Abstract
Background:
Despite the increasing use of colistin in clinical practice, the optimal dosing, and administration route have not been established. This study aimed to evaluate the clinical outcome and safety of intravenous (IV) colistin with a loading dose (LD) and adjunctive aerosolized (AS) colistin administration in critically ill patients with hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) caused by carbapenem-resistant gram-negative bacteria (CRGNB).
Methods:
We retrospectively reviewed 191 critically ill patients who received colistin for the treatment of HAP or VAP caused by CRGNB. Patients were divided into three groups: non-LD IV (patients received only IV colistin without LD), LD IV (patients received only IV colistin with LD), and AS–LD (patients received IV colistin with LD and adjunctive AS colistin).
Results:
There was no difference in clinical response between the three groups. However, the rate of microbiological eradication was significantly higher in the AS–LD group (60%) than in the non-LD IV (31%), and LD IV (33%) groups (p = 0.010). Patients treated with adjunctive AS colistin in combination with LD IV had significantly lower 30-day mortality rates than patients treated with IV colistin alone (p = 0.027). After adjusting for potential confounding factors, adjunctive AS colistin was still significantly associated with lower mortality (adjusted OR 0.338, CI 95% 0.132–0.864, p = 0.024). However, nephrotoxicity did not change according to the use of LD regimen and AS colistin administration (p = 0.100).
Conclusions:
Adjunctive AS colistin in combination with IV colistin with LD was related to an improved 30-day mortality and microbiological outcome without an increase in nephrotoxicity in critically ill patients with HAP and VAP caused by CRGNB.
The reviews of this paper are available via the supplemental material section.
Keywords: carbapenem-resistant Enterobacteriaceae, colistin, critical illness, inhalation administration, ventilator-associated pneumonia
Introduction
Colistin is a bactericidal antibiotic that was first used in the 1950s but was withdrawn for several decades due to concerns about potential adverse effects, including nephrotoxicity and neurotoxicity.1,2 Recently, colistin use has re-emerged in response to associated nosocomial infections associated with carbapenem-resistant gram-negative bacteria (CRGNB).1,3 Despite the increasing use in clinical practice, no standardized method has been established for the optimal dosing and administration route of colistin.
Recent pharmacokinetics/pharmacodynamics studies have demonstrated that the dosing regimen recommended in the package insert is not an appropriate strategy because it results in a subtherapeutic concentration and a delayed time to steady-state, especially in critically ill patients.4,5 Therefore, intravenous (IV) colistin with a loading dose (LD) was proposed as a way to achieve therapeutic concentrations more quickly. The LD regimen was associated with improved outcomes in several clinical studies,6–8 but the clinical efficacy and renal toxicity of such regimens have yet to be assessed.9–11
In particular, the optimal colistin treatment strategy in patients with hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) is unclear because of the inadequate permeation of colistin into the lung parenchyma.12,13 To overcome the limitation of IV colistin, aerosolized (AS) colistin was suggested as a promising approach for drug delivery in pulmonary infections.13,14 The guidelines from the American Thoracic Society/Infectious Diseases Society of America suggest adjunctive AS colistin for patients with highly resistant organisms or for patients who are not responding to IV antibiotics alone.15 However, the European Society of Clinical Microbiology and Infectious Diseases guidelines recommend avoiding the use of adjunctive AS antibiotics.16 In addition, three recent meta-analyses on the role of AS colistin have reported mixed results.17–19 Therefore, this study aimed to evaluate the clinical outcome and safety of IV colistin with LD and adjunctive AS colistin administration in critically ill patients with HAP or VAP caused by CRGNB.
Methods
Study design and population
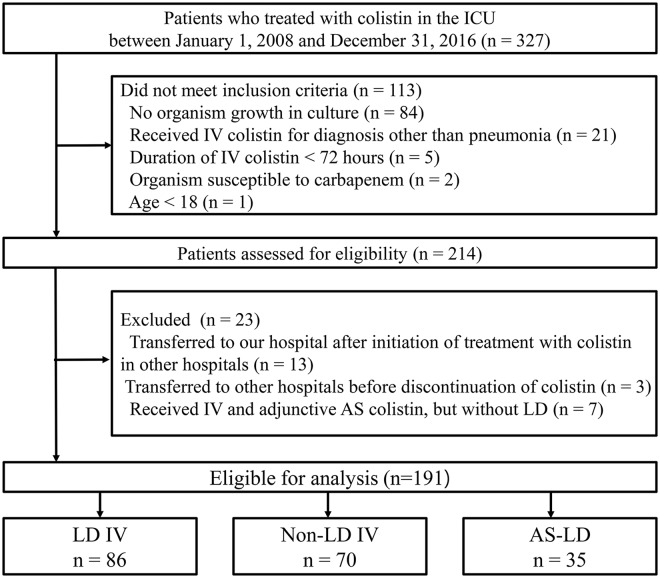
We retrospectively reviewed adult patients diagnosed with HAP or VAP and treated with IV colistin at Samsung Medical Center, an university-affiliated, tertiary referral hospital with 1989 beds including 112 adult intensive care unit (ICU) beds in Seoul, Korea. Patients were included if all of the following conditions were satisfied: admitted to the ICU between 1 January 2008 and 31 December 2016; CRGNB, which is resistant to all tested carbapenems and susceptible to colistin, was identified as a pathogen in microbiological tests; and received IV colistin for treatment of pneumonia for at least 72 h. For patients treated with colistin multiple times during the study period, only the first episode was included in this analysis. Patients with any of the following criteria were excluded: transferred to our hospital after initiation of treatment with colistin in other hospitals (n = 13); transferred to other hospitals before discontinuation of colistin (n = 3); or received IV and adjunctive AS colistin, but without LD (n = 7). Finally, eligible patients were divided into three groups based on treatment regimen: non-LD IV (patients received only IV colistin without LD), LD IV (patients received only IV colistin with LD), and AS–LD (patients received IV colistin with LD and adjunctive AS colistin).
The Institutional Review Board at Samsung Medical Center approved the study and waived the requirement for informed consent because of the observational nature of the study.
Diagnosis of pneumonia and microbiological tests
Pneumonia was diagnosed when a new and progressive pulmonary infiltrate on chest radiography was accompanied by clinical evidence including fever, purulent sputum, leukocytosis, or a decrease in oxygenation.15 HAP was defined as pneumonia that developed more than 48 h after admission. VAP was defined as pneumonia that developed more than 48 h after endotracheal intubation.
If a patient was diagnosed with pneumonia, respiratory specimens for quantitative culture were obtained prior to initiation of new antibiotic therapy. Respiratory specimens included sputum, transtracheal aspirate, bronchoalveolar lavage fluid, and pleural fluid. Clinical and Laboratory Standards Institute interpretive criteria were used to determine antimicrobial susceptibilities. Follow-up cultures were performed at least 72 h after initiation of colistin treatment to assess the microbiological response.
Treatment regimens
In our hospital, before the hospital guidelines were established, patients received IV colistin using a physician-selected colistin dosage regimen without a LD. Since the hospital guidelines for colistin dosing were developed in October 2013 (Table 1), physicians have used a LD that targeted an average colistin steady-state plasma concentration of 2.5 mg/L. Our guideline recommended a 5 mg/kg colistin base activity (CBA) LD [equivalent to 150,000 IU/kg colistimethate sodium (CMS)]20 followed by 150 mg CBA (equivalent to 4.5 million IU CMS) every 12 h, adjusted for renal function. The LD never exceeded 300 mg (equivalent to 9 million IU CMS), even if the patient weighed over 60 kg.
Table 1.
Colistin dosing guidelines.
| (1) Loading dose: 5.0 × body weight (kg), to not exceed 300 mg* | |
| (2) Maintenance dose (mg of colistin base activity) | |
| Creatinine clearance ⩾50 | 150 mg every 12 h |
| 20< Creatinine clearance <50 | 150 mg every 24 h |
| Creatinine clearance ⩽20 | 150 mg every 48 h |
| Intermittent hemodialysis | 75 mg every 24 h (plus extra 37.5 mg after dialysis) |
| Continuous renal replacement therapy. | |
| Effluent flow rate <2500 ml/h | 150 mg every 12 h |
| Effluent flow rate ⩾2500 ml/h | 150 mg every 8 h |
Conversion factor: 1 million IU colistimethate sodium (CMS) corresponds to approximately 33 mg colistin base activity (CBA).20
In January 2014 adjunctive AS colistin regimen was incorporated in the hospital guidelines. AS colistin was administered 150 mg CBA every 8 h only in patients receiving mechanical ventilation. Colistin was mixed with 10 ml of normal saline or sterile water immediately before inhalation. Patients receiving mechanical ventilation used an ultrasonic nebulizer for administration of AS colistin, and AS colistin administration then continued using a jet nebulizer when the patients extubated. We routinely performed nebulized bronchodilator therapy with ipratropium 15 min before administration of AS colistin to prevent bronchoconstriction. The decision to use adjunctive AS colistin and concomitant antibiotics was left to the individual physician’s discretion.
Data collection and clinical outcomes
The clinical, laboratory, and outcome data were collected using a retrospective review of electronic hospital records. Demographic data, including age, sex, body mass index, comorbidity, immune state, sequential organ failure assessment (SOFA) score, causative microorganism, and antibiotic susceptibility were recorded on the first day of administration of IV colistin. The SOFA score was calculated using the most extreme values within 24 h of IV colistin use. Immunocompromised was defined as one of the following medical conditions: hematological malignancies, solid tumor with neutropenia after chemotherapy, solid-organ transplantation, high-dose or long-term corticosteroid and/or immunosuppressant use, and human immunodeficiency virus infection.21 Data about use of concomitant nephrotoxic agents and other antibiotics and initiation of renal replacement therapy (RRT) during IV colistin therapy were also collected.
The primary outcome was 30-day all-cause mortality. The secondary outcomes included clinical responses, microbiological responses, rate of nephrotoxicity during colistin therapy, and initiation rates of RRT, ICU length of stay, and 90-day all-cause mortality. Clinical responses were classified as clinical cure (improvement of all signs and symptoms associated with pneumonia), clinical failure (persistence or worsening of signs, symptoms, or both, associated with pneumonia, symptoms, signs of pneumonia, or both, occurring again within 3 days after termination of treatment), and recurrence (occurrence of a new event of pneumonia after 72 h of antibiotic discontinuation). The clinical response of all patients was assessed by two authors who were not aware of which treatment was given to the patient. In the event of a discrepancy, two reviewers discussed the results and reached a consensus. Microbiological responses were classified as microbiological eradication (absence of the baseline pathogen in the final culture of specimens during hospitalization), colonization (persistence of the baseline pathogen but clinically cured), microbiological failure (persistence of the baseline pathogen and not clinically cured), or microbiological recurrence (regrowth of the baseline pathogen irrespective of the clinical outcome). Nephrotoxicity was defined as a risk, injury, failure, loss of kidney function, and end-stage kidney disease (RIFLE) classification of injury or more, with injury defined as a greater than two-fold increase in serum creatinine, a greater than 50% reduction in glomerular filtration rate compared with the value at the start of treatment, or oliguria (⩽0.5 ml/kg/h) for ⩾12 h.22,23 Patients on RRT at the time of IV colistin initiation were excluded from the nephrotoxicity analysis.
Statistical analysis
Data are presented as median and interquartile ranges for continuous variables and as numbers (percentages) for categorical variables. The baseline characteristics and outcome measures of interest were then compared between the three groups: non-LD IV group, LD IV group, and AS–LD group. Data were compared using the Kruskal–Wallis test for continuous variables and the Chi-square or Fisher’s exact test for categorical variables. Multiple comparisons were performed to compare each group using the Wilcoxon signed-rank test, and the Bonferroni correction was used to determine whether multiple comparisons were significant.
Logistic regression models were used to adjust for potential confounding factors in the association between AS–LD and 30-day all-cause mortality. Variables with a p < 0.2 on univariate analyses,24 as well as a priori variables that were clinically relevant were entered into the forward stepwise multiple logistic regression model. Three models were constructed: model 1 was adjusted for age and gender; model 2 was additionally adjusted for SOFA score, malignancy, and immunocompromised; and in addition, model 3 included duration of intravenous colistin, intravenous LD, and combination with carbapenem. Data are presented as odds ratios (ORs) with their 95% confidence intervals (CIs). Additional logistic regression analysis was performed to identify the variables associated with nephrotoxicity. In addition, predictors of the cumulative incidence of nephrotoxicity were identified with the Fine and Gray model to consider death as a competing risk for nephrotoxicity.25 Two-tailed testing with p < 0.05 was considered statistically significant. All analyses were performed using the STATA 14.2 software program (Stata Corp LLC, College Station, TX, USA).
Results
Baseline clinical characteristics
Of the 191 patients who were eligible for analysis, 156 received only IV colistin (70 without LD and 86 with LD) and 35 received adjunctive AS colistin added to IV colistin with LD (Figure 1). The baseline clinical characteristics of the patients are summarized in Table 2. Age and body mass index (BMI) were similar among the three groups, but the proportion of men was significantly higher in the AS–LD group (89%) than in the non-LD IV group (66%) (p = 0.013). Patients with chronic kidney disease were more common in the non-LD IV group (31%) than in the LD IV (12%) group (p = 0.003), but there was no difference between the groups in median glomerular filtration rate of the patients not receiving RRT (p = 0.100) or proportion of patients receiving RRT on the first day of colistin treatment (p = 0.773). The proportions of patients who had a malignancy and who were immunocompromised among the three groups were not different.
Figure 1.
Study flow diagram.
Table 2.
Baseline characteristics of 191 patients with HAP or VAP caused by CRGNB and who were treated with colistin in the ICU.
| Variables | Non-LD IV (n = 70) | LD IV (n = 86) | AS–LD (n = 35) | p value |
|---|---|---|---|---|
| Age, years | 68 (62–74) | 63 (54–75) | 67 (54–76) | 0.214 |
| Male | 46 (66) | 64 (74) | 31 (89) | 0.042‡ |
| BMI, kg/m2 | 22.8 (19.1–24.8) | 21.0 (17.9–24.3) | 21.0 (18.7–23.7) | 0.530 |
| SOFA score | 7 (5–10) | 8 (4–11) | 8 (4–12) | 0.411 |
| Estimated GFRa, ml/min/1.73 m2 | 86 (44–116) | 94 (67–130) | 76 (43–95) | 0.100 |
| Underlying disease | ||||
| Diabetes mellitus | 23 (33) | 16 (19) | 13 (37) | 0.048 |
| Malignancy | 22 (31) | 28 (33) | 17 (49) | 0.178 |
| Chronic kidney disease | 22 (31) | 10 (12) | 4 (11) | 0.003* |
| Immunocompromised | 18 (26) | 19 (22) | 15 (43) | 0.063 |
| RRT at baseline | 20 (29) | 25 (29) | 8 (23) | 0.773 |
| Microorganism | ||||
| Acinetobacter baumannii | 59 (84) | 76 (88) | 34 (97) | 0.151 |
| Pseudomonas eruginosa | 17 (24) | 19 (22) | 2 (6) | 0.063 |
| Klebsiella pneumoniae | 0 (0) | 1 (1) | 0 (0) | NA |
| Type of pneumonia | 0.702 | |||
| VAP | 49 (70) | 64 (74) | 27 (77) | |
| HAP | 21 (30) | 22 (26) | 8 (23) | |
| Combination therapy | ||||
| Carbapenem | 17 (24) | 37 (43) | 10 (29) | 0.038* |
| Piperacillin/Tazobactam | 6 (9) | 12 (14) | 3 (9) | 0.497 |
| Minocycline | 1 (1) | 6 (7) | 3 (9) | 0.125 |
| Tigecycline | 3 (4) | 4 (5) | 2 (6) | >0.999 |
| Number of nephrotoxins | 1.0 (0.8–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–1.0) | 0.121 |
| Vancomycin | 29 (41) | 22 (26) | 10 (29) | 0.096 |
| Aminoglycoside | 10 (14) | 2 (2) | 0 (0) | 0.003* |
| Loop diuretics | 31 (44) | 28 (33) | 11 (31) | 0.248 |
| Amphotericin B | 1 (1) | 1 (1) | 3 (9) | 0.099 |
| Contrast | 4 (6) | 12 (14) | 4 (11) | 0.242 |
| Othersb | 1 (1) | 7 (8) | 2 (6) | 0.141 |
| Mechanical ventilation | 65 (93) | 80 (93) | 35 (100) | 0.297 |
| Duration of IV colistin, days | 14 (10–15) | 14 (9–15) | 14 (12–17) | 0.171 |
| Dose of IV colistin, mg/kg/day | 2.9 (2.1–4.3) | 3.9 (2.9–5.0) | 3.1 (2.2–4.1) | 0.002*$ |
AS, aerosolized; BMI, body mass index; CRGNB, carbapenem-resistant gram-negative bacteria; GFR, glomerular filtration rate; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IV, intravenous; IQR, interquartile range; LD, loading dose; RRT, renal replacement therapy; SOFA, sequential organ failure assessment; VAP, ventilator-associated pneumonia.
, $, ‡indicate significant differences (p < 0.017) between the non-LD IV group and the LD IV group, LD IV group and AS–LD group, and the non-LD IV group and the AS–LD group, respectively.
Only those who did not need RRT at the time of IV colistin initiation were analyzed.
IV voriconazole, tacrolimus, cyclosporine, angiotensin-converting-enzyme inhibitors, and nonsteroidal anti-inflammatory drugs were included.
Microorganism and treatment characteristics
About three-quarters of the patients were diagnosed with VAP (Table 2). Acinetobacter baumannii was the dominant causative organism in 88% of the overall group, and the remaining cases were related to Pseudomonas aeruginosa except for one patient with Klebsiella pneumonia. The percentage of patients with each pathogen was similar among the three groups. The median duration of IV colistin treatment was 14 days in all three groups, and the daily median dose of IV colistin was significantly higher in the LD IV group than in the other two groups (2.9 mg/kg/day versus 3.9 mg/kg/day versus 3.1 mg/kg/day, p = 0.002). In the AS–LD group, the median duration of AS colistin treatment was 12 (6–16) days. As a combination therapy for CRGNB, carbapenem (33.5%), penicillin (11.0%), minocycline (5.2%), and tigecycline (4.7%) were used together with colistin. Almost all patients required mechanical ventilation during colistin treatment (93% versus 93% versus 100%, p = 0.297).
Clinical outcomes
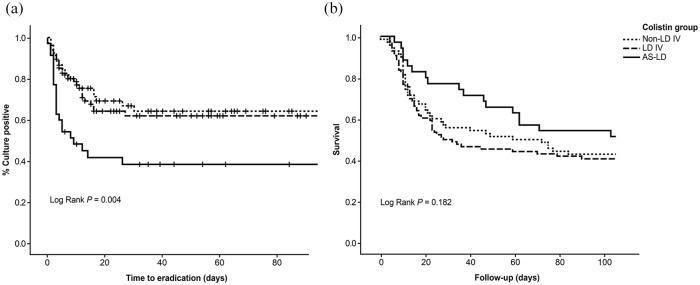
Clinical outcomes of patients who received colistin therapy for pneumonia caused by CRGNB are shown in Table 3. There was no difference in clinical response between the three groups. However, the rate of microbiological eradication was significantly higher in the AS–LD group (60%) than in the non-LD IV (31%), and LD IV (33%) groups (p = 0.010) [Figure 2(a)]. In addition, 30-day mortality was lower in the AS–LD group (23%) than in the non-LD IV (46%), and LD IV (49%) groups (p = 0.027). After adjusting for potential confounding factors, the AS–LD group was still significantly associated with lower mortality (adjusted OR 0.338, CI 95% 0.132–0.864, p = 0.024) [Table 4, Figure 2(b)].
Table 3.
Clinical outcomes of 191 patients with HAP or VAP caused by CRGNB, who were treated with colistin in the ICU.
| Variables | Non-LD IV (n = 70) | LD IV (n = 86) | AS–LD (n = 35) | p value |
|---|---|---|---|---|
| Clinical response | ||||
| Clinical cure | 32 (46) | 36 (42) | 17 (49) | 0.764 |
| Recurrence | 4 (6) | 10 (12) | 4 (11) | 0.414 |
| Clinical failure | 34 (49) | 40 (47) | 14 (40) | 0.724 |
| Microbiological response | ||||
| Eradication | 21/67 (31) | 27/81 (33) | 21/35 (60) | 0.010$‡ |
| Recurrence | 9/67 (13) | 12/81 (15) | 7/35 (20) | 0.663 |
| Colonization | 20/67 (30) | 22/81 (27) | 3/35 (9) | 0.047$ |
| Microbiological failure | 17/67 (25) | 20/81 (25) | 4/35 (11) | 0.222 |
| Duration of ICU stay, days | 13 (8–21) | 12 (8–18) | 20 (10–33) | 0.013$ |
| Mortality | ||||
| 30-day mortality | 32 (46) | 42 (49) | 8 (23) | 0.027$ |
| 90-day mortality | 41 (59) | 50 (58) | 16 (46) | 0.396 |
| Nephrotoxicity | 27/50 (54) | 23/61 (38) | 16/27 (59) | 0.100 |
| Initiation rates of RRT | 8/50 (16) | 5/61 (8) | 6/27 (22) | 0.151 |
AS, aerosolized; CRGNB, carbapenem-resistant gram-negative bacteria; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IV, intravenous; LD, loading dose; RRT, renal replacement therapy; VAP, ventilator-associated pneumonia.
, $, ‡indicate significant differences (p < 0.017) between the non-LD IV group and the LD IV group, LD IV group and AS–LD group, and the non-LD IV group and the AS–LD group, respectively.
Figure 2.
Kaplan–Meier curves of the probability of sputum culture positive (a) and survival (b) for three groups based on treatment regimen: patients received only intravenous (IV) colistin without loading (LD) (Non-LD IV); patients received only intravenous colistin with LD (LD IV); and patients received IV colistin with LD and adjunctive aerosolized (AS) colistin (AS–LD).
Table 4.
Associations between administration of AS colistin and 30-day all-cause mortality after adjustments for potential confounding factors.
| Administrations of AS colistin | Variables in the equation |
||||
|---|---|---|---|---|---|
| Coefficient | SE | p value | OR | CI 95% | |
| Crude state | −1.114 | 0.433 | 0.010 | 0.328 | 0.140–0.768 |
| Adjusted statea | |||||
| Model 1 | −1.132 | 0.439 | 0.010 | 0.322 | 0.136–0.761 |
| Model 2 | −1.121 | 0.448 | 0.012 | 0.326 | 0.135–0.785 |
| Model 3 | −1.084 | 0.479 | 0.024 | 0.338 | 0.132–0.864 |
AS, aerosolized; CI, confidence interval; OR, odds ratio; SE, standard error; SOFA, sequential organ failure assessment.
Model 1 was adjusted for age and gender. Model 2 was, in addition, adjusted for SOFA score, malignancy, and immunocompromised. Model 3, in addition, included intravenous colistin duration, intravenous loading dose, and combination with carbapenem.
Nephrotoxicity
The results of univariable and multivariable analyses with the multiple logistic regression model for the probability of nephrotoxicity are presented in Table 5. After adjusting for potential confounding factors, older age (adjusted OR 1.031, CI 95% 1.001–1.063, p = 0.044) and use of vancomycin (adjusted OR 2.623, CI 95% 1.146–6.004, p = 0.022) were independently associated with nephrotoxicity in patients treated with IV colistin for CRGNB pneumonia. In addition, after accounting for the competing risk of death with the Fine and Gray competing risk regression models, the only predictor of increased cumulative incidence of nephrotoxicity was use of vancomycin (HR 1.686, CI 95% 1.053–2.700, p = 0.030).
Table 5.
Univariable and multivariable analyses with logistic regression model for probability of nephrotoxicity.
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| OR (CI 95%) | p value | Adjusted OR (CI 95%) | p value | |
| Age, per year | 1.023 (0.995–1.051) | 0.103 | 1.031 (1.001–1.063) | 0.044 |
| Sex, male | 1.675 (0.760–3.691) | 0.201 | ||
| Vancomycin | 2.631 (1.241–5.576) | 0.012 | 2.623 (1.146–6.004) | 0.022 |
| Aerosolized colistin | 1.775 (0.756–4.168) | 0.188 | ||
| IV colistin duration, per day | 1.065 (1.004–1.129) | 0.035 | ||
| IV colistin dose, per mg/kg/day | 0.996 (0.992–1.001) | 0.116 | ||
CI, confidence interval; OR, odds ratio.
Discussion
In this study, we investigated the association of each method of colistin treatment with clinical response, microbiological response, mortality, and nephrotoxicity in 191 critically ill patients with HAP or VAP caused by CRGNB. Our results demonstrated that patients treated with adjunctive AS colistin in combination with LD had significantly lower 30-day mortality than patients treated with IV colistin alone, and adjunctive AS colistin therapy was an independent prognostic factor of 30-day mortality in a multivariate logistic regression model. In addition, microbiological eradication was more frequently achieved in patients using AS colistin as an adjunctive therapy to the LD regimen. Clinical response and nephrotoxicity did not differ according to the use of LD regimen and AS colistin administration.
In critically ill patients, optimal colistin dosing has not previously been clearly defined.26 Unfortunately, colistin has not gone through all of the modern drug development procedures and requirements.27 Therefore, there are limited data to determine its optimal use. In addition, recent pharmacokinetic studies have highlighted the inadequacies of colistin dosing based on package insert recommendations.4,5 The need for a colistin LD was initially proposed after the evaluation of the pharmacokinetics of colistimethate sodium and colistin in critically ill patients,28 and four subsequent studies have assessed this suggestion.6–9 Colistin LD was associated with improved outcomes in several clinical studies.6–8 However, studies that have evaluated the administration of colistin LD were mostly small descriptive studies,6,7 which may limit the generalizability of the findings. Only three studies were conducted to compare the clinical outcomes of colistin LD regimens and standard regimens without LD.8–10 The use of colistin LD was associated with a higher cure rate in one study by Trifi and colleagues 8 no improvement in clinical outcomes was reported in the other studies.9,10 Although the clinical cure rates of 66–67% with the use of a LD regimen reported by Elefritz and colleageus9 and Katip and colleagues10 are comparable to the 63% clinical cure rate reported by Trifi and colleagues8 a statistically significant difference in clinical outcomes could not be achieved. Therefore, it is likely that the clinical responses from the comparative groups were higher than that of the study reporting a significant difference between the two regimens. However, in this study, which included a relatively large number of patients with VAP caused by CRGNB, a colistin LD regimen did not significantly improve clinical cure or other clinical outcomes. The reason for this is not clear, but it may be because most patients in our study population had VAP, unlike the previous studies. In addition, the potential benefit of the colistin LD regimen may have been concealed by the inherent complexity of our patients and the severity of their underlying illness.
Pharmacokinetic studies, in addition, showed that IV administration of colistin may result in undetectable, to more than sufficient concentrations in lung tissue or epithelial lining fluid.12,13 In this context, AS administration of colistin is intended to maximize its transport to the target site and to limit the systemic adverse effects of antibiotics.13,14 Previous studies that demonstrated a high concentration in lung parenchyma with a low systemic concentration following administration of AS colistin and favorable clinical outcomes in cystic fibrosis appear to support the use of AS colistin.29,30 Since the early 2000s several clinical studies have evaluated AS colistin treatment for patients with pneumonia caused by CRGNB. However, individual studies did not find any mortality benefit when AS colistin was added to IV colistin in patients with HAP/VAP.31,32 Similarly, meta-analyses of unadjusted data highlighted higher clinical success and microbiological eradication rates and lower mortality with AS colistin in combination with IV colistin.17,18 In addition, a recent meta-analysis reported that a combination of AS and IV colistin could improve clinical effectiveness but not mortality.19 However, translating clinical success to survival in critically ill patients with HAP/VAP may be difficult and requires adjusting for possible confounders contributing to death, which was not carried out in these analyses. Most of the previous studies failed to show an improvement in mortality with AS colistin.19 In this study, however, adjunctive AS colistin in combination with LD was found to be independently associated with lower mortality after adjusting for potential confounding factors. Although higher dose of AS colistin (150 mg CBA every 8 h) used in this study compared with previous studies (75 mg CBA every 12 h) might be associated with lower mortality,33 therefore, our results support the benefit of the combination of AS and IV colistin in critically ill patients with HAP/VAP.
Nephrotoxicity is an important adverse effect of colistin that leads physicians to hesitate to prescribe colistin or to withhold it outright. Therefore, nephrotoxicity is another concern when determining the optimal treatment regimen. Several previous studies demonstrated that high-dose IV colistin is associated with higher incidence of nephrotoxicity.34,35 However, these studies did not adjust for potential confounding factors affecting nephrotoxicity. In our study, there was no significant difference in incidence of nephrotoxicity and need for RRT during colistin treatment between the non-LD IV group and the LD IV group. Although nephrotoxicity occurred in approximately half of the overall population, which was a higher incidence than in previous studies, LD and longer duration of IV colistin treatment were not associated with nephrotoxicity in the multivariable analysis. In addition, adjunctive AS colistin did not increase the risk of nephrotoxicity. These results are consistent with those of previous studies that showed no significant association between adjunctive AS colistin and nephrotoxicity.31,36–40 However, death is a competing risk which precludes the possibility of experiencing the nephrotoxicity. Therefore, we performed the Fine and Gray competing risk regression model. However, the only predictor of increased cumulative incidence of nephrotoxicity was use of vancomycin.
This study has several limitations. First, our study was conducted in a single center and was retrospective and observational in nature with a relatively small sample size of patients treated with AS colistin. There was no standardization regarding the timing of microbiological sampling, therefore, there is a potential risk for measurement and selection bias even though we performed a multiple logistic regression analysis to control for potential confounding factors. Second, the patients in each group received colistin treatment for different time periods according to our treatment guidelines, and the difference’s potential influence among the three groups could not be excluded. Third, we only evaluated nephrotoxicity as an indicator of safety for colistin treatment. Because we routinely performed bronchodilation before administration of AS colistin and sedation during mechanical ventilation, it was difficult to accurately assess the incidence of bronchospasm neurotoxicity, which are both associated with colistin.
Conclusion
In critically ill patients with HAP and VAP caused by CRGNB, adjunctive AS colistin in combination with IV colistin with LD was related to improved microbiological outcome and 30-day mortality without an increase in nephrotoxicity. Our results indicate that adjunctive AS colistin should be considered as a treatment option in this population. However, further prospective, randomized studies are required to validate these results and to establish the safest and most efficient strategies for colistin treatment in patients with HAP and VAP.
Supplemental Material
Supplemental material, Author_Response_1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.2 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.2 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_3_v.1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors thank Keumhee C. Carriere, and Joonghyun Ahn, at the Samsung Biomedical Research Institute for their excellent statistical support.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from Samsung Medical Center grant (OTA1602901).
Conflict of interest statement: The authors declare that there are no conflicts of interest.
ORCID iD: Kyeongman Jeon  https://orcid.org/0000-0002-4822-1772
https://orcid.org/0000-0002-4822-1772
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Junsu Choe, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
You Min Sohn, Department of Pharmaceutical Services, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Suk Hyeon Jeong, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Hyo Jung Park, Department of Pharmaceutical Services, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Soo Jin Na, Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Kyungmin Huh, Division of Infectious Diseases, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Gee Young Suh, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Kyeongman Jeon, Department of Critical Care Medicine and Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Republic of Korea.
References
- 1. Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006; 6: 589–601. [DOI] [PubMed] [Google Scholar]
- 2. Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother 1964; 23: 552–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51(Suppl. 1): S81–S87. [DOI] [PubMed] [Google Scholar]
- 4. Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55: 3284–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care 2011; 1: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalfino L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54: 1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalfino L, Puntillo F, Ondok MJ, et al. Colistin-associated acute kidney injury in severely ill patients: a step toward a better renal care? A prospective cohort study. Clin Infect Dis 2015; 61: 1771–1777. [DOI] [PubMed] [Google Scholar]
- 8. Trifi A, Abdellatif S, Daly F, et al. Efficacy and toxicity of high-dose colistin in multidrug-resistant gram-negative bacilli infections: a comparative study of a matched series. Chemotherapy 2016; 61: 190–196. [DOI] [PubMed] [Google Scholar]
- 9. Elefritz JL, Bauer KA, Jones C, et al. Efficacy and safety of a colistin loading dose, high-dose maintenance regimen in critically ill patients with multidrug-resistant gram-negative pneumonia. J Intensive Care Med 2017; 32: 487–493. [DOI] [PubMed] [Google Scholar]
- 10. Katip W, Meechoui M, Thawornwittayakom P, et al. Efficacy and safety of high loading dose of colistin in multidrug-resistant Acinetobacter baumannii: a prospective cohort study. J Intensive Care Med. Epub ahead of print 1 January 2017. DOI: 885066617725694. [DOI] [PubMed] [Google Scholar]
- 11. Vardakas KZ, Rellos K, Triarides NA, et al. Colistin loading dose: evaluation of the published pharmacokinetic and clinical data. Int J Antimicrob Agents 2016; 48: 475–484. [DOI] [PubMed] [Google Scholar]
- 12. Imberti R, Cusato M, Villani P, et al. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 2010; 138: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 13. Boisson M, Jacobs M, Gregoire N, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 2014; 58: 7331–7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Athanassa ZE, Markantonis SL, Fousteri MZ, et al. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 2012; 38: 1779–1786. [DOI] [PubMed] [Google Scholar]
- 15. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis 2016; 63: e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rello J, Sole-Lleonart C, Rouby JJ, et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect 2017; 23: 629–639. [DOI] [PubMed] [Google Scholar]
- 17. Valachis A, Samonis G, Kofteridis DP. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit Care Med 2015; 43: 527–533. [DOI] [PubMed] [Google Scholar]
- 18. Liu D, Zhang J, Liu HX, et al. Intravenous combined with aerosolised polymyxin versus intravenous polymyxin alone in the treatment of pneumonia caused by multidrug-resistant pathogens: a systematic review and meta-analysis. Int J Antimicrob Agents 2015; 46: 603–609. [DOI] [PubMed] [Google Scholar]
- 19. Vardakas KZ, Mavroudis AD, Georgiou M, et al. Intravenous plus inhaled versus intravenous colistin monotherapy for lower respiratory tract infections: a systematic review and meta-analysis. J Infect 2018; 76: 321–327. [DOI] [PubMed] [Google Scholar]
- 20. Nation RL, Li J, Cars O, et al. Consistent global approach on reporting of colistin doses to promote safe and effective use. Clin Infect Dis 2014; 58: 139–141. [DOI] [PubMed] [Google Scholar]
- 21. Jeong BH, Jeon EJ, Yoo H, et al. Comparison of severe healthcare-associated pneumonia with severe community-acquired pneumonia. Lung 2014; 192: 313–320. [DOI] [PubMed] [Google Scholar]
- 22. Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 2009; 35: 1692–1702. [DOI] [PubMed] [Google Scholar]
- 23. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004; 8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989; 129: 125–137. [DOI] [PubMed] [Google Scholar]
- 25. Barnett A, Graves N. Competing risks models and time-dependent covariates. Crit Care 2008; 12: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy 2015; 35: 11–16. [DOI] [PubMed] [Google Scholar]
- 27. Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis 2009; 22: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009; 53: 3430–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yapa SWS, Li J, Patel K, et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 2014; 58: 2570–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beringer P. The clinical use of colistin in patients with cystic fibrosis. Curr Opin Pulm Med 2001; 7: 434–440. [DOI] [PubMed] [Google Scholar]
- 31. Kofteridis DP, Alexopoulou C, Valachis A, et al. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: a matched case-control study. Clin Infect Dis 2010; 51: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 32. Demirdal T, Sari US, Nemli SA. Is inhaled colistin beneficial in ventilator associated pneumonia or nosocomial pneumonia caused by Acinetobacter baumannii? Ann Clin Microbiol Antimicrob 2016; 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jang JY, Kwon HY, Choi EH, et al. Efficacy and toxicity of high-dose nebulized colistin for critically ill surgical patients with ventilator-associated pneumonia caused by multidrug-resistant Acinetobacter baumannii. J Crit Care 2017; 40: 251–256. [DOI] [PubMed] [Google Scholar]
- 34. Benattar YD, Omar M, Zusman O, et al. The effectiveness and safety of high-dose colistin: prospective cohort study. Clin Infect Dis 2016; 63: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 35. Vicari G, Bauer SR, Neuner EA, et al. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant gram-negative bacteremia. Clin Infect Dis 2013; 56: 398–404. [DOI] [PubMed] [Google Scholar]
- 36. Rattanaumpawan P, Lorsutthitham J, Ungprasert P, et al. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother 2010; 65: 2645–2649. [DOI] [PubMed] [Google Scholar]
- 37. Korbila IP, Michalopoulos A, Rafailidis PI, et al. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin Microbiol Infect 2010; 16: 1230–1236. [DOI] [PubMed] [Google Scholar]
- 38. Tumbarello M, De Pascale G, Trecarichi EM, et al. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest 2013; 144: 1768–1775. [DOI] [PubMed] [Google Scholar]
- 39. Kalin G, Alp E, Coskun R, et al. Use of high-dose IV and aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia: do we really need this treatment? J Infect Chemother 2012; 18: 872–877. [DOI] [PubMed] [Google Scholar]
- 40. Korkmaz Ekren P, Toreyin N, Sayiner A, et al. The role of aerolized colistin in the treatment of hospital-acquired pneumonia: experience of multicenter from Turkey. Crit Care Med 2016; 44: e304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria by Junsu Choe, You Min Sohn, Suk Hyeon Jeong, Hyo Jung Park, Soo Jin Na, Kyungmin Huh, Gee Young Suh and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease