Abstract
Diabetes mellitus in Asia accounts for more than half of the global prevalence. There is a high prevalence of cardiovascular disease (CVD) in the region among people with type 2 diabetes mellitus (T2DM) and it is often associated with multiple risk factors including hypertension, renal disease and obesity. The early onset of T2DM and the eventual long disease duration portends an increasing proportion of the population to premature CVD. In addition to lowering blood glucose, sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors exert favourable effects on multiple risk factors (including blood pressure, body weight and renal function) and provide an opportunity to reduce the risk of CVD in patients with T2DM. In this article, we consolidated the existing literature on SGLT‐2 inhibitor use in Asian patients with T2DM and established contemporary guidance for clinicians. We extensively reviewed recommendations from international and regional guidelines, published data from clinical trials in the Asian population (dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, luseogliflozin and tofogliflozin), CVD outcomes trials (EMPAREG‐OUTCOME, CANVAS and DECLARE‐TIMI 58) and real‐world evidence studies (CVD‐REAL, EASEL, CVD‐REAL 2 and OBSERVE‐4D). A series of clinical recommendations on the use of SGLT‐2 inhibitors in Asian patients with T2DM was deliberated among experts with multiple rounds of review and voting. Based on the available evidence, we conclude that SGLT‐2 inhibitors represent an evidence‐based therapeutic option for the primary prevention of heart failure hospitalization and secondary prevention of CVD in patients with T2DM, and should be considered early on in the treatment algorithm for patients with multiple risk factors, or those with established CVD.
Keywords: cardiovascular, diabetes, gliflozins, sodium‐glucose co‐transporter‐2 inhibitors, type 2 diabetes mellitus
1. INTRODUCTION
Cardiovascular disease (CVD) is the most common cause of death globally,1 and patients with type 2 diabetes mellitus (T2DM) have a 2‐ to 3‐fold increased risk of CVD.2, 3 T2DM is also one of the major causes of premature mortality besides CVD, cancer and chronic pulmonary diseases.4 About 40% of deaths in patients with T2DM are attributed to CVD. In addition, there is a high incidence of non‐fatal cardiovascular (CV) events in patients with T2DM, with heart failure (HF) hospitalizations accounting for up to 33% of non‐fatal CV events.5, 6, 7, 8 The high prevalence of T2DM in Asia will lead to an epidemic of CVD and HF, thus prevention of CV complications is of great importance in managing patients with T2DM.9 Despite the close link between hyperglycaemia and CV complications, the effects of intensive glucose lowering on reducing CV outcomes remain inconclusive.10, 11, 12 Early intensive glucose lowering among newly diagnosed T2DM may have a legacy effect that is only translated into CV benefits 10 to 20 years later.13 Among patients with long‐term T2DM with high risk of CVD, intensive glucose lowering has not shown benefits on CV outcomes and could be harmful.10, 11, 12
Patients with T2DM have concomitant multiple risk factors for CVD, such as high blood pressure (BP), dyslipidaemia and albuminuria. To this end, control of multiple risk factors has been shown to confer CV protection.9, 14, 15 In the Steno‐2 study, multifactorial management of hyperglycaemia, hypertension, hyperlipidaemia and microalbuminuria halved the risk of CV morbidity and mortality in patients with T2DM.16, 17 A glucose‐lowering drug (GLD) with multifactorial effects on CV risk factors has long been desired but not forthcoming, with none of the GLDs showing definitive CV protective effects in T2DM. Traditionally, GLDs are designed primarily to improve hyperglycaemia; however, following the observation of increased risk of myocardial infarction (MI) with rosiglitazone in patients with T2DM, all new GLDs are required to show non‐inferiority against placebo for the risk of major adverse cardiac events (MACE) in an adequately powered cardiovascular outcomes trial (CVOT) in high‐risk T2DM patients. For example, the CVOTs of dipeptidyl peptidase‐4 inhibitors showed non‐inferiority against placebo for the risk of MACE, but a signal for higher HF hospitalizations was observed with saxagliptin and alogliptin.18, 19 Conversely, four large CVOTs of sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors and glucagon‐like peptide‐1 (GLP‐1) analogues showed significant benefits on different types of CV outcomes depending on the trial populations and mode of action of the drugs: empagliflozin (EMPA‐REG Outcome trial, 2015),6 liraglutide (LEADER trial, 2016),8 canagliflozin (CANVAS trials, 2017)7 and dapagliflozin (DECLARE‐TIMI 58).20 A meta‐analysis of three CVOTs of SGLT‐2 inhibitors showed a significant reduction in MACE and hospitalization for HF, and delayed the progression of renal complications among patients with T2DM with multiple risk factors or established CVD.21 The findings are corroborated by several real‐world evidence studies comprising over 1.5 million patients with T2DM that reported a significant reduction in CV outcomes with the use of SGLT‐2 inhibitors,22, 23, 24, 25 suggesting a potential class effect. In the recently completed CREDENCE trial, the SGLT‐2 inhibitor canagliflozin significantly reduced the risk of disease progression (a composite of end stage renal disease (ESRD), doubling of the serum creatinine level from baseline for at least 30 days, or death from renal or CV disease) by 30% in patients with diabetic kidney disease.26 Compared with GLP‐1 analogues, SGLT‐2 inhibitors exhibit additional benefits, including reduced risk of HF hospitalizations, favourable tolerability profile, insulin‐independent glucose‐lowering effects and once‐daily oral administration. Given the epidemic of T2DM and CVD in Asia, a group of experts curated, reviewed and consolidated the current literature on SGLT‐2 inhibitors in this population to produce a series of clinical recommendations on their clinical use.
2. METHODOLOGY
An expert panel comprising 12 endocrinologists from China, Hong Kong, India, Indonesia, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand and Vietnam met four times (in Bangkok in November 2017, in Shanghai in March 2018, in Orlando in June 2018, and in Kuala Lumpur in November 2018) to review the clinical evidence and to develop expert clinical recommendations on the clinical use of SGLT‐2 inhibitors in Asian patients with T2DM. A literature search was conducted in the MEDLINE database for articles published up to May 15, 2018, using the search terms “canagliflozin” OR “dapagliflozin” OR “empagliflozin” OR “ipragliflozin” OR “luseogliflozin” OR “tofogliflozin” AND “type 2 diabetes.” A list of studies and reviews were screened for efficacy and safety studies of SGLT‐2 inhibitors conducted in Asia. The panel also critically analyzed recommendations from international guidelines, as well as results from CVOTs and real‐world evidence studies. Following discussion, the panel reached consensus on a series of recommendations supported by scientific evidence and experts' clinical opinions.
3. EPIDEMIOLOGY AND ASSOCIATED COMPLICATIONS OF T2DM IN ASIA
In 2017, the International Diabetes Federation (IDF) estimated that 158.8 million (9.5%) and 82 million (8.5%) adults aged 20 to 79 years in the western Pacific and south‐east Asia regions have diabetes, respectively,27 accounting for 56.7% of the global prevalence. China has the largest affected population (114.4 million) followed by India (72.9 million), with over 90% of them affected by T2DM.9 By 2045, the population with diabetes is expected to increase by 22% in the western Pacific to 193.3 million (prevalence: 10.3%) and by 85% in south‐east Asia to 151.4 million (prevalence: 11.1%).27 Furthermore, more than half of the patients with T2DM remain undiagnosed (57.6% in south‐east Asia and 54% in the western Pacific).27
In the Global Burden of Disease study, CVD in the east, south and south‐east Asia regions and the high‐income Asia Pacific countries (Brunei, Japan, South Korea and Singapore) accounted for ~50% of the global burden, with a total of 210 million people having CVD.28 Alarmingly, there is a high prevalence of HF in the Asian countries (ranging from 1.3 to 6.7%), with rates rising to >30% among the elderly (aged ≥70 years).29, 30 The coexistence of multiple risk factors leads to premature CV morbidity and mortality in patients with T2DM. Individuals without hypertension, obesity and diabetes were found to have up to 85% lower risk of incident HF and lived 3 to 15 years longer free of HF compared with those with one, two or all three risk factors.31 According to IDF estimates, 47 per 1000 patients with T2DM experience a CV event every year.32 In the recent CVOTs in patients with T2DM, CV deaths accounted for 60% to 74% of total deaths while HF hospitalizations accounted for up to 33% of non‐fatal CV events.5, 6, 7, 8 Asian patients with T2DM also have a higher prevalence of ischaemic stroke and renal disease compared with their European counterparts.33 CVD and HF pose a huge burden on healthcare systems in Asian countries,34, 35, 36, 37 thus there is an urgent need for the early detection and effective management of CV risk in patients with T2DM.
4. ASIAN T2DM PHENOTYPE
Apart from interethnic differences in genetic predisposition, Asian patients with T2DM have several distinctive features compared with their Caucasian counterparts. Asian populations have a high prevalence of young‐onset diabetes,38, 39 metabolic syndrome, β‐cell dysfunction,40 and a higher degree of insulin resistance (particularly in south Asians).41, 42, 43 They also have lower lean muscle mass, higher visceral fat mass, lower circulating adiponectin levels,44, 45, 46 and are more likely to exhibit postprandial hyperglycaemia.47, 48 East Asian populations (in China, Korea and Japan) also have reduced insulin response to metabolic stress such as obesity.41, 49, 50, 51 In south Asian populations (in India), undernutrition or overnutrition in utero (pregnant mothers with diabetes) or during infancy increases the risk of impaired glucose tolerance or diabetes in young adulthood. Exposure to poor nutrition in utero or during infancy may lead to foetal programming that promotes fat preservation. In these predisposed individuals, positive energy balance later in life results in accelerated accumulation of adiposity with increased insulin resistance and β‐cell dysfunction.52 In the Joint Asia Diabetes Evaluation (JADE) register, ~18% of Asian patients with T2DM were diagnosed before the age of 40 years (vs 13.8% in the USA population aged 18‐44 years53), with a mean age of 32.2 years at diagnosis.38 Compared with late‐onset disease, T2DM diagnosed before the age of 40 years is associated with a higher risk of CVD, which is attributed to the longer duration of disease.54
5. SGLT‐2 INHIBITORS
Most of the plasma glucose (99%) filtered through the glomerulus is reabsorbed through SGLT in the luminal membrane of proximal renal tubules. Two distinct isoforms of SGLT have been identified. The low‐capacity, high‐affinity SGLT‐1 transporters are found in various tissues, including the small intestine, heart, skeletal muscle and kidney. The high‐capacity, low‐affinity SGLT‐2 transporters are located almost exclusively in the kidney and are responsible for 90% of the glucose reabsorption from the S1 and S2 segments of the proximal convoluted tubule. SGLT‐2 inhibitors reduce blood glucose through selective and reversible inhibition of the SGLT‐2, thereby preventing the renal reabsorption of glucose and increasing its urinary excretion,55, 56 a mechanism independent of β‐cell function and insulin resistance. Also, SGLT‐2 inhibitors have favourable effects on multiple risk factors such as BP, body weight and insulin sensitivity.55, 56, 57 The reduction in body weight with SGLT‐2 inhibitors is comparable with that observed with GLP‐1 analogues.58
In most Asian countries, four SGLT‐2 inhibitors (canagliflozin, dapagliflozin, empagliflozin and luseogliflozin) have been approved for clinical use in patients with T2DM while additional agents such as ipragliflozin and tofogliflozin have been approved in Japan (Table 1).
Table 1.
Sodium‐glucose co‐transporter‐2 inhibitors: Drug profile
| Drug name | Dosage | Elimination T1/2 | Availability in Asia |
|---|---|---|---|
| Dapagliflozin | 5 mg; 10 mg once daily | ~13 h (10 mg) | Most of the Asian countries |
| Canagliflozin | 100 mg; 300 mg once daily | ~11 h (100 mg); | Most of the Asian countries |
| ~13 h (300 mg) | |||
| Empagliflozin | 10 mg; 25 mg once daily | ~12 h | Most of the Asian countries |
| Ipragliflozin | 25 mg; 50 mg once daily | ~15 h (50 mg) | Japan |
| Luseogliflozin | 2.5 mg; 5 mg once daily | ~9 h (2.5 mg); | Japan, Malaysia, Thailand |
| ~10 h (5 mg) | |||
| Tofogliflozin | 20 mg once daily | 5‐6 h | Japan |
Abbreviation: T1/2, half‐life.
5.1. Current guidelines for the use of SGLT‐2 inhibitors
The European Association for the Study of Diabetes (EASD), the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE), the National Institute for Health and Care Excellence (NICE) and Diabetes Canada guidelines recommend SGLT‐2 inhibitors at any stage of T2DM as a combination therapy with other glucose‐lowering therapies.59, 60, 61, 62 The guidelines also recommend SGLT‐2 inhibitors as an acceptable alternative to metformin as initial therapy when metformin is contraindicated or not tolerated. Similar recommendations have been made in clinical practice guidelines for T2DM in Asian countries.63, 64, 65 In 2019, the American Diabetes Association (ADA) Standard of Care provided a treatment algorithm based on the presence of established CVD or chronic kidney disease (CKD).66 In patients with predominant atherosclerotic CVD, GLP‐1 analogues or SGLT‐2 inhibitors are recommended as add‐on therapy to metformin. In patients with predominant HF or CKD, an SGLT‐2 inhibitor is the recommended therapy after metformin.66
The 2016 European Society of Cardiology (ESC) guidelines for CVD prevention recommend that SGLT‐2 inhibitors should be considered early on in the clinical course to reduce all‐cause and CV death in patients with T2DM and CVD (Class IIa recommendation).67 The 2016 ESC guidelines for the management of chronic HF recommend that empagliflozin should be considered in patients with T2DM to prevent or delay the onset of HF and to prolong survival (Class IIa recommendation).68 It should be noted that these guidelines require an update following the availability of evidence from the CANVAS and DECLARE‐TIMI 58 trials. The more recent Canadian practice guidelines recommend the addition of SGLT‐2 inhibitors to reduce the risk of major CV events, HF hospitalization, and progression of nephropathy in patients with T2DM.69, 70, 71
6. SGLT‐2 INHIBITORS IN ASIAN PATIENTS WITH T2DM
6.1. Effect on hyperglycaemia
Table S1 summarizes the antihyperglycaemic effects of SGLT‐2 inhibitors as a monotherapy or in combination with other GLDs or insulin in Asian patients with T2DM. In placebo‐controlled trials (duration up to 24 weeks) on treatment‐naïve Asian patients with T2DM, SGLT‐2 inhibitor monotherapy reduced the HbA1c level by up to −1.11% from baseline, with a greater reduction observed in patients with high baseline HbA1c levels.72, 73, 74, 75, 76, 77 In Asian patients with uncontrolled T2DM, SGLT‐2 inhibitors as add‐on therapy to other GLDs further reduced HbA1c, ranging from −0.44% to −1.26% (study duration up to 52 weeks).78, 79, 80, 81, 82, 83 In insulin‐treated patients, SGLT‐2 inhibitors reduced HbA1c, ranging from −0.55% to −1.09%, and led to insulin dose reductions (study duration up to 52 weeks).84, 85, 86, 87, 88
6.2. Safety
SGLT‐2 inhibitors are well tolerated, with low risk of hypoglycaemia, even when combined with other oral GLDs. In insulin‐treated Asian patients, most SGLT‐2 inhibitors increased the risk of hypoglycaemia, except for dapagliflozin, which was not associated with increased risk of hypoglycaemia compared with placebo.84, 85, 86, 87, 88, 89, 90, 91 The most common adverse event (AE) reported with SGLT‐2 inhibitors is mycotic genital infections, which can be managed with standard treatment and good personal hygiene. In addition, urinary tract infections and volume depletion‐related AEs (especially in patients with impaired renal function, elderly patients, and those receiving diuretics) have also been reported.89, 90, 91
A higher risk of euglycaemic diabetic ketoacidosis (DKA) was observed with SGLT‐2 inhibitors, albeit with a very low overall event rate.92 The risk of euglycaemic DKA can be minimized through patient education and by avoiding trigger situations, such as a very low carbohydrate diet or excessive alcohol intake, and suspension of insulin treatment. SGLT‐2 inhibitors should be discontinued at least 24 hours before metabolically stressful events such as scheduled surgeries or extreme sports.92 Increased risks of bone fractures and lower extremity amputations were only observed in the CANVAS trial (canagliflozin).7 In a real‐world evidence study (OBSERVE‐4D) of four USA administrative claims databases (N = 714 582; 142 800 new users of canagliflozin, 110 897 new users of other SGLT‐2 inhibitors, 460 885 new users of non‐SGLT‐2 inhibitor GLDs), neither canagliflozin nor other SGLT‐2 inhibitors were associated with increased risk of below‐knee amputations compared with non‐SGLT‐2 inhibitor therapy in patients with T2DM, including those with established CVD.25
6.3. Use in special populations
A higher risk of volume depletion‐related AEs, renal impairment or urinary tract infections has been reported in elderly patients (aged ≥65 years); however, no dose adjustments have been recommended.89, 90, 91 The Malaysian clinical practice guidelines recommend moving the dose timing to the post sunset (evening) meal during Ramadan fasting with no specific indication on dose adjustments.63
7. ROLE OF SGLT‐2 INHIBITORS IN PATIENTS WITH T2DM AND MULTIPLE RISK FACTORS
Besides glucose lowering, SGLT‐2 inhibitors exert beneficial effects on multiple CV risk factors, including insulin resistance, obesity, BP and albuminuria. These factors, together with increased haematocrit, may contribute to the CV and renoprotective effects of SGLT‐2 inhibitors.55, 56 In Asian patients with T2DM, several studies have reported the beneficial metabolic and vascular effects of SGLT‐2 inhibitors. In the following sections, we summarize these clinical benefits in Asian studies, as well as data from CVOTs and real‐world evidence studies.
7.1. Effect on glycaemic variables
Apart from HbA1c, indices of insulin resistance and hyperinsulinaemia are associated with impaired myocardial contractility, cardiac hypertrophy and atherosclerosis93, 94; an association of postprandial hyperglycaemia with oxidative stress and endothelial dysfunction has also been reported.95 In Asian patients with T2DM, SGLT‐2 inhibitors alone or in combination with oral GLDs improved insulin sensitivity and β‐cell function, reduced insulin and proinsulin levels, and decreased postprandial glucose levels.72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83
7.2. Effect on BP, pulse pressure and arterial stiffness
Hypertension is a well‐established risk factor for CVD, and up to 87% of patients with T2DM have concomitant hypertension with half of these patients having uncontrolled BP.96, 97 Hypertensive T2DM patients have a 90% higher risk of CV death and a 57% higher risk of any CV event, compared with normotensive T2DM patients. Hence, optimal BP control is a major treatment goal to reduce CV risk.9 SGLT‐2 inhibitors reduce systolic BP by ~3 to 5 mmHg and diastolic BP by ~2 to 3 mmHg,98 with similar findings for 24‐hour systolic and diastolic BP and pulse pressure.99, 100 In Asian patients with T2DM, similar reductions in systolic and diastolic BP have been reported (range: SBP, −1.2 to −7.9 mmHg; DBP, −0.8 to −6.1 mmHg; Table S2). The exact mechanism of BP lowering by SGLT‐2 inhibitors is probably related to the osmotic diuretic and mild natriuretic effects.55 In addition, empagliflozin and dapagliflozin have been shown to reduce arterial stiffness and to improve vascular endothelial function, respectively, which may contribute to BP lowering.101, 102
Arterial stiffness is associated with an increased risk of CV death, especially in patients with T2DM and those at high CV risk.103 In a pooled analysis of five Phase III trials (duration up to 24 weeks) in patients with T2DM (N = 3300; 823 patients with hypertension), treatment with empagliflozin improved the markers of arterial stiffness (pulse pressure and arterial stiffness index) and vascular resistance (mean arterial pressure).104 The DEFENCE study was a 16‐week, prospective, randomized, open‐label, parallel‐group study designed to evaluate the effect of dapagliflozin on endothelial function, assessed by flow‐mediated dilation (FMD), in Japanese patients with T2DM treated with metformin.102 In patients with HbA1c ≥ 7%, treatment with dapagliflozin improved FMD with a mean (SD) change from baseline of 1.05% (2.59; P = 0.041). Notably, every 1% increase in FMD is associated with a 12% risk reduction in CV events.105
7.3. Effect on body weight and fat mass
Asians have a higher visceral adiposity than Caucasians for the same body mass index. By inducing glycosuria, SGLT‐2 inhibitors produce a calorie deficit with a net weight loss of 2 to 3 kg over 52 weeks of therapy,98 with a similar effect in Asian populations (range: −1.29 to −3.9 kg; Table S2). Similarly, waist circumference, a marker for visceral adiposity, was reduced by SGLT‐2 inhibitors (ranging from −1.2 to −3.8 cm; Table S2).
In a proof‐of‐concept study involving 27 obese Japanese patients with T2DM, treatment with dapagliflozin (5 mg) increased adiponectin, ketone bodies, and reduced high‐sensitivity C‐reactive protein (CRP) levels.106 The increase in adiponectin levels correlated with reductions in HbA1c and body weight, supporting the benefits of SGLT‐2 inhibitors for adipocyte biology.
In epidemiological surveys, epicardial fat volume (EFV) is shown to be positively correlated with the risk of atherosclerosis and coronary events.107, 108 In Japanese patients with T2DM with or without obesity, ipragliflozin and luseogliflozin reduced EFV (measured by magnetic resonance imaging) at 12 weeks (change from baseline: luseogliflozin, 6 cm3, P = 0.048; ipragliflozin, 13 cm3; P = 0.008),109, 110 which correlated with reduction in circulating CRP levels.110
7.4. Effect on lipids
As in Caucasians, treatment with SGLT‐2 inhibitors increased high‐density lipoprotein cholesterol (HDL‐C) and low‐density lipoprotein cholesterol (LDL‐C), and reduced triglyceride levels among Asian patients with T2DM (Table S2). While there was an increase in LDL‐C levels with SGLT‐2 inhibitor therapy, no increase in the atherogenic LDL‐C levels was observed, and there was a negligible effect on the LDL‐C to HDL‐C ratio. In an open‐label 12‐week study of Japanese patients with T2DM (N = 80) comparing the effects of dapagliflozin and sitagliptin on lipid subfractions, treatment with dapagliflozin reduced the atherogenic small dense LDL by 20% from the baseline levels (P < 0.01)111 and increased the less atherogenic large buoyant LDL by 18% (P < 0.05), while no changes were observed with sitagliptin.111
7.5. Effect on haematocrit
Haematocrit is an independent predictor of CVD, and the risk of CVD follows a U‐shaped relationship with an increased risk at the lowest and highest quartiles of haematocrit.112, 113 In the EMPA‐REG OUTCOME trial, changes in haematocrit and haemoglobin were the most important mediators of CV death risk reduction in an exploratory analysis (mediating 51.8% and 48.9%, respectively, of the effect of empagliflozin vs placebo on the risk of CV death).114 In Asian patients with T2DM, SGLT‐2 inhibitor treatment has been shown to increase haematocrit (ranging from +0.59% to +5.5%; Table S2). In a meta‐analysis of 25 randomized controlled trials, treatment with SGLT‐2 inhibitors was associated with an increase in haematocrit by 1.4% (95% CI, 0.2‐2.7; P < 0.05 vs placebo).115 Possible mechanisms of this effect include enhancement of erythropoiesis via increased production of erythropoietin (through a reduction in the workload of the proximal tubules and restoration of tubulointerstitial function) and haemoconcentration (secondary to diuresis).116
7.6. Effect on CV outcomes
Three CVOTs have now confirmed the CV benefits of SGLT‐2 inhibitors (empagliflozin, canagliflozin and dapagliflozin) in patients with T2DM with established CVD on optimal therapy or those with multiple risk factors.6, 7, 20 In a meta‐analysis of these trials, which included 34 322 patients with T2DM (60% with established CVD), SGLT‐2 inhibitors significantly reduced the risk of MACE (a composite of CV death, non‐fatal MI and non‐fatal stroke) by 11% compared with placebo [hazard ratio (HR) 0.89, 95% confidence interval (CI): 0.83‐0.96; P = 0.0014].21 The beneficial effect on MACE was more pronounced in patients with established CVD (HR, 0.86; 95% CI: 0.80‐0.93), with a neutral effect on patients with multiple CV risk factors (HR, 1.00; 95% CI: 0.87‐1.16; P for interaction = 0.0501). SGLT‐2 inhibitors significantly reduced the risk of CV death or HF hospitalization by 23% (HR, 0.77; 95% CI: 0.71‐0.84; P < 0.0001) irrespective of history of CVD (P for interaction = 0.41) or HF (P for interaction = 0.51). The risk of HF hospitalization was also reduced by 31% (HR, 0.69; 95% CI: 0.61‐0.79; P < 0.0001) irrespective of history of HF (P for interaction = 0.76).21 Table 2 summarizes the results from these three CVOTs.6, 7, 20
Table 2.
Cardiovascular outcomes trials with sodium‐glucose co‐transporter‐2 inhibitors
| HR (95% CI) | P‐value | |
|---|---|---|
| EMPA‐REG OUTCOME (empagliflozin vs placebo), N = 7020 | ||
| MACE | 0.86 (0.74‐0.99) | 0.04 |
| CV death | 0.62 (0.49‐0.77) | <0.001 |
| HF hospitalization | 0.65 (0.50‐0.85) | 0.002 |
| Fatal or non‐fatal myocardial infarctiona | 0.87 (0.70‐1.09) | 0.23 |
| Fatal or non‐fatal stroke | 1.18 (0.89‐1.56) | 0.26 |
| CANVAS (canagliflozin vs placebo), N = 10 142 | ||
| MACE | 0.86 (0.75‐0.97) | 0.02 |
| CV death | 0.87 (0.72‐1.06) | — |
| HF hospitalization | 0.67 (0.52‐0.87) | — |
| Fatal or non‐fatal myocardial infarction | 0.89 (0.73‐1.09) | — |
| Fatal or non‐fatal stroke | 0.87 (0.69‐1.09) | — |
| DECLARE‐TIMI 58 (dapagliflozin vs placebo), N = 17 160 | ||
| MACE | 0.93 (0.84‐1.03) | 0.17 |
| CV death or HF hospitalization | 0.83 (0.73‐0.95) | 0.005 |
| CV death | 0.98 (0.82‐1.17) | — |
| HF hospitalization | 0.73 (0.61‐0.88) | — |
| Myocardial infarction | 0.89 (0.77‐1.01) | — |
| Ischaemic stroke | 1.01 (0.84‐1.21) | — |
Abbreviations: CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiovascular events, which are a composite of CV death, non‐fatal myocardial infarction and non‐fatal stroke.
Excluding silent myocardial infarction.
In the EMPA‐REG OUTCOME trial, which recruited adults (N = 7020) with T2DM and established CVD receiving standard care,6 after a median follow‐up of 3.1 years, treatment with empagliflozin (10 or 25 mg once daily) significantly reduced the relative risk of primary endpoint three‐point MACE by 14% (P = 0.04 for superiority) and CV death by 38% (P < 0.001) compared with placebo. However, there was no significant between‐group difference for the risk of non‐fatal MI and non‐fatal stroke, indicating that the risk reduction in MACE was driven primarily by the reduction in CV death. Treatment with empagliflozin also reduced the risk of HF hospitalizations by 35% (P = 0.002) and all‐cause death by 32% (HR 0.68, 95% CI: 0.57‐0.82; P < 0.001) although there was no significant between‐group difference for the secondary outcomes of a four‐point MACE, including hospitalization for unstable angina (HR 0.89, 95% CI: 0.78‐1.01; P < 0.001 for non‐inferiority; P = 0.08 for superiority).6 In a subgroup analysis of Asian patients (N = 1517), consistent results were found, with reduced risk of the primary outcome in the empagliflozin group with no heterogeneity in treatment effect by race (HR 0.68, 95% CI: 0.48‐0.95; P‐value for the interaction of treatment effect by race = 0.0872).117
The CANVAS programme integrated data from two trials (CANVAS and CANVAS‐Renal), which recruited 10 142 participants with T2DM and high CV risk.7 The mean follow‐up period for the pooled analysis was 188.2 weeks (295.9 weeks in CANVAS and 108.0 weeks in CANVAS‐R). The risk of the primary endpoint (three‐point MACE) was significantly reduced by 14% in the canagliflozin group compared with placebo (P = 0.02 for superiority). There was no significant between‐group difference for all‐cause death, CV death, non‐fatal MI and non‐fatal stroke. The risk of HF hospitalizations was significantly lower in canagliflozin versus the placebo group (Table 2).7 In a prespecified analysis, canagliflozin consistently reduced the risk of MACE in patients with or without a history of CVD for both primary and secondary prevention (interaction P = 0.18).118
The DECLARE‐TIMI 58 was the largest of the three SGLT‐2 inhibitor CVOTs, which recruited 17 160 patients with T2DM with or without atherosclerotic CVD (6974 with CVD and 10 186 with multiple risk factors for CVD), with a median follow‐up duration of 4.2 years.20 In the primary safety outcome analysis, dapagliflozin met the prespecified criterion for non‐inferiority to placebo with respect to MACE (P < 0.001 for non‐inferiority). The two co‐primary efficacy endpoints were MACE and a composite of CV death or HF hospitalization. Treatment with dapagliflozin did not reduce the risk of MACE (P = 0.17), but significantly reduced the risk of CV death or HF hospitalization compared with placebo (HR 0.83, 95% CI: 0.73‐0.95; P = 0.005). The individual component analysis revealed that the effect of dapagliflozin was mainly driven by a reduction in HF hospitalization (HR 0.73, 95% CI: 0.61‐0.88), with no between‐group difference for the risk of CV death (HR 0.98, 95% CI: 0.82‐1.17). The beneficial effects on CV death or HF hospitalization were consistent in patients with established CVD (HR 0.83, 95% CI: 0.71‐0.98) and in those with multiple risk factors (HR 0.84, 95% CI: 0.67‐1.04; P for interaction = 0.99), among those with or without a history of HF (HR 0.79 and 0.84, 95% CI: 0.63‐0.99 and 0.72‐0.99, respectively) and across the estimated glomerular filtration rate (eGFR) subgroups (≥90, ≥60 to <90, and < 60 mL/min/1.73 m2). The risk of HF hospitalization alone was significantly reduced in patients with established CVD (HR 0.78, 95% CI: 0.63‐0.97) and in those with multiple risk factors (HR 0.64, 95% CI: 0.46‐0.88). There was no between‐group difference for the risk of all‐cause death (HR 0.93, 95% CI: 0.82‐1.04). The risk of renal composite outcome (≥40% decrease in eGFR to <60 mL/min/1.73 m2 or end‐stage renal disease, or death from renal or CV cause) was reduced with dapagliflozin, compared with placebo (HR 0.76, 95% CI: 0.67‐0.87).20
In addition to the CVOTs, four large real‐world studies have reported the CV outcomes of SGLT‐2 inhibitors in patients with T2DM (Table 3).22, 23, 24, 25 In the Comparative Effectiveness of Cardiovascular Outcomes (CVD‐REAL), the Evidence for cArdiovascular outcomes with Sodium‐glucose co‐transporter 2 inhibitors in the rEal worLd (EASEL), and the OBSERVE‐4D studies, the risk of HF hospitalization was significantly reduced by up to 61% with SGLT‐2 inhibitors compared with other non‐SGLT‐2 inhibitor GLDs.23, 24, 25
Table 3.
Real‐world evidence studies with sodium‐glucose co‐transporter‐2 inhibitors
| HR (95% CI) | P‐value | |
|---|---|---|
| CVD‐REAL (initiation of SGLT‐2 inhibitor vs other GLDs), N = 309 056 | ||
| HF hospitalization | 0.61 (0.51‐0.73) | <0.001 |
| All‐cause death | 0.49 (0.41‐0.57) | <0.001 |
| HF hospitalization or all‐cause death | 0.54 (0.48‐0.60) | <0.001 |
| EASEL (initiation of SGLT‐2 inhibitor vs other GLDs), N = 25 258 | ||
| HF hospitalization | 0.57 (0.45‐0.73) | <0.0001 |
| All‐cause death | 0.57 (0.49‐0.66) | <0.0001 |
| HF hospitalization or all‐cause death | 0.57 (0.50‐0.65) | <0.0001 |
| MACE | 0.67 (0.60‐0.75) | <0.0001 |
| Non‐fatal stroke | 0.85 (0.66‐1.10) | 0.2190 |
| Non‐fatal myocardial infarction | 0.81 (0.64‐1.03) | 0.0888 |
| CVD‐REAL 2 (initiation of SGLT‐2 inhibitor vs other GLDs), N = 470 128 | ||
| HF hospitalization | 0.64 (0.50‐0.82) | 0.001 |
| All‐cause death | 0.51 (0.37‐0.70) | <0.001 |
| HF hospitalization or all‐cause death | 0.60 (0.47‐0.76) | <0.001 |
| Myocardial infarction | 0.81 (0.74‐0.88) | <0.001 |
| Stroke | 0.68 (0.55‐0.84) | <0.001 |
| OBSERVE‐4D, N = 714 582 | ||
| Canagliflozin versus other GLDs | 0.39 (0.26‐0.60) | 0.01 |
| HF hospitalization | ||
| Other SGLT‐2 inhibitors versus other GLDs | 0.43‐ (0.30‐0.62) | 0.01 |
| HF hospitalization | ||
Abbreviations: CI, confidence interval; CV, cardiovascular; GLD, glucose‐lowering drug; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiovascular events, which are a composite of CV death, non‐fatal myocardial infarction and non‐fatal stroke; SGLT‐2, sodium‐glucose co‐transporter‐2.
The CVD‐REAL 2 study (real‐world data) evaluated CV outcomes in propensity‐matched patients with T2DM from the Asia‐Pacific (South Korea, Japan, Singapore and Australia), Middle East (Israel) and North American (Canada) regions, who initiated treatment with SGLT‐2 inhibitors or other oral GLDs (470 128 instances of treatment initiation in 408 807 patients).22 Results from Asian countries (South Korea, Japan and Singapore) showed that treatment with SGLT‐2 inhibitors was associated with a significant reduction in the risk of all‐cause death (by 25%‐44%), HF hospitalization (13%‐38%), MI (19%‐25%) and stroke (18%‐66%) compared with other oral GLDs.22
8. POTENTIAL MECHANISMS MEDIATING THE CARDIORENAL BENEFITS OF SGLT‐2 INHIBITORS
Several possible mechanisms have been proposed to explain the effects of SGLT‐2 inhibitors on CV risk reduction (Figure 1). These include: (a) diuretic and natriuretic effects leading to reduced plasma volume and thus cardiac preload; (b) greater reduction in extracellular fluid volume compared with that in blood volume through electrolyte‐free water clearance (as opposed to other diuretics); (c) BP lowering leading to reduced cardiac afterload and improved arterial compliance; (d) weight loss and reduced body fat; (e) shift in cardiac energy substrate from fat and glucose oxidation to more efficient ketone bodies; (f) anti‐inflammatory effects and reduced epicardial fat volume leading to reduced cardiac fibrosis and enhanced contractility; (g) reno‐protective effects such as improved salt and water homeostasis, reduced sympathetic activation and renin‐angiotensin‐aldosterone system (RAAS) activity (note that increased sodium reabsorption can lead to reduced delivery of sodium chloride to the macula densa, resulting in glomerular hyperfiltration. By reducing sodium reabsorption at the proximal tubules, SGLT‐2 inhibitors restore the tubuloglomerular feedback mechanism by delivering more sodium chloride to the macula densa, resulting in vasoconstriction of afferent arterioles, reduction of hyperfiltration and normalization of transglomerular perfusion pressures, which complement the vasodilation of efferent arterioles by RAAS inhibitors for renoprotection); (h) increase in haematocrit with improved oxygen delivery at tissue level; and (i) inhibition of sodium‐hydrogen exchanger (NHE) in the heart and vasculature (NHE1 isoform) and the kidneys (NHE3 isoform), which are implicated in sodium retention, cardiac hypertrophy, injury, fibrosis (leading to the progression of HF) and pathogenesis of diabetic nephropathy.55, 56, 119, 120, 121
Figure 1.
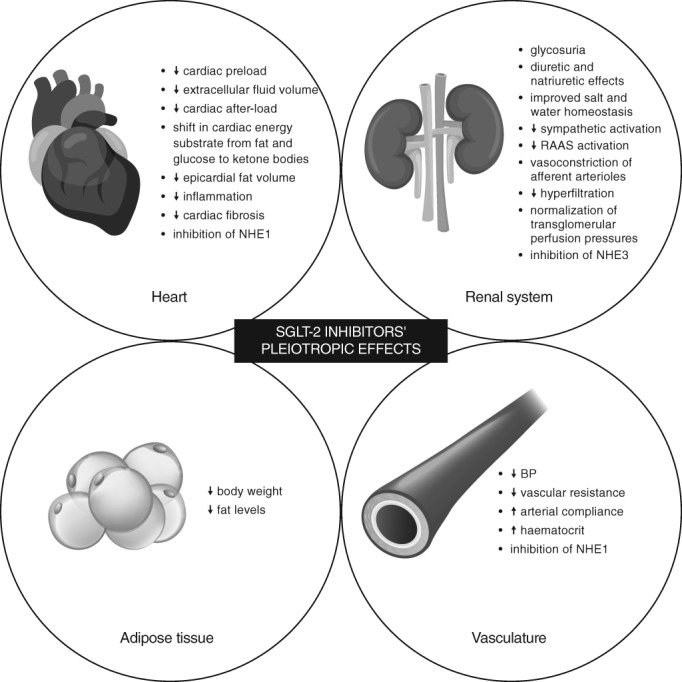
Pleiotropic effects of sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors. BP, blood pressure; NHE1, sodium‐hydrogen exchanger 1; NHE3, sodium‐hydrogen exchanger 3; RAAS, renin‐angiotensin aldosterone system
9. THE FUTURE ROLE OF SGLT‐2 INHIBITORS IN THE MANAGEMENT OF T2DM AND CVD
We are entering an exciting era where a GLD could modify the natural history of diabetes mellitus by reducing the risk of CVD, HF hospitalization and renal disease. The clinical indications for SGLT‐2 inhibitors have been updated with recommendations to initiate these agents in patients with T2DM and established CV disease for the reduction of CV death or major adverse CV events. We eagerly await the policies and guidelines regarding the use of SGLT‐2 inhibitors in the treatment of HF or CKD in patients with or without diabetes. There are now several ongoing studies that aim to evaluate the effects of SGLT‐2 inhibition in patients with HF, CKD and hypertension, and these will define the place of SGLT‐2 inhibitors in CV disease management.
Here are some of the large, ongoing outcome trials with SGLT‐2 inhibitors:
Heart failure: EMPEROR‐Reduced (empagliflozin; NCT03057977); EMPEROR‐Preserved (empagliflozin; NCT03057951); Dapa‐HF (dapagliflozin; NCT03036124); DELIVER (dapagliflozin; NCT03619213);
Diabetic nephropathy: Dapa‐CKD (dapagliflozin; NCT03036150); EMPA‐KIDNEY (empagliflozin; NCT03594110);
Hypertension/prehypertension: PREHYPD (empagliflozin; NCT01001962).
Also, there are several ongoing studies to determine the mechanisms of CV and renal benefits with SGLT‐2 inhibitors (Figure 2). These include studies evaluating the effects of SGLT‐2 inhibitors on heart function and metabolism, energy metabolism and fuel handling, endothelial function and vascular compliance, kidney function, and metabolic regulation of fluid and sodium balance. Furthermore, SGLT‐2 inhibitors have been shown to reduce the liver fat content in randomized and open‐label studies and may provide liver protection in patients with non‐alcoholic fatty liver disease (NAFLD) and non‐alcoholic steatohepatitis (NASH).122 The effects of SGLT‐2 inhibitors in patients with NAFLD and those at risk of NASH remain to be confirmed in long‐term randomized controlled studies.
Figure 2.
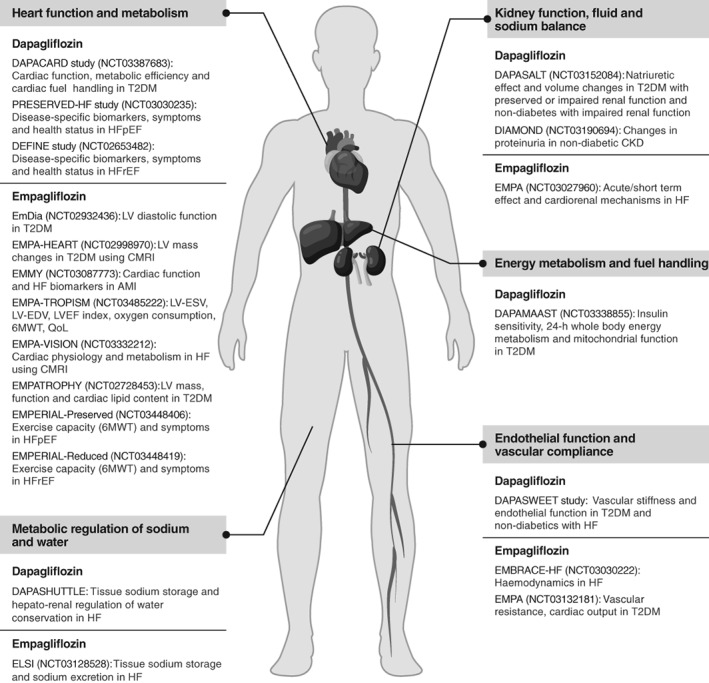
Ongoing mechanistic studies of sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors. 6MWT, 6‐minute walk test; AMI, acute myocardial infarction; CKD, chronic kidney disease; CMRI, cardiac magnetic resonance imaging; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; LV‐EDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LV‐ESV, left ventricular end‐systolic volume; QoL, quality of life; T2DM, type 2 diabetes mellitus
10. CONCLUSIONS AND RECOMMENDATIONS
With the increasing prevalence of T2DM and heightened risk of CVD in Asia, SGLT‐2 inhibitors represent a novel, key therapeutic agent for clinicians in the management of patients with diabetes who have established CVD or who are at high risk of CV disease. Given their consistent benefits in the primary prevention of HF hospitalization and secondary prevention of CVD, SGLT‐2 inhibitors should be considered early on in the treatment algorithm for patients with multiple risk factors or for those with established CVD. Based on the available evidence on SGLT‐2 inhibitors in the Asian population, results from CVOTs as well as clinical experience, a series of clinical recommendations has been developed for the use of SGLT‐2 inhibitors in this population (Table 4). The ongoing mechanistic studies and other outcomes trials in patients with HF and CKD will eventually define the cardiorenal protective role of SGLT‐2 inhibitors above and beyond glucose lowering.
Table 4.
Clinical recommendations on the clinical use of sodium‐glucose co‐transporter‐2 inhibitors for the management of Asian patients with type 2 diabetes mellitus
| CV risk burden in Asian patients with T2DM | |
| 1 | The current prevalence rates of T2DM and CVD and the estimated future risk are alarmingly high in Asia; this calls for effective measures directed towards prevention of disease onset and effective management of T2DM and CV disease. |
| Glycaemic efficacy of SGLT‐2 inhibitors in Asian patients with T2DM | |
| 2 | The glycaemic efficacy (HbA1c reduction) of SGLT‐2 inhibitors in Asian patients with T2DM is consistent with that reported in Caucasian populations; SGLT‐2 inhibitors are equally effective as a monotherapy or in combination with other oral GLDs. |
| 3 | The glycaemic efficacy of SGLT‐2 inhibitors is consistent across subgroups, including gender, age, race, duration of disease and baseline BMI. |
| Safety and tolerability of SGLT‐2 inhibitors in Asian patients with T2DM | |
| 4 | The risk of hypoglycaemia is low with SGLT‐2 inhibitors when used as monotherapy or in combination with other oral GLDs. The risk of hypoglycaemia should be monitored when SGLT‐2 inhibitors are used in combination with insulin. SGLT‐2 inhibitors have insulin‐sparing effects; adjustment of the insulin dose may be required. |
| 5 | SGLT‐2 inhibitors may increase the risk of mycotic genital infections (that can be managed with standard treatment and maintenance of perineal hygiene), bacterial urinary tract infections, and euglycaemic DKA (treatment with SGLT‐2 inhibitors should be discontinued at least 24 h before metabolically stressful events such as scheduled surgeries or extreme sports). |
| 6 | As a result of diuretic and mild natriuretic effects, SGLT‐2 inhibitors may cause intravascular volume contraction; there is a risk of volume contraction‐related AEs (such as symptomatic hypotension, dizziness, acute kidney disease), especially in patients with impaired renal function, elderly patients, and those receiving diuretics. |
| 7 | An increased risk of bone fractures and lower limb amputations is reported only with canagliflozin in the CANVAS trial. |
| Use in special populations | |
| 8 | Elderly: No dosage adjustments are required in elderly patients; the risk of volume depletion‐related AEs, renal impairment or urinary tract infection is higher in patients aged ≥65 y. |
| 9 | Risk of euglycaemic DKA is higher in lean patients, those with reduced β‐cell reserves, and those receiving a ketogenic diet. |
| 10 | Ramadan: No dose adjustments are required; move dose timing to the post sunset (evening) meal. |
| Non‐glycaemic metabolic effects of SGLT‐2 inhibitors | |
| 11 | Treatment with SGLT‐2 inhibitors is associated with clinically relevant reductions in BP and arterial stiffness, improvement in endothelial function, and an increase in haematocrit. |
| 12 | SGLT‐2 inhibitors are associated with a reduction in body weight, visceral adiposity and atherogenic small dense LDL‐C, an increase in HDL‐C, and less atherogenic large buoyant LDL‐C. |
| Effects on CV outcomes | |
| 13 | SGLT‐2 inhibitors are recommended for use in patients with T2DM with multiple risk factors to prevent and reduce hospitalization for HF. |
| 14 | SGLT‐2 inhibitors are recommended for use in patients with T2DM with established CV disease to reduce the risk of CV death. |
| 15 | The CV benefits of SGLT‐2 inhibitors have been shown in multiple randomized controlled trials and real‐world evidence studies, suggesting a class effect of SGLT‐2 inhibitors on CV outcomes. |
| Potential mechanism of CV effects | |
| 16 | The beneficial CV effects of SGLT‐2 inhibitors can be attributed to their impact on multiple risk factors, including a reduction in cardiac preload via diuresis and natriuresis, reduction in BP, body weight and visceral adiposity, shift in energy substrate from fat and glucose to ketone bodies, and an increase in haematocrit. |
| Place in the treatment algorithm for patients with T2DM | |
| 17 | Considering their beneficial CV and metabolic effects, SGLT‐2 inhibitors are the preferred second‐line therapy after metformin for:
|
Abbreviations: AE, adverse event; BMI, body mass index; BP, blood pressure; CV, cardiovascular; DKA, diabetic ketoacidosis; GLD, glucose‐lowering drug; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; LDL‐C, low‐density lipoprotein cholesterol; SGLT‐2, sodium‐glucose co‐transporter‐2; T2DM, type 2 diabetes mellitus.
CONFLICT OF INTEREST
C.D. has received honoraria as the speaker or advisor or research grant from AstraZeneca, Boehringer Ingelheim, Janssen, Bayer, Eli Lilly, Abbott, Novartis, Pfizer, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda. S.P.C. has received honoraria as speaker and advisor for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Servier. B.J.M. has received honoraria and CME grants from AstraZeneca, Boehringer Ingelheim, Lilly, Merck, Multicare, MSD, NatraPharm, Novartis, Novo Nordisk, Pfizer, Sanofi, Servier and Torrent Pharma. W.H.‐H.S. has been an advisor and/or speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals, Daiichi‐Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi‐Aventis and Takeda Pharmaceutical Company. J.C. is the Chief Executive Officer (on pro‐bono basis) of Asia Diabetes Foundation, a charitable foundation established under The Chinese University of Hong Kong Foundation for developing the JADE Technology; she has received honoraria and travelling support for consultancy or giving lectures and her affiliated institutions have received research and educational grants from Amgen, Ascencia, AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Daiichi‐Sankyo, Eli‐Lilly, GlaxoSmithKline, Medtronic, Merck Serono, Merck Sharp & Dohme, Novo Nordisk, Pfizer and Sanofi. N.H.M. has no conflict of interest to declare. K.S. has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, Sanofi, Merck and Servier. A.L. is a member of advisory boards for AstraZeneca, Boehringer Ingelheim, Sanofi and Amgen; she has received research grants from Boehringer Ingelheim, MSD, Sanofi and Amgen, and travel grants from AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Novo Nordisk and Sanofi. C.M.K. has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Sanofi. K.‐H.Y. has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda, and research support from AstraZeneca and Takeda. A.M. has received honoraria as a speaker and advisor from AstraZeneca, Abbott, Boehringer Ingelheim, Cipla, Dr. Reddy's, Eli Lilly, Glenmark, Glaxosmithkline, Ipca, Janssen, Lupin, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi, Serdia Servier, Sun Pharma, Torrent, Wockhardt and Zydus Nutrition. J.L. is a member of the DISCOVER Scientific Committee and received support from AstraZeneca to attend DISCOVER planning and update meetings; he has also received honoraria from Eli Lilly, Bristol‐Myers Squibb, Novartis, Novo Nordisk, Bayer, Merck Sharp & Dohme, Takeda, Sanofi, Roche, Boehringer Ingelheim and AstraZeneca, and research support from Roche, Sanofi, Merck Sharp & Dohme, AstraZeneca, Novartis, Eli Lilly and Bristol‐Myers Squibb.
AUTHOR CONTRIBUTIONS
All authors participated in the design, literature analysis and data interpretation. All authors participated in the manuscript preparation, critically reviewed the draft manuscript and approved the final version of the manuscript for publication.
Supporting information
Table S1. Glycemic efficacy of SGLT‐2 inhibitors in Asian patients with T2DM.
Table S2. Non‐glycemic effects of SGLT‐2 inhibitors in Asian patients with T2DM.
ACKNOWLEDGMENTS
Medical writing assistance was provided by Syed Abdul Haseeb (MS, CMPP) of MediTech Media, Asia Pacific, and was funded by AstraZeneca Ltd.
Deerochanawong C, Chan SP, Matawaran BJ, et al. Use of sodium‐glucose co‐transporter‐2 inhibitors in patients with type 2 diabetes mellitus and multiple cardiovascular risk factors: An Asian perspective and expert recommendations. Diabetes Obes Metab. 2019;21:2354–2367. 10.1111/dom.13819
Peer Review: The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13819.
Funding information The development of these expert recommendations was supported by an unrestricted educational grant from AstraZeneca, who had no influence on the content. All authors have read, approved, and take full responsibility for the accuracy of the content
REFERENCES
- 1. WHO . The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/. Updated January 2017. Accessed April 2, 2019.
- 2. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta‐analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emerging Risk Factors Collaboration , Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375(9733):2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Noncommunicable diseases. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. Updated June 2018. Accessed April 2, 2019.
- 5. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Authors/Task Force Members , Ryden L, Grant PJ, Anker SD, et al. ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035‐3087. [DOI] [PubMed] [Google Scholar]
- 10. ACCORD Study Group , Buse JB, Bigger JT, Byington RP, et al. Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i‐33i. [DOI] [PubMed] [Google Scholar]
- 11. ADVANCE Collaborative Group , Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 12. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129‐139. [DOI] [PubMed] [Google Scholar]
- 13. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 14. del Canizo Gomez FJ, Moreira Andres MN. Strict control of modifiable cardiovascular risk factors in patients with type 2 diabetes mellitus. Med Clin (Barc). 2008;130(17):641‐644. [DOI] [PubMed] [Google Scholar]
- 15. Li YH, Sheu WH, Lee IT. Effects of retinopathy and chronic kidney disease on long‐term mortality in type 2 diabetic inpatients with normal urinary albumin or protein: a retrospective cohort study. BMJ Open. 2018;8(7):e021655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580‐591. [DOI] [PubMed] [Google Scholar]
- 17. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383‐393. [DOI] [PubMed] [Google Scholar]
- 18. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 19. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double‐blind trial. Lancet. 2015;385(9982):2067‐2076. [DOI] [PubMed] [Google Scholar]
- 20. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 21. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 22. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628‐2639. [DOI] [PubMed] [Google Scholar]
- 23. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation. 2017;136(3):249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor: results from the EASEL population‐based cohort study (evidence for cardiovascular outcomes with sodium glucose cotransporter 2 inhibitors in the real world). Circulation. 2018;137(14):1450‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non‐SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real‐world meta‐analysis of 4 observational databases (OBSERVE‐4D). Diabetes Obes Metab. 2018;20(11):2585‐2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 27. International Diabetes Federation . IDF Diabetes Atlas, 8th edition. https://www.idf.org/component/attachments/attachments.html?id=1405&task=download. Updated 2017. Accessed April 2, 2019.
- 28. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J. 2013;77(9):2209‐2217. [DOI] [PubMed] [Google Scholar]
- 31. Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd‐Jones DM, Wilkins JT. Hypertension, obesity, diabetes, and heart failure‐free survival: the cardiovascular disease lifetime risk pooling project. JACC Heart Fail. 2016;4(12):911‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. International Diabetes Federation . Diabetes and Cardiovascular Disease report. https://www.idf.org/component/attachments/attachments.html?id=409&task=download. Updated 2016. Accessed April 2, 2019.
- 33. Clarke PM, Glasziou P, Patel A, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010;7(2):e1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cho H, Oh SH, Lee H, Cho HJ, Kang HY. The incremental economic burden of heart failure: a population‐based investigation from South Korea. PLoS One. 2018;13(12):e0208731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rijal A, Adhikari TB, Khan JAM, Berg‐Beckhoff G. The economic impact of non‐communicable diseases among households in South Asia and their coping strategy: a systematic review. PLoS One. 2018;13(11):e0205745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang J, Yin H, Zhang M, Ni Q, Xuan J. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J Med Econ. 2017;20(5):549‐553. [DOI] [PubMed] [Google Scholar]
- 37. Deerochanawong C, Ferrario A. Diabetes management in Thailand: a literature review of the burden, costs, and outcomes. Global Health. 2013;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young‐onset type 2 diabetes in Asia (the JADE programme): a cross‐sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2(12):935‐943. [DOI] [PubMed] [Google Scholar]
- 39. Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care. 2008;31(5):893‐898. [DOI] [PubMed] [Google Scholar]
- 40. Moller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37(3):796‐804. [DOI] [PubMed] [Google Scholar]
- 41. Dickinson S, Colagiuri S, Faramus E, Petocz P, Brand‐Miller JC. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr. 2002;132(9):2574‐2579. [DOI] [PubMed] [Google Scholar]
- 42. Liew CF, Seah ES, Yeo KP, Lee KO, Wise SD. Lean, nondiabetic Asian Indians have decreased insulin sensitivity and insulin clearance, and raised leptin compared to Caucasians and Chinese subjects. Int J Obes Relat Metab Disord. 2003;27(7):784‐789. [DOI] [PubMed] [Google Scholar]
- 43. Liew CF, Wise SD, Yeo KP, Lee KO. Insulin‐like growth factor binding protein‐1 is independently affected by ethnicity, insulin sensitivity, and leptin in healthy, glucose‐tolerant young men. J Clin Endocrinol Metab. 2005;90(3):1483‐1488. [DOI] [PubMed] [Google Scholar]
- 44. Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross‐sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(suppl 1):53‐61. [DOI] [PubMed] [Google Scholar]
- 45. Deurenberg P, Deurenberg‐Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141‐146. [DOI] [PubMed] [Google Scholar]
- 46. Abate N, Chandalia M, Snell PG, Grundy SM. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab. 2004;89(6):2750‐2755. [DOI] [PubMed] [Google Scholar]
- 47. Henry CJ, Lightowler HJ, Newens K, et al. Glycaemic index of common foods tested in the UKand India. Br J Nutr. 2008;99(4):840‐845. [DOI] [PubMed] [Google Scholar]
- 48. Sheu WH, Rosman A, Mithal A, et al. Addressing the burden of type 2 diabetes and cardiovascular disease through the management of postprandial hyperglycaemia: an Asian‐Pacific perspective and expert recommendations. Diabetes Res Clin Pract. 2011;92(3):312‐321. [DOI] [PubMed] [Google Scholar]
- 49. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta‐analysis. Diabetes Care. 2013;36(6):1789‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yabe D, Seino Y. Type 2 diabetes via beta‐cell dysfunction in east Asian people. Lancet Diabetes Endocrinol. 2016;4(1):2‐3. [DOI] [PubMed] [Google Scholar]
- 51. Yoon KH, Ko SH, Cho JH, et al. Selective beta‐cell loss and alpha‐cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88(5):2300‐2308. [DOI] [PubMed] [Google Scholar]
- 52. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129‐2140. [DOI] [PubMed] [Google Scholar]
- 53. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999‐2010. N Engl J Med. 2013;368(17):1613‐1624. [DOI] [PubMed] [Google Scholar]
- 54. Huo X, Gao L, Guo L, et al. Risk of non‐fatal cardiovascular diseases in early‐onset versus late‐onset type 2 diabetes in China: a cross‐sectional study. Lancet Diabetes Endocrinol. 2016;4(2):115‐124. [DOI] [PubMed] [Google Scholar]
- 55. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752‐772. [DOI] [PubMed] [Google Scholar]
- 56. Inzucchi SE, Zinman B, Wanner C, et al. SGLT‐2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marx N, McGuire DK. Sodium‐glucose cotransporter‐2 inhibition for the reduction of cardiovascular events in high‐risk patients with diabetes mellitus. Eur Heart J. 2016;37(42):3192‐3200. [DOI] [PubMed] [Google Scholar]
- 58. Cai X, Ji L, Chen Y, et al. Comparisons of weight changes between sodium‐glucose cotransporter 2 inhibitors treatment and glucagon‐like peptide‐1 analogs treatment in type 2 diabetes patients: a meta‐analysis. J Diabetes Investig. 2017;8(4):510‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1‐203. [DOI] [PubMed] [Google Scholar]
- 60. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429‐442. [DOI] [PubMed] [Google Scholar]
- 61. NICE Guidelines . Type 2 diabetes in adults: Management. https://www.nice.org.uk/guidance/ng28. Updated 2017. Accessed April 2, 2019.
- 62. Diabetes Canada Clinical Practice Guidelines Expert Committee , Lipscombe L, Booth G, Butalia S, et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes. 2018;42(suppl 1):S88‐S103. [DOI] [PubMed] [Google Scholar]
- 63. Malaysian Clinical Practice Guidelines . Management of type 2 diabetes mellitus. http://www.acadmed.org.my/view_file.cfm?fileid=763. Updated 2015. Accessed April 2, 2019.
- 64. DAROC . Clinical Practice Guidelines for diabetes care ‐ 2018, Taiwan, Diabetes Association of the R.O.C., 2018. http://www.endo-dm.org.tw/dia/direct/index.asp?BK_KIND=29¤t=2018%E7%B3%96%E5%B0%BF%E7%97%85%E8%87%A8%E5%BA%8A%E7%85%A7%E8%AD%B7%E6%8C%87%E5%BC%95+++++++++++++++++. Updated 2018. Accessed April 2, 2019. [DOI] [PubMed]
- 65. Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. American Diabetes Association . Standards of medical care in diabetes ‐ 2019. Diabetes Care. 2019;42(suppl 1):S1‐S193.30559224 [Google Scholar]
- 67. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129‐2200. [DOI] [PubMed] [Google Scholar]
- 69. Diabetes Canada Clinical Practice Guidelines Expert Committee , McFarlane P, Cherney D, Gilbert RE, Senior P. Chronic kidney disease in diabetes. Can J Diabetes. 2018;42(suppl 1):S201‐S209. [DOI] [PubMed] [Google Scholar]
- 70. Diabetes Canada Clinical Practice Guidelines Expert Committee , Stone JA, Houlden RL, Lin P, Udell JA, Verma S. Cardiovascular protection in people with diabetes. Can J Diabetes. 2018;42(suppl 1):S162‐S169. [DOI] [PubMed] [Google Scholar]
- 71. Diabetes Canada Clinical Practice Guidelines Expert Committee , Connelly KA, Gilbert RE, Liu P. Treatment of diabetes in people with heart failure. Can J Diabetes. 2018;42(suppl 1):S196‐S200. [DOI] [PubMed] [Google Scholar]
- 72. Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter‐2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo‐controlled, double‐blind, parallel‐group comparative study. Cardiovasc Diabetol. 2014;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seino Y, Kaku K, Inagaki N, et al. Fifty‐two‐week long‐term clinical study of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Endocr J. 2015;62(7):593‐603. [DOI] [PubMed] [Google Scholar]
- 74. Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab. 2015;17(3):304‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kadowaki T, Haneda M, Inagaki N, et al. Efficacy and safety of empagliflozin monotherapy for 52 weeks in Japanese patients with type 2 diabetes: a randomized, double‐blind, parallel‐group study. Adv Ther. 2015;32(4):306‐318. [DOI] [PubMed] [Google Scholar]
- 76. Ji L, Ma J, Li H, et al. Dapagliflozin as monotherapy in drug‐naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther. 2014;36(1):84‐100.e9. [DOI] [PubMed] [Google Scholar]
- 77. Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24‐week, randomized, double‐blind, placebo‐controlled, phase III study. Expert Opin Pharmacother. 2014;15(11):1501‐1515. [DOI] [PubMed] [Google Scholar]
- 78. Ikeda S, Takano Y, Cynshi O, et al. A novel and selective sodium‐glucose cotransporter‐2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17(10):984‐993. [DOI] [PubMed] [Google Scholar]
- 79. Seino Y, Inagaki N, Haneda M, et al. Efficacy and safety of luseogliflozin added to various oral antidiabetic drugs in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2015;6(4):443‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lu CH, Min KW, Chuang LM, Kokubo S, Yoshida S, Cha BS. Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: results of a phase 3 randomized, placebo‐controlled, double‐blind, multicenter trial. J Diabetes Investig. 2016;7(3):366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Araki E, Tanizawa Y, Tanaka Y, et al. Long‐term treatment with empagliflozin as add‐on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17(7):665‐674. [DOI] [PubMed] [Google Scholar]
- 82. Kaku K, Maegawa H, Tanizawa Y, et al. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open‐label study. Diabetes Ther. 2014;5(2):415‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ji L, Han P, Liu Y, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17(1):23‐31. [DOI] [PubMed] [Google Scholar]
- 84. Terauchi Y, Tamura M, Senda M, Gunji R, Kaku K. Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J‐STEP/INS): results of a 16‐week randomized, double‐blind, placebo‐controlled multicentre trial. Diabetes Obes Metab. 2017;19(10):1397‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seino Y, Sasaki T, Fukatsu A, Imazeki H, Ochiai H, Sakai S. Efficacy and safety of luseogliflozin added to insulin therapy in Japanese patients with type 2 diabetes: a multicenter, 52‐week, clinical study with a 16‐week, double‐blind period and a 36‐week, open‐label period. Curr Med Res Opin. 2018;34(6):1‐35. [DOI] [PubMed] [Google Scholar]
- 86. Ishihara H, Yamaguchi S, Nakao I, Okitsu A, Asahina S. Efficacy and safety of ipragliflozin as add‐on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi‐centre, randomized, placebo‐controlled, double‐blind study. Diabetes Obes Metab. 2016;18(12):1207‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang W, Ma J, Li Y, et al. Dapagliflozin as add‐on therapy in Asian patients with type 2 diabetes inadequately controlled on insulin with or without oral antihyperglycemic drugs: a randomized controlled trial. J Diabetes. 2018;10(7):589‐599. [DOI] [PubMed] [Google Scholar]
- 88. Inagaki N, Harashima S, Maruyama N, Kawaguchi Y, Goda M, Iijima H. Efficacy and safety of canagliflozin in combination with insulin: a double‐blind, randomized, placebo‐controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dapagliflozin . US FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202293s012lbl.pdf. Updated 2018. Accessed April 2, 2019.
- 90. Empagliflozin . US FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204629s016lbl.pdf. Updated 2018. Accessed April 2, 2019.
- 91. Canagliflozin . US FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204042s026lbl.pdf. Updated 2018. Accessed April 2, 2019.
- 92. Handelsman Y, Henry RR, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT‐2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22(6):753‐762. [DOI] [PubMed] [Google Scholar]
- 93. Ilercil A, Devereux RB, Roman MJ, et al. Associations of insulin levels with left ventricular structure and function in American Indians: the strong heart study. Diabetes. 2002;51(5):1543‐1547. [DOI] [PubMed] [Google Scholar]
- 94. Howard G, O'Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis; the insulin resistance atherosclerosis study (IRAS) investigators. Circulation. 1996;93(10):1809‐1817. [DOI] [PubMed] [Google Scholar]
- 95. Ceriello A, Taboga C, Tonutti L, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short‐ and long‐term simvastatin treatment. Circulation. 2002;106(10):1211‐1218. [DOI] [PubMed] [Google Scholar]
- 96. Nilsson PM, Cederholm J, Zethelius BR, Eliasson BR, Eeg‐Olofsson K, Gudbj Rnsdottir S. Trends in blood pressure control in patients with type 2 diabetes: data from the Swedish National Diabetes Register (NDR). Blood Press. 2011;20(6):348‐354. [DOI] [PubMed] [Google Scholar]
- 97. Tseng LN, Tseng YH, Jiang YD, et al. Prevalence of hypertension and dyslipidemia and their associations with micro‐ and macrovascular diseases in patients with diabetes in Taiwan: an analysis of nationwide data for 2000‐2009. J Formos Med Assoc. 2012;111(11):625‐636. [DOI] [PubMed] [Google Scholar]
- 98. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med. 2013;159(4):262‐274. [DOI] [PubMed] [Google Scholar]
- 99. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420‐428. [DOI] [PubMed] [Google Scholar]
- 101. Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shigiyama F, Kumashiro N, Miyagi M, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early‐stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta‐analysis. Heart Fail Rev. 2015;20(3):291‐303. [DOI] [PubMed] [Google Scholar]
- 104. Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta‐analysis. J Am Heart Assoc. 2015;4(11):1‐15. 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Okamoto A, Yokokawa H, Sanada H, Naito T. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R D. 2016;16(3):255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf recall study. J Am Coll Cardiol. 2013;61(13):1388‐1395. [DOI] [PubMed] [Google Scholar]
- 108. Liang KW, Tsai IC, Lee WJ, et al. MRI measured epicardial adipose tissue thickness at the right AV groove differentiates inflammatory status in obese men with metabolic syndrome. Obesity (Silver Spring). 2012;20(3):525‐532. [DOI] [PubMed] [Google Scholar]
- 109. Fukuda T, Bouchi R, Terashima M, et al. Ipragliflozin reduces epicardial fat accumulation in non‐obese type 2 diabetic patients with visceral obesity: a pilot study. Diabetes Ther. 2017;8(4):851‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bouchi R, Terashima M, Sasahara Y, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017;16(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low‐density lipoprotein‐cholesterol and increases high‐density lipoprotein 2‐cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gotoh S, Hata J, Ninomiya T, et al. Hematocrit and the risk of cardiovascular disease in a Japanese community: the Hisayama study. Atherosclerosis. 2015;242(1):199‐204. [DOI] [PubMed] [Google Scholar]
- 113. Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease‐‐the Framingham study: a 34‐year follow‐up. Am Heart J. 1994;127(3):674‐682. [DOI] [PubMed] [Google Scholar]
- 114. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41(2):356‐363. [DOI] [PubMed] [Google Scholar]
- 115. Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co‐transport‐2 inhibitors in type 2 diabetes: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(5):457‐466. [DOI] [PubMed] [Google Scholar]
- 116. Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium‐glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kaku K, Lee J, Mattheus M, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease‐ results from EMPA‐REG OUTCOME([R]). Circ J. 2017;81(2):227‐234. [DOI] [PubMed] [Google Scholar]
- 118. Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (canagliflozin cardiovascular assessment study). Circulation. 2018;137(4):323‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kimura G. Diuretic action of sodium‐glucose cotransporter 2 inhibitors and its importance in the management of heart failure. Circ J. 2016;80(11):2277‐2281. [DOI] [PubMed] [Google Scholar]
- 120. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME trial: a "thrifty substrate" hypothesis. Diabetes Care. 2016;39(7):1108‐1114. [DOI] [PubMed] [Google Scholar]
- 121. Packer M. Activation and inhibition of sodium‐hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation. 2017;136(16):1548‐1559. [DOI] [PubMed] [Google Scholar]
- 122. Scheen AJ. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: a common comorbidity associated with severe complications. Diabetes Metab. 2019;45(3):213‐223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Glycemic efficacy of SGLT‐2 inhibitors in Asian patients with T2DM.
Table S2. Non‐glycemic effects of SGLT‐2 inhibitors in Asian patients with T2DM.


