Abstract
The aim of this study was to evaluate blood transfusion services (BTS) at the main blood banks (BBs) of the Sana’a Capital. The 4 main BBs at Sana’a Capital were evaluated according to the safe World Health Organization BTS standards. Qualitative and quantitative data were collected using semi-structured questionnaires covering 6 components: activities, quality assurance system (QAS) and training, donation, grouping and compatibility testing, components, and screening for transfusion-transmitted infections (TTIs). An overall mean percent score for BTS was calculated where <60% is considered unsatisfactory, 60% to 79.9% satisfactory, and ≥80% highly satisfactory. The 4 BBs screen for HIV, hepatitis B, and hepatitis C and perform all functions except therapeutic transfusion. While 75% of the staff in BBs had received training in biosafety and half of the staff had received training in Standard Operating Procedures (SOPs), no QAS in place at any of the 4 BBs. The 4 BBs depended on 71% of their transfusions on family donors. Two BBs do not perform reverse grouping and do not keep patient/donor samples for the required minimum 5 days. Only one BB achieved an overall high satisfactory score and one achieved a satisfactory score. Findings highlight the increasing challenges facing BTS in Sana’a Capital especially the lack of therapeutic transfusion, poor QAS, and predominant dependence on the family donors. Therefore, there is a need to develop and train staff on QAS and to increase awareness among public on importance of voluntary donation. A wider scale evaluation of BTS in Sana’a is recommended.
Keywords: blood transfusion services, evaluation, quality, field epidemiology training program, Yemen
What do we already know about this topic?
Although blood transfusion services (BTS) can save millions of lives each year, poor BTS can lead to adverse consequences including acute and delayed complications.
How does your research contribute to the field?
This is one of few studies that identify shortcomings in procedures and policies of BTS to improve them.
What are your research’s implications toward theory, practice, or policy?
The findings will be used to ensure BTS safety through enhancing quality system and raise awareness regarding the importance of voluntary blood donation.
Introduction
Blood transfusion is an essential component of modern health care that saves millions of lives each year. According to the World Health Organization (WHO) estimate, more than 5 million people die from violence and injury and 536 000 women die during pregnancy or childbirth each year, with most deaths that could be saved through blood transfusion.1
Every country needs to meet its requirements for blood and blood products and ensure that blood supplies are free from HIV, hepatitis viruses and other life-threatening infections that can be transmitted through unsafe transfusion. Furthermore, blood safety is an integral part of the WHO HIV/AIDS plan to accelerate the prevention of HIV infection and achieve its health-related goals.2,3 According to WHO reports, the prevalence of hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV infections among blood donors in different parts of the world varies from 0.008% to 6.08% for HBV, 0.004% to 1.96% for HCV, and 0.0004% to 2.0% for HIV.4
About 108 million blood donations are collected worldwide, and more than half of these are collected in high-income countries.4 A stable base of regular, voluntary, unpaid blood donors can assure an adequate and reliable supply of safe blood.4 Voluntary donors have been reported to be the safest group of donors because they usually have better health-seeking behavior than the replacement blood donors.5 The World Health Assembly Resolution urges all member states to develop national blood systems based on voluntary unpaid donation and work toward the goal of self-sufficiency.4
A primary goal of blood transfusion services (BTS) is to promote high standards of quality in all aspects of production, patient care, and service. A quality system for BTS includes the organizational structure, responsibilities, policies, processes, procedures, and resources established by the executive management to achieve quality.6
In Yemen, BTS are provided mainly by the National Blood Transfusion and Research Center (NBTRC) and the National Center of Public Health Laboratories (NCPHL) as well as their governorate branches. They are also provided by public and private hospitals’ blood banks.
However, BTS in Yemen face many problems due to the current war. Shortage of blood bags, reagents, and fuel necessary to run the generators that support work of the centers are the main challenges. Furthermore, weak quality control and lack of standard operating procedures (SOPs) are a major drawback.7 The recent increasing pressure on BTS due to war and upsurge of casualties and injuries may increase the possibility of transfusion-transmitted infections (TTIs) among recipients through using low sensitivity and specificity reagents and rapid method tests due to conflict, siege, and low resources. Studies that were conducted in Sana’a City, Aden, and Hodeidah revealed that HBV, HCV, and HIV are a major issue among blood donors. The prevalence of HBV, HCV, HIV, and syphilis according to those studies varies from 2.1% to 5.1%, 0.79% to 3%, 0.34% to 0.39 and 0.34% to 0.75%, respectively.8–11 More than 78 336 suspected malaria cases and 31 791 laboratory-confirmed cases were reported in 2015.12
Therefore, evaluating BTS is the corner stone to ensure its proper delivery and that related problems are monitored efficiently and effectively. The main objective of this study is to evaluate BTS in the 4 main blood banks at Sana’a Capital regarding activities, quality system and training, blood collection, screening, and components preparation.
Method
Study Design and Data Collection
This is a descriptive study, where the 4 main blood banks providing BTS in Sana’a Capital (Table 1) were evaluated based on WHO standards for safe BTS in the period from October 2016 to December 2016.13 Pertinent literature and blood banks relevant documents were reviewed. Qualitative and quantitative data were collected through in-depth interviews with directors, officers, and supervisors of blood bank departments using semistructured questionnaires. The questionnaire comprised of 6 domains including blood transfusion activities, quality system and training, blood donors and blood collection, blood group serology and compatibility testing, blood components, and screening for TTIs. For example, 5 questions were used for the evaluation of blood transfusion activities such as availability of donation area, conducting blood grouping and cross matching tests, therapeutic transfusion services availability, preparation of blood components (eg, packed red blood cells [RBCs]) and screening for TTIs using a commercially available electro-chemi-luminescence immunoassay method.14 In addition, the samples were screened for syphilis and malaria antibodies by rapid immune-chromatographic assay method.15,16
Table 1.
The Main Centers and Blood Banks of Blood Transfusion Services in Sana’a Capital, Yemen, 2016.
| Centers and blood banks | Description |
|---|---|
| NBTRC | The NBTRC, established in 2005, has the legal entity responsible for BTS safety in the country including collecting, screening, storing and ensuring safety, and adequacy of blood. |
| NCPHL | The NCPHL was established in 1977 as referential, research, educational, diagnostic laboratory and provides of BTS. |
| KUBB | The KUBB is a 250-bed hospital, and the blood bank section in hospital is responsible for providing BTS to the hospital’s admissions. |
| USTBB | The USTBB is affiliated with the biggest private hospital in Yemen. The hospital has 200 beds and a comprehensive clinical service. The blood bank provides blood for patients admitted to the hospital. |
Note. NBTRC = National Blood Transfusion and Research Center; BTS = blood transfusion services; NCPHL = National Center of Public Health Laboratories; KUBB = Kuwait University Blood Bank; USTBB = University of Sciences and Technology Blood Bank.
Permission was secured from NBTRC as well as from each blood bank authority. The names of the respondent were anonymous.
Data Coding, Entry, and Analysis
Each question in the questionnaire was scored as 1 for yes and 0 for no. The mean score for each domain was calculated by dividing the number of correct answers by the total number of questions measuring that domain. Similarly, the overall mean percent for each blood bank was calculated by summing up all individuals domains mean percent and dividing by the total number of domains. The overall mean percent score was categorized as follows: <60% (unsatisfactory), 60% to 79.9% (satisfactory), and ≥80% (highly satisfactory). Data were analyzed using Epi Info (version 7.2) developed by Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia (US) and were presented as percentages and presented using figures.
Results
Description of Blood Banks
Four BTS centers and blood banks in Sana’a city were assessed during the period from October 2016 to September 2016. The centers and blood banks perform different activities including blood collection, screening, grouping, and cross matching, but none performs therapeutic transfusion. The NCPHL also does not perform blood components preparation. Only NBTRC has 3 shifts, and the other blood banks have 2 shifts. The median number of blood bank staff responsible for blood collection and screening was 12 (range: 4-56), where it was the highest in NBTRC (n = 56) and the lowest in the University of Sciences and Technology Blood Bank (USTBB; n=4). Male-to-female ratio for technical staff was 3:1 (Table 2).
Table 2.
Activities Staff Frequently and Number of Shifts in Centers Blood Bank, Sana’a Capital, Yemen, 2016.
| Indicator | Public blood banks |
Private blood bank |
||
|---|---|---|---|---|
| National Blood Transfusion and Research Center | National Center of Public Health Laboratories | Kuwait University Blood Bank | University of Sciences and Technology Blood Bank | |
| Activities of blood banks | ||||
| Collection of blood donors | Yes | Yes | Yes | Yes |
| Screening of blood donors | Yes | Yes | Yes | Yes |
| Blood grouping and cross-matching | Yes | Yes | Yes | Yes |
| Component preparation | Yes | No | Yes | Yes |
| Therapeutic transfusion | No | No | No | No |
| Staff numbers | ||||
| Physician | 1 | 1 | 0 | 0 |
| Laboratory technician | 12 | 6 | 2 | 0 |
| Laboratory specialist | 43 | 7 | 6 | 4 |
| Total number of staff | 56 | 15 | 8 | 4 |
| Staff gender | ||||
| Male | 46 | 7 | 4 | 4 |
| Female | 10 | 8 | 4 | 0 |
| Number of shifts | 3 | 2 | 2 | 2 |
Quality System and Training
The different quality system indicators for each blood bank were assessed. Organizational structure was documented in 3 blood banks. The 4 blood banks had no quality assurance system (QAS). While technical staff received training on biosafety in 3 blood banks, only they received training on SOPs in 2 blood banks. All staff in all blood banks wear gloves and coats. Only one of the blood banks had SOPs for waste management and disposal. Two blood banks have a system for management of biohazards waste and infectious blood (Table 3).
Table 3.
Quality System Status and Training Indicators in the 4 Blood Banks, Sana’a Capital, Yemen, 2016.
| Indictors | Public blood banks |
Private blood bank |
||
|---|---|---|---|---|
| National Blood Transfusion and Research Center | National Center of Public Health Laboratories | Kuwait University Blood Bank | University of Sciences and Technology Blood Bank | |
| Documentation of the organizational structure | YES | YES | NO | YES |
| Management is technically separated | YES | YES | NO | NO |
| Received training on biosafety | YES | YES | NO | YES |
| Received training on SOP | YES | YES | NO | NO |
| Quality assurance system | NO | NO | NO | NO |
| Staff are wearing coats and gloves | YES | YES | YES | YES |
| Have SOPs for waste management and disposal | NO | YES | NO | NO |
| Management system for biohazards waste and infectious blood | Incineration | Not known | General waste | Incineration |
Note. SOP = standard operating procedures.
Donation area
The 4 blood banks collected 20 660 blood units during 2015. Most collections were from men (98%) and replacement family donors (71%). The percentage of volunteer donor is the highest in NBTRC and the lowest in the Kuwait University Blood Bank (KUBB; 45% vs 2%). Staff in blood banks, except KUBB, use a unique ID number to identify the donor and fill the blood donor questionnaire. All blood banks had donor records that are maintained for 5 years. Three blood banks use only 70% alcohol for disinfecting vein puncture site, while NBTRC use both alcohol and iodine. After phlebotomy process, only NBTRC labels the donor blood bags with date, number, and time of donation, but other blood banks label them with date and number only. Mobile blood-collection operations were conducted only by NBTRC (Figure 1).
Figure 1.
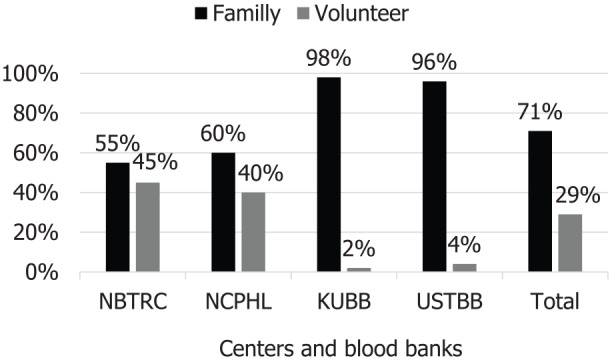
Type of blood donors in 2015, Sana’a capital, Yemen (n = 20 660).
Note. NBTRC = National Blood Transfusion and Research Center; NCPHL = National Center of Public Health Laboratories; KUBB = Kuwait University Blood Bank; USTBB = University of Sciences and Technology Blood Bank.
Blood Group Serology and Compatibility Testing
All blood banks use standardized procedures and have SOPs to perform compatibility tests. The most frequently used technique for compatibility tests was tube method. None of the blood banks performs antibodies screening test or gel method. Two blood banks do not perform reverse grouping and do not keep patient/donor samples for the required minimum 5 days. Beside the regular indications for blood transfusion (eg, hemolytic anemia, leukemia, and renal failure), there is an increase in emergency blood transfusion for surgical purposes such as injuries, amputations, and shock due to the ongoing conflict (Table 4).
Table 4.
Frequently Used Techniques and Methods of Compatibility Tests in Blood Banks, Sana’a Capital, Yemen, 2016.
| Indicators | Public blood banks |
Private blood bank |
||
|---|---|---|---|---|
| National Blood Transfusion and Research Center | National Center of Public Health Laboratories | Kuwait University Blood Bank | University of Sciences and Technology Blood Bank | |
| Preform test technique | ||||
| ABO, Rh D | Tube | Tube | Slide | Slide |
| Reverse grouping | Tube | Tube | Not done | Not done |
| Cross matching (CXM) | Tube | Tube | Tube | Tube |
| Other preformed test | ||||
| D weak (Du) | Yes | Yes | No | Yes |
| Antibody screening | No | No | No | No |
| Samples keeping | ||||
| Keep patient and donor samples at least (5 days) | Yes | No | No | Yes |
Blood Component Preparation Area
Packed RBCs, fresh-frozen plasma, and platelet concentrates were prepared in 3 blood banks, except the NCPHL, that only provide whole blood. Although 3 blood banks have components preparation department, only NBTRC had SOPs for blood components preparation. Furthermore, there was no document of quality control for blood component in the 3 blood banks. Blood component refrigerators were used to be checked for temperature during the day. In all blood banks that have blood component department, records of discarded blood units were found (Table 5).
Table 5.
Status of Blood Components Area in Blood Banks, Sana’a Capital, Yemen, 2016.
| Indicators | Public blood banks |
Private blood bank |
||
|---|---|---|---|---|
| National Blood Transfusion and Research Center | National Center of Public Health Laboratories | Kuwait University Blood Bank | University of Sciences and Technology Blood Bank | |
| Standard operating procedures (SOPs) | Yes | The department is not available | Yes | Yes |
| Quality control documents | Yes | No | No | |
| Check of refrigerator temperature | Yes | Yes | Yes | |
| Thermometer in refrigerator | No | No | Yes | |
| Quarantine refrigerator | No | No | Yes | |
| Discard blood units record | Yes | Yes | Yes | |
Screening of Blood for TTIs
According to statistical data in 2015 of NBTRC, the prevalence rates of hepatitis B surface antigen (HBsAg), anti–hepatitis B core (HBc), HCV, HIV, syphilis, antimalaria were 2.3%, 12.8%, 0.9%, 0.9%, 0.3%, and 1.2%, respectively. The 4 blood banks screen for HIV, HBV, and HCV using enzyme-linked immunosorbent assay (ELISA) method. Only NBTRC performs HBc test. Three blood banks screen for syphilis, where the NCPHL uses ELISA and the other two use rapid test. Two blood banks perform malaria screening using rapid test. None of the blood banks use nucleic acid tests for confirmation of reactive samples. Only, two blood banks repeat reactive TTIs samples testing. However, currently there is no counseling or referral system for those who found to be reactive (Table 6).
Table 6.
Serological Methods Used for TTIs Tests by Blood Banks, Sana’a Capital, Yemen, 2016.
| Indicator | Public blood banks |
Private blood bank |
||
|---|---|---|---|---|
| National Blood Transfusion and Research Center | National Center of Public Health Laboratories | Kuwait University Blood Bank | University of Sciences and Technology Blood Bank | |
| Perform TTIs by ELISA | ||||
| HBsAg | Yes | Yes | Yes | Yes |
| HBc | Yes | No | No | No |
| HCV | Yes | Yes | Yes | Yes |
| HIV | Yes | Yes | Yes | Yes |
| Rapid test method | ||||
| Syphilis | Yes | ELISA | No | Yes |
| Malaria | Yes | No | No | Yes |
| NAT method of TTIs | No | No | No | No |
| Repeat reactive TTIs samples testing | Yes | No | No | No |
| Counseling or referral system of reactive donors | No | No | No | No |
Note. TTIs = transfusion-transmitted infections; HBsAg = hepatitis B surface antigen; HBc = hepatitis B core; HCV = hepatitis C virus; NAT = nucleic acid test.
Overall Scoring of the Evaluated Blood Banks
The performance of the evaluated blood banks was highly satisfactory for NBTRC (84%), satisfactory (61%) for USTBB, and unsatisfactory for the NCPHL and KUBB (Table 7).
Table 7.
Quality of Services Provided in Blood Banks Based on Composite Scoring Index, Sana’a Capital, Yemen- 2016.
| Areas | Public blood banks |
Private blood bank |
||
|---|---|---|---|---|
| National Blood Transfusion and Research Center | National Center of Public Health laboratories | Kuwait University Blood Bank | University of Sciences and Technology Blood Bank | |
| Scores obtained for each evaluated area in percentage | ||||
| Blood transfusion activities | 80 | 60 | 80 | 80 |
| Quality system and training | 76 | 52 | 59 | 41 |
| Donation area | 92 | 69 | 58 | 62 |
| Blood group serology and compatibility testing | 89 | 67 | 44 | 67 |
| Blood components | 71 | 0.0 | 43 | 43 |
| Screening for TTIs | 93 | 71 | 42 | 71 |
| Average Score | 84 | 53 | 54 | 61 |
Note. Note. TTIs = transfusion-transmitted infections. <60% = unsatisfactory; 60-79.9% = satisfactory; ≥ 80% = highly Satisfactory.
Discussion
Blood safety depends on the recruitment and retention of blood donors. It is important to promote high standards of quality in all aspects of production, patient care, and service to ensure no risk of transmitting infection, safe blood collection procedures, correct testing for TTIs, accurate blood grouping and compatibility testing and the appropriate use and safe administration of blood.13
Therefore, evaluating BTS is the corner stone to ensure proper delivery of BTS and performing the aforementioned tasks.
Ideally, blood banks should perform the following 5 essential function: blood collection, preparation of blood components, blood grouping and cross-matching tests, screening of TTIs, and therapeutic transfusion. While blood bank evaluations in neighboring countries showed that the main blood transfusion centers in these countries had achieved all activities,17,18 we found that none of the main blood banks in Sana’a Capital performs therapeutic transfusion. Although, the previous blood banks evaluation by WHO showed that blood component preparation was not available in any of the blood bank in Yemen,19 our study showed that 3 blood banks perform blood components preparation, which indicates some improvement in BTS in Yemen.
The number of staff showed marked variation between different blood banks. Although this may reflect workload and donors number, there is a need to standardize staff number per 1000 donors—which is currently not available—to ensure proper preforming of activities with high quality.
Evaluation of BTS quality system is mandatory to ensure its quality. Our study showed the absence of quality-assurance procedures in the 4 blood banks, and the staff had received training on SOPs in only 2 blood banks. Previous evaluation of BTS in Bhutan also showed the lack of quality system.20 Therefore, trainings on quality assurance in all blood banks is highly needed.
In our study, most blood donors (95 %) were men, which is nearly similar to the findings of previous studies.21–23 This gender imbalance might be due to the fact that in Yemeni society, men are more proactive and independently make decisions. In addition, there may be misbelieve that women are less suitable for donating blood due to their physiological characteristics and repeated blood loss because of pregnancy and menstruation.
The WHO advocates and recommends developing national BTS based on voluntary nonremunerated regular blood donation to ensure safe and adequate blood supply.24 Our finding showed that only 29% of blood donors were volunteers who may reflects poor awareness and negative attitude among the Yemeni community regarding voluntary donation. A 100% voluntary donation was reported from Oman, Iran, and Qatar.17
Therefore, there is a need to launch awareness activities and motivation to shift from replacement to voluntary donation.
Blood grouping and compatibility testing were performed in 3 blood banks according to regular procedures, but no blood banks made antibodies screening test (which is the standard procedure for final compatibility testing) due to shortage in reagents and equipment. Regarding preparation of blood components, our finding revealed that blood banks prepared blood components (RBCs, plasma, and platelets) without applying quality procedures. This may be due to the lack of standards/guidelines and trainings.
On the contrary, to the previous BTS evaluation in Yemen,10 most blood banks preformed HIV, HBV, and HCV tests for blood donors samples by rapid methods. This reflects growing better understanding of the importance of TTIs prevention and control. However, our finding showed that 3 blood banks were not performing HBc test. Routine blood donor screening for anti-HBc is important for decreasing the risk of posttransfusion HBV infection. Furthermore in Yemen, HBV-DNA was detected in 4.8% of positive anti-HBc-IgG subjects who were negative for HBsAg.25,26
Transfusion-transmitted malaria is a real public health problem in malaria-endemic countries such as Yemen, where more than 78% of the population lives in at-risk areas and 25% lives in high-risk areas.12,27 In our findings, 2 blood banks were not performing malaria screening, and 3 are not repeating positive results that may increase the spread of infectious diseases among blood recipients, which still need to be improved.
Our findings showed that none of the blood banks uses NAT for the confirmation of reactive samples due to the lack of financial resources in Yemen, compared to findings from Saudi Arabia that uses NAT for confirmation.28 The overall blood banks service quality score showed that only NBTRC achieved highly satisfactory (84%) score. Therefore, more efforts are needed to improve service quality in blood banks.
One should consider important limitations of this study when interpreting the study findings. The selection of the 4 blood banks and the timeframe of the evaluation were not representative, and this might affect the external validity of the data. Questions for quality system failed to cover all its important indicators like screening of blood units, waste management, and waste disposal.
In conclusion, the findings highlight the increasing challenges facing BTS in Sana’a Capital especially the lack of therapeutic transfusion, poor QAS, and predominant dependence on the family donors. Therefore, there is a need to develop and train blood banks staff on QAS and to increase awareness among public on the importance of voluntary donation. A wider scale evaluation of BTS in whole Sana’a city is recommended.
Acknowledgments
The authors would like to acknowledge the EMPHNET for their technical support. They would also like to acknowledge the support of TEPHNET, the CDC, and the WHO.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mohammed Abdullah Alamad  https://orcid.org/0000-0002-1566-4964
https://orcid.org/0000-0002-1566-4964
References
- 1. Cheraghali AM. Blood safety concerns in the Eastern Mediterranean region. Hepat Mon. 2011;11(6):422-426. [PMC free article] [PubMed] [Google Scholar]
- 2. Mohammed Y, Bekele A. Seroprevalence of transfusion transmitted infection among blood donors at Jijiga blood bank, Eastern Ethiopia: retrospective 4 years study. BMC Res Notes. 2016;9:129. doi: 10.1186/s13104-016-1925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Universal access to safe blood transfusion. Global strategic plan 2008–2015. http://www.who.int/bloodsafety/StrategicPlan2008-2015AccessSafeBloodTransfusion.pdf. Accessed August 5, 2019.
- 4. World Health Organization. Blood safety and availability WHO factsheet. No. 279.http://www.who.int/mediacentre/factsheets/fs279/en/. Published June, 2015. Accessed August 5, 2019.
- 5. Motayo BO, Faneye AO, Udo UA, Olusola BA, Ezeani I, Ogiogwa JI. Seroprevalence of transfusion transmissible infections (TTI), in first time blood donors in Abeokuta, Nigeria. Afr Health Sci. 2015;15(1):19-24. doi: 10.4314/ahs.v15i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva MA, ed. Standards for Blood Banks and Transfusion Services. 23rd ed. Bethesda, MD: American Association of Blood Banks (AABB); 2005. [Google Scholar]
- 7. World Health Organization. Yemen’s national blood transfusion center in Sana’a faces threat of closure. http://www.emro.who.int/yem/yemen-news/blood-transfusion-centre-in-sanaa-threatens-to-shut-down.html. Published 2015. Accessed August 5, 2019.
- 8. Alodini AQ. Prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections among blood donors at Al-Thawra Hospital Sana’a City-Yemen. Yemeni J Med Sci. 2014;6:16-20. https://ust.edu/ojs/index.php/yjmp/article/view/22. Accessed August 5, 2019. [Google Scholar]
- 9. Al-Waleedi AA, Khader YS. Prevalence of hepatitis B and C infections and associated factors among blood donors in Aden city, Yemen. East Mediterr Health J, 2012;18(6):624-629. [DOI] [PubMed] [Google Scholar]
- 10. Saghir SA, Al–Hassan FM, Alsalahi OS, Abdul-Alaziz AE, Baqir HS. Frequencies of HBV, HCV, HIV, and Syphilis Markers Among Blood Donors: A Hospital-Based Study in Hodeidah, Yemen. Tropical J Pharmaceutical Research. 2012;11(1):132-136. [Google Scholar]
- 11. Saghir SAM, Alsalahi OSA, Zabad AAM, Al-Hassan M. HIV and syphilis among blood donors in Sana’a, Yemen. Biohealth Sci Bull. 2012;4(1):24-27 [Google Scholar]
- 12. World Health Organization. WHO Scales up malaria response in Yemen. http://www.emro.who.int/yem/yemen-news/who-scales-up-response-for-increase-in-malaria-in-yemen.html. Published 2016. Accessed August 5, 2019.
- 13. World Health Organization. Blood transfusion safety. http://www.who.int/bloodsafety/en/Blood_Transfusion_Safety.pdf. Published 2016. Accessed August 5, 2019.
- 14. Roche Diagnostics. Products and solutions, 2015. http://www.cobas.com/content/dam/cobascom/pdf. Accessed August 5, 2019.
- 15. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of test for syphilis. Clin Microbiol Rev. 1995;8:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. United Nations Children’s Fund. Guidance for choosing a malaria rapid diagnostic test (RDT). https://www.unicef.org/french/supply/files/Guidance_for_malaria_rapid_tests.pdf. Published 2007. Accessed August 5, 2019.
- 17. World Health Organization, EMRO. The regional status report on blood safety and availability, 2016. applications.emro.who.int/docs/EMROPub_2017_EN_18907.pdf. Accessed August 5, 2019. [Google Scholar]
- 18. SoSec Consulting Services. End project evaluation of GFATM financed NGO run blood bank services. http://www.nacp.gov.pk/library/reports/Surveillance%20&%20Research/Final%20Report%20of%20Sosec%20on%20Safe%20Blood.pdf. Published 2008. Accessed August 5, 2019.
- 19. Sibinga CTS. Assignment report of blood transfusion in Yemen. WHO consultancy in blood transfusion; January 3-22, 2004; Sana’a, Yemen. [Google Scholar]
- 20. World Health Organization. Comprehensive assessment study of blood transfusion service, Bhutan. http://www.who.int/bloodsafety/transfusion_services/BhutanNationalStandardsBTServices.pdf. Published 2011. Accessed August 5, 2019.
- 21. Saghir SAM, Alsalahi OSA, Zabad AAM, Al-Hassan M. HIV and syphilis among blood donors in Sana’a, Yemen. Biohealth Sci Bull. 2012;4(1):24-27. [Google Scholar]
- 22. Gelaw B, Mengistu Y. The prevalence of HBV, HCV and malaria among blood donors, Amhara and Tigray regional states. Ethiop J Health Dev. 2007;22(1):3-7. [Google Scholar]
- 23. World Health Organization. Voluntary blood donation. http://www.who.int/bloodsafety/voluntary_donation/en/. Published 2010. Accessed August 5, 2019. [PubMed]
- 24. Saghir SA, Al-Hassan FM, Alsalahi OS, Abdul-Alaziz AE, Baqir HS. Frequencies of HBV, HCV, HIV, and syphilis markers among blood donors: a hospital-based study in Hodeidah, Yemen. Trop J Pharm Res. 2012;11(1):132-136. [Google Scholar]
- 25. Kleinman S, Kuhns M, Todd D, et al. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion. 2003;43(6):696-704. [DOI] [PubMed] [Google Scholar]
- 26. Al-Moyed KA, Al-Haddad AM, Sallam WA. Detection of anti-hepatitis B core antibodies among hepatitis B surface antigen negative blood donors in Sana’a city, Yemen. Alandalus J Appl Sci. 2014;391(3556):1-15. [Google Scholar]
- 27. Oladeinde BH, Omoregie R, Osakue EO, Onaiwu TO. Asymptomatic malaria among blood donors in Benin city Nigeria. Iran J Parasitol. 2014;9(3):415-422. [PMC free article] [PubMed] [Google Scholar]
- 28. Elbjeirami WM, Arsheed NM, Al-Jedani HM, et al. Prevalence and trends of HBV, HCV, and HIV serological and NAT markers and profiles in Saudi blood donors. J Blood Disord Transfus. 2015;6:280. [Google Scholar]


