Abstract
Background
Chemerin is an adipokine that signals through the G protein‐coupled receptor ChemR23 and is associated with inflammation, glucose homeostasis, lipid metabolism and renal function, all of which strongly influence cardiovascular risk. However, elevated chemerin provides a survival advantage in patients with chronic kidney disease (CKD), but how this relates to the cardiovascular phenotype is unknown.
Objectives
The aim of the present study was to establish the association of chemerin with coronary calcification and to determine the effects of chemerin signalling, through ChemR23, in vascular smooth muscle cell (VSMC) calcification.
Methods
Plasma chemerin was measured in 113 patients with CKD and 50 healthy controls. All patients underwent computed tomography to determine coronary artery calcium (CAC) score. VSMCs were isolated from wild‐type and ChemR23 knock‐out mice and treated with chemerin.
Results
Multivariate analyses established creatinine, cholesterol, body mass index and tumour necrosis factor as significant confounders for circulating chemerin levels. Despite these positive associations with renal function, cardiometabolic risk factors and inflammation, chemerin was inversely associated with CAC both in an age‐ and sex‐adjusted analysis and in a multivariate analysis adjusting for the aforementioned confounders. In addition, circulating chemerin levels were associated with the calcification inhibitors matrix gla protein (MGP) and fetuin‐A. Finally, chemerin significantly reduced phosphate‐induced calcification and increased MGP expression in VSMCs, whereas chemerin was devoid of these effects in VSMCs lacking ChemR23.
Conclusion
In conclusion, these results suggest that chemerin signalling through ChemR23 in VSMCs protects against vascular calcification in CKD.
Keywords: adipokines, arterial stiffness, biomarkers, coronary artery disease, inflammation
Introduction
Chemerin is an adipokine associated with inflammation, glucose homeostasis, lipid metabolism and renal function, all of which strongly influence cardiovascular risk. However, the role of chemerin in the pathogenesis of cardiovascular disease is still a matter of debate 1. Studies of chemerin as a biomarker of coronary artery disease (CAD) have generated conflicting results with either increased levels 2 or no difference 3 in subjects with or without CAD. Such conflicting results may relate to a differential risk factor profile of the cohort studied. Indeed, the association of circulating chemerin levels with atherosclerotic plaques on contrast‐enhanced CT coronary angiography is lost after adjustment for traditional CV risk factors 4. However, to the best of our knowledge, no previous study has examined the association between chemerin and coronary calcium as detected and quantified by coronary artery calcification (CAC) score.
Importantly, in patients with CAD, chemerin is significantly and independently associated with the glomerular filtration rate (GFR) 3, and similar findings have been reported also in the general population 5 and in subjects with diabetes 6. Furthermore, chemerin levels are higher in subjects with chronic kidney disease (CKD) as compared with control subjects 7, 8, 9 and normalize after kidney transplantation 7, 8. Given the importance of renal function as a cardiovascular risk factor, the emerging role of chemerin as a biomarker has merited particular attention in this patient group.
Enigmatically, elevated chemerin is associated with a survival advantage in incident dialysis patients, despite associations with increased inflammation and dyslipidemia 10. However, the underlying associations for this apparent contradiction have remained hitherto unexplored. The signature cardiovascular phenotype in CKD is a premature vascular ageing with vascular calcification and increased arterial stiffness 11.
Chemerin is one of the ligands of the G protein‐coupled receptor ChemR23. We recently reported that another ChemR23 ligand, the proresolving lipid mediator resolvin (Rv) E1, inhibits vascular smooth muscle cell (VSMC) calcification 12. Previous studies have also indicated a determinative role of ChemR23 for VSMC phenotype 12 as well as vascular inflammation in atherosclerosis and intimal hyperplasia 13, 14. Importantly, ChemR23 is expressed in vessels derived from patients with CKD 12. However, whether chemerin alters vascular calcification through ChemR23 remains unexplored.
Based on the above, the aims of the present study were to specifically establish the association of chemerin with coronary calcification and arterial stiffness in subjects with CKD and to determine the direct effects of chemerin signalling through ChemR23 in VSMC calcification.
Materials and methods
Patient cohort
Adult CKD stage 5 patients undergoing living donor transplantation at the Department of Transplantation Surgery at Karolinska University Hospital were invited to participate in the study, which was approved by the regional committee of ethics in Stockholm and adhered to the statutes of the Declaration of Helsinki Principles. Basic characteristics of the 113 included patients are outlined in Table 1.
Table 1.
Patient characteristics and stratification according to chemerin above or below median
| All (n = 113) | Low (n = 56) | High (n = 57) | P | |
|---|---|---|---|---|
| Sex (female) | 0.35 | 0.31 | 0.39 | 0.43 |
| Age (years) | 45 (19 to 75) | 45 (20 to 75) | 45 (19 to 69) | 0.86 |
| Chemerin (ng mL−1) | 154 (68 to 257) | 124 (68 to 151) | 183 (153 to 257) | <0.001 |
| CAC | 363 (0 to 4278) | 524 (0 to 4278) | 204 (0 to 1946) | 0.032 |
| Aortic Aix (%) | 19 (−15 to 57) | 20 (−10 to 52) | 18 (−15 to 57) | 0.34 |
| Creatinine (μmol/L) | 735 (285 to 1453) | 690 (285 to 1124) | 780 (431 to 1453) | 0.032 |
| Prevalent CVD (%) | 0.14 | 0.20 | 0.08 | 0.11 |
| SBP (mmHg) | 143 (100 to 215) | 148 (100 to 215) | 139 (107 to 186) | 0.019 |
| DBP (mmHg) | 83 (59 to 110) | 85 (60 to 110) | 81 (59 to 107) | 0.064 |
| BMI (kg m−2) | 24.6 (18.7 to 38.9) | 24.0 (19.1 to 32.0) | 25.1 (18.7 to 38.9) | 0.089 |
| P‐cholesterol (mmol L−1) | 4.5 (2.5 to 8.0) | 4.3 (2.5 to 6.9) | 4.7 (2.8 to 8.0) | 0.026 |
| Diabetes (%) | 8 | 19 | 2 | 0.016 |
| B‐Hba1c (mmol/mol) | 34 (15 to 72) | 37 (16 to 72) | 32 (15 to 48) | 0.023 |
| hsCRP (mg L−1) | 2.7 (0.2 to 58) | 3.2 (0.2 to 58) | 2.2 (0.2 to 15) | 0.457 |
| IL‐6 (pg mL−1) | 1.9 (0.01 to 21) | 1.6 (0.01 to 21) | 2.1 (0.0 to 11.5) | 0.39 |
| TNF (pg mL−1) | 12.1 (4.2 to 24) | 11.0 (4.2 to 21) | 13.0 (7.3 to 24) | 0.0037 |
Aix, augmentation index; CAC, coronary artery calcium; TNF, tumour necrosis factor.
Data are expressed as mean and ranges. p<0.05 represented as bold values.
Control group
Circulating chemerin concentrations were measured in a subset of 50 healthy individuals included in a population‐based cohort of randomly selected individuals in the Stockholm region (Statistics Sweden, Stockholm, Sweden; http://www.scb.se). The median age of the controls was 64 (46–80) years, median GFR 84 (68–104) mL min−1 1.73 m−2, and 15 individuals (30%) were females.
Coronary artery calcification imaging and quantification
All patients underwent a noncontrast multidetector cardiac CT (LightSpeed VCT or Revolution CT; GE Healthcare, Milwaukee, WI, USA) with standard ECG‐gated protocol. CAC was measured using a semi‐automatic software (syngo.via ct cascoring; Siemens Healthcare, Forchheim, Germany) and assessed as a lesion with an area >1 mm2 and a peak intensity >130 Hounsfield Unit (HU) based on the Agatston method and expressed in Agatston units (AU) 15. The Agatston score is a product of density (HU) and area (mm2) of the coronary calcification and is calculated using a weighted measurement to the highest density of calcification in a coronary artery. The density (HU) is scored as 1 = 130–199 HU, 2 = 200–299 HU, 3 = 300–399 HU and 4 = ≥400 HU. The Agatston score of each plaque is then summed for all image slices of the heart to determine the total CAC score. Total CAC score was defined as the sum of the CAC scores in the left main artery, the left anterior descending artery, the left circumflex artery and the right coronary artery.
Augmentation index measurement
Assessment of arterial stiffness was performed noninvasively with SphygmoCor® system (AtCor Medical, Sydney, NSW, Australia). Augmentation index (Aix) was derived from pulse wave analyses and normalized for heart rate of 75 bpm was used in accordance with Wilkinson et al. 16. SphygmoCor adjusts the AIx at an inverse rate of 4.8% for each 10 bpm increment.
Biochemical measurements
Plasma concentrations of chemerin were measured in patients and controls using a Colorimetric Sandwich ELISA Kit (Human Chemerin Quantikine® ELISA; R&D Systems, Minneapolis, MN, USA) according to manufacturer's instructions. Analyses of high‐sensitivity C‐reactive protein (hsCRP), plasma cholesterol and creatinine were performed at the Clinical Chemical Laboratory of Karolinska University Hospital, Stockholm, Sweden. Interleukin‐6 (IL‐6) and tumour necrosis factor α (TNF) were analysed by immunometric assays on an Immulite 1000 Analyzer (Siemens Healthcare Diagnostics, Los Angeles, CA, USA) using commercial kits according to the manufacturer's instructions. Matrix gla protein (MGP) and fetuin‐A were measured as previously described 17 using an antibody against dephosphorylated uncarboxylated MGP 18.
Clinical assessments
Prevalent CVD was defined based on clinical history or signs of ischemic cardiac disease, and/or presence of peripheral vascular disease and/or cerebrovascular disease. Supine blood pressure was measured using a manual sphygmomanometer. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres.
SMC isolation and stimulation
C57BL/6 and ChemR23‐deficient mice on a C57BL/6 background were bred as previously described 12, 13. Aortic VSMCs were isolated from 8‐ to 12‐week‐old male mice according to previously published protocols 12, 13. All experiments were performed between passage 3 and 5 with an initial cell density of 10 000 cells cm−2 in 5% FBS. In vitro calcification was induced by culturing VSMCs in the absence and presence of Chemerin (50 μg mL−1; R&D) for 9 days in cell culture media supplemented with 2.6 mmol L−1 inorganic phosphate (Sigma, St. Louis, MO, USA) as previously described 12.
Alizarin red staining and quantification
Calcification was assessed by staining VSMCs with alizarin red. In brief, 2% alizarin red was diluted in dH2O, filtered and pH adjusted to 4.1–4.3 with either 10% ammonium hydroxide or HCl. After calcification, VSMCs were washed twice with PBS, fixed in 4% formaldehyde, washed in water, stained in 2% alizarin red and washed. Alizarin red staining extraction and quantification of in vitro cultured cells was performed according to previously published protocol using an acetic acid based extraction method 19.
TaqMan real‐time PCR
Reverse transcription was performed using High Capacity RNA‐to‐cDNA Kit (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative real‐time PCR reaction was developed on a 7900HT Fast Real‐Time PCR system (Life Technologies) using Taqman Assay‐on‐Demand from Life Technologies (Runx2: Mm00501584_m1; MGP: Mm00485009_m1). The relative mRNA expression of the target genes was quantified by the 2−ΔCT or 2−ΔΔCT method using TATA‐binding protein (TBP: Mm01277042_m1) as endogenous control.
Statistical analyses
Data are expressed as either mean or median. Statistical significance was set at the level of P < 0.05. Comparisons between two groups were assessed with either a Student's t‐test or a nonparametric Wilcoxon test for continuous variables. Chi‐square test was used for nominal variables. Nonparametric Spearman rank correlation analysis was used to determine associations between variables. Age‐ and sex‐adjusted multivariable regression analysis was performed. Statistical analyses were performed using statistical software sigmaplot for Windows version 12.5 (Systat Software, San Jose, CA, USA).
Results
Chemerin levels in CKD stage 5 compared with healthy controls
The comparison between the patient cohort and the healthy controls revealed significantly increased chemerin levels in CKD stage 5 (Fig. 1). The baseline characteristics of the CKD stage 5 are shown in Table 1. Stratification of the latter cohort according to chemerin above and below median revealed decreased CAC and increased creatinine, respectively, as well as significant differences in several cardiometabolic risk factors and inflammation (Table 1).
Figure 1.
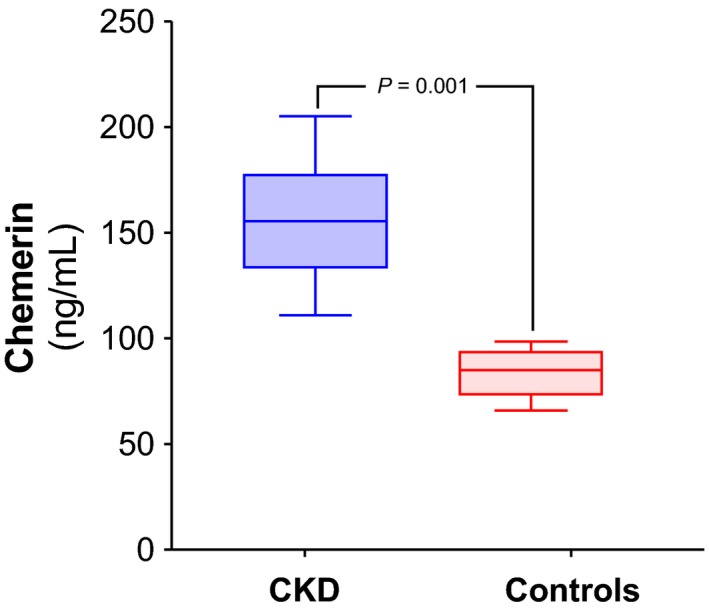
Chemerin levels in chronic kidney disease (CKD) stage 5 compared with healthy controls. Plasma chemerin was measured in 50 healthy controls and in 113 patients with CKD stage 5.
Chemerin inversely associated with CAC but not Aix
The distribution of CAC in relation to chemerin levels is shown in Fig. 2(a). Age‐ and sex‐adjusted univariate analysis revealed a weak, but significant, inverse correlation between CAC and chemerin (β = −0.19; P = 0.048). After exclusion of the 52 patients with CAC score 0 (Fig. 2b), the association between CAC and chemerin was stronger (β = −0.33; P = 0.011). In contrast, no significant association between chemerin and Aix was observed (Fig. 2c).
Figure 2.
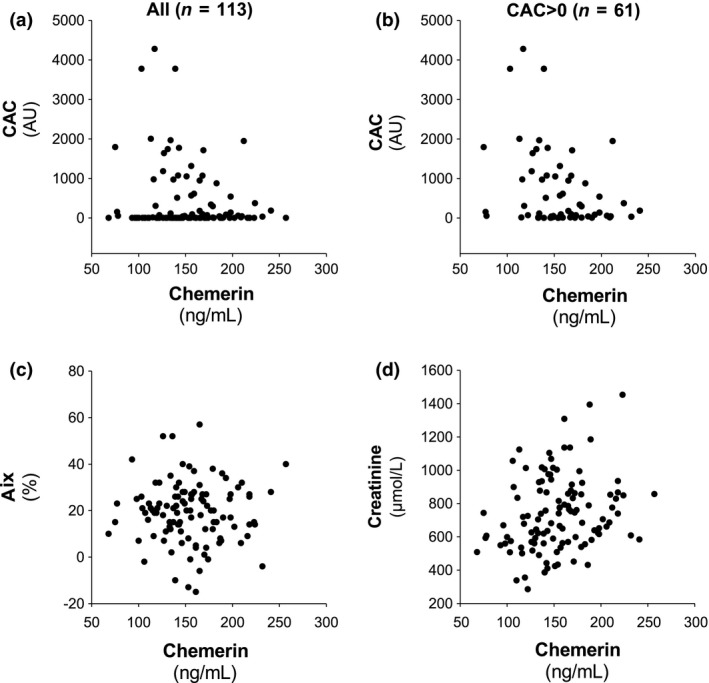
Chemerin levels in relation to coronary artery calcium score (CAC) in the whole cohort (a) and in subjects with CAC > 0 (b). The lack of association for chemerin with aortic augmentation index (Aix) is shown in panel (c). Panel (d) shows the association between chemerin and creatinine.
Renal function, cholesterol, BMI and inflammation are significantly associated with chemerin in CKD
The stratified analysis (Table 1) indicated that subjects with worsened renal function exhibited increased chemerin levels as well as increased cardiometabolic risk factors and increased inflammation (Table 1). Since all these factors may be associated with increased CAC, we next addressed these separately to determine the major confounders for the association of chemerin with CAC and Aix. The association between chemerin and creatinine is shown in Fig. 2(d). In an age‐ and sex‐adjusted analysis, chemerin was significantly associated with creatinine (β = 0.35, P < 0.001).
Since prevalent CVD and the presence of cardiometabolic risk factors may affect both chemerin levels and CAC, a multivariate analysis was performed identifying cholesterol (β = 0.21, P = 0.010) and BMI (β = 0.23, P = 0.010) as independently associated with chemerin levels, whereas prevalent CVD, systolic blood pressure, diastolic blood pressure, prevalent diabetes and Hba1c % did not significantly add to the model. Next, the three measured inflammatory mediators were evaluated in a multivariate analysis, identifying TNF (β = 0.32; P < 0.001) but not hsCRP or IL‐6 as independently associated with chemerin.
Based on the above‐mentioned associations, a multivariate model was constructed to examine the association of chemerin with the degree of coronary calcification in the cohort with CAC > 0 (Fig. 2b). Adjusting for age, sex, creatinine, cholesterol, BMI and TNF, chemerin was significantly associated with lower CAC (β: −0.40; P = 0.006). In accordance with the aforementioned results, the associations for Chemerin with Aix remained nonsignificant (P = 0.905).
Chemerin associates with calcification inhibitors MGP and fetuin‐A
To examine possible pathways involved in the observed inverse relation between chemerin and CAC, we subsequently examined the association between chemerin and calcification inhibitors. In a subset of CKD patients, MGP (n = 54) and fetuin‐A (n = 47) were analysed. In univariate analysis, chemerin was significantly and positively associated with both MGP and fetuin‐A (Fig. 3).
Figure 3.
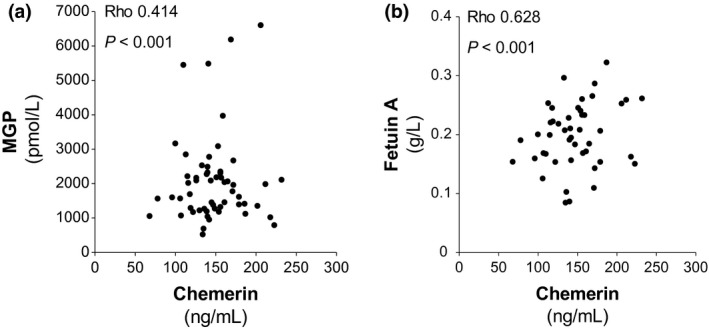
Associations between chemerin and the calcification inhibitors matrix gla protein (MGP; a) and fetuin‐A (b).
Chemerin inhibits VSMC calcification and induces MGP expression through ChemR23
To the determine the causality behind the observed associations between chemerin and calcification, murine VSMCs were cultured under calcifying conditions and treated with or without chemerin. Chemerin‐treated VSMCs exhibited significantly less calcification compared with control cells (Fig. 4a), this reduction in calcification was accompanied by a significant decrease in the expression of the pro‐osteogenic runt‐related transcription factor (Runx2) (Fig. 4a). To assess the mechanism by which chemerin reduced calcification, VSMCs from ChemR23+/+ and ChemR23−/− were treated with chemerin. In contrast to ChemR23+/+ VSMCs, chemerin did not significantly reduce phosphate‐induced calcification in ChemR23−/− VSMCs (Fig. 4b). Gene expression analysis revealed that chemerin increased MGP mRNA levels by 1.32 ± 0.19‐fold in ChemR23+/+ VSMC, which was significantly higher compared with the chemerin‐induced changes in ChemR23−/− VSMC (Fig. 4b).
Figure 4.
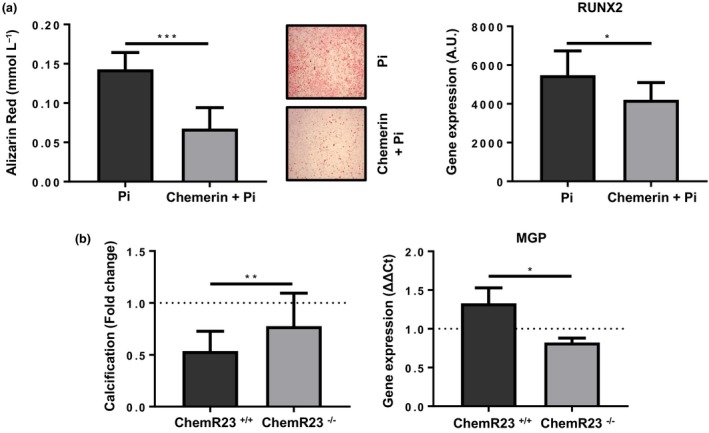
Chemerin induces matrix gla protein (MGP) expression and inhibits calcification through ChemR23 in vascular smooth muscle cells (VSMCs) (a) Quantification of phosphate‐induced calcification after 9 days and representative images n = 6. Runx2 mRNA expression in VSMCs after treatment with chemerin (50 μg mL−1) after 9 days in culture n = 5. (b) Quantification of phosphate‐induced calcification after 9 days in ChemR23+/+ and ChemR23−/− VSMCs. MGP mRNA expression in ChemR23+/+ and ChemR23−/− VSMCs after treatment with chemerin (50 μg mL−1) after 9 days in culture n = 3. Data represented as media ± SD. *P < 0.05; **P < 0.01, ***P < 0.001.
Discussion
The present study points to chemerin as a marker of lower CAC in CKD and as an inhibitor of calcification, based on the observations that chemerin levels were associated with lower CAC in CKD patients, despite positive associations with renal function, cardiometabolic risk factors and inflammation. These results were accompanied by a significant association of chemerin with two calcification inhibitors; fetuin‐A and MGP. Finally, in murine VSMC, chemerin increased MGP levels and decreased calcification through ChemR23. Taken together, these results reveal a vascular protective potential of chemerin through ChemR23 in CKD.
A striking finding in this cohort of CKD stage 5 patients was that more than 50% had prevalent CAD as defined by a CAC score >0, which is in the same range as observed in another CKD cohort 18, emphasizing the potential of the uraemic milieu to accelerate coronary calcification. Chemerin was significantly increased in CKD stage 5 compared with healthy controls and associated with creatinine in the CKD cohort in the present and previous studies 7, 8, 9, 10.
In terms of cardiovascular risk factors, chemerin has been associated with BMI 4, 9, 20, 21, 22, 23, 24, lipid levels 4, 6, 20, 21, 22, 24, 25 and hypertension 4, 20, 22, 25. Among the cardiometabolic risk factors examined in the present study, only cholesterol and BMI were independently associated with chemerin levels. This is in line with our in vivo studies that showed increased chemerin levels in mice fed a high‐fat diet 14. Furthermore, although hsCRP, TNF and IL‐6 exhibit established associations with chemerin 4, 6, 22, 24, 25, only TNF remained significantly associated with chemerin in our multivariate analysis. A causal link between TNF and chemerin has been suggested based on the observation that anti‐TNF treatment with adalimumab lowers chemerin levels 26.
The association of chemerin with lower CAC in the present study is unexpected given the association of this biomarker with decreased renal function, inflammation and other CV risk factors. Indeed, taking these identified confounders into consideration, chemerin remained independently associated with lower CAC in the subgroup with CAC > 0. Importantly, CAC is associated with arterial medial calcification in other vascular beds in this cohort 11. Thus, although chemerin associated with cardiovascular risk factors and inflammation, it may transduce protection against vascular calcification.
In favour of a causal role of chemerin behind these associations, chemerin inhibited phosphate‐induced calcification of VSMCs, a model which to some extent mimics the hyperphosphatemic conditions in CKD. Those findings were similar to the recently reported protective effects on vascular calcification by the lipid mediator RvE1 12, which shares the ChemR23 receptor with chemerin for the transduction of biological effects 27. The lack of chemerin‐induced effects on calcification in VSMC lacking the ChemR23 receptor indeed supports that ChemR23 signalling inhibits vascular calcification in response to its ligands chemerin and RvE1. Importantly, our observation of a positive correlation for chemerin with the calcification inhibitor MGP in CKD patients was accompanied by an increase in MGP mRNA levels in response to chemerin in VSMC. Since chemerin did not alter MGP levels in ChemR23‐deficient VSMCs, these results point to a causal relationship in which chemerin increases the calcification inhibitor MGP through ChemR23 locally within the vascular wall, resulting in reduced calcification and osteogenic differentiation, as exemplified by reduced VSMC levels of Runx2 in the present study. Nevertheless, also systemic effects may be involved since we also found a significant correlation between chemerin and the liver‐derived calcification inhibitor fetuin‐A 28 in the present study. These two calcification inhibitors contribute to a decreased vascular calcification 17, 29, but their association with chemerin in this context has not previously been reported. Nevertheless, the association between chemerin and fetuin‐A has been implicated in hepatic steatosis and obesity in haemodialysis patients 23. Interestingly, a genetic variant of ChemR23, protects obese subjects from excessive inflammatory burden 30, further reinforcing the notion that chemerin signalling under certain conditions may be beneficial.
A specific effect on calcification as opposed to other vascular changes is further supported by the lack of association of chemerin with Aix which in addition to calcification also depends on other structural changes of the vasculature, such as fibrosis and endothelial dysfunction 31 as well as diastolic heart function 32. However, our finding that chemerin did not associate with arterial stiffness is in contrast to studies in nonrenal patients 21, 25, suggesting a differential association between chemerin not only between CAC and Aix but possibly also between renal and nonrenal patients.
The present results hence raise a novel notion of chemerin as a marker of lower CAC in CKD and as an inhibitor of calcification. There are however certain limitations that should be acknowledged. First, although we performed an extensive phenotyping of the patients and performed multivariate analysis to adjust for several factors, it cannot be excluded that the observed association was driven by unknown confounding. Furthermore, calcification inhibitor measurements were available only in a subset of patients, and the proportion of activated MGP was not determined. It should finally be appreciated that this cohort consists of a selected group of CKD stage 5 patients selected for living donor renal transplantation. Thus, the cohort may not be fully representative of the typical older and frail haemodialysis patient cohort.
Summary and conclusions
In summary, the present study shows increased levels of chemerin in CKD patients, in which renal function, cholesterol, BMI and the inflammatory mediator TNF were major confounders. Despite the latter findings, our results indicate an inverse correlation with CAC. This protective effect of chemerin was transduced through ChemR23 in VSMCs and associated with increasing levels of calcification inhibitors such as MGP. In conclusion, these results provide evidence for a protective role of chemerin against vascular calcification, which potentially could contribute to the positive prognostic value of this marker in CKD patients 10.
Conflict of interest statement
None declared.
Funding statement
Supported by the Swedish Research Council (PS, MB), the Swedish Heart and Lung Foundation (PS, MB), Njurfonden (PS), Westmans Foundation (PS), Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse (MB) and the Stockholm County Council (PS, MB).
Supporting information
Carracedo M, Witasp A, Qureshi AR, Laguna‐Fernandez A, Brismar T, Stenvinkel P, Bäck M (Karolinska Institutet; Karolinska Institutet, Campus Flemingsberg; Karolinska University Hospital, Stockholm, Sweden). Chemerin inhibits vascular calcification through ChemR23 and is associated with lower coronary calcium in chronic kidney disease. J Intern Med 2019;286:449–457.
References
- 1. Ferland DJ, Watts SW. Chemerin: a comprehensive review elucidating the need for cardiovascular research. Pharmacol Res 2015; 99: 351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern Med 2011; 50: 1093–7. [DOI] [PubMed] [Google Scholar]
- 3. Leiherer A, Muendlein A, Kinz E et al High plasma chemerin is associated with renal dysfunction and predictive for cardiovascular events ‐ insights from phenotype and genotype characterization. Vascul Pharmacol 2016; 77: 60–8. [DOI] [PubMed] [Google Scholar]
- 4. Lehrke M, Becker A, Greif M et al Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol 2009; 161: 339–44. [DOI] [PubMed] [Google Scholar]
- 5. Zylla S, Rettig R, Volzke H, Endlich K, Nauck M, Friedrich N. Serum chemerin levels are inversely associated with renal function in a general population. Clin Endocrinol (Oxf) 2018; 88: 146–53. [DOI] [PubMed] [Google Scholar]
- 6. Hu W, Feng P. Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res Clin Pract 2011; 91: 159–63. [DOI] [PubMed] [Google Scholar]
- 7. Blaszak J, Szolkiewicz M, Sucajtys‐Szulc E et al High serum chemerin level in CKD patients is related to kidney function, but not to its adipose tissue overproduction. Ren Fail 2015; 37: 1033–8. [DOI] [PubMed] [Google Scholar]
- 8. Rutkowski P, Sledzinski T, Zielinska H et al Decrease of serum chemerin concentration in patients with end stage renal disease after successful kidney transplantation. Regul Pept 2012; 173: 55–9. [DOI] [PubMed] [Google Scholar]
- 9. Pfau D, Bachmann A, Lossner U et al Serum levels of the adipokine chemerin in relation to renal function. Diabetes Care 2010; 33: 171–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamamoto T, Qureshi AR, Anderstam B et al Clinical importance of an elevated circulating chemerin level in incident dialysis patients. Nephrol Dial Transplant 2010; 25: 4017–23. [DOI] [PubMed] [Google Scholar]
- 11. Mukai H, Dai L, Chen Z et al Inverse J‐shaped relation between coronary arterial calcium density and mortality in advanced chronic kidney disease. Nephrol Dial Transplant 2018. 10.1093/ndt/gfy352 [DOI] [PubMed] [Google Scholar]
- 12. Carracedo M, Artiach G, Witasp A et al The G‐protein coupled receptor ChemR23 determines smooth muscle cell phenotypic switching to enhance high phosphate‐induced vascular calcification. Cardiovasc Res 2018. 10.1093/cvr/cvy316 [DOI] [PubMed] [Google Scholar]
- 13. Artiach G, Carracedo M, Claria J, Laguna‐Fernandez A, Back M. Opposing effects on vascular smooth muscle cell proliferation and macrophage‐induced inflammation reveal a protective role for the proresolving lipid mediator receptor ChemR23 in intimal hyperplasia. Front Pharmacol 2018; 9: 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laguna‐Fernandez A, Checa A, Carracedo M et al ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low‐density lipoprotein uptake and phagocytosis in macrophages. Circulation 2018; 138: 1693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827–32. [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000; 525(Pt 1): 263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suliman ME, Garcia‐Lopez E, Anderstam B, Lindholm B, Stenvinkel P. Vascular calcification inhibitors in relation to cardiovascular disease with special emphasis on fetuin‐A in chronic kidney disease. Adv Clin Chem 2008; 46: 217–62. [DOI] [PubMed] [Google Scholar]
- 18. Meuwese CL, Olauson H, Qureshi AR et al Associations between thyroid hormones, calcification inhibitor levels and vascular calcification in end‐stage renal disease. PLoS One 2015; 10: e0132353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red‐based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 2004; 329: 77–84. [DOI] [PubMed] [Google Scholar]
- 20. Bozaoglu K, Bolton K, McMillan J et al Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007; 148: 4687–94. [DOI] [PubMed] [Google Scholar]
- 21. Yoo HJ, Choi HY, Yang SJ et al Circulating chemerin level is independently correlated with arterial stiffness. J Atheroscler Thromb 2012; 19: 59–66; discussion 7‐8. [DOI] [PubMed] [Google Scholar]
- 22. Gu P, Jiang W, Lu B, Shi Z. Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin Exp Hypertens 2014; 36: 326–32. [DOI] [PubMed] [Google Scholar]
- 23. Chen HY, Lin CC, Chiu YL et al Serum fetuin A and chemerin levels correlate with hepatic steatosis and regional adiposity in maintenance hemodialysis patients. PLoS One 2012; 7: e38415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sell H, Divoux A, Poitou C et al Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 2010; 95: 2892–6. [DOI] [PubMed] [Google Scholar]
- 25. Gu P, Cheng M, Hui X, Lu B, Jiang W, Shi Z. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J Hypertens 2015; 33: 1624–32. [DOI] [PubMed] [Google Scholar]
- 26. Herenius MM, Oliveira AS, Wijbrandts CA, Gerlag DM, Tak PP, Lebre MC. Anti‐TNF therapy reduces serum levels of chemerin in rheumatoid arthritis: a new mechanism by which anti‐TNF might reduce inflammation. PLoS One 2013; 8: e57802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Back M, Powell WS, Dahlen SE et al Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR review 7. Br J Pharmacol 2014; 171: 3551–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carracedo M, Back M. Fetuin A in aortic stenosis and valve calcification: not crystal clear. Int J Cardiol 2018; 265: 77–8. [DOI] [PubMed] [Google Scholar]
- 29. Leroux‐Berger M, Queguiner I, Maciel TT, Ho A, Relaix F, Kempf H. Pathologic calcification of adult vascular smooth muscle cells differs on their crest or mesodermal embryonic origin. J Bone Miner Res 2011; 26: 1543–53. [DOI] [PubMed] [Google Scholar]
- 30. Lopez‐Vicario C, Rius B, Alcaraz‐Quiles J et al Association of a variant in the gene encoding for ERV1/ChemR23 with reduced inflammation in visceral adipose tissue from morbidly obese individuals. Sci Rep 2017; 7: 15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benetos A, Waeber B, Izzo J et al Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens 2002; 15: 1101–8. [DOI] [PubMed] [Google Scholar]
- 32. Sarajlic P, Friden C, Lund LH et al Enhanced ventricular‐arterial coupling during a 2‐year physical activity programme in patients with rheumatoid arthritis: a prospective substudy of the physical activity in rheumatoid arthritis 2010 trial. J Intern Med 2018; 284: 664–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


