Abstract
Spastic tetraplegia, thin corpus callosum, and progressive microcephaly is a recently described very rare autosomal recessive neurodevelopmental disorder. This disease was first described in 2015 in several families from the Ashkenazi Jewish ancestry with a founder mutation in SLC1A4 (p.E256K) as the underlying genetic cause. SLC1A4 gene encodes for the amino acid transporter ASCT1 that is necessary for serine cellular transport to neurons. We clinically evaluated 2 Pakistani siblings with severe global developmental delay, progressive microcephaly, and seizure disorder. We performed exome sequencing, Sanger sequencing, and segregation analysis to identify the genetic cause of the phenotype followed by in silico analysis to evaluate the pathogenicity of the identified mutation. We identified a novel homozygous variant (c.573T>G) in both patients. The mutation is predicted to cause nonsense mutation (p.Y191*) in the ASCT1 protein. Here, we report the fifth disease causing mutation in SLC1A4 gene and review all previously reported cases.
Keywords: developmental delay, epileptic encephalopathy, infantile spasms, next-generation sequencing, spasticity
Spastic tetraplegia, thin corpus callosum, and progressive microcephaly is a very rare, recently described, autosomal recessive neurodevelopmental disorder (MIM: 616657). It is characterized by severe early global developmental delay with progressive microcephaly. It has been shown to be caused by homozygous loss-of-function mutations in SLC1A4 gene, which encodes the neutral amino acid transporter (ASCT1). Most patients described so far were unable to achieve independent walking or speaking.1-4 The phenotype itself was first described by Damseh and colleagues in 2015 in 11 patients from unrelated families descending from the Ashkenazi Jewish population. All patients presented with an inherited form of severe intellectual disabilities of unknown genetic cause. Using whole-exome sequencing, they were able to detect a founder mutation p.E256K in ASCT1 in 10 of the 11 patients and the R457W and p.L314Hfs*42 mutations in the 11th patient. Segregation analysis in these families confirmed the autosomal recessive mode of inheritance. The founder mutation was found to be highly represented in the Ashkenazi Jewish population with a carrier rate of 0.7%.1 This mutation was also detected in 2 families described by Heimer et al2 and Srour et al.3 Both families were of the Ashkenazi Jewish ancestry, and the patients presented with a similar phenotype.2,3 More recently, Conroy and colleagues4 reported a novel SLC1A4 mutation in an Irish child presenting with infantile spasms, ruling out the possibility that SPACCM is population-specific disease. Here, we describe another novel nonsense mutation in a Pakistani family with 2 siblings presenting with similar phenotype.
Patients and Methods
Patients’ Consents—Ethical Clearance
This study has been approved by Al-Ain Medical Human Research Ethics Committee according to the national regulations (Protocol number 10/09). The parents have provided an informed written consent prior to research and publication.
We ascertained 1 Pakistani consanguineous family with 2 affected children, both presented early with progressive microcephaly, global developmental delay, and spasticity. No other remarkable family history was reported. Clinical data were obtained by thorough history and clinical examination. Growth parameters are standardized using the National Center for Health Services/World Health Organization global child growth reference database. Peripheral blood samples were collected from all family members in EDTA tubes.
DNA Extraction and Whole-Exome Sequencing
The affected siblings and their parents’ DNA were extracted from peripheral blood cells using Flexigene DNA extraction kit (Qiagen Gmbh, Germany) according to the manufacturer’s protocol. The whole-exome sequencing was carried out by CENTOGENE AG laboratory in Rostock, Germany (www.centogene.com). The sample has been processed on the Ion Proton Platform (Life Technologies, Renfrew, United Kingdom). Approximately 33 Mb of coding exons were converted as described by Consensus Coding Sequences. Highly multiplied primers pools were used to construct the amplicon library using polymerase chain reaction–based targeted amplification. Following the base calling and primary filtering of low-quality reads, standard bioinformatics pipeline was implemented to annotate detected variants and to filter out probable artifacts. In addition, molecular testing was performed on the affected sister and their parents with Sanger DNA sequencing.
In Silico Variant Analysis
The variants were compared to the controls in dbSNP build 147 (https://www.ncbi.nlm.nih.gov/projects/SNP/), the 1000 genome project (http://1000genomes.org), the Arab Genotype Frequency Database (http://galaxc.sengenics.com), and the Exome Aggregation Consortium database (http://exac.broadinstitute.org). The functional impact of the variants identified was assessed with Alamut visual v.2.9.0 interactive biosoftware, which uses many in silico pathogenicity prediction tools such as mutation taster, Sorting Intolerant From Tolerant, and Align GVGD. The conservation of the variant reference allele was assessed with the UCSC multiple sequence alignment for 100 vertebrates.5 The protein domains affected were assessed using the reference protein structure in UniProt.6 Modeling of the wild-type and mutated hypothetical proteins was done using SWISS-MODEL (www.swissmodel.expasy.org).7
Results and Discussion
We have ascertained 1 Pakistani consanguineous family referred to the genetics clinic in Tawam hospital with 2 affected children, both presented early with progressive microcephaly, global developmental delay, and spasticity (Figure 1A). No other remarkable family history was reported. The first child is currently 7 years old and is born by normal vaginal delivery with a pregnancy history complicated by oligohydramnios and decreased fetal movements. His birth weight was 2800 g. Head circumference was 31.1 cm (2.4 standard deviation [SD]). The early neonatal period passed uneventfully, and at 1 month of age the parents noted that he developed increased irritability and excessive crying. At 6 months of age he developed seizures that were generalized tonic with uprolling of eyes lasting 2 to 3 minutes occurring 2 to 3 times per day. He was treated with oxcarbazepine initially, then sodium valporate was added and he showed reasonable response. Seizure frequency improved to once every 2 to 3 months. Abnormal repeated hand movements were noticed at age 1.5 years. These included pulling hair, beating himself, and washing hands movement, all improved with time. He also has continuous drooling of saliva which responded to scopolamine 1.5 mg. He still has chewing and swallowing difficulties and has a history of aspiration pneumonia. Examination at 6 years of age showed a head circumference of 35.5 cm (−2.7 SD) with some dysmorphic features including hypertelorism, depressed nasal bridge, synophrys, and prominent ears. Consent for photos publishing was declined. He has spasticity of all limbs with exaggerated reflexes. There was clonus in the lower limbs with positive Babinski reflex. He was delayed in all aspects of development. He was able to smile to stimuli, had some head control, and was unable to roll over, sit, or stand. He coos and babbles, but he does not have a single word and has no bladder control. Hearing and vision tests were normal. Electroencephalography (EEG) showed bilateral hemispheric dysfunction with asymmetry, with independent focal epileptic discharges in the left hemisphere. Magnetic resonance imaging (MRI) of the brain revealed moderate-to-severe bilateral cerebral atrophy with marked atrophy of corpus callosum (Figure 1B). Several investigations were normal including very-long-chain fatty acid and phytanic acid, carnitine and acylcarnitine, ammonia, lactate, amino acids profile and urine organic acid, transferrin isoelectric focusing, microarray, and microcephaly genetic panel screening.
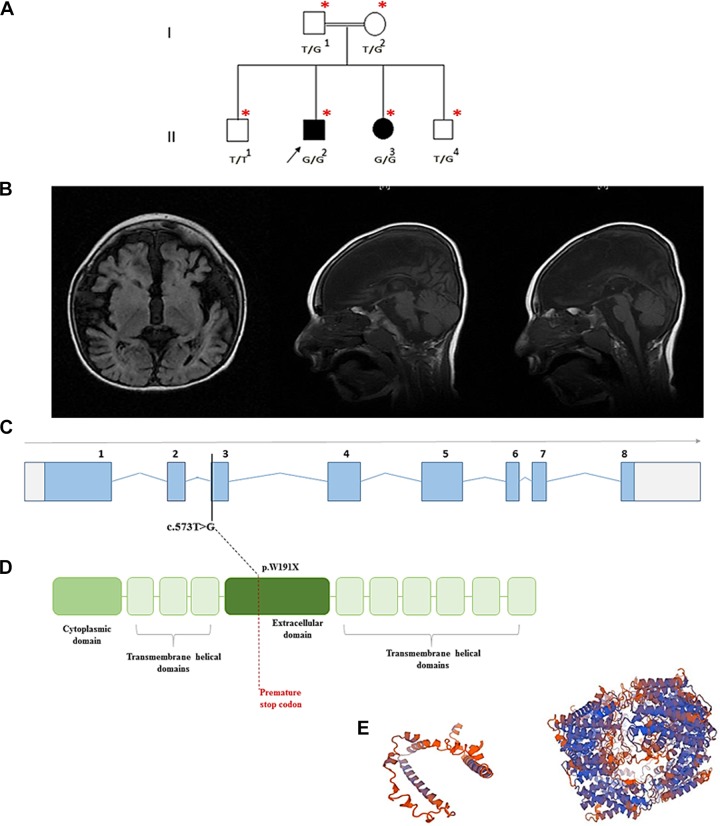
Figure 1.
A, The family pedigree of the affected siblings. B, Magnetic resonance imaging (MRI) findings for the affected male, at 4 years of age. Note the absent corpus callosum, the hypomyelination of the cerebral hemispheres and brain atrophy. C, The SLC1A4 transcript demonstrating the mutation site. D, The protein topological structure and site of the premature stop codon. E, The predicted mutated protein 3D structure modeled by SWISSMODEL/ExPASy prediction tool to the left, compared to the modeled wild-type protein (right).
His younger sister, 3 years old, was born by normal vaginal delivery with uneventful antenatal and postnatal period. Head circumferenc was 31 cm (z score = −2.4SD) at birth. She was evaluated by neurology at 7 months of age due to abnormal brief and frequent movements up to 10 times per day in form of clonic movements of her upper and lower limbs, turning her head and body to the right side followed by sleeping for seconds. Electroencephalography showed persistent focal slowing in the right anterior to mid-temporal region which is suggestive of an underlying structural abnormality. She was started on Trileptal. At 1 year of age, the seizures increased in frequency and included focal seizure, and her EEG was suggestive of epileptic encephalopathy. She started to have spasms 2 to 3 times per day at 3 years of age. Electroencephalography did not show hypsarhythmia, and she was started on Vigabatrine and the response was reasonable. She has global developmental delay, and at age 3 years, she controls her head but is unable to roll or sit. She babbles with no single words, she does not fix or follow with her eyes, or smile. Neurological examination revealed cogwheel rigidity in all limbs with exaggerated reflexes and positive Babinski reflex. Clonus was noticed in lower limbs.
We performed whole-exome sequencing on the index case and a homozygous variant in SLC1A4 gene NM_003038.4: c.573T>G, Chr2(GRCh37): g.65231089T>G, p.(Tyr191*) was detected. The variant c.573T>G is a nonsense variant that leads to the substitution of the tyrosine in position 191 with a premature stop codon (Figure 1C and D). This variant that is located on exon 3 was also detected by Sanger sequencing in a homozygous state in the affected sister and heterozygous in both parents and a younger healthy sibling. It has not been reported in the Exome Aggregation Consortium, 1000 genome, Ensembl, or the dbSNP databases. In silico prediction reported this mutation as Class 3–Unknown pathogenicity in Alamut visual report. It was found in MutationTaster (www.mutationtastr.org) that the disease affected the stability of the messenger RNA, leading to nonsense-mediated decay. It is predicted that this gene will not be translated, yet, even if the variant did not affect the messenger RNA stability and somehow was translated, the hypothetical mutant protein would be dysfunctional, as 6 of the 9 transmembrane helical domains of ASCT1 protein encoded by sequence downstream to the mutation would accordingly be lost. In addition, the extracellular topological domain (AA140-AA216) is partially lost in addition to the loss of Na+ binding sites Na1 and Na3 at AA380 and AA467 (Figure 1D and E). Added to that, 6 of the 9 transmembrane helical domains would also be lost. Also, 5 of the 6 posttranslational modifications sites (glycosylation at Asn201 and Asn206 and phosphorylation at Ser507, Ser527, and Ser530) would be lost.
ASCT1 is an Na-dependent neutral amino acid transporter for serine, alanine, cysteine, and threonine coded by the solute carrier family 1 member 4 gene (SLC1A4).8 l-Serine is a nonessential amino acid that can be derived from diet, glycine, and protein degradation. It can also be biosynthesized de novo from 3-phosphoglycerate, an intermediate in the glycolytic pathway, to be utilized for the synthesis of proteins, nucleotides, heme, and other amino acids.8 Despite being a nonessential amino acid, its de novo synthesis in the brain is essential due to its critical roles in neurons and its poor permeability through the blood–brain barrier.9
l-Serine is particularly important in the brain, as it is a precursor for the synthesis of l-cysteine, phosphatidyl-l-serine, sphingolipids, and neuromodulators like d-serine, an activator of the N-methyl-d-aspartate–selective glutamate receptor.10 Synthesis of l-serine in the brain is confined to the astrocytes and is transported to neurons by the amino acid transporter family 1 (ASCT1). Therefore, the expression and function of ASCT1 in neurons is essential for early neuronal development and function.
Serine supplementation therapies have been tried for several serine biosynthesis defects.11 However, the benefit of serine administration is doubtful in serine transportation defects given that the neurological defects occurred early during intrauterine life. It is worth studying in the future whether high dosage administration of serine, enough to cross the blood–brain barrier for the neuronal uptake, would be safe and could limit the progression of the disease.
Three loss-of-function mutations in ASCT1 (p.E256K, p.L314Hfs*42, and p.R457 W) have been reported in families from Ashkenazi Jewish population to cause spastic tetraplegia, thin corpus callosum, and progressive microcephaly. Infants present early with spastic tetraplegia, microcephaly, and global developmental delay.1-3 Brain MRI of the affected children show thin corpus callosum, progressive demyelination, and white matter abnormalities. It has been noted that the carrier rate for SLC1A4 mutations among the Ashkenazi Jewish population is 0.7%, and it was recommended to be added to the carrier-screening panel in this community.1 Conroy and colleagues have reported the first European patient with spastic tetraplegia, thin corpus callosum, and progressive microcephaly, an Irish child with infantile spasms in a family carrying the nonsense mutation (p.W453X). A review of the clinical presentation of all previously described patients is summarized in Table 1. As illustrated in Table 1, besides reporting the dysmorphic features of the patients with SPACCM and grand mal seizures, with generalized tonic–clonic convulsions with eye-rolling and followed by loss of consciousness, the location of nonsense mutation reported here lies in the SLC1A4 transcript upstream to all the previously reported pathogenic mutations, and in addition to the phenotypic overlapping, we concluded that this indeed is a disease-causing mutation. It is important to note that due to the high consanguinity in the family history, the coexistence of multiple recessive variants is possible. We tried to use the patient sample available (peripheral blood) to test the nonsense-mediated decay hypothesis, but the transcript was undetectable on the patients’ and control complementary DNA. It would be interesting to test the functional impact of this mutation on the transcript fate.
Table 1.
The Clinical Profile of All Reported Patients With SLC1A4 Mutations.
| Patient/Variant Reports | Damseh et al1 | Damseh et al1 | Damseh et al (9 Patients)1 | Srour et al3 | Srour et al3 | Heimer et al2 | Heimer et al2 | Conroy et al4 | Pironti et al | Current | Current |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 3.5 years | 4.5 years | 3-15 years | 11 years | 4 years | 6 years | 4.5 years | 3 years | 7 years | 7 years | 3 years |
| Gender | Female | Female | 4 males/5 females | Female | Male | Female | Female | Male | Male | Male | Female |
| Ethnic group | AJ | AJ | AJ | AJ | AJ | AJ | AJ-Iraqi | Irish | Italian | Pakistani | Pakistani |
| Pregnancy events | NA | NA | NA | − | − | − | − | NA | − | oligohydramnios | − |
| Dysmorphic features | − | − | − | − | − | − | − | NA | Large nose root, low-implanted, and wide auricles | hypertelorism, synophrys, depressed nasal bridge, and prominent ears | hypertelorism, synophrys, depressed nasal bridge, and prominent ears |
| Microcephaly | Acquired | Primary | 4 primary, 4 acquired, 1 NA | Acquired | Acquired | Acquired | Acquired | Primary | Acquired | Primary | Primary |
| Age of epilepsy onset | − | NA | NA | − | − | 1 years | 11 months | 5 months | 4 months | 6 months | 9 months |
| Type of seizure | − | Infantile spasms | 2 starring episodes, 1 infantile spasms, rest no seizures | − | − | NA | Myoclonic | Focal motor and dyscognitive seizures | Tonic extensor spasms and left eye derivation | Generalized tonic–clonic seizures with eye uprolling and loss of consciousness | Generalized tonic–clonic seizures with eye uprolling and loss of consciousness |
| Deep reflexes | Increased | Increased | Increased | Increased | Increased | Increased | Increased | Increased | Increased | Increased | Increased |
| Tone | Hypotonic | Hypotonic | 5 hypotonic, 4 hypertonic, 1 normotonic | Peripheral hypertonia | Mild hypertonia | Peripheral hypertonia | Hypertonic | Hypotonic | Central hypotonia, peripheral hypertonia | Hypertonic | Central hypotonia, peripheral hypertonia |
| Clonus | − | − | − | + | − | − | − | − | NA | + | + |
| Swallowing difficulties | + | NA | NA | − | − | − | − | + | + | + | + |
| Bladder control | NA | NA | NA | NA | NA | NA | NA | NA | NA | − | − |
| Motor delay | None | Can sit, not standing | All delayed, variable degrees | Can crawl, stand with support, cannot walk | Can stand, cannot cruise | Crawls on hands and feet, walk with assistance | Can crawl on tummy, no sitting | Can roll, no sitting | No head control | Some head control, no rolling, sitting, or standing | Head control, no rolling, sitting, or standing |
| Speech delay | Babbles, no words | Nonverbal | 5 nonverbal, 3 babble, 2 speak few word phrases | Nonverbal | Nonverbal | Babbles, no words | No babbling | No babbling | Nonverbal | Babble, no single words | Babble, no single word |
| Abnormal movements Hair pulling Stereotypies Irritability Hyperactivity Sleep disorder |
− | − | − | NA | NA |
+ + + + |
+ + + + |
NA | NA |
+ + + NA |
+ + + NA |
| MRI signs | Brain atrophy and hypomyelination | Brain atrophy | Thin CC, hypomyelination, brain atrophy | Thin CC, nonspecific white matter abnormalities | Mildly thinned CC, mild myelination delay | Mild cerebral atrophy, thin CC | Thin CC, delayed myelination, cerebral atrophy | Hypomyelination, thin CC | Hypoplastic CC, enlarged anterior commissure, cerebral, and brain stem atrophy | Bilateral cerebral atrophy, atrophied CC | NA |
| Variant | p.Arg457Trp | p.Glu256Lys and p.Leu315Hisfs*42 | p.Glu256Lys | p.Glu256Lys | p.Glu256Lys | p.Glu256Lys | p.Glu256Lys and p.Leu315Hisfs*42 | p.Trp453* | p.Gly381Arg | p.Tyr191* | p.Tyr191* |
Abbreviations: AJ, Ashkenazi Jews; CC, corpus callosum; MRI, magnetic resonance imaging; NA, not available.
Based on our findings and the population ancestry analysis results of the Exome Aggregation Consortium database,4 we suggest that ASCT1 deficiency should be investigated regardless of ethnicity in any child with unexplained early-onset epileptic encephalopathy or global developmental delay.
Acknowledgments
The authors would like to sincerely thank the family for taking part in this study.
Authors’ Note: All coauthors listed hereby declare that they have no affiliations with /or involvement with any organization or entity with any financial interest of any kind. Dr Hanadi Abdelrahman, Dr Aisha AL Shamsi, Mrs Anne John, Prof Bassam Ali, and Prof Lihadh Al-Gazali.
Authors contribution: HAA and AA-S contributed to conception and design. HAA contributed to acquisition, analysis, and interpretation. HAA drafted the manuscript. AA-S and AJ contributed to acquisition. BRA and LA-G critically revised manuscript and gave final approval. All authors agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project is funded by the UAEU-UPAR grant (31M241).
Ethical Approval: HAA and AA-S obtained a written informed consent from the parents for the patients for research and publication.
References
- 1. Damseh N, Simonin A, Jalas C, et al. Mutations in SLC1A4, encoding the brain serine transporter, are associated with developmental delay, microcephaly and hypomyelination. J Med Genet. 2015;52(8):541–547. doi:10.1136/jmedgenet-2015-103104. [DOI] [PubMed] [Google Scholar]
- 2. Heimer G, Marek-Yagel D, Eyal E, et al. SLC1A4 mutations cause a novel disorder of intellectual disability, progressive microcephaly, spasticity and thin corpus callosum. Clin Genet. 2015;88(4):327–335. doi:10.1111/cge.12637. [DOI] [PubMed] [Google Scholar]
- 3. Srour M, Hamdan FF, Gan-Or Z, et al. A homozygous mutation in SLC1A4 in siblings with severe intellectual disability and microcephaly. Clin Genet. 2015;88:E1–E4. doi:10.1111/cge.12605. [DOI] [PubMed] [Google Scholar]
- 4. Conroy J, Allen NM, Gorman K, et al. Novel European SLC1A4 variant: infantile spasms and population ancestry analysis. J Hum Genet. 2016;61(8):761–764. doi:10.1038/jhg.2016.44. [DOI] [PubMed] [Google Scholar]
- 5. Kent WJ, Sugnet CW, Furey TS, Roskin KM. The human genome browser at UCSC W. J Med Chem. 1976;19(10):1228–1231. doi:10.1101/gr.229102.186604 [Google Scholar]
- 6. Wasmuth EV, Lima CD. UniProt: the universal protein knowledgebase. Nucleic Acid Res. 2016;45:1–12. doi:10.1093/nar/gkw1152.27899559 [Google Scholar]
- 7. Biasini M, Bienert S, Waterhouse A, et al. ‘Swiss-model: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42(W1):252–258. doi:10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hofmann K, Duker M, Fink T, Lichter P, Stoffel W. Human neutral amino acid transporter ASCT1: structure of the gene (SLC1A4) and localization to chromosome 2p13-p15. Genomics. 1994;24(1):20–26. doi:10.1006/geno.1994.1577. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto T, Nishizaki I, Nukada T, et al. Functional identification of ASCT1 neutral amino acid transporter as the predominant system for the uptake of L-serine in rat neurons in primary culture. Neurosci Res. 2004;49(1):101–111. doi:10.1016/j.neures.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 10. Foster AC, Farnsworth J, Lind GE, et al. D-serine is a substrate for neutral amino acid transporters ASCT1/SLC1A4 and ASCT2/SLC1A5, and is transported by both subtypes in rat hippocampal astrocyte cultures. PLoS One. 2016;11(6):1–18. doi:10.1371/journal.pone.0156551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El-Hattab AW. Serine biosynthesis and transport defects. Mol Genet Metab. 2016;118(3):153–159. doi:10.1016/j.ymgme.2016.04.010. [DOI] [PubMed] [Google Scholar]