can have mixed outcomes ranging from positive to unsatisfactory.121, 167, 168, 169 The efficacy of most chemical cleaning products is limited to the application, duration, and thoroughness of cleaning, the latter of which is subjective to each person.120 While cleaning practices can be mandated in work environments, it is impossible to ensure total compliance to stringent cleaning policies. Even if practices are rigorously followed, built environments are difficult to maintain due to consistent traffic of individuals. This high rate of individual occupant turnover increases the rate of microorganismal and chemical exchange between the built environment and outside environment, making it difficult for cleaning protocols to serve a consistent environment.
While it is undeniable that the act of cleaning can be effective at reducing the microbial load on surfaces, it is an understatement to say that the influence of the act of cleaning is variable among methods. Many of the products and practices reviewed here and, elsewhere, may be effective for specific purposes but still require significant investigation to be understood in the context of human health or microbial ecology. Notably, the effectiveness of many cleaning products or behaviors is highly situational, a nuance which may be lost on consumers. Moreover, the action against monocultured organisms may not accurately reflect the action of a chemical against whole communities of microorganisms, highlighting the need for more study utilizing complex communities and broader genomic targets (ie, shotgun metagenomics). The disruption of the microbiome, not only human but that of the built environment, needs to be considered within the developing context of cleanliness and beneficial microbiota.
Many of our current practices, products, and ideology center around the context of “scorched‐earth cleaning”—sterilizing and removing everything—leading to growing concern among the scientific community about the potential for evolutionary effects on microorganisms. Not only is the complete removal or sterilization of the indoor environment potentially unachievable, but recent work into host‐associated microbial communities reveals this to be contrary to proper immune system development and health, though it is unclear, and outside the scope of this review, what role the microbial community of the built environment may play in the development of the human immune system. The antithesis to sterilization, fostering a surface microbial community or using probiotic cleaners, presents some promising results but are too nascent and in situ testing has proved difficult for evaluating these in controlled settings. We are only just beginning to understand the complexities that result from our symbiotic relationships with our resident microorganisms, and even more recently, to unravel the intricacies of our microbiome and health with respect to microbial and chemical exposures mediated by the built environment.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
SI conceived of scope and contributed to writing and editing. SV performed literature review, writing, and editing. KVDW, WG, LD, MF, PH, and SN contributed to writing and editing, and JH, JS, CB, YX, and EM contributed to editing and provided valuable insight.
ACKNOWLEDGEMENTS
The authors would like to thank Julia May for her graphical contributions. This work was funded by grants from the Alfred P. Sloan Foundation to the Biology and the Built Environment Center at the University of Oregon. Jiaxian Shen is supported by the CDC via contract number 75D30118C02915. Jinglin Hu and Dr Hartmann are supported in part by the Searle Leadership Fund.
Velazquez S, Griffiths W, Dietz L, et al. From one species to another: A review on the interaction between chemistry and microbiology in relation to cleaning in the built environment. Indoor Air. 2019;29:880–894. 10.1111/ina.12596
REFERENCES
- 1. Speltini G, Passini S. Cleanliness/dirtiness, purity/impurity as social and psychological issues. Cult Psychol. 2014;20:203‐219. [Google Scholar]
- 2. Best M, Neuhauser D. Ignaz Semmelweis and the birth of infection control. Qual Saf Health Care. 2004;13:233‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith PW, Watkins K, Hewlett A. Infection control through the ages. Am J Infect Control. 2012;40:35‐42. [DOI] [PubMed] [Google Scholar]
- 4. Taylor DL, Kahawita TM, Cairncross S, Ensink J. The impact of water, sanitation and hygiene interventions to control cholera: A systematic review. PLoS ONE. 2015;10:e0135676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tumwebaze IK, Mosler H‐J. Why clean the toilet if others don’t? Using a social dilemma approach to understand users of shared toilets' collective cleaning behaviour in urban slums: a review. J Water Sanit Hyg Dev. 2014;4:359‐370. [Google Scholar]
- 6. World Health Organization . WHO Guidelines on Hand Hygiene in Health Care. Geneva, Switzerland: World Health Organization; 2009:1–270. [Google Scholar]
- 7. Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231‐252. [DOI] [PubMed] [Google Scholar]
- 8. Jones AP. Indoor air quality and health. Atmos Environ. 1999;33:4535‐4564. [Google Scholar]
- 9. Steinemann A. Fragranced consumer products: exposures and effects from emissions. Air Qual Atmos Health. 2016;9:861‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinemann AC, MacGregor IC, Gordon SM, et al. Fragranced consumer products: chemicals emitted, ingredients unlisted. Environ Impact Assess Rev. 2011;31:328‐333. [Google Scholar]
- 11. Weschler CJ, Carslaw N. Indoor chemistry. Environ Sci Technol. 2018;52:2419‐2428. [DOI] [PubMed] [Google Scholar]
- 12. Cakmak S, Dales RE, Liu L, et al. Residential exposure to volatile organic compounds and lung function: results from a population‐based cross‐sectional survey. Environ Pollut. 2014;194:145‐151. [DOI] [PubMed] [Google Scholar]
- 13. Zhong L, Su F‐C, Batterman S. Volatile Organic Compounds (VOCs) in conventional and high performance school buildings in the U.S. Int J Environ Res Public Health. 2017;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adisesh A, Murphy E, Barber CM, Ayres JG. Occupational asthma and rhinitis due to detergent enzymes in healthcare. Occup Med. 2011;61:364‐369. [DOI] [PubMed] [Google Scholar]
- 15. Parnes CA. Efficacy of sodium hypochlorite bleach and ‘alternative’ products in preventing transfer of bacteria to and from inanimate surfaces. J Environ Health. 1997;59:14. [Google Scholar]
- 16. Bi C, Maestre JP, Li H, et al. Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low‐income homes: association with season, building characteristics, and childhood asthma. Environ Int. 2018;121:916‐930. [DOI] [PubMed] [Google Scholar]
- 17. Lucattini L, Poma G, Covaci A, de Boer J, Lamoree MH, Leonards P. A review of semi‐volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere. 2018;201:466‐482. [DOI] [PubMed] [Google Scholar]
- 18. Corsi RL, Siegel JA, Chiang C. Particle resuspension during the use of vacuum cleaners on residential carpet. J Occup Environ Hyg. 2008;5:232‐238. [DOI] [PubMed] [Google Scholar]
- 19. Corsi RL, Siegel J, Karamalegos A, Simon H, Morrison GC. Personal reactive clouds: introducing the concept of near‐head chemistry. Atmos Environ. 2007;41:3161‐3165. [Google Scholar]
- 20. Just AC, Miller RL, Perzanowski MS, et al. Vinyl flooring in the home is associated with children’s airborne butylbenzyl phthalate and urinary metabolite concentrations. J Expo Sci Environ Epidemiol. 2015;25:574‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolkoff P, Schneider T, Kildesø J, Degerth R, Jaroszewski M, Schunk H. Risk in cleaning: chemical and physical exposure. Sci Total Environ. 1998;215:135‐156. [DOI] [PubMed] [Google Scholar]
- 22. Siracusa A, De Blay F, Folletti I, et al. Asthma and exposure to cleaning products – a European Academy of Allergy and Clinical Immunology task force consensus statement. Allergy. 2013;68:1532‐1545. [DOI] [PubMed] [Google Scholar]
- 23. Trantallidi M, Dimitroulopoulou C, Wolkoff P, Kephalopoulos S, Carrer P. EPHECT III: health risk assessment of exposure to household consumer products. Sci Total Environ. 2015;536:903‐913. [DOI] [PubMed] [Google Scholar]
- 24. Korpi A, Pasanen A‐L, Pasanen P, Kalliokoski P. Microbial growth and metabolism in house dust. Int Biodeterior Biodegradation. 1997;40:19‐27. [Google Scholar]
- 25. Ng TW, Chan PY, Chan TT, Wu H, Lai KM. Skin squames contribute to ammonia and volatile fatty acid production from bacteria colonizing in air‐cooling units with odor complaints. Indoor Air. 2017;28:258‐265. [DOI] [PubMed] [Google Scholar]
- 26. Wessén B, Schoeps K‐O. Microbial volatile organic compounds—what substances can be found in sick buildings? Analyst. 1996;121:1203‐1205. [DOI] [PubMed] [Google Scholar]
- 27. Bennett JW, Inamdar AA. Are some fungal volatile organic compounds (VOCs) mycotoxins? Toxins. 2015;7:3785‐3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilkins K, Larsen K. Variation of volatile organic compound patterns of mold species from damp buildings. Chemosphere. 1995;31:3225‐3236. [Google Scholar]
- 29. Adams RI, Bhangar S, Dannemiller KC, et al. Ten questions concerning the microbiomes of buildings. Build Environ. 2016;109:224‐234. [Google Scholar]
- 30. Wieslander G, Norbäck D, Venge P. Changes of symptoms, tear film stability and eosinophilic cationic protein in nasal lavage fluid after re‐exposure to a damp office building with a history of flooding. Indoor Air. 2007;17:19‐27. [DOI] [PubMed] [Google Scholar]
- 31. Mendell MJ, Macher JM, Kumagai K. Measured moisture in buildings and adverse health effects: a review. Indoor Air. 2018;28:488‐499. [DOI] [PubMed] [Google Scholar]
- 32. Kercsmar CM, Dearborn DG, Schluchter M, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspect. 2006;114:1574‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller JD, Sun M, Gilyan A, Roy J, Rand TG. Inflammation‐associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chem Biol Interact. 2010;183:113‐124. [DOI] [PubMed] [Google Scholar]
- 34. Araki A, Kawai T, Eitaki Y, et al. Relationship between selected indoor volatile organic compounds, so‐called microbial VOC, and the prevalence of mucous membrane symptoms in single family homes. Sci Total Environ. 2010;408:2208‐2215. [DOI] [PubMed] [Google Scholar]
- 35. Täubel M, Hyvaerinen A.Chapter 18 – occurrence of mycotoxins in indoor environments In: Viegas C, Pinheiro AC, Sabino R, Viegas S, Brandão J, Veríssimo C, eds. Environmental Mycology in Public Health: Fungi and Mycotoxins Risk Assessment and Management. London, UK: Academic Press; 2015:299‐323. [Google Scholar]
- 36. Pasanen A‐L, Korpi A, Kasanen J‐P, Pasanen P. Critical aspects on the significance of microbial volatile metabolites as indoor air pollutants. Environ Int. 1998;24:703‐712. [Google Scholar]
- 37. Al horr Y, Arif M, Katafygiotou M, Mazroei A, Kaushik A, Elsarrag E. Impact of indoor environmental quality on occupant well‐being and comfort: a review of the literature. Int J Sustain Built Environ. 2016;5:1‐11. [Google Scholar]
- 38. Bope A, Haines SR, Hegarty B, Weschler CJ, Peccia J,Dannemiller KC. Degradation of phthalate esters in floor dust at elevated relative humidity. Environ Sci Process Impacts. 2019;21:1268‐1279. [DOI] [PubMed] [Google Scholar]
- 39. Dannemiller KC, Weschler CJ, Peccia J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air. 2017;27:354‐363. [DOI] [PubMed] [Google Scholar]
- 40. Adams RI, Lymperopoulou D.Lessons learned when looking for non‐neutral ecological processes in the built environment: the bacterial and fungal microbiota of shower tiles. bioRxiv September 2018:413773. September 11, 2018. https://www.biorxiv.org/content/early/2018/09/11/413773. Accessed September 12, 2018.
- 41. Singh R. Microbial biotransformation: a process for chemical alterations. J Bacteriol Mycol Open Access. 2017;4:47‐51. [Google Scholar]
- 42. Rainey PB, Beaumont H, Ferguson GC, et al. The evolutionary emergence of stochastic phenotype switching in bacteria. Microb Cell Fact. 2011;10:S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kwan SE, Shaughnessy RJ, Hegarty B, Haverinen‐Shaughnessy U, Peccia J. The reestablishment of microbial communities after surface cleaning in schools. J Appl Microbiol. 2018;125:897‐906. [DOI] [PubMed] [Google Scholar]
- 44. Tamburini E, Donegà V, Marchetti MG, Pedrini P, Monticelli C, Balbo A. Study on microbial deposition and contamination onto six surfaces commonly used in chemical and microbiological laboratories. Int J Environ Res Public Health. 2015;12:8295‐8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhai Y, Li X, Wang T, Wang B, Li C, Zeng G. A review on airborne microorganisms in particulate matters: composition, characteristics and influence factors. Environ Int. 2018;113:74‐90. [DOI] [PubMed] [Google Scholar]
- 46. Fahimipour AK, Ben Mamaar S, McFarland AG, et al. Antimicrobial chemicals associate with microbial function and antibiotic resistance indoors. mSystems. 2018;3:e00200‐e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hartmann EM, Hickey R, Hsu T, et al. Antimicrobial chemicals are associated with elevated antibiotic resistance genes in the indoor dust microbiome. Environ Sci Technol. 2016;50:9807‐9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li S‐J, Hua Z‐S, Huang L‐N, et al. Microbial communities evolve faster in extreme environments. Sci Rep. 2014;4:6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gaze WH, Krone SM, Joakim Larsson DG, et al. Influence of Humans on Evolution and Mobilization of Environmental Antibiotic Resistome. Centers for Disease Control and Prevention; 2013. http://wwwnc.cdc.gov/eid/article/19/7/12-0871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N. A review on antibiotic resistance: alarm bells are ringing. Cureus. 2017;9:e1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huet AA, Raygada JL, Mendiratta K, Seo SM, Kaatz GW. Multidrug efflux pump overexpression in Staphylococcus aureus after single and multiple in vitro exposures to biocides and dyes. Microbiology. 2008;154:3144‐3153. [DOI] [PubMed] [Google Scholar]
- 52. Larson EL, Lin SX, Gomez‐Pichardo C, Della‐Latta P. Effect of antibacterial home cleaning and handwashing products on infectious disease symptoms: a randomized, double‐blind trial. Ann Intern Med. 2004;140:321‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Food and Drug Administration . Consumer Updates – Antibacterial Soap? You Can Skip It, Use Plain Soap and Water. FDA. September 2, 2016. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm378393.htm. Accessed December 5, 2018.
- 54. Han JH, Sullivan N, Leas BF, Pegues DA, Kaczmarek JL, Umscheid CA. Cleaning hospital room surfaces to prevent health care‐associated infections: a technical brief. Ann Intern Med. 2015;163:598‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sitzlar B, Deshpande A, Fertelli D, Kundrapu S, Sethi AK, Donskey CJ. An environmental disinfection odyssey: Evaluation of sequential interventions to improve disinfection of Clostridium difficile isolation rooms. Infect Control Hosp Epidemiol. 2013;34:459‐465. [DOI] [PubMed] [Google Scholar]
- 56. Singh MM, Mullin GE. Environmental chemicals, and the gut microbiome In: Cohen A, vom Saal FS, eds. Integrative Environmental Medicine. Oxford University Press; 2017:115–140. [Google Scholar]
- 57. Lankester J, Patel C, Cullen MR, Ley C, Parsonnet J. Urinary triclosan is associated with elevated body mass index in NHANES. PLoS ONE. 2013;8:e80057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang H, Wang W, Romano KA, et al. A common antimicrobial additive increases colonic inflammation and colitis‐associated colon tumorigenesis in mice. Sci Transl Med. 2018;30:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Environmental Protection Agency . Selected EPA‐registered Disinfectants. EPA Pesticide Registration. September 28, 2015. https://www.epa.gov/pesticide-registration/selected-epa-registered-disinfectants. Accessed December 8, 2018.
- 60. Yoo JH. Review of disinfection and sterilization – back to the basics. Infect Chemother. 2018;50:101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Toyofuku C, Alam MS, Yamada M, et al. Enhancement of bactericidal effects of sodium hypochlorite in chiller water with food additive grade calcium hydroxide. J Vet Med Sci. 2017;79:1019‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Menalled FD, Peterson R, Smith RG, Curran WS, Páez DJ, Maxwell BD. The eco‐evolutionary imperative: revisiting weed management in the midst of an herbicide resistance crisis. Sustain Sci Pract Policy. 2016;8:1297. [Google Scholar]
- 63. Greatorex JS, Page RF, Curran MD, et al. Effectiveness of common household cleaning agents in reducing the viability of human influenza A/H1N1. PLoS ONE. 2010;5:e8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marinella MA, Pierson C, Chenoweth C. The stethoscope. A potential source of nosocomial infection? Arch Intern Med. 1997;157:786‐790. [DOI] [PubMed] [Google Scholar]
- 65. Bjornsdottir K, Breidt F Jr, McFeeters RF. Protective effects of organic acids on survival of Escherichia coli O157:H7 in acidic environments. Appl Environ Microbiol. 2006;72:660‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gerba CP. Quaternary ammonium biocides: efficacy in application. Appl Environ Microbiol. 2014;81:464‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pidot SJ, Gao W, Buultjens AH, et al. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci Transl Med. 2018;10:eaar6115. [DOI] [PubMed] [Google Scholar]
- 68. La Duc MT, Dekas A, Osman S, Moissl C, Newcombe D, Venkateswaran K. Isolation and characterization of bacteria capable of tolerating the extreme conditions of clean room environments. Appl Environ Microbiol. 2007;73:2600‐2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Linley E, Denyer SP, McDonnell G, Simons C, Maillard J‐Y. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67:1589‐1596. [DOI] [PubMed] [Google Scholar]
- 70. Ölmez H, Kretzschmar U. Potential alternative disinfection methods for organic fresh‐cut industry for minimizing water consumption and environmental impact. LWT Food Sci Technol. 2009;42:686‐693. [Google Scholar]
- 71. Gonzalez EA, Nandy P, Lucas AD, Hitchins VM. Ability of cleaning‐disinfecting wipes to remove bacteria from medical device surfaces. Am J Infect Control. 2015;43:1331‐1335. [DOI] [PubMed] [Google Scholar]
- 72. Gil MI, Selma MV, López‐Gálvez F, Allende A. Fresh‐cut product sanitation and wash water disinfection: problems and solutions. Int J Food Microbiol. 2009;134:37‐45. [DOI] [PubMed] [Google Scholar]
- 73. Singer BC, Destaillats H, Hodgson AT, Nazaroff WW. Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air. 2006;16:179‐191. [DOI] [PubMed] [Google Scholar]
- 74. Megahed A, Aldridge B, Lowe J. The microbial killing capacity of aqueous and gaseous ozone on different surfaces contaminated with dairy cattle manure. PLoS ONE. 2018;13:e0196555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Klánová K, Lajèíková A. Use of ozone to reduce bacteria and moulds in the air and on surfaces. Indoor Built Environ. 2006;15:81‐84. [Google Scholar]
- 76. Weschler CJ, Hodgson AT, Wooley JD. Indoor chemistry: ozone, volatile organic compounds, and carpets. Environ Sci Technol. 1992;26:2371‐2377. [Google Scholar]
- 77. Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10:269‐288. [DOI] [PubMed] [Google Scholar]
- 78. Gerster FM, Vernez D, Wild PP, Hopf NB. Hazardous substances in frequently used professional cleaning products. Int J Occup Environ Health. 2014;20:46‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hancox LR, Le Bon M, Dodd C, Mellits KH. Inclusion of detergent in a cleaning regime and effect on microbial load in livestock housing. Vet Rec. 2013;173:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baker Z, Harrison RW, Miller BF. Action of synthetic detergents on the metabolism of bacteria. J Exp Med. 1941;73:249‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tripathi VS, Tripathi P. Detergent effect on metabolic changes in microorganisms. A review. Zentralbl Bakteriol Naturwiss. 1980;135:510‐514. [DOI] [PubMed] [Google Scholar]
- 82. Jaska JM, Fredell DL. Impact of detergent systems on bacterial survival on laundered fabrics. Appl Environ Microbiol. 1980;39:743‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rudel RA, Perovich LJ. Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ. 2009;43:170‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Velmurugan G, Ramprasath T, Gilles M, Swaminathan K, Ramasamy S. Gut microbiota, endocrine‐disrupting chemicals, and the diabetes epidemic. Trends Endocrinol Metab. 2017;28:612‐625. [DOI] [PubMed] [Google Scholar]
- 85. Owino VO, Cornelius C, Loechl CU. Elucidating adverse nutritional implications of exposure to endocrine‐disrupting chemicals and mycotoxins through stable isotope techniques. Nutrients. 2018;10:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang H, Wang Y, Zhu H, Fei Z, Cao J. Binding mechanism of triclocarban with human serum albumin: effect on the conformation and activity of the model transport protein. J Mol Liq. 2017;247:281‐288. [Google Scholar]
- 87. US Environmental Protection Agency . Triclosan facts. US Environmental Protection Agency; March 2010. https://archive.epa.gov/pesticides/reregistration/web/html/triclosan_fs.html. Accessed April 2, 2019. [Google Scholar]
- 88. Teplova VV, Belosludtsev KN, Kruglov AG. Mechanism of triclosan toxicity: Mitochondrial dysfunction including complex II inhibition, superoxide release and uncoupling of oxidative phosphorylation. Toxicol Lett. 2017;275:108‐117. [DOI] [PubMed] [Google Scholar]
- 89. Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol. 2011;45:3109‐3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sreevidya VS, Lenz KA, Svoboda KR, Ma H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol – three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ Pollut. 2018;235:814‐824. [DOI] [PubMed] [Google Scholar]
- 91. Luby SP, Agboatwalla M, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366:225‐233. [DOI] [PubMed] [Google Scholar]
- 92. Aiello AE, Marshall B, Levy SB, Della‐Latta P, Lin SX, Larson E. Antibacterial cleaning products and drug resistance. Emerg Infect Dis. 2005;11:1565‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Aiello AE, Larson EL, Levy SB. Consumer antibacterial soaps: effective or just risky? Clin Infect Dis. 2007;45(Suppl 2):S137‐S147. [DOI] [PubMed] [Google Scholar]
- 94. Zhu L, Bi H, Ma J, et al. The two functional enoyl‐acyl carrier protein reductases of Enterococcus faecalis do not mediate triclosan resistance. MBio. 2013;4:e00613‐e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ribado JV, Ley C, Haggerty TD, Tkachenko E, Bhatt AS, Parsonnet J. Household triclosan and triclocarban effects on the infant and maternal microbiome. EMBO Mol Med. 2017;9:1732‐1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carey DE, McNamara PJ. The impact of triclosan on the spread of antibiotic resistance in the environment. Front Microbiol. 2014;5:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Walsh LJ. Safety issues relating to the use of hydrogen peroxide in dentistry. Aust Dent J. 2000;45:257‐269; quiz 289. [DOI] [PubMed] [Google Scholar]
- 98. Kotay S, Chai W, Guilford W, Barry K, Mathers AJ. Spread from the sink to the patient: in situ study using green fluorescent protein (GFP)‐expressing Escherichia coli to model bacterial dispersion from hand‐washing sink‐trap reservoirs. Appl Environ Microbiol. 2017;83:e03327‐e3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu S‐S, Qu H‐M, Yang D, et al. Chlorine disinfection increases both intracellular and extracellular antibiotic resistance genes in a full‐scale wastewater treatment plant. Water Res. 2018;136:131‐136. [DOI] [PubMed] [Google Scholar]
- 100. Rutala WA, Weber DJ. Surface disinfection: should we do it? J Hosp Infect. 2001;48(Suppl A):S64‐S68. [DOI] [PubMed] [Google Scholar]
- 101. Stiefel P, Mauerhofer S, Schneider J, Maniura‐Weber K, Rosenberg U, Ren Q. Enzymes enhance biofilm removal efficiency of cleaners. Antimicrob Agents Chemother. 2016;60:3647‐3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hesnawi R, Dahmani K, Al‐Swayah A, Mohamed S, Mohammed SA. Biodegradation of municipal wastewater with local and commercial bacteria. Procedia Eng. 2014;70:810‐814. [Google Scholar]
- 103. Caselli E. Hygiene: microbial strategies to reduce pathogens and drug resistance in clinical settings. Microb Biotechnol. 2017;10:1079‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can J Microbiol. 2004;50:1‐17. [DOI] [PubMed] [Google Scholar]
- 105. Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis . Mol Microbiol. 2008;67:254‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gupta KK, Rana D. Evaluation of antagonistic activities of Bacillus Spp. against certain bacteria of medical importance. Arch Agric Environ Sci. 2017;2:353‐356. [Google Scholar]
- 107. Sharif M, Yazdani M, Almas Z, Ghias W, Hussain M Bacillus species found antagonistic against bacteria isolated from currency notes in local circulation. Biomed Lett. 2016;2:2‐86. [Google Scholar]
- 108. Suva MA, Sureja VP, Kheni DB. Novel insight on probiotic Bacillus subtilis: mechanism of action and clinical applications. J Curr Res Sci Med. 2016;2:65. [Google Scholar]
- 109. Arvanitakis G, Temmerman R, Spök A. Development and use of microbial‐based cleaning products (MBCPs): current issues and knowledge gaps. Food Chem Toxicol. 2018;116:3‐9. [DOI] [PubMed] [Google Scholar]
- 110. Thavasi R, Jayalakshmi S, Balasubramanian T, Banat IM. Production and characterization of a glycolipid biosurfactant from Bacillus megaterium using economically cheaper sources. World J Microbiol Biotechnol. 2008;24:917‐925. [Google Scholar]
- 111. Rosenberg G, Steinberg N, Oppenheimer‐Shaanan Y, et al. Not so simple, not so subtle: the interspecies competition between Bacillus simplex and Bacillus subtilis and its impact on the evolution of biofilms. NPJ Biofilms Microbiomes. 2016;2:15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. van Gestel J, Weissing FJ, Kuipers OP, Kovács AT. Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms. ISME J. 2014;8:2069‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stubbendieck RM, Straight PD. Escape from lethal bacterial competition through coupled activation of antibiotic resistance and a mobilized subpopulation. PLoS Genet. 2015;11:e1005722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bado M, Kwende S, Shishodia S, Rosenzweig JA. Impact of dust exposure on mixed bacterial cultures and during eukaryotic cell co‐culture infections. Appl Microbiol Biotechnol. 2017;101:7027‐7039. [DOI] [PubMed] [Google Scholar]
- 115. Vandini A, Temmerman R, Frabetti A, et al. Hard surface biocontrol in hospitals using microbial‐based cleaning products. PLoS ONE. 2014;9:e108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. La Fauci V, Costa GB, Anastasi F, Facciolà A, Go C, Squeri R. An innovative approach to hospital sanitization using probiotics: in vitro and field trials. J Microb Biochem Technol. 2015;7:5. [Google Scholar]
- 117. Caselli E, Brusaferro S, Coccagna M, et al. Reducing healthcare‐associated infections incidence by a probiotic‐based sanitation system: a multicentre, prospective, intervention study. PLoS ONE. 2018;13:e0199616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Caselli E, D’Accolti M, Vandini A, et al. Impact of a probiotic‐based cleaning intervention on the microbiota ecosystem of the hospital surfaces: focus on the resistome remodulation. PLoS ONE. 2016;11:e0148857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Carling PC, Bartley JM. Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. Am J Infect Control. 2010;38:S41‐50. [DOI] [PubMed] [Google Scholar]
- 120. Ramphal L, Suzuki S, McCracken IM, Addai A. Improving hospital staff compliance with environmental cleaning behavior. Proc (Bayl Univ Med Cent). 2014;27:88‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Carling PC, Parry MF, Bruno‐Murtha LA, Dick B. Improving environmental hygiene in 27 intensive care units to decrease multidrug‐resistant bacterial transmission. Crit Care Med. 2010;38:1054‐1059. [DOI] [PubMed] [Google Scholar]
- 122. Durkin MJ, Dicks KV, Baker AW, et al. Seasonal variation of common surgical site infections: does season matter? Infect Control Hosp Epidemiol. 2015;36:1011‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Furuya‐Kanamori L, McKenzie SJ, Yakob L, et al. Clostridium difficile infection seasonality: patterns across hemispheres and continents – a systematic review. PLoS ONE. 2015;10:e0120730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. D’Accolti M, Soffritti I, Piffanelli M, Bisi M, Mazzacane S, Caselli E. Efficient removal of hospital pathogens from hard surfaces by a combined use of bacteriophages and probiotics: potential as sanitizing agents. Infect Drug Resist. 2018;11:1015‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rusin P, Maxwell S, Gerba C. Comparative surface‐to‐hand and fingertip‐to‐mouth transfer efficiency of gram‐positive bacteria, gram‐negative bacteria, and phage. J Appl Microbiol. 2002;93:585‐592. [DOI] [PubMed] [Google Scholar]
- 126. Hannigan GD, Duhaime MB, Koutra D, Schloss PD. Biogeography and environmental conditions shape bacteriophage‐bacteria networks across the human microbiome. PLoS Comput Biol. 2018;14:e1006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Al‐Marzooq F, Al Bayat S, Sayyar F, et al. Can probiotic cleaning solutions replace chemical disinfectants in dental clinics? Eur J Dent. 2018;12:532‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. OECD . Harmonisation of Regulatory Oversight in Biotechnology Biosafety and the Environmental Uses of Micro‐Organisms Conference Proceedings: Conference Proceedings. Paris, France: OECD Publishing; 2015. [Google Scholar]
- 129. Tuladhar E, Hazeleger WC, Koopmans M, Zwietering MH, Beumer RR, Duizer E. Residual viral and bacterial contamination of surfaces after cleaning and disinfection. Appl Environ Microbiol. 2012;78:7769‐7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Andersen BM, Rasch M, Kvist J, et al. Floor cleaning: effect on bacteria and organic materials in hospital rooms. J Hosp Infect. 2009;71:57‐65. [DOI] [PubMed] [Google Scholar]
- 131. Sattar SA, Springthorpe S, Mani S, et al. Transfer of bacteria from fabrics to hands and other fabrics: development and application of a quantitative method using Staphylococcus aureus as a model. J Appl Microbiol. 2001;90:962‐970. [DOI] [PubMed] [Google Scholar]
- 132. Mettler E, Carpentier B. Variations over time of microbial load and physicochemical properties of floor materials after cleaning in food industry premises. J Food Prot. 1998;61:57‐65. [DOI] [PubMed] [Google Scholar]
- 133. Jang H, Rusconi R, Stocker R. Biofilm disruption by an air bubble reveals heterogeneous age‐dependent detachment patterns dictated by initial extracellular matrix distribution. NPJ Biofilms Microbiomes. 2017;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mahfoud C, El Samrani A, Mouawad R, et al. Disruption of biofilms from sewage pipes under physical and chemical conditioning. J Environ Sci. 2009;21:120‐126. [DOI] [PubMed] [Google Scholar]
- 135. Fahimipour AK, Hartmann EM, Siemens A, et al. Daylight exposure modulates bacterial communities associated with household dust. Microbiome. 2018;6:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sandhu BS, Singh CK. Relationship of sunlight and humidity on the virulence of street rabies virus in saliva. Indian J Anim Sci. 2009;79:24‐25. [Google Scholar]
- 137. Nerandzic MM, Thota P, Sankar CT, et al. Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare‐associated pathogens in hospital rooms. Infect Control Hosp Epidemiol. 2015;36:192‐197. [DOI] [PubMed] [Google Scholar]
- 138. Takada A, Matsushita K, Horioka S, Furuichi Y, Sumi Y. Bactericidal effects of 310 nm ultraviolet light‐emitting diode irradiation on oral bacteria. BMC Oral Health. 2017;17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012;10:185‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Goldman RP, Travisano M. Experimental evolution of ultraviolet radiation resistance in Escherichia coli . Evolution. 2011;65:3486‐3498. [DOI] [PubMed] [Google Scholar]
- 141. Farrell FM, Fitch TM, Bicking M. Analysis of chemical and physical effects of ultraviolet bulbs on cooking emissions. J Air Waste Manag Assoc. 2011;61:1005‐1014. [DOI] [PubMed] [Google Scholar]
- 142. Lu Y, Sathasivam S, Song J, Crick CR, Carmalt CJ, Parkin IP. Repellent materials. Robust self‐cleaning surfaces that function when exposed to either air or oil. Science. 2015;347:1132‐1135. [DOI] [PubMed] [Google Scholar]
- 143. Bonetta S, Bonetta S, Motta F, Strini A, Carraro E. Photocatalytic bacterial inactivation by TiO2‐coated surfaces. AMB Express. 2013;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Arango‐Santander S, Pelaez‐Vargas A, Freitas SC, García C. A novel approach to create an antibacterial surface using titanium dioxide and a combination of dip‐pen nanolithography and soft lithography. Sci Rep. 2018;8:15818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tuson HH, Weibel DB. Bacteria‐surface interactions. Soft Matter. 2013;9:4368‐4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011;77:1541‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Espírito Santo C, Lam EW, Elowsky CG, et al. Bacterial killing by dry metallic copper surfaces. Appl Environ Microbiol. 2011;77:794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schmidt MG, Attaway HH, Sharpe PA, et al. Sustained reduction of microbial burden on common hospital surfaces through introduction of copper. J Clin Microbiol. 2012;50:2217‐2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Airey P, Verran J. Potential use of copper as a hygienic surface; problems associated with cumulative soiling and cleaning. J Hosp Infect. 2007;67:271‐277. [DOI] [PubMed] [Google Scholar]
- 150. Bruins MR, Kapil S, Oehme FW. Microbial resistance to metals in the environment. Ecotoxicol Environ Saf. 2000;45:198‐207. [DOI] [PubMed] [Google Scholar]
- 151. Molteni C, Abicht HK, Solioz M. Killing of bacteria by copper surfaces involves dissolved copper. Appl Environ Microbiol. 2010;76:4099‐4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Blossey R. Self‐cleaning surfaces–virtual realities. Nat Mater. 2003;2:301‐306. [DOI] [PubMed] [Google Scholar]
- 153. Xu Q, Zhang W, Dong C, Sreeprasad TS, Xia Z. Biomimetic self‐cleaning surfaces: synthesis, mechanism and applications. J R Soc Interface. 2016;13:20160300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ragesh P, Anand Ganesh V, Nair SV, Sreekumaran Nair A. A review on “self‐cleaning and multifunctional materials”. J Mater Chem A Mater Energy Sustain. 2014;2:14773‐14797. [Google Scholar]
- 155. Ball P. Engineering Shark skin and other solutions. Nature. 1999;400:507. [Google Scholar]
- 156. Casey AL, Adams D, Karpanen TJ, et al. Role of copper in reducing hospital environment contamination. J Hosp Infect. 2010;74:72‐77. [DOI] [PubMed] [Google Scholar]
- 157. Kumar A, Vemula PK, Ajayan PM, John G. Silver‐nanoparticle‐embedded antimicrobial paints based on vegetable oil. Nat Mater. 2008;7:236‐241. [DOI] [PubMed] [Google Scholar]
- 158. Vandegrift R, Bateman AC, Siemens KN, et al. Cleanliness in context: reconciling hygiene with a modern microbial perspective. Microbiome. 2017;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Xiao S, Jones RM, Zhao P, Li Y. The dynamic fomite transmission of Methicillin‐resistant Staphylococcus aureus in hospitals and the possible improved intervention methods. Build Environ. 2019;161:106246. [Google Scholar]
- 160. Kwan SE, Peccia J, Simonds J, Haverinen‐Shaughnessy U, Shaughnessy RJ. Comparing bacterial, fungal, and human cell concentrations with rapid adenosine triphosphate measurements for indicating microbial surface contamination. Am J Infect Control. 2018;47:671‐676. [DOI] [PubMed] [Google Scholar]
- 161. Dharan S, Mourouga P, Copin P, Bessmer G, Tschanz B, Pittet D. Routine disinfection of patients’ environmental surfaces. Myth or reality? J Hosp Infect. 1999;42:113‐117. [DOI] [PubMed] [Google Scholar]
- 162. Payne G, Payne J. The Hawthorne effect In: Key Concepts in Social Research. SAGE Key Concepts. London, UK: SAGE; 2004:108–111. [Google Scholar]
- 163. Fabian MP, Adamkiewicz G, Stout NK, Sandel M, Levy JI. A simulation model of building intervention impacts on indoor environmental quality, pediatric asthma, and costs. J Allergy Clin Immunol. 2014;133:77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Murray SG, Yim J, Croci R, et al. Using spatial and temporal mapping to identify nosocomial disease transmission of Clostridium difficile . JAMA Intern Med. 2017;177:1863‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Carling PC, Von Beheren S, Kim P, Woods C, Healthcare Environmental Hygiene Study Group . Intensive care unit environmental cleaning: an evaluation in sixteen hospitals using a novel assessment tool. J Hosp Infect. 2008;68:39‐44. [DOI] [PubMed] [Google Scholar]
- 166. Hübner N‐O, Hübner C, Kramer A, Assadian O. Survival of bacterial pathogens on paper and bacterial retrieval from paper to hands: preliminary results. Am J Nurs. 2011;111:30‐34; quiz 35–6. [DOI] [PubMed] [Google Scholar]
- 167. Johnson DL, Mead KR, Lynch RA, Hirst D. Lifting the lid on toilet plume aerosol: a literature review with suggestions for future research. Am J Infect Control. 2013;41:254‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Donskey CJ. Does improving surface cleaning and disinfection reduce health care‐associated infections? Am J Infect Control. 2013;41:S12‐S19. [DOI] [PubMed] [Google Scholar]
- 169. Dancer SJ. Controlling hospital‐acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27:665‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]


