Abstract
OBJECTIVES
Older adult patients are underrepresented in clinical trials comparing non–vitamin K antagonist oral anticoagulants (NOACs) and warfarin. This subgroup analysis of the ARISTOPHANES study used multiple data sources to compare the risk of stroke/systemic embolism (SE) and major bleeding (MB) among very old patients with nonvalvular atrial fibrillation (NVAF) prescribed NOACs or warfarin.
DESIGN
Retrospective observational study.
SETTING
The Centers for Medicare & Medicaid Services and three US commercial claims databases.
PARTICIPANTS
A total of 88 582 very old (aged ≥80 y) NVAF patients newly initiating apixaban, dabigatran, rivaroxaban, or warfarin from January 1, 2013, to September 30, 2015.
MEASUREMENTS
In each database, six 1:1 propensity score matched (PSM) cohorts were created for each drug comparison. Patient cohorts were pooled from all four databases after PSM. Cox proportional hazards models were used to estimate hazard ratios (HRs) of stroke/SE and MB.
RESULTS
The patients in the six matched cohorts had a mean follow‐up time of 7 to 9 months. Compared with warfarin, apixaban (HR = .58; 95% confidence interval [CI] = .49‐.69), dabigatran (HR = .77; 95% CI = .60‐.99), and rivaroxaban (HR = .74; 95% CI = .65‐.85) were associated with lower risks of stroke/SE. For MB, apixaban (HR = .60; 95% CI = .54‐.67) was associated with a lower risk; dabigatran (HR = .92; 95% CI = .78‐1.07) was associated with a similar risk, and rivaroxaban (HR = 1.16; 95% CI = 1.07‐1.24) was associated with a higher risk compared with warfarin. Apixaban was associated with a lower risk of stroke/SE and MB compared with dabigatran (stroke/SE: HR = .65; 95% CI = .47‐.89; MB: HR = .60; 95% CI = .49‐.73) and rivaroxaban (stroke/SE: HR = .72; 95% CI = .59‐.86; MB: HR = .50; 95% CI = .45‐.55). Dabigatran was associated with a lower risk of MB (HR = .77; 95% CI = .67‐.90) compared with rivaroxaban.
CONCLUSION
Among very old NVAF patients, NOACs were associated with lower rates of stroke/SE and varying rates of MB compared with warfarin. J Am Geriatr Soc 67:1662–1671, 2019
Keywords: oral anticoagulants, atrial fibrillation, stroke, major bleeding, older adults
The presence of atrial fibrillation (AF) is an independent risk factor for stroke, and the percentage of stroke events that could be attributed to AF increases significantly with age.1 The stroke and major bleeding (MB) risk stratification schemas, CHA2DS2‐VASc and HAS‐BLED, consider age as a risk factor for stroke/thromboembolism and MB, respectively, in patients with AF.2, 3
Clinical evidence favors treatment with oral anticoagulants (OACs) to prevent stroke/systemic embolism (SE) in very old adults given that the benefits are considered to outweigh the risk of MB.4, 5 Randomized clinical trials (RCTs) demonstrated that non–vitamin K antagonist oral anticoagulants (NOACs), including apixaban, dabigatran, edoxaban, and rivaroxaban, have a lower frequency of stroke/SE and a noninferior risk of MB compared with conventional therapy, such as vitamin K antagonists (VKAs), among patients aged 75 years and older.6, 7, 8
The 2018 European Heart Rhythm Association Practical Guide suggests that use of NOACs rather than VKA led to a larger risk reduction among older patients9 due to the higher risk for stroke/SE and MB in this population. A systematic review among AF patients (aged 65 y or older) comparing NOACs with VKAs suggested that NOACs have favorable results for hemorrhagic stroke and intracranial hemorrhage (ICH).10 Using the Fit‐for‐the‐Aged (FORTA) classification and Delphi process, warfarin, dabigatran, edoxaban, and rivaroxaban were labeled B (beneficial; safely and effectively treat AF), and apixaban was labeled A (absolutely; most beneficial risk‐benefit ratio) for the treatment of AF in patients aged 65 years or older.11
Although OACs are recommended for patients with AF and a high CHA2DS2‐VASc score, it was consistently reported that less than 50% of patients aged 80 to 89 years are treated with OACs, with reasons pertaining to safety concerns rather than related to efficacy, such as fear of bleeding, perceived harm greater than benefit, poor health, and geriatric syndromes.12 Moreover, the financial burden and health plan restrictions related to the prescription of NOACs might also serve as potential barriers to treatment. More than 50% of the nonvalvular atrial fibrillation (NVAF) patients are 80 years or older, yet only one‐third of the patients enrolled in the four landmark NVAF trials of the NOACs were 75 years of age or older.13 As the older adult US population increases, this becomes an increasingly important group to study.
This analysis of older patients (aged ≥80 y) in the ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled analysis on Health Outcomes and Experience of Patients [NCT03087487]) study aimed to provide complementary information for this underrepresented population by evaluating and comparing the rates of stroke/SE and MB among NVAF patients newly prescribed apixaban, dabigatran, rivaroxaban, or warfarin.
METHODS
Data Sources
The details of the data description and pooling process of the ARISTOPHANES study were published previously.14, 15 In brief, data in this study were pooled from the US Centers for Medicare & Medicaid Services (CMS) database and three commercial claims databases in the United States: the IMS PharMetrics Plus Database (“PharMetrics”), the Optum Clinformatics Data Mart (“Optum”), and the Humana Research Database (“Humana”). Collectively, the four data sets cover more than 123 million beneficiaries annually that account for approximately 38% of the US population. The Truven MarketScan Commercial Claims and Encounter database, used in the ARISTOPHANES study, was not included in this subgroup analysis because all patients are working‐age adults who are younger than 65 years.15 Also, the MarketScan Medicare Supplemental and Coordination of Benefits Database (patients >65 y) was not included in the ARISTOPHANES study to avoid potential duplicates of beneficiaries with both CMS Medicare fee‐for‐service and Medicare supplementary insurance. In addition, the analysis was conducted using CMS data individually when examining outcomes or subgroups for which other commercial data sets do not have comprehensive information.
Patient Selection
This subgroup analysis of the ARISTOPHANES study focused on very old (≥80 y) NVAF patients newly treated with apixaban, dabigatran, rivaroxaban, or warfarin. AF patients with an OAC pharmacy claim between January 1, 2013, and September 30, 2015 (identification period) were selected. The first NOAC pharmacy claim during the identification period was designated as the index date for patients with any NOAC claim(s). For those without a NOAC claim, the first warfarin prescription date was designated as the index date.16 The baseline period was defined as 12 months before or on the index date in an effort to restrict the population to new initiators.
Patients were excluded if they were treated with an OAC within 12 months before the index date, had evidence of valvular heart disease, venous thromboembolism, transient AF (pericarditis, hyperthyroidism, thyrotoxicity), or heart valve replacement/transplant during the baseline period; were pregnant during the study period; or had hip or knee replacement surgery within 6 weeks before the index date. Detailed selection criteria can be found in Figure 1.
Figure 1.
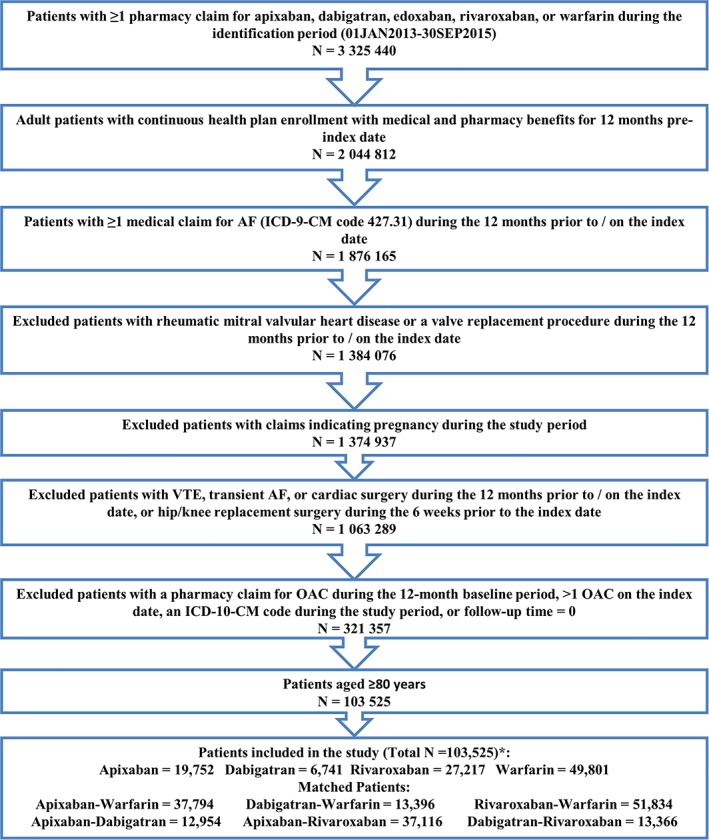
Patient selection criteria. AF, atrial fibrillation; ICD‐9/10‐CM, International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification; OAC, oral anticoagulant; VTE, venous thromboembolism. *Edoxaban was not included in the study given the recent Food and Drug Administration approval in 2015, and hence the small sample size (N = 14). [Late correction added May 28, 2019, after first online publication. Figure 1 legend was missing the footnote for *.]
Outcome Measures
Stroke/SE and MB were the primary outcomes. Stroke/SE and MB were identified based on hospitalizations with stroke/SE or MB as the principal (Medicare and Optum) or first listed (Humana and PharMetrics) diagnosis. Stroke/SE was stratified by ischemic stroke, hemorrhagic stroke, and SE; MB was stratified by gastrointestinal (GI) bleeding, ICH, and MB at other key sites (Table S1).17, 18 All‐cause mortality was evaluated using the CMS data, given that only CMS data provide reliable validated death information from the Social Security Administration.
The follow‐up period was from the day after the index date to the earliest of the following: 30 days after the discontinuation date, switch date, death (only inpatient death for the commercial databases and all‐cause death for the Medicare database), end of continuous medical or pharmacy plan enrollment, or the end of the study period (September 30, 2015).
Statistical Methodology
Propensity score matching (PSM) was chosen for the comparative analysis of the effectiveness and safety profiles among very old NVAF patients initiated on OACs. The rationale of PSM and the detailed matching process can be found in previous publications.14, 15 Six PSM pairs were created between NOACs and warfarin (apixaban vs warfarin, dabigatran vs warfarin, and rivaroxaban vs warfarin) and between the NOACs (apixaban vs dabigatran, apixaban vs rivaroxaban, and dabigatran vs rivaroxaban).
The propensity scores were generated using logistic regressions with treatment cohorts and baseline characteristics as covariates. The baseline covariates included demographics, Charlson Comorbidity Index score,19 bleeding and stroke/SE history, comorbidities, and baseline co‐medications (complete list of covariates in Table S2). After PSM, the balance of covariates was checked based on standardized differences with a threshold of 10%.20 Patients in each arm of the comparison were pooled for analysis after PSM in each data set. The P values <.05 were considered statistically significant.
Cox proportional hazard models with robust sandwich estimates were used to evaluate the comparative risk of stroke/SE and MB in each PSM cohort in the pooled population, and all‐cause mortality in each PSM cohort in the Medicare population.21 Given that all the baseline covariates were balanced after matching, only OAC treatment was included as an independent variable in the Cox models.
Subgroup and Sensitivity Analyses
In the first subgroup analysis, the risk of stroke/SE and MB were examined separately in standard dose NOAC (apixaban 5 mg, dabigatran 150 mg, rivaroxaban 20 mg) and lower dose NOAC (apixaban 2.5 mg, dabigatran 75 mg, rivaroxaban 15 mg/10 mg) patients based on the index prescription dosage. PSM was reconducted in each data set stratified by dose of NOACs before pooling. After PSM and the pooling process, the same statistical methods were used as the main analysis.
The second subgroup analysis was conducted to examine the risk of stroke/SE, MB, and all‐cause mortality associated with different age categories in the very old population. Only CMS data provide comprehensive age information that would allow this analysis, whereas commercial data sets do not include specific age data beyond a maximum age (eg, 84 y) due to privacy policies. Age strata (80‐84, 85‐89, and ≥ 90 y) were created in the CMS post‐PSM population. In each age stratum, the post‐PSM baseline covariates with standardized differences more than 10% were included in the Cox proportional hazards model. The statistical significance of the interaction between age and treatment was evaluated in the Cox models based on P value with a threshold of .10.
Two sensitivity analyses were conducted to examine the robustness of the comparative risk of stroke/SE and MB. In the first sensitivity analysis, Cox proportional hazards models were separately conducted for CMS and the pooled commercial data populations. In the second sensitivity analysis, all‐cause mortality was included in the models as a competing risk in the CMS population using the Fine and Gray method.22
Because this study did not involve the collection, use, or transmittal of individual identifiable data, institutional review board approval was not required. Both the data sets and the security of the offices where analysis was completed (and where the data sets are kept) met the requirements of the Health Insurance Portability and Accountability Act of 1996.
RESULTS
After applying the selection criteria, a total of 103 511 NVAF patients 80 years or older were identified including 19 752 (19.1%) apixaban, 6741 (6.5%) dabigatran, 27 217 (26.3%) rivaroxaban, and 49 801 (48.1%) warfarin patients (Figure 1). More than 80% of the patients had CHA2DS2‐VASc of 4 or higher, and more than 70% had HAS‐BLED of 3 or higher. For apixaban, dabigatran, and rivaroxaban patients, 52% (2.5 mg), 37% (75 mg), and 51% (43% on 15 mg and 8% on 10 mg) had lower dosage regimens, respectively (Table S2).
The unadjusted incidence rate of stroke/SE was 1.8 (apixaban), 2.2 (dabigatran), 2.2 (rivaroxaban), and 2.8 (warfarin) per 100 person‐years. The unadjusted incidence rate of MB was 4.9 (apixaban), 6.4 (dabigatran), 8.6 (rivaroxaban), and 7.4 (warfarin) per 100 person‐years (Table S2).
After PSM, 88 582 unique patients were included, with 18 897 apixaban‐warfarin, 6698 dabigatran‐warfarin, 25 917 rivaroxaban‐warfarin, 6477 apixaban‐dabigatran, 18 558 apixaban‐rivaroxaban, and 6683 dabigatran‐rivaroxaban PSM pairs. The median follow‐up time was 5 to 6 months for the matched cohorts. Select baseline characteristics of the matched populations are shown in Tables 1 and 2 . The complete baseline characteristics can be found in Tables S3 and S4.
Table 1.
PSM Baseline Characteristics for Apixaban vs Warfarin, Dabigatran vs Warfarin, and Rivaroxaban vs Warfarin
| Apixaban (N = 18 897) | Warfarin (N = 18 897) | Dabigatran (N = 6698) | Warfarin (N = 6698) | Rivaroxaban (N = 25 917) | Warfarin (N = 25 917) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | |
| Agea | 85.3 | 4.0 | 85.3 | 4.0 | 84.8 | 3.8 | 84.8 | 3.8 | 85.1 | 3.9 | 85.1 | 3.9 |
| 80‐83 | 7362 | 39.0% | 7335 | 38.8% | 2978 | 44.5% | 2978 | 44.5% | 10 774 | 41.6% | 10 771 | 41.6% |
| ≥84 | 11 535 | 61.0% | 11 562 | 61.2% | 3720 | 55.5% | 3720 | 55.5% | 15 143 | 58.4% | 15 146 | 58.4% |
| Sex | ||||||||||||
| Male | 7800 | 41.3% | 7742 | 41.0% | 2826 | 42.2% | 2794 | 41.7% | 10 854 | 41.9% | 10 804 | 41.7% |
| Female | 11 097 | 58.7% | 11 155 | 59.0% | 3872 | 57.8% | 3904 | 58.3% | 15 063 | 58.1% | 15 113 | 58.3% |
| Baseline comorbidity | ||||||||||||
| Deyo‐Charlson Comorbidity Index | 3.5 | 2.7 | 3.6 | 2.7 | 3.2 | 2.6 | 3.2 | 2.6 | 3.4 | 2.7 | 3.3 | 2.7 |
| CHA2DS2‐VASc score | 4.8 | 1.4 | 4.8 | 1.4 | 4.7 | 1.4 | 4.7 | 1.4 | 4.7 | 1.4 | 4.7 | 1.4 |
| HAS‐BLED scoreb | 3.4 | 1.2 | 3.4 | 1.2 | 3.3 | 1.2 | 3.3 | 1.2 | 3.3 | 1.2 | 3.3 | 1.2 |
| Index prescription dose | ||||||||||||
| Standard dosec | 9044 | 47.9% | 4195 | 62.6% | 12 576 | 48.5% | ||||||
| Lower dosed | 9853 | 52.1% | 2503 | 37.4% | 13 341 | 51.5% | ||||||
| Follow‐up time, d | 209.7 | 187.4 | 253.9 | 232.8 | 255.2 | 248.1 | 261.1 | 237.8 | 251.5 | 237.7 | 255.2 | 233.9 |
| Median | 144 | 164 | 148 | 171 | 156 | 164 | ||||||
Abbreviations: ACE/ARB, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers; NSAIDs, nonsteroidal anti‐inflammatory drugs; PSM, propensity score matching; SD, standard deviation; SE, systemic embolism.
The maximum age in PharMetrics is 84; the maximum age in Optum and Humana is 89 years. Patients older than these thresholds are set to the maximum age due to privacy concerns.
Because the international normalized ratio value is not available in the databases, a modified HAS‐BLED score was calculated with a range of 0 to 8.
Standard dose: 5 mg apixaban, 150 mg dabigatran, 20 mg rivaroxaban.
Lower dose: 2.5 mg apixaban, 75 mg dabigatran, 10 or 15 mg rivaroxaban. A total of 2162 patients in the rivaroxaban‐warfarin cohort received 10 mg rivaroxaban.
Table 2.
PSM Baseline Characteristics for Apixaban vs Dabigatran, Apixaban vs Rivaroxaban, and Dabigatran vs Rivaroxaban
| Apixaban (N = 6477) | Dabigatran (N = 6477) | Apixaban (N = 18 558) | Rivaroxaban (N = 18 558) | Dabigatran (N = 6683) | Rivaroxaban (N = 6683) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | |
| Agea | 84.9 | 3.9 | 84.9 | 3.9 | 85.3 | 4.0 | 85.3 | 4.0 | 84.8 | 3.8 | 84.8 | 3.9 |
| 80‐83 | 2864 | 44.2% | 2779 | 42.9% | 7329 | 39.5% | 7345 | 39.6% | 2972 | 44.5% | 2972 | 44.5% |
| ≥84 | 3613 | 55.8% | 3698 | 57.1% | 11 229 | 60.5% | 11 213 | 60.4% | 3711 | 55.5% | 3711 | 55.5% |
| Sex | ||||||||||||
| Male | 2681 | 41.4% | 2702 | 41.7% | 7596 | 40.9% | 7470 | 40.3% | 2817 | 42.2% | 2838 | 42.5% |
| Female | 3796 | 58.6% | 3775 | 58.3% | 10 962 | 59.1% | 11 088 | 59.7% | 3866 | 57.8% | 3845 | 57.5% |
| Baseline comorbidity | ||||||||||||
| Deyo‐Charlson Comorbidity Index | 3.3 | 2.6 | 3.2 | 2.6 | 3.5 | 2.7 | 3.5 | 2.7 | 3.2 | 2.6 | 3.2 | 2.6 |
| CHA2DS2‐VASc score | 4.7 | 1.4 | 4.7 | 1.4 | 4.8 | 1.4 | 4.8 | 1.4 | 4.7 | 1.4 | 4.7 | 1.4 |
| HAS‐BLED scoreb | 3.3 | 1.2 | 3.3 | 1.2 | 3.4 | 1.2 | 3.4 | 1.2 | 3.3 | 1.2 | 3.3 | 1.2 |
| Index prescription dose | ||||||||||||
| Standard dosec | 3310 | 51.1% | 4038 | 62.3% | 8961 | 48.3% | 8746 | 47.1% | 4194 | 62.8% | 3374 | 50.5% |
| Lower dosed | 3167 | 48.9% | 2439 | 37.7% | 9597 | 51.7% | 9812 | 52.9% | 2489 | 37.2% | 3309 | 49.5% |
| Follow‐up time, d | 217.0 | 192.2 | 254.9 | 247.9 | 210.8 | 187.9 | 248.1 | 235.8 | 255.3 | 248.2 | 255.7 | 242.7 |
| Median | 149 | 148 | 145 | 153 | 148 | 154 | ||||||
Abbreviations: ACE/ARB, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers; DOAC, direct oral anticoagulant; NSAIDs, nonsteroidal anti‐inflammatory drugs; PSM, propensity score matching; SD, standard deviation; SE, systemic embolism.
The maximum age in PharMetrics is 84; the maximum age in Optum and Humana is 89 years. Patients older than these thresholds are set to the maximum age due to privacy concerns.
Because the international normalized ratio value is not available in the databases, a modified HAS‐BLED score was calculated with a range of 0 to 8.
Standard dose: 5 mg apixaban, 150 mg dabigatran, 20 mg rivaroxaban.
Lower dose: 2.5 mg apixaban, 75 mg dabigatran, 10 or 15 mg rivaroxaban. A total of 1550 and 516 patients in the apixaban‐rivaroxaban and dabigatran‐rivaroxaban cohorts, respectively, received 10 mg rivaroxaban.
The pre‐ and post‐PSM baseline characteristics in the very old CMS population meeting all eligibility criteria are shown in Tables S5 to S7. The CMS patient population was older, but other baseline characteristics generally had a similar trend compared with the pooled population.
NOAC‐Warfarin Comparisons
The Kaplan‐Meier curves for cumulative incidence rates of stroke/SE and MB in the matched populations are shown in Figure S1A and S1B.
In the comparisons with warfarin, all NOACs were associated with a lower risk of stroke/SE: apixaban (hazard ratio [HR] = .58; 95% confidence interval [CI] = .49‐.69), dabigatran (HR = .77; 95% CI = .60‐.99), and rivaroxaban (HR = .74; 95% CI = .65‐.85). Ischemic stroke was the most prevalent type of stroke/SE, of which the risk was lower in apixaban and rivaroxaban patients compared with warfarin patients. All NOACs were associated with a lower risk of hemorrhagic stroke vs warfarin.
Apixaban (HR = .60; 95% CI = .54‐.67) was associated with a lower risk of MB compared with warfarin. Dabigatran (HR = .92; 95% CI = .78‐1.07) was associated with a similar risk, and rivaroxaban (HR = 1.16; 95% CI = 1.07‐1.24) was associated with a higher risk of MB compared with warfarin. GI bleeding was most prevalent, which showed the same trend as the overall MB. All NOACs were associated with a lower risk of ICH vs warfarin (Figure 2A).
Figure 2.
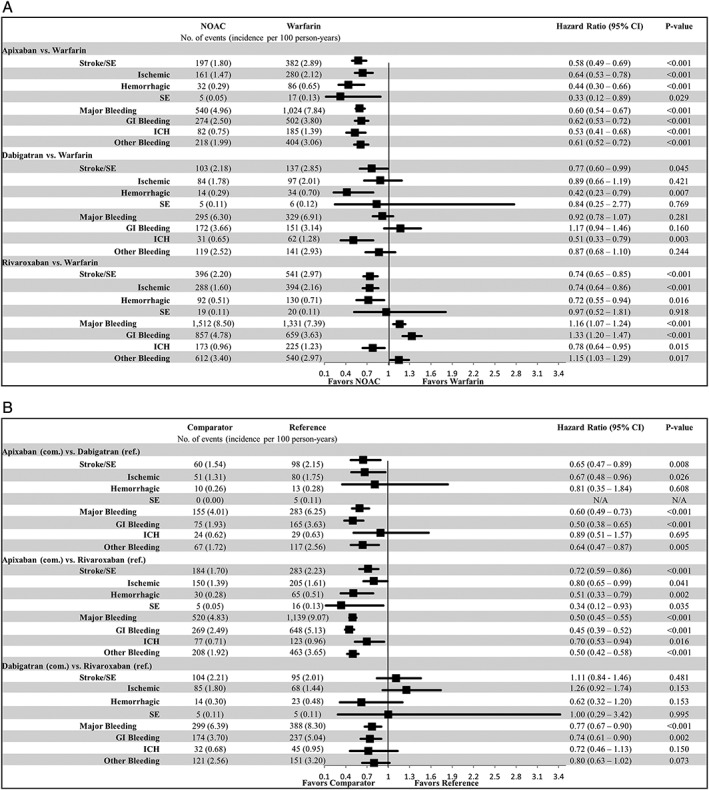
Propensity score matched incidence rates and hazard ratios of stroke/systemic embolism (SE) and major bleeding for A, non–vitamin K antagonist oral anticoagulants (NOACs) vs warfarin and B, NOAC vs NOAC. CI, confidence interval; GI, gastrointestinal; ICH, intracranial hemorrhage; SE, systemic embolism. [Late correction added May 22, 2019, after first online publication. Figure 2, Part A was replaced to correct a statistic related to NOAC incidence of major bleeding.]
NOAC‐NOAC Comparisons
In the comparisons between NOACs, apixaban was associated with a lower risk of stroke/SE and MB compared with dabigatran (stroke/SE: HR = .65; 95% CI = .47‐.89; MB: HR = .60; 95% CI = .49‐.73) and rivaroxaban (stroke/SE: HR = .72; 95% CI = .59‐.86; MB: HR = .50; 95% CI = .45‐.55). Dabigatran was associated with a similar risk of stroke/SE (HR = 1.11; 95% CI = .84‐1.46), and lower risk of MB (HR = .77; 95% CI = .67‐.90) compared with rivaroxaban (Figure 2B).
All‐Cause Mortality
In the CMS population, compared with warfarin, all NOACs were associated with a lower risk of all‐cause mortality: apixaban (HR = .61; 95% CI = .56‐.67), dabigatran (HR = .87; 95% CI = .75‐.99), and rivaroxaban (HR = .87; 95% CI = .81‐.93). Apixaban was associated with a lower risk of all‐cause mortality compared with dabigatran (HR = .78; 95% CI = .66‐.91) and rivaroxaban (HR = .71; 95% CI = .64‐.77). Dabigatran was associated with a similar risk of all‐cause mortality (HR = .95; 95% CI = .82‐1.09) compared with rivaroxaban (Figure S2).
Subgroup and Sensitivity Analyses
In the dose subgroup analysis among the pooled population, the pre‐ and post‐PSM baseline characteristics are shown in Tables S8 to S13. After PSM, both lower and standard dose patients showed broadly consistent results to the main analysis (Figure S3).
In the age subgroup analysis among the CMS population, the results for stroke/SE, MB, and all‐cause mortality were generally consistent with the main analysis. Several significant interactions were found for all‐cause mortality. For example, compared with warfarin, the risk of all‐cause mortality was lower for rivaroxaban patients aged 80 to 84 years but was similar for those older than 85 years (Figure S4).
The two sensitivity analyses showed generally consistent results as the main analysis that supported the robustness of the findings for comparative risk of stroke/SE and MB (Tables S14 and S15).
DISCUSSION
This comparative effectiveness and safety analysis among patients aged 80 years or older in the ARISTOPHANES study showed that very old NVAF patients who initiated apixaban, dabigatran, or rivaroxaban were associated with lower rates of stroke/SE compared with very old patients who initiated warfarin, but the safety results varied across NOACs. In the very old CMS Medicare population, all NOACs were associated with a lower risk of all‐cause mortality compared with warfarin.
Very old subjects were underrepresented in the pivotal phase III NOAC RCTs. Subgroup analyses by age in the RCTs showed that older patients with NVAF who were treated with OACs could have a distinct effectiveness and safety profile compared with younger patients.23, 24, 25 For example, the analysis of the RE‐LY trial showed a significant interaction between MB and age among NVAF patients treated with dabigatran and warfarin: while 110 mg and 150 mg twice/day dabigatran were associated with a lower risk of MB among patients younger than 75 years, they were associated with a similar risk in older patients (≥75 y).23 The 110 mg and 150 mg twice/day dabigatran were associated with a lower risk of stroke/SE compared with warfarin for both young and older NVAF patients.23 Similar trends were observed in our analysis of patients aged 80 years or older; dabigatran was associated with a similar risk of MB and lower risk of stroke/SE compared with warfarin. In the ARISTOTLE and ROCKET AF trials, no interactions between stroke/SE or MB and age were found for patients younger than 75 years and older (≥75 y) patients.24, 25 In the ROCKET AF trial, 20 mg and 15 mg once/day rivaroxaban showed similar risk of stroke/SE and MB compared with warfarin in both age cohorts.24 In the ARISTOTLE trial, patients prescribed apixaban had a lower risk of stroke/SE and MB in both younger and older patients.25
In addition to RCTs, very few real‐world studies have been conducted to compare the safety and effectiveness between OACs focusing on very old NVAF patients.13, 26, 27, 28 Using different age categories and real‐world data, these studies provide supplementary information on the comparative efficacy and safety between NOACs and warfarin in clinical practice. A population‐based analysis on linked claims data among patients aged 80 years or older in northeastern Italy found numerically lower risks of ischemic stroke and MB among NOACs (dabigatran, rivaroxaban, or apixaban) compared with warfarin users.26 Similarly, a study among patients aged 90 years or older using the National Health Insurance Research Database in Taiwan found that NOACs were associated with a lower risk of ICH with no difference in ischemic stroke.27 A retrospective claims study using US MarketScan data comparing rivaroxaban and warfarin found that, among NVAF patients aged 80 years or older, rivaroxaban was associated with a lower risk of stroke/SE and a similar risk of MB compared with warfarin.13 This is also evident in a meta‐analysis including both real‐world studies and RCTs, where Bai et al concluded that among patients aged 65 years or older, NOACs were associated with a decrease in risk of MB and stroke/SE compared with warfarin.29
Several real‐world studies were conducted with subgroup analysis by age including age 80 years or older or 85 years, as subcategories.30, 31, 32 Consistent with previous real‐world studies, our study shows generally more favorable outcomes for NOACs vs warfarin in very old patients.
This study is by far the largest retrospective observational study examining the comparative effectiveness and safety between OACs with the focus of very old NVAF patients. In addition to the comparisons between NOACs and warfarin, which would supplement the results of the RCTs for each NOAC, comparisons between each NOAC were also conducted. Moreover, the CMS Medicare data were also used individually for the analysis of all‐cause mortality and the age subgroup analysis. By pooling four data sets and including a comprehensive comparison of the OACs, this study was able to add supplementary information to the literature in assisting the decision of treatment selection for stroke prevention among very old NVAF patients.
Limitations
As with many real‐world studies, our study has several limitations. This study was designed to examine the associations between clinical outcomes and OAC treatment, so causal relationships cannot be evaluated. As is the nature with retrospective observational studies, our study was subject to confounders. Although PSM with a comprehensive list of covariates was used, this study remains bound by the limitation of claims data; variables such as over‐the‐counter use of aspirin, serum creatinine/creatinine clearance, and laboratory values are unavailable and thus were not controlled for in the model. International Classification of Diseases, Ninth Revision, Clinical Modification, codes were used to identify baseline characteristics and outcomes that may lack clinical accuracy. Moreover, age is top coded in several data sets that may have caused the underestimation of the mean age. Additionally, we are unable to determine time in therapeutic range for patients prescribed warfarin. The functional characteristics of patients are also unknown. Nevertheless, by analyzing the real‐world data, our study reflects the quality of anticoagulation experienced by patients in clinical practice. For example, given that very old patients are likely to have a poorer measurement for the international normalized ratio in real‐world clinical practice, this may in part explain the higher risk of stroke/SE for warfarin users in our study. Due to the lack of data on renal function and body weight, it is not clear whether patients used a lower dose of NOACs appropriately. In addition, at the time of the study, no reversal agents were available on the market for NOACs for patients with life‐threatening bleeding or requiring urgent surgery, which may have impacted the choice of OAC treatment and the safety results. Lastly, although the main and the additional subgroup analyses added healthcare outcome evidence related to the very old NVAF patient population who were newly prescribed OACs, limited generalizability of the results to a different population, such as an institutionalized older NVAF population, may be expected.
In conclusion, this retrospective observational study among very old (≥80 y) NVAF patients newly initiated on OACs showed that, compared with warfarin, NOACs were associated with lower risks of stroke/SE and all‐cause mortality, and various comparative risks of MB. This study adds to the growing body of evidence in a population that is vulnerable and also at high risk of NVAF‐related stroke.
Supporting information
Table S1. International Classification of Diseases, Ninth Revision, Clinical Modification, codes for stroke/systemic embolism and major bleeding
Table S2. Baseline characteristics and outcomes for prematched apixaban, dabigatran, rivaroxaban, and warfarin patients
Table S3. Propensity score matched baseline characteristics for apixaban vs warfarin, dabigatran vs warfarin, and rivaroxaban vs warfarin
Table S4. Propensity score matched baseline characteristics for apixaban vs dabigatran, apixaban vs rivaroxaban, and dabigatran vs rivaroxaban
Table S5. Baseline characteristics and outcomes for pre‐matched apixaban, dabigatran, rivaroxaban, and warfarin patients in the CMS population
Table S6. Baseline characteristics for the propensity score matched population in CMS data: non–vitamin K antagonist oral anticoagulants vs warfarin
Table S7. Baseline characteristics for the propensity score matched population in CMS data: non–vitamin K antagonist oral anticoagulant (NOAC) vs NOAC
Table S8. Baseline and outcome characteristics for the pre–propensity score matched population of the standard dose patients
Table S9. Baseline characteristics for the propensity score matched population of the standard dose patients: non–vitamin K antagonist oral anticoagulants vs warfarin
Table S10. Baseline characteristics for the propensity score matched population of the standard dose patients: non–vitamin K antagonist oral anticoagulant (NOAC) vs NOAC
Table S11. Baseline and outcome characteristics for the pre– propensity score matched population of the lower dose patients
Table S12. Baseline characteristics for the propensity score matched population of the lower dose: non–vitamin K antagonist oral anticoagulants vs warfarin
Table S13. Baseline characteristics for the propensity score matched population of the lower dose patients: non–vitamin K antagonist oral anticoagulants (NOAC) vs NOAC
Table S14. Hazard ratios of stroke/systemic embolism and major bleeding in the CMS vs commercial populations (sensitivity analysis)
Table S15. Hazard ratios of stroke/systemic embolism and major bleeding, accounting for death as a competing event in the CMS population (sensitivity analysis)
Figure S1. A, Cumulative incidence of stroke/systemic embolism (SE) and major bleeding in non–vitamin K antagonist oral anticoagulants (NOAC)‐warfarin propensity score matched cohorts. B, Cumulative incidence of stroke/SE and major bleeding in NOAC‐NOAC propensity score matched cohorts
Figure S2. Propensity score matched incidence rates and hazard ratios of all‐cause mortality
Figure S3. Propensity score matched incidence rates and hazard ratios of stroke/systemic embolism and major bleeding for dose subgroup analysis
Figure S4. Propensity score matched incidence rates and hazard ratios of stroke/systemic embolism, major bleeding, and all‐cause mortality for age subgroup analysis
ACKNOWLEDGMENTS
Conflict of Interest
Steven Deitelzweig is a consultant for Bayer/Janssen, Bristol Myers Squibb Company/Pfizer Inc., Daiichi‐Sankyo, Portola, and Boehringer Ingelheim, and he has been on the speakers’ bureau for Janssen, Bristol Myers Squibb Company/Pfizer Inc., and Boehringer Ingelheim. Allison Keshishian is a paid employee of STATinMED Research, which is a paid consultant to Pfizer and Bristol‐Myers Squibb in connection with this study and the development of this manuscript. Xiaoyan Li, Amiee Kang, Amol Dhamane, Neeraja Balachander, Lisa Rosenblatt, Xianying Pan, Anagha Nadkarni, and Alessandra B. Garcia Reeves are paid employees of Bristol‐Myers Squibb with ownership of stocks in Bristol‐Myers Squibb Company. Xuemei Luo, Jack Mardekian, and Manuela Di Fusco are paid employees of Pfizer Inc., with ownership of stocks in Pfizer Inc. Huseyin Yuce has nothing to disclose. Gregory Y.H. Lip is a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi‐Sankyo. He is a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees are directly received personally.
Author Contributions
Interpretation of the data and substantially contributed to critical revision of the intellectual content: All authors. Analyzed the data, contributed to the acquisition of the data, and wrote the manuscript: Keshishian.
Sponsor's Role
This study was funded by Bristol‐Myers Squibb and Pfizer Inc. The funders provided support in the form of salaries for authors Li, Kang, Dhamane, Luo, Balachander, Rosenblatt, Mardekian, Pan, Nadkarni, Di Fusco, and Garcia Reeves. The funders also provided consultation fees for author Kang of STATinMED Research. However, the funders did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke. 2013;44(11):3103‐3108. [DOI] [PubMed] [Google Scholar]
- 2. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263‐272. [DOI] [PubMed] [Google Scholar]
- 3. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the euro heart survey. Chest. 2010;138(5):1093‐1100. [DOI] [PubMed] [Google Scholar]
- 4. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121‐1201. [DOI] [PubMed] [Google Scholar]
- 5. Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision‐making. Thromb Haemost. 2017;117(7):1230‐1239. [DOI] [PubMed] [Google Scholar]
- 6. Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta‐analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857‐864. [DOI] [PubMed] [Google Scholar]
- 7. Kim IS, Kim HJ, Kim TH, et al. Non‐vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: a systematic review and meta‐analysis. J Cardiol. 2018;72(2):105‐112. [DOI] [PubMed] [Google Scholar]
- 8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383(9921):955‐962. [DOI] [PubMed] [Google Scholar]
- 9. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330‐1393. [DOI] [PubMed] [Google Scholar]
- 10. Sommerauer C, Schlender L, Krause M, et al. Effectiveness and safety of vitamin K antagonists and new anticoagulants in the prevention of thromboembolism in atrial fibrillation in older adults ‐ a systematic review of reviews and the development of recommendations to reduce inappropriate prescribing. BMC Geriatr. 2017;17(Suppl 1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wehling M, Collins R, Gil VM, et al. Appropriateness of oral anticoagulants for the long‐term treatment of atrial fibrillation in older people: results of an evidence‐based review and international consensus validation process (OAC‐FORTA 2016). Drugs Aging. 2017;34(7):499‐507. [DOI] [PubMed] [Google Scholar]
- 12. Bo M, Grisoglio E, Brunetti E, Falcone Y, Marchionni N. Oral anticoagulant therapy for older patients with atrial fibrillation: a review of current evidence. Eur J Intern Med. 2017;41:18‐27. [DOI] [PubMed] [Google Scholar]
- 13. Coleman CI, Weeda ER, Nguyen E, Bunz TJ, Sood NA. Effectiveness and safety of rivaroxaban vs. warfarin in patients 80+ years of age with non‐valvular atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2018;4(4):328‐329. [DOI] [PubMed] [Google Scholar]
- 14. Li XS, Deitelzweig S, Keshishian A, et al. Effectiveness and safety of apixaban versus warfarin in non‐valvular atrial fibrillation patients in “real‐world” clinical practice. Thromb Haemost. 2017;117(6):1072‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among non‐valvular atrial fibrillation patients. Stroke. 2018;49(12):2933‐2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(6):e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thigpen JL, Dillon C, Forster KB, et al. Validity of International Classification of Disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8(1):8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray W. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 20. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28(25):3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496‐509. [Google Scholar]
- 23. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123(21):2363‐2372. [DOI] [PubMed] [Google Scholar]
- 24. Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;130(2):138‐146. [DOI] [PubMed] [Google Scholar]
- 25. Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35(28):1864‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zoppellaro G, Zanella L, Denas G, et al. Different safety profiles of oral anticoagulants in very elderly non‐valvular atrial fibrillation patients. A retrospective propensity score matched cohort study. Int J Cardiol. 2018;265:103‐107. [DOI] [PubMed] [Google Scholar]
- 27. Chao TZ, Liu CJ, Lin YJ, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation. 2018;138(1):37‐47. [DOI] [PubMed] [Google Scholar]
- 28. Lai CL, Chen HM, Liao MT, Lin TT. Dabigatran, rivaroxaban, and warfarin in the oldest adults with atrial fibrillation in Taiwan. J Am Geriatr Soc. 2018;66(8):1567‐1574. [DOI] [PubMed] [Google Scholar]
- 29. Bai Y, Guo SD, Deng H, et al. Effectiveness and safety of oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta‐regression analysis. Age Ageing. 2018;47(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 30. Forslund T, Wettermark B, Andersen M, Hjemdahl P. Stroke and bleeding with non‐vitamin K antagonist oral anticoagulant or warfarin treatment in patients with non‐valvular atrial fibrillation: a population‐based cohort study. Europace. 2018;20(3):420‐428. [DOI] [PubMed] [Google Scholar]
- 31. Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for non‐valvular atrial fibrillation. Circulation. 2015;131(2):157‐164. [DOI] [PubMed] [Google Scholar]
- 32. Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176(11):1662‐1671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, Ninth Revision, Clinical Modification, codes for stroke/systemic embolism and major bleeding
Table S2. Baseline characteristics and outcomes for prematched apixaban, dabigatran, rivaroxaban, and warfarin patients
Table S3. Propensity score matched baseline characteristics for apixaban vs warfarin, dabigatran vs warfarin, and rivaroxaban vs warfarin
Table S4. Propensity score matched baseline characteristics for apixaban vs dabigatran, apixaban vs rivaroxaban, and dabigatran vs rivaroxaban
Table S5. Baseline characteristics and outcomes for pre‐matched apixaban, dabigatran, rivaroxaban, and warfarin patients in the CMS population
Table S6. Baseline characteristics for the propensity score matched population in CMS data: non–vitamin K antagonist oral anticoagulants vs warfarin
Table S7. Baseline characteristics for the propensity score matched population in CMS data: non–vitamin K antagonist oral anticoagulant (NOAC) vs NOAC
Table S8. Baseline and outcome characteristics for the pre–propensity score matched population of the standard dose patients
Table S9. Baseline characteristics for the propensity score matched population of the standard dose patients: non–vitamin K antagonist oral anticoagulants vs warfarin
Table S10. Baseline characteristics for the propensity score matched population of the standard dose patients: non–vitamin K antagonist oral anticoagulant (NOAC) vs NOAC
Table S11. Baseline and outcome characteristics for the pre– propensity score matched population of the lower dose patients
Table S12. Baseline characteristics for the propensity score matched population of the lower dose: non–vitamin K antagonist oral anticoagulants vs warfarin
Table S13. Baseline characteristics for the propensity score matched population of the lower dose patients: non–vitamin K antagonist oral anticoagulants (NOAC) vs NOAC
Table S14. Hazard ratios of stroke/systemic embolism and major bleeding in the CMS vs commercial populations (sensitivity analysis)
Table S15. Hazard ratios of stroke/systemic embolism and major bleeding, accounting for death as a competing event in the CMS population (sensitivity analysis)
Figure S1. A, Cumulative incidence of stroke/systemic embolism (SE) and major bleeding in non–vitamin K antagonist oral anticoagulants (NOAC)‐warfarin propensity score matched cohorts. B, Cumulative incidence of stroke/SE and major bleeding in NOAC‐NOAC propensity score matched cohorts
Figure S2. Propensity score matched incidence rates and hazard ratios of all‐cause mortality
Figure S3. Propensity score matched incidence rates and hazard ratios of stroke/systemic embolism and major bleeding for dose subgroup analysis
Figure S4. Propensity score matched incidence rates and hazard ratios of stroke/systemic embolism, major bleeding, and all‐cause mortality for age subgroup analysis


