Abstract
Background:
In recent years, understanding of the anatomy of the ulnar collateral ligament (UCL) has evolved, demonstrating that the insertional footprint of the UCL on the ulna is more elongated and distally tapered than previously described. Current UCL reconstruction configurations do not typically re-create this native anatomy, which may represent a potential area for improvement.
Purpose/Hypothesis:
The purposes of this study were (1) to describe a novel anatomic UCL reconstruction technique designed to better replicate the native UCL anatomy and (2) to biomechanically compare this with the docking technique. The hypothesis was that the ultimate load to failure for the anatomic technique would not be inferior to the docking technique.
Study Design:
Controlled laboratory study.
Methods:
A total of 16 fresh-frozen cadaveric upper extremities (8 matched pairs) were utilized. One elbow in each pair was randomized to receive UCL reconstruction via the docking technique or the novel anatomic UCL reconstruction technique with palmaris tendon autograft. Following reconstruction, biomechanical testing was performed by applying valgus rotational torque at a constant rate of 5 deg/s until ultimate mechanical failure of the construct occurred. Maximal torque (N·m), rotation stiffness (N·m/deg), and mode/location of failure were recorded for each specimen.
Results:
The mean ultimate load to failure for elbows in the docking technique group was 23.8 ± 6.1 N·m, as compared with 31.9 ± 8.4 N·m in the anatomic technique group (P = .045). Mean rotational stiffness was 1.9 ± 0.7 versus 2.3 ± 0.9 N·m/deg for the docking and anatomic groups, respectively (P = .338). The most common mode of failure was suture pullout from the graft, which occurred in all 8 (100%) docking technique specimens and 7 of 8 (88%) specimens that underwent the anatomic UCL reconstruction technique.
Conclusion:
Ultimately, the anatomic UCL reconstruction technique demonstrated superior strength and resistance to valgus torque when compared with the docking technique, and this was comparable with that of the native UCL from prior studies. Increased initial strength may allow for earlier initiation of throwing postoperatively and potentially shorten return-to-play times.
Clinical Relevance:
Current UCL reconstruction techniques do not accurately reproduce the UCL insertional anatomy on the ulna. The novel anatomic technique described may result in more natural joint kinematics. This study demonstrated load-to-failure rates that are significantly higher than with the docking technique and consistent with the native ligament, as reported from previous studies. These findings may serve as a foundation for future clinical study and optimization of this technique.
Keywords: elbow, medial ulnar collateral ligament, anatomic UCL reconstruction, novel, docking technique, modified Jobe technique
The ulnar collateral ligament (UCL) is the primary static stabilizer to valgus stress at the elbow, and it may become attenuated or ruptured when experiencing excessive or repetitive forces.3,33 Injuries to the UCL are most commonly associated with overhead-throwing athletes as a result of the repetitive valgus forces incurred from sports such as baseball or javelin throw.37 The medial UCL of the elbow experiences up to 34.6 N·m of torque during maximum-effort throwing.26 Prior biomechanical work has demonstrated that load to failure of the native UCL is approximately 22.7 N·m to 34.0 N·m1,28; therefore, it is no surprise that the rates of UCL injuries and reconstructive surgical procedures are on the rise.6,7,13,15 UCL injuries and reconstruction are now a common occurrence among professional baseball players, with primary and revision operations increasing annually from 1974 to 2016 based on a study of 1429 professional baseball pitchers.6 A number of other recent studies have demonstrated rising rates of medial UCL injuries among all levels of baseball.13,14,24,29,37 Studies of national and statewide databases have demonstrated rising rates of UCL reconstruction surgery for all age groups, and the most notable increases have been in patients aged 15 to 20 years.24,29 As UCL injuries continue to occur at increasing rates in this population, the need for surgical intervention will likely continue to rise in a corresponding manner.
Given the rising injury rates and the increased need for UCL reconstruction, various surgical techniques, rehabilitation regimens, and clinical outcomes have been studied.2,8,17,18,22 A number of surgical techniques2,17,44 have been developed since the first successful operation was described by Jobe et al31 in 1986. Currently, the most commonly used techniques are the modified Jobe technique and the docking technique.10 The initial descriptions of the modified Jobe technique were first published in 2000 and 2001,4,48 and the procedure was further modified to the docking technique, originally described in 2002.44 Potential benefits of the docking technique as compared with the modified Jobe include decreased bone removal, flexor-pronator preservation, avoidance of routine ulnar nerve transposition, and robust graft tensioning.8
Prior studies have shown that 80% to 97% of athletes return to their previous levels of play or higher following UCL reconstruction with these techniques.18,22,23,38,44 While this return-to-play (RTP) rate is quite favorable, the mean time to RTP is 12 to 18 months.6,7,13,27,46 This extended time frame is problematic for patients, as they miss at least 1 full season of play and often miss 2 full seasons following surgery. Additionally, Erickson et al21 demonstrated that there is no significant difference in the time to return to sport between professional baseball pitchers who required a revision UCL reconstruction and those who did not. For comparative purposes, the mean time for National Football League athletes to RTP after surgery for a multiligament knee injury is 12.7 months.5 This suggests that there may be potential for significant improvement in RTP times for athletes who undergo UCL reconstruction. Accordingly, one option for improving this recovery time may be to optimize the strength and healing potential of the graft at the time of surgery.
In recent years, our understanding of the anatomy of the UCL has evolved. Dugas et al19 and Farrow et al25 demonstrated that the insertional footprint of the UCL on the ulna is more elongated and distally tapered than had been previously described. This finding was confirmed in a study by Camp et al,9 who found the mean length of the insertional footprint of the anterior bundle from its most proximal to distal aspect to be 29.7 mm and the total area of the footprint to be 187.6 mm2. These anatomic findings have the potential for novel implications in UCL reconstruction surgery, which has led some to question reconstruction techniques that were developed before this large tapered footprint was described. Some authors are now advocating for distalizing the ulnar tunnel when performing UCL reconstruction39; however, this still may not recapitulate normal anatomy.
Both the modified Jobe technique and the docking technique rely on a tunnel on the ulnar side, resulting in a 2-tailed graft that is separated by 7 to 10 mm as it inserts on the ulna.8 Although the native UCL consists of 2 bands (anterior and posterior) that are re-created during these techniques, the native bands are adjacent to each other rather than divergent, as they are after UCL reconstruction with an ulnar tunnel. Because these 2 bands take up differing loads depending on the degree of elbow flexion, their orientation to each other is likely important.30 If the distance between the bands is widened (as occurs with UCL reconstruction with the modified Jobe and docking techniques), this relationship may be significantly altered. Additionally, use of a single tunnel or socket on the ulna does not permit re-creation of the large attachment site surface area recently described for the native UCL on the ulna.9
The nonanatomic nature of current reconstruction configurations may represent an area for improvement and reduced RTP times. Numerous studies performed on the reconstruction of other commonly injured ligaments (eg, the anterior cruciate ligament [ACL]) have demonstrated improved results and more natural joint kinematics when ligaments are reconstructed in a more anatomic fashion.16,32,35,41,49 Accordingly, the primary purposes of this study were to (1) describe a novel anatomic UCL reconstruction technique with palmaris tendon autograft, designed to better replicate native UCL anatomy and (2) biomechanically compare this with the docking technique. Our hypothesis was that the ultimate load to failure for the anatomic technique would not be inferior to the docking technique.
Methods
Specimen Preparation
A total of 16 fresh-frozen cadaveric upper extremities (8 matched pairs; mean ± SD age, 55.9 ± 7.9 years; 7 male, 1 female) were utilized for this study. Cadaveric specimens were purchased from an accredited tissue bank (Science Care). Specimens were allowed to thaw overnight prior to surgery. The palmaris longus tendon was procured from all specimens and were subsequently stripped of all soft tissues other than the elbow joint capsule and ligamentous structures. The proximal humerus and wrist were transected, and each was potted in fiberglass resin (402 Bondo; 3M) for later biomechanical testing. The proximal UCL was completely detached from the medial epicondyle on all specimens to simulate a complete UCL disruption. The ligament was then split centrally in line with its fibers down to the level of the ulnohumeral joint. Afterward, the specimens were randomized to receive UCL reconstruction via the docking technique (group 1) or the novel anatomic UCL reconstruction technique with the palmaris autograft (group 2). Specimens were randomized so that within each matched pair, one side would receive the docking technique and the contralateral side would receive the anatomic technique. To maintain consistency across specimens, a UCL-specific set of guides was used for the drilling of humeral sockets for both techniques and the ulnar tunnel for the docking technique.
Docking Technique
The docking technique was performed as previously described.8 In brief, a 4.0-mm socket was drilled to a depth of 15 mm at the humeral footprint, and 2 smaller (2 mm) perforating tunnels were created from the anterior humerus to the base of this socket. Looped passing sutures were passed through these small perforating tunnels out to the large socket. On the ulnar side, two 3.5-mm sockets were drilled 7 mm apart, anterior and posterior to the sublime tubercle so that they converged at their base. These tunnels were created approximately 10 mm distal to the joint line. A No. 2-0 absorbable suture was used for repair of the native capsule and ligament. The suture was placed prior to graft passage but was not tied until after the graft was passed. A reinforced nonabsorbable suture (Arthrex Inc) was run in a Krakow fashion in one end of the palmaris autograft. The graft was passed through the ulnar tunnel, and the sutured end was docked into the humeral socket. Maximal manual tension was applied to the graft as the elbow was cycled 5 times through an arc of flexion and extension, and the free end of the graft was marked at the level of the humeral socket. An additional Krakow suture was placed at this level, and excess graft was excised. The free end was then shuttled into the socket and docked. The graft was once again tensioned and cycled. With the arm in 30° of flexion and a varus load applied, the graft was secured by tying the sutures over the bone bridge on the humerus. The suture previously placed in the native capsule and ligament was then tied to ensure that the graft remained extra-articular (Figure 1).
Figure 1.
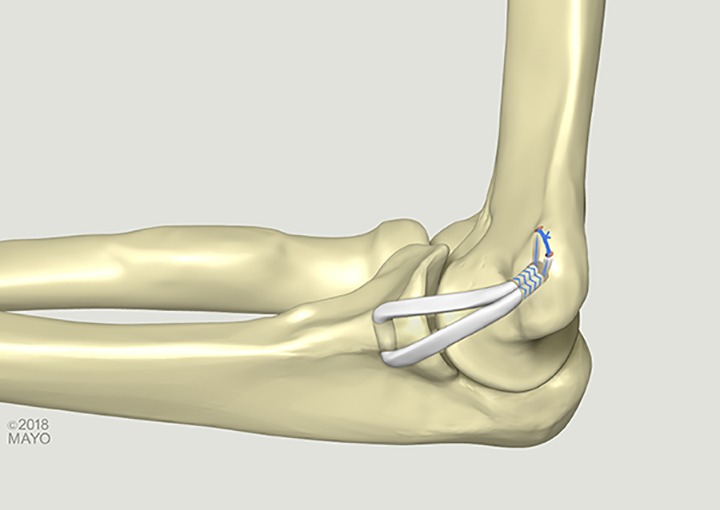
Ulnar collateral ligament reconstruction with the docking technique.
Anatomic UCL Reconstruction Technique
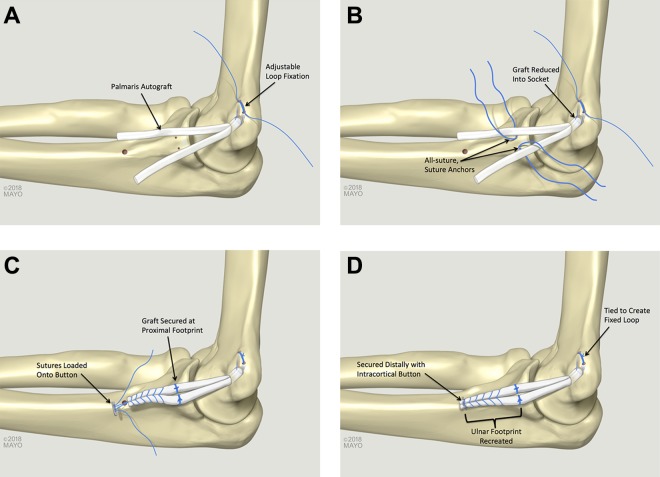
A 4.0-mm socket with a depth of 15 mm with 2 small (2-mm) perforating tunnels was created in the humerus in the same fashion as the docking technique. Two separate shuttling sutures were used to pass the free ends of an unassembled all-suture adjustable suspensory loop (Arthrex Inc) from the smaller 2-mm tunnels out through the larger 4-mm socket. The palmaris graft was folded over in half, and the suspensory loop was assembled around the midportion of the graft. The suspensory loop was tensioned so that the graft was reduced 10 mm into the humeral socket (two-thirds of total socket depth to allow for additional space for sequential tensioning after the graft was fixed on the ulna) (Figure 2A). Attention was turned to the ulna. Just distal to the joint line (5 mm), two 1.3-mm all-suture anchors (FiberTak; Arthrex Inc) were placed in the anterior and posterior aspects of the native ligament footprint. These were spaced approximately 5 mm apart, tagged, and laid aside (Figure 2B). A No. 2-0 absorbable suture was used for repair of the native capsule and ligament. The suture was placed prior to graft passage but was not tied until after the graft was passed.
Figure 2.
Ulnar collateral ligament (UCL) reconstruction with the novel anatomic UCL reconstruction technique. (A) The graft is fixed into a socket on the humerus via adjustable loop fixation. (B) All-suture suture anchors are placed in the ulna and (C) tied to secure the graft at the proximal UCL footprint. (C) A looped suture is used to run a whipstitch in the graft, and this suture is loaded onto a cortical button, (D) which is secured at the distal aspect of the native UCL footprint.
The 2 distal limbs of the graft were sutured together with a closed-loop No. 0 nonabsorbable suture (FiberLoop; Arthrex, Inc) in a whipstitch fashion, and excess graft was excised. The looped suture was cut to create 2 free suture ends, which were loaded onto an intramedullary cortical suspensory button (Arthrex Inc) (Figure 2C). The sutures from the 2 anchors near the joint line were passed around each limb of the graft (anterior sutures passed around the anterior limb and posterior sutures around the posterior limb). The graft was tensioned and cycled. With the arm in 30° of flexion and a varus load applied, the sutures from the anchors were tied around each limb of the graft to secure it at the proximal aspect of the triangular-shaped native UCL footprint on the ulna. For fixation at the distal apex of the ulnar footprint, a 3.2-mm drill hole was placed in a unicortical fashion. The suspensory button was inserted into the intramedullary canal and deployed, and sutures were tensioned to reduce the graft to the ulna. Sutures were tied over the top of the graft to create a closed-loop construct. The suspensory loop on the humeral side was tensioned once again, and the suture ends were tied over the bone bridge to create a closed-loop construct (Figure 2D). The suture previously placed in the native capsule and ligament was then tied to ensure that the graft remained extra-articular.
Biomechanical Analysis
For testing, all specimens were loaded onto an Instron Model E10000 Electropuls Dynamic Test Instrument with a combination force and torque load cell with 10-kN and 100-N·m capacity. Similar to previous investigations, samples were oriented at 90° of elbow flexion with the humerus oriented vertically and secured inline with the system actuator (Figure 3).11,12,36,45 A valgus rotational torque was applied to the humerus at a constant rate of 5 deg/s while the forearm was held stationary. The elbow was loaded at this constant rate until ultimate mechanical failure of the construct occurred. Maximal torque (N·m), rotation stiffness (N·m/deg), and mode/location of failure were recorded for each specimen.
Figure 3.
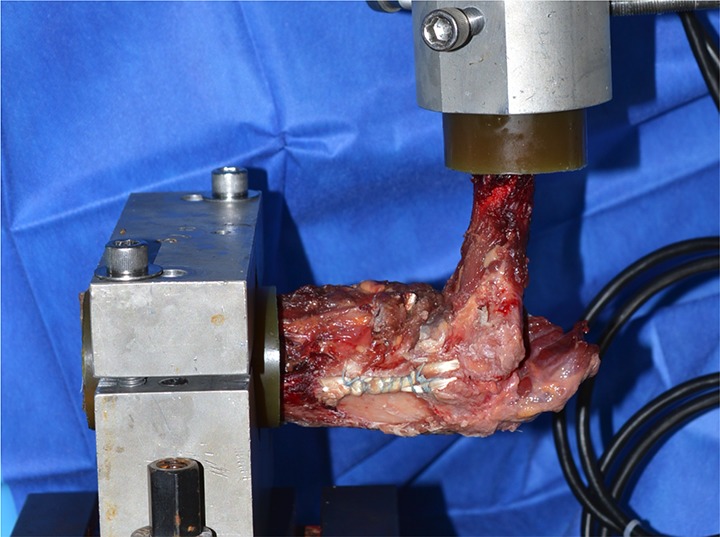
Biomechanical testing apparatus.
Data Analysis
Summary statistics such as mean ± SD, median, ranges, and standard error of measurement are provided for each reconstruction technique. For the comparison of continuous variables, a 2-tailed Student t test was used to assess differences in maximum load to failure and rotational stiffness between the study groups. These results are reported with their corresponding mean differences, 95% CIs, and P values. Only P values <.05 were considered to represent statistical significance.
Results
The results of biomechanical testing are provided in Table 1. In brief, the mean ultimate load to failure for elbows in the docking technique group was 23.8 ± 6.1 N·m, as opposed to 31.9 ± 8.4 N·m in the anatomic technique group (mean difference, 8.1 N·m; 95% CI, 0.23-15.97; P = .045). Mean rotational stiffness was 1.9 ± 0.7 versus 2.3 ± 0.9 N·m/deg for the docking and anatomic groups, respectively (mean difference, 0.4 N·m/deg; 95% CI, –0.47 to 1.27; P = .338). In all 8 (100%) docking technique specimens, the mode of failure was suture pullout from the graft on the humeral side. For the anatomic technique, 7 of 8 (88%) specimens failed secondary to suture pullout from the graft on the ulnar side, while 1 of 8 (12%) failed because of a medial epicondyle fracture.
TABLE 1.
Biomechanical Testing for the Docking and Anatomic Techniques
| Mean | SD | Range | Median | Mean Difference | 95% CI | P Value | |
|---|---|---|---|---|---|---|---|
| Ultimate load to failure, N·m | 8.1 | 0.228 to 15.972 | .045 | ||||
| Docking | 23.8 | 6.1 | 16.1 to 31.2 | 22.7 | |||
| Anatomic | 31.9 | 8.4 | 16.6 to 46.2 | 31.9 | |||
| Rotational stiffness, N·m/deg | 0.4 | –0.465 to 1.2265 | .338 | ||||
| Docking | 1.9 | 0.7 | 0.8 to 2.6 | 2.2 | |||
| Anatomic | 2.3 | 0.9 | 0.8 to 3.8 | 2.4 |
Discussion
Although other ligament reconstruction procedures (eg, ACL and medial collateral ligament [MCL] reconstructions) have been refined to mimic native anatomy, this is not yet the case for the UCL of the elbow.16,32,35,41,49 Given the complex and dynamic forces through the UCL during the throwing motion, a more anatomic reconstruction geometry that more closely mirrors native anatomy may be of benefit. Accordingly, the purpose of this study was to (1) describe a novel anatomic UCL reconstruction technique designed to better replicate native UCL anatomy and (2) biomechanically compare this with the docking technique. The primary findings of this study were that the mean load to failure for elbows in the docking technique group was 23.8 ± 6.1 versus 31.9 ± 8.4 N·m in the anatomic technique group. This ultimate load to failure of 32 N·m is greater than that of other described UCL reconstruction/repair techniques12,20,36,42,45 and similar to that of the native ligament.1,28 While the most common mechanism of failure was suture pullout from the graft, this occurred on the ulnar side of the anatomic technique, as opposed to the humeral side in the docking technique. In both cases, this is the site of the free ends of the graft. The primary difference is that the free ends of the graft in the anatomic technique are primarily secured with a looped whipstitch, and backup fixation is provided by the more proximal all-suture anchors.
Previous biomechanical work on the native UCL has demonstrated a load to failure of approximately 22.7 to 34 N·m.1,28 The ideal reconstruction technique would be able to provide similar strength to that of the native ligament. Unfortunately, current techniques have been unable to mimic this. In biomechanical studies where the docking technique was performed on cadaveric elbows and then subjected to biomechanical testing, the load to failure averaged 4.85 to 18.86 N·m.12,36,42,45 In studies where biomechanical testing was performed on cadaveric elbows after the modified Jobe technique, the load to failure was found to average 8.9 to 20.9 N·m.20,42 Dugas et al20 investigated the load to failure of UCL repair with internal bracing and found it to be a mean of 23.6 N·m. These load-to-failure rates are lower than what has previously been described for the native ligament. This study, however, has shown that by utilizing the anatomic technique described, the load-to-failure rate averages 31.9 N·m. This result is superior to those of the most commonly used techniques as well as that demonstrated with ligament repair and internal bracing, and the load to failure is most similar to that of the native ligament.
This newly described anatomic technique provides several unique advantages over the most commonly used UCL reconstruction techniques (Table 2). The anatomic-based reconstruction approach has been very successful for other injured ligaments in the body. Previous studies comparing anatomic and nonanatomic ACL reconstruction demonstrated that patients with the anatomic reconstruction had better clinical and functional outcomes,32 as well as stability that more closely resembled the native ligament.35,41 Similarly, contemporary knee MCL repair or reconstruction techniques have been designed to more closely replicate native anatomy, and these have demonstrated favorable outcomes.16,34 While anatomic reconstructions have been very successful for ligaments of the knee, the elbow has its own unique biomechanical characteristics. As a result, it is currently unclear if a similar approach to the elbow would also produce superior outcomes. Further investigation is necessary to determine if the observations of ligament reconstructions of the knee are applicable to the elbow as well. However, the anatomic UCL technique may allow for more robust initial fixation strength, which may allow for some improvement in rehabilitation and RTP times. Although the initial strength may improve RTP times, a multitude of factors likely affects RTP rates and times: surgical technique, concomitant procedures, player motivation, quality of rehabilitation, interval thrower program, individual thrower biomechanics, and psychological factors.
TABLE 2.
Potential Advantages of Utilizing the Novel Anatomic Technique for Ulnar Collateral Ligament Reconstruction
| Ulnar Side | Humeral Side |
|---|---|
| Increased tendon-to-bone contact Multipoint fixation Larger surface area (may be target for biologic augmentation) No need to drill ulnar tunnel (may reduce risk to ulnar nerve injury) Potential for larger graft size without additional bone removal For revision setting, prior ulnar tunnels can be spanned and avoided Sequential retensioning of graft after fixation |
Decreased suture burden in the socket Allows for measurement of graft diameter prior to drilling the socket Increased tendon-to-bone contact in the humeral socket, which may promote healing Sequential retensioning of graft after fixation |
The novel anatomic technique relies on cortical surface healing on the ulna rather than bone tunnel healing, as in the docking and Jobe techniques. Additionally, although bone-to-tendon contact was not directly measured, it was observed to have increased in the anatomic technique as compared with the docking technique because of the enlarged ulnar footprint that mimics that of the native ligament . Recent studies on tendon-to-bone healing following biceps tenodesis have suggested that surface healing is as good as, if not better than, bone tunnel healing.40,43,47 Tan et al47 performed a study comparing the tendon-to-bone healing for bone tunnel and cortical surface techniques in a rabbit model and found no significant difference between the groups in terms of mean failure loads, stiffness, and mean volume of newly formed bone. Histological analysis at 8 weeks demonstrated direct tendon-bone interdigitation and early fibrocartilaginous zone formation at the tendon-bone interface on the outer cortical surface. In the specimens with intracortical fixation, only 5% of new bone formed in the bone tunnel, while 95% of new bone formed on the cortical surface, suggesting that surface healing may be the primary mode of healing even for intracortical grafts. Similarly, Park et al43 found no significant difference in clinical outcomes when comparing interference screw and suture anchor techniques for biceps tenodesis, but there was a much higher failure rate with the interference screw method than with the suture anchor method, especially in patients with a high work level.
This new technique maintains the same benefits that the docking technique has relative to the Jobe technique—namely, decreased bone removal, flexor-pronator preservation, avoidance of routine ulnar nerve transposition, and robust graft tensioning.8 Additionally, the anatomic technique more closely replicates native ligament anatomy (in terms of ulnar footprint and overall geometry), increased tendon-to-bone contact on the ulna, multipoint fixation on the ulna, and a larger surface area on the ulna. This larger surface area may also provide a target for biologic augmentation. Additionally, there is no need to drill tunnels on the ulna, which may reduce risk to ulnar nerve injury as compared with techniques that require an ulnar tunnel. Because an ulnar tunnel is not required, a larger-diameter graft could potentially be utilized without having to remove additional ulnar bone. This technique may also prove valuable in revision settings where prior ulnar tunnels have compromised proximal ulnar bone stock. With the anatomic UCL reconstruction technique, prior ulnar tunnels can be spanned and completely avoided.
On the humeral side, the flipped configuration of the graft (vs a traditional docking technique) has a number of potential benefits as well. This arrangement decreases the suture burden in the socket, and it allows for measurement of graft diameter prior to drilling of the socket, allowing the surgeon to create a more precisely sized socket. Both of these advancements increase tendon-to-bone contact in the humeral socket and may promote healing. Also, the adjustable suspensory loop fixation on both sides allows for sequential retensioning of the graft after fixation.
There were several limitations to this study. Similar to that of other cadaveric studies, the mean age of specimens in this study was 56 years, which is older than the typical patient who undergoes UCL reconstruction. This study did not investigate laxity data during loading. There were multiple variables between techniques, so it is difficult to know which is most important in determining load-to-failure strength (adjustable loop on humeral side, increased fixation point on ulna, button vs tunnel, etc). The native ligament was not evaluated in this study, which prevents a direct comparison of the ultimate load to failure for the anatomic technique to the native ligament for these specimens. Future studies could be performed to directly compare the native ligament with the anatomic technique. Additionally, this study assessed load to failure at time zero and did not include cyclic loading testing. Therefore, this study did not account for the tendon-to-bone healing that would be anticipated in the postoperative period. Finally, similar to other works, this study relied on a biomechanical load-to-failure model that did not replicate the natural mechanism of injury (the rapid throwing motion). Clinical correlation is needed to further assess this newly designed anatomic technique.
In addition to these limitations, there are potential disadvantages to the anatomic technique. Although there was not any difficulty flipping the intracortical button in these cadaveric specimens, this could be a difficulty for some surgeons in the clinical setting. Finally, this technique requires an increased number of fixation devices as compared with the docking technique, which has the potential to add significant financial cost to the procedure.
Conclusion
Ultimately, the anatomic UCL reconstruction technique demonstrated higher load to failure from valgus torque as compared with the docking technique, and this load to failure was comparable with that of the native UCL demonstrated in previous studies. Increased initial strength may allow for earlier initiation of throwing postoperatively. There are additional potential benefits conferred by this technique, and future clinical study and technique optimization are needed.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by a research grant from Arthrex. C.L.C. has received educational support from Arthrex and hospitality payments from Arthrex and Zimmer Biomet. B.B. and J.K. are employed by Arthrex. D.W.A. has received consulting fees from Stryker and hospitality payments from Arthrex. J.S.D. has received consulting fees from Arthrex, Trice, Linvatec, DePuy, Wright Medical, and Merck Sharp & Dohme; has received research support from Arthrex; receives royalties from Linvatec and Wolters Kluwer Health; is a paid speaker/presenter for Arthrex; and has received hospitality payments from Horizon Pharma. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332–337. [DOI] [PubMed] [Google Scholar]
- 2. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atanda A, Jr, Buckley PS, Hammoud S, Cohen SB, Nazarian LN, Ciccotti MG. Early anatomic changes of the ulnar collateral ligament identified by stress ultrasound of the elbow in young professional baseball pitchers. Am J Sports Med. 2015;43(12):2943–2949. [DOI] [PubMed] [Google Scholar]
- 4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16–23. [DOI] [PubMed] [Google Scholar]
- 5. Bakshi NK, Khan M, Lee S, et al. Return to play after multiligament knee injuries in National Football League athletes. Sports Health. 2018;10(6):495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camp CL, Conte S, D’Angelo J, Fealy SA. Epidemiology of ulnar collateral ligament reconstruction in Major and Minor League Baseball pitchers: comprehensive report of 1429 cases. J Shoulder Elbow Surg. 2018;27(5):871–878. [DOI] [PubMed] [Google Scholar]
- 7. Camp CL, Dines JS, van der List JP, et al. Summative report on time out of play for Major and Minor League Baseball: an analysis of 49,955 injuries from 2011 through 2016. Am J Sports Med. 2018;46(7):1727–1732. [DOI] [PubMed] [Google Scholar]
- 8. Camp CL, Dines JS, Voleti PB, James EW, Altchek DW. Ulnar collateral ligament reconstruction of the elbow: the docking technique. Arthrosc Tech. 2016;5(3):e519–e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camp CL, Jahandar H, Sinatro AM, Imhauser CW, Altchek DW, Dines JS. Quantitative anatomic analysis of the medial ulnar collateral ligament complex of the elbow. Orthop J Sports Med. 2018;6(3):2325967118762751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Camp CL, Klinger CE, Lazaro LE, et al. Osseous vascularity of the medial elbow after ulnar collateral ligament reconstruction: a comparison of the docking and modified Jobe techniques. Orthop J Sports Med. 2018;6(4):2325967118763153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chronister JE, Morris RP, Andersen CR, Buford WL, Jr, Bennett JM, Mehlhoff TL. A biomechanical comparison of 2 hybrid techniques for elbow ulnar collateral ligament reconstruction. J Hand Surg Am. 2014;39(10):2033–2040. [DOI] [PubMed] [Google Scholar]
- 12. Cohen SB, Woods DP, Siegler S, Dodson CC, Namani R, Ciccotti MG. Biomechanical comparison of graft fixation at 30 degrees and 90 degrees of elbow flexion for ulnar collateral ligament reconstruction by the docking technique. J Shoulder Elbow Surg. 2015;24(2):265–272. [DOI] [PubMed] [Google Scholar]
- 13. Conte S, Camp CL, Dines JS. Injury trends in Major League Baseball over 18 seasons: 1998-2015. Am J Orthop (Belle Mead NJ). 2016;45(3):116–123. [PubMed] [Google Scholar]
- 14. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764–1769. [DOI] [PubMed] [Google Scholar]
- 15. Degen RM, Camp CL, Bernard JA, Dines DM, Altchek DW, Dines JS. Current trends in ulnar collateral ligament reconstruction surgery among newly trained orthopaedic surgeons. J Am Acad Orthop Surg. 2017;25(2):140–149. [DOI] [PubMed] [Google Scholar]
- 16. DeLong JM, Waterman BR. Surgical techniques for the reconstruction of medial collateral ligament and posteromedial corner injuries of the knee: a systematic review. Arthroscopy. 2015;31(11):2258–2272. [DOI] [PubMed] [Google Scholar]
- 17. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039–2044. [DOI] [PubMed] [Google Scholar]
- 18. Dines JS, Jones KJ, Kahlenberg C, Rosenbaum A, Osbahr DC, Altchek DW. Elbow ulnar collateral ligament reconstruction in javelin throwers at a minimum 2-year follow-up. Am J Sports Med. 2012;40(1):148–151. [DOI] [PubMed] [Google Scholar]
- 19. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657–660. [DOI] [PubMed] [Google Scholar]
- 20. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735–741. [DOI] [PubMed] [Google Scholar]
- 21. Erickson BJ, Chalmers PN, Bach BR, Jr, et al. Length of time between surgery and return to sport after ulnar collateral ligament reconstruction in Major League Baseball pitchers does not predict need for revision surgery. J Shoulder Elbow Surg. 2017;26(4):699–703. [DOI] [PubMed] [Google Scholar]
- 22. Erickson BJ, Chalmers PN, Bush-Joseph CA, Verma NN, Romeo AA. Ulnar collateral ligament reconstruction of the elbow: a systematic review of the literature. Orthop J Sports Med. 2015;3(12):2325967115618914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erickson BJ, Cvetanovich GL, Frank RM, et al. Do clinical results and return-to-sport rates after ulnar collateral ligament reconstruction differ based on graft choice and surgical technique? Orthop J Sports Med. 2016;4(11):2325967116670142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: a retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770–1774. [DOI] [PubMed] [Google Scholar]
- 25. Farrow LD, Mahoney AJ, Stefancin JJ, Taljanovic MS, Sheppard JE, Schickendantz MS. Quantitative analysis of the medial ulnar collateral ligament ulnar footprint and its relationship to the ulnar sublime tubercle. Am J Sports Med. 2011;39(9):1936–1941. [DOI] [PubMed] [Google Scholar]
- 26. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233–239. [DOI] [PubMed] [Google Scholar]
- 27. Gibson BW, Webner D, Huffman GR, Sennett BJ. Ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2007;35(4):575–581. [DOI] [PubMed] [Google Scholar]
- 28. Hechtman KS, Tjin ATEW, Zvijac JE, Uribe JW, Latta LL. Biomechanics of a less invasive procedure for reconstruction of the ulnar collateral ligament of the elbow. Am J Sports Med. 1998;26(5):620–624. [DOI] [PubMed] [Google Scholar]
- 29. Hodgins JL, Vitale M, Arons RR, Ahmad CS. Epidemiology of medial ulnar collateral ligament reconstruction: a 10-year study in New York State. Am J Sports Med. 2016;44(3):729–734. [DOI] [PubMed] [Google Scholar]
- 30. Jackson TJ, Jarrell SE, Adamson GJ, Chung KC, Lee TQ. Biomechanical differences of the anterior and posterior bands of the ulnar collateral ligament of the elbow. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2319–2323. [DOI] [PubMed] [Google Scholar]
- 31. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158–1163. [PubMed] [Google Scholar]
- 32. Kilinc BE, Kara A, Oc Y, et al. Transtibial vs anatomical single bundle technique for anterior cruciate ligament reconstruction: a retrospective cohort study. Int J Surg. 2016;29:62–69. [DOI] [PubMed] [Google Scholar]
- 33. Labott JR, Aibinder WR, Dines JS, Camp CL. Understanding the medial ulnar collateral ligament of the elbow: review of native ligament anatomy and function. World J Orthop. 2018;9(6):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laprade RF, Wijdicks CA. Surgical technique: development of an anatomic medial knee reconstruction. Clin Orthop Relat Res. 2012;470(3):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim HC, Yoon YC, Wang JH, Bae JH. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: a cadaveric study of comparison of knee stability. Clin Orthop Surg. 2012;4(4):249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lynch JL, Pifer MA, Maerz T, et al. The GraftLink ulnar collateral ligament reconstruction: biomechanical comparison with the docking technique in both kinematics and failure tests. Am J Sports Med. 2013;41(10):2278–2287. [DOI] [PubMed] [Google Scholar]
- 37. Mahure SA, Mollon B, Shamah SD, Kwon YW, Rokito AS. Disproportionate trends in ulnar collateral ligament reconstruction: projections through 2025 and a literature review. J Shoulder Elbow Surg. 2016;25(6):1005–1012. [DOI] [PubMed] [Google Scholar]
- 38. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: a review of 147 cases. Am J Sports Med. 2014;42(6):1323–1332. [DOI] [PubMed] [Google Scholar]
- 39. Marshall T, Frangiomore S, Schickendantz M. Medial ulnar collateral ligament reconstruction: restoring the ulnar footprint. Techniques in Shoulder and Elbow Surgery. 2017;18:62–64. [Google Scholar]
- 40. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murawski CD, Wolf MR, Araki D, Muller B, Tashman S, Fu FH. Anatomic anterior cruciate ligament reconstruction: current concepts and future perspective. Cartilage. 2013;4(3):27S–37S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paletta GA, Jr, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599–1603. [DOI] [PubMed] [Google Scholar]
- 43. Park JS, Kim SH, Jung HJ, Lee YH, Oh JH. A prospective randomized study comparing the interference screw and suture anchor techniques for biceps tenodesis. Am J Sports Med. 2017;45(2):440–448. [DOI] [PubMed] [Google Scholar]
- 44. Rohrbough JT, Altchek DW, Hyman J, Williams RJ, 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541–548. [DOI] [PubMed] [Google Scholar]
- 45. Ruland RT, Hogan CJ, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565–1570. [DOI] [PubMed] [Google Scholar]
- 46. Saper M, Shung J, Pearce S, Bompadre V, Andrews JR. Outcomes and return to sport after ulnar collateral ligament reconstruction in adolescent baseball players. Orthop J Sports Med. 2018;6(4):2325967118769328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tan H, Wang D, Lebaschi AH, et al. Comparison of bone tunnel and cortical surface tendon-to-bone healing in a rabbit model of biceps tenodesis. J Bone Joint Surg Am. 2018;100(6):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10(2):152–157. [DOI] [PubMed] [Google Scholar]
- 49. Zhang H, Qiu M, Zhou A, Zhang J, Jiang D. Anatomic anterolateral ligament reconstruction improves postoperative clinical outcomes combined with anatomic anterior cruciate ligament reconstruction. J Sports Sci Med. 2016;15(4):688–696. [PMC free article] [PubMed] [Google Scholar]