Abstract
Aims
The efficacy of flash glucose monitoring (flash GM) systems has been demonstrated by improvements in glycaemia; however, during high rates of glucose flux, the performance of continuous glucose monitoring systems was impaired, as detailed in previous studies. This study aimed to determine the performance of the flash GM system during daily‐life glycaemic challenges such as carbohydrate‐rich meals, bolus insulin‐induced glycaemic disturbances and acute physical exercise in individuals with type 1 diabetes.
Materials and methods
This study comprised four randomized trial visits with alternating pre‐ and post‐exercise bolus insulin doses. Throughout the four 14‐hour inpatient phases, 19 participants received three carbohydrate‐rich meals and performed moderate‐intensity exercise. Venous blood glucose and capillary blood glucose during exercise was compared to interstitial glucose concentrations. Flash GM accuracy was assessed by median absolute relative difference (MARD) (interquartile range [IQR]) using the Bland–Altman method and Clark error grid, as well as according to guidelines for integrated CGM approvals (Class II–510(K)).
Results
The overall MARD (IQR) during inpatient phases was 14.3% (6.9%–22.8%), during hypoglycaemia (≤3.9 mmol/L) was 31.6% (16.2%–46.8%), during euglycaemia (4.0 mmol/L − 9.9 mmol/L) was 16.0% (8.5%–24.0%) and during hyperglycaemia (≥10 mmol/L) was 9.4% (5.1%–15.7%). Overall Bland–Altman analysis showed a bias (95% LoA) of 1.26 mmol/L (−1.67 to 4.19 mmol/L). The overall MARD during acute exercise was 29.8% (17.5%–39.8%), during hypoglycaemia was 45.1% (35.2%–51.1%), during euglycaemia was 30.7% (18.7%–39.2%) and during hyperglycaemia was 16.3% (10.0%–22.8%).
Conclusion
Flash GM interstitial glucose readings were not sufficiently accurate within the hypoglycaemic range and during acute exercise and require confirmatory blood glucose measurements.
Keywords: continuous glucose monitoring (CGM), exercise intervention, hypoglycaemia, type 1 diabetes
1. INTRODUCTION
In July 2018 the US Food and Drug Administration (FDA) approved the Freestyle Libre flash glucose monitoring (flash GM) system (Abbott Diabetes Care Inc, USA) to monitor interstitial glucose concentration without obtaining a capillary blood sample from the fingertip for management of type 1 and type 2 diabetes in individuals 18 years of age and older.1 The beneficial effects of integrating flash GM technology into diabetes management include: reduced time spent in hypoglycaemia, improved glycaemic variability,2 lower HbA1c levels3 and increased numbers of readings per day4 in individuals with type 1 diabetes.5
Although the flash GM system demonstrated good efficacy in chronic glucose monitoring settings, the accuracy of acute continuous glucose monitoring (CGM) systems was impaired during periods of high rates of change in glucose.6 From a physiological point of view, a time lag was observed in the interval needed for glucose to diffuse from the bloodstream into the interstitium.6 In general, use of CGM systems involves a struggle with sensor accuracy during hypoglycaemia and exercise, as shown for both professional (iPro2, Enlite 2, Medtronic, USA) and personal (Minimed 640G, Medtronic; Freestyle Libre 1, Abbott, USA; Dexcom G4 Platinum, Dexcom, USA) CGM systems.7, 8, 9 The performance of the flash GM system sensor was found to be accurate, with an overall mean absolute relative difference of approximately 13% under routine environmental conditions.8 During acute glycaemic challenges such as physical exercise or following carbohydrate‐rich meals and high doses of exogenous insulin, the interstitial glucose response may be further delayed. Pleus et al. showed that during periods of rapidly changing blood glucose concentrations of more than −0.2 mmol/L/minute and +0.2 mmol/L/minute the mean absolute relative difference deteriorated from 12.6% and 11.3% to 24.9% and 29.6% for the Dexcom G4 Platinum CGM system (Dexcom, USA).10 Taking this information into account, there is a need to investigate flash GM performance during exogenously induced glucose excursions to ensure patient safety. Therefore, the aim of this study was to determine the sensor accuracy of the Freestyle Libre flash GM system in individuals with type 1 diabetes during the acute glycaemic challenges of carbohydrate‐rich meal ingestion, bolus insulin administration and aerobic physical exercise.
2. METHODS
This study is an analysis of a predefined secondary outcome of a clinical trial registered at the German Clinical Trials Register (DRKS.de; DRKS.de; DRKS00013509). This single‐centre, randomized, open‐label, four‐period 14‐hour inpatient cross‐over trial was performed in line with Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the ethics committee of Health and Care Research Wales, UK (16/WA/0394) and the local health authority. All participants gave written informed consent prior to any trial‐related activities.
2.1. Eligibility criteria
Inclusion criteria are as follows: diagnosis of type 1 diabetes at least 12 months previously; age 18 to 65 years; body mass index of 18.0 to 29.4 kg/m2; use of multiple daily injections (MDI) of insulin for at least 12 months; mass‐specific peak oxygen uptake (VO2peak) of more than 20 mL/kg/minute; and status of being physically active as assessed by the International Physical Activity Questionnaire Short Form (IPAQ‐SF). Main exclusion criteria were: presence of a life‐threatening disease; proliferative retinopathy or maculopathy; severe neuropathy; recurrent severe hypoglycaemia (more than one severe hypoglycaemia event during the previous 12 months); hypoglycaemia unawareness as judged by the investigator; hospitalization for diabetic ketoacidosis during the previous 6 months; and any other condition that would interfere with trial participation or evaluation of results as judged by the investigator.
2.2. Screening visit
Anthropometry, body composition, resting cardiovascular markers and HbA1c were measured. Participants performed a peak cardio‐pulmonary exercise (CPX) test using a semi‐recumbent cycle ergometer (Corival Recumbent, Lode, Groningen, The Netherlands).11 CPX testing comprised a 3‐minute resting period without pedalling, followed by a 3‐minute warm‐up phase with pedalling at 20 W (W). Thereafter, the exercise workload increased at the end of each minute by 10, 15 or 20 W, dependent on the anticipated functional capacity of the participant, as assessed by an experienced exercise physiologist. After reaching maximum volitional exhaustion, participants performed an active cool‐down for 3 minutes at 20 W, followed by a 3‐minute passive cool‐down period without pedalling. Maximum volitional exhaustion was defined by one of the following parameters: a lactate concentration greater than10 mmol/L; a respiratory exchange ratio (RER) greater than 1.1; a plateau in oxygen uptake (VO2); or inability to maintain a pedalling cadence of more than 50 rpm (rpm) for 5 seconds.12 During CPX testing, respiration measurement (METAMAX 3B; Cortex Biophysik GmbH, Leipzig, Germany), heart rate measurement (S410, Polar Electro, Kmpele, Finland) and electrocardiogram measurement (eMotion Faros 180°, Bittium Biosignals Ltd, Oulu, Finland) were ongoing. Capillary blood glucose and blood lactate from earlobe sampling (20 μL) were taken as follows: at the end of the passive and active warm‐up periods; at the end of each incremental step in exercise; and at the end of the active and passive cool‐down periods (Biosen C‐line, EKF Diagnostic, Barleben, Germany). Blood glucose was measured to minimize the risk of hypoglycaemia and blood lactate was evaluated to prescribe exercise intensity by means of the midpoint of the first (LTP1) and the second lactate turn points (LTP2) (~65% of VO2peak). If participants were using insulins other than insulin aspart (Novo Nordisk, A/S, Denmark) and insulin degludec U100 (Novo Nordisk, A/S), they were switched over to these during a maximum period of 28 days prior to the study. This ensured a homogenous study cohort and allowed for a stable therapy, defined as a pre‐breakfast self‐measured blood glucose concentration between 4.0 and 7.0 mmol/L over three consecutive days. If participants were using insulin detemir (Novo Nordisk, A/S) or insulin glargine U100 (Sanofi, France) prior to the trial, the first dose of insulin degludec (Novo Nordisk, A/S) was 80% of their pre‐study total basal insulin dose. Participants using insulin glargine U300 (Sanofi) were switched over to insulin degludec (Novo Nordisk, A/S) with a 1:1 dose at the beginning. If participants were already using insulin degludec, they were also expected to achieve the titration target within 28 days. If adjustment of the dose of insulin degludec (Novo Nordisk, A/S) was required, this was undertaken every 3 days. Participants received an unblinded flash GM system (FreeStyle Libre, Abbott Diabetes Care Inc., USA) and spare sensors. Participants were trained in use of the system and the first flash GM sensor was inserted under instruction by the research team. Participants were told to change the sensor at least 48 hours before each trial visit to avoid sensor expiration during the research period and to avoid assessment of flash GM performance during the initial warm‐up period.
2.3. Trial visits
Trial visits were separated by at least five working days. Participants were randomized to the following alternating pre‐ and post‐exercise bolus insulin doses:
50%‐reduced pre‐exercise and 50%‐reduced post‐exercise dose of insulin aspart with a carbohydrate‐rich meal.
Regular pre‐exercise and regular post‐exercise dose of insulin aspart.
50%‐reduced pre‐exercise and regular post‐exercise dose of insulin aspart.
Regular pre‐exercise and 50%‐reduced post‐exercise dose of insulin Aspart.
Throughout each of the four 14‐hour inpatient phases, participants received three carbohydrate‐rich meals and venous blood glucose concentration was compared to interstitial glucose concentration (Figure 1). During exercise, only capillary blood was obtained from the earlobe, with resultant glucose concentrations compared to interstitial glucose concentration; these values were analysed separately because of collection from a different compartment. In this study, only scanned data were used for assessment of the flash GM system; at the time a blood sample was taken, a scan was performed using the flash GM system and these data were assessed for accuracy.13 Both values were recorded in a case report form.
Figure 1.
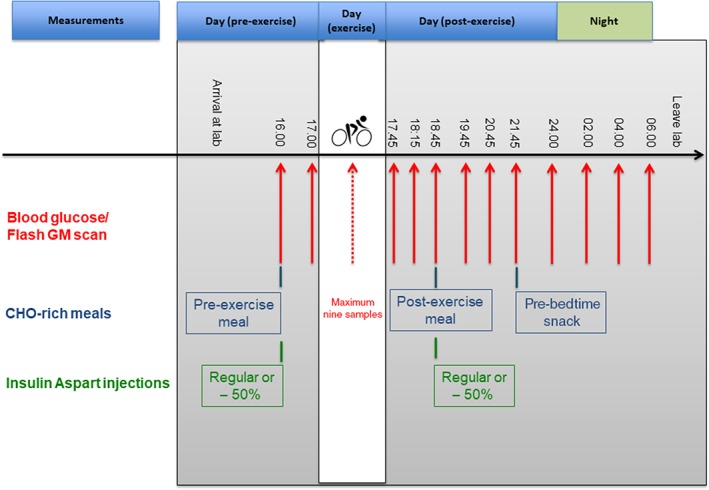
Study flow chart detailing time points of blood glucose collection, carbohydrate (CHO)‐rich meals and insulin aspart injections. Red lines = venous blood glucose sampling. Red dotted line = capillary blood glucose sampling obtained from earlobe during exercise
At the pre‐ and post‐exercise meal, participants consumed 1 g of carbohydrates per kilogram of bodyweight, with a regular dose or 50% dose of bolus insulin. Additionally, a pre‐bedtime snack, consisting of 0.4 g of carbohydrates per kilogram of bodyweight, was consumed without a dose of bolus insulin.14 The basal insulin dose remained unchanged for the purpose of this study. A total of 13 samples were collected from an antecubital vein and analysed using the fully enzymatic Biosen C‐Line system (EKF Diagnostic). During exercise testing, capillary samples were collected from the earlobe and analysed using the Biosen C‐Line system. Exercise blood glucose testing comprised nine sample time points; however, there were fewer time points in the case of a level 1 hypoglycaemia episode (blood glucose ≤3.9 mmol/L).15
2.4. Moderate‐intensity exercise testing
Exercise testing comprised four 45‐minute moderate‐intensity exercise sessions, defined as exercise intensity at the midpoint of LTP1 and LTP2, each session separated by at least five working days. In the case of level 1 hypoglycaemia during exercise testing, participants received 10 g of carbohydrates via a glucose gel and exercise testing was discontinued. Initiation of exercise testing was delayed by 10 minutes if blood glucose concentration was below 6 mmol/L, and 10 g of carbohydrates were given. This pre‐exercise procedure was repeated as often as required to reach a blood glucose concentration above 6 mmol/L before initiation of exercise. Capillary blood glucose was measured at the end of the warm‐up period and every 7 minutes during exercise testing. CPX testing variables were measured continuously, as detailed during maximum CPX testing.
2.5. Statistical analyses
Data were analysed for normal distribution using Shapiro–Wilk testing to assess whether median or mean absolute relative difference must be shown. Flash GM sensor performance was analysed using median absolute relative difference (MARD) (IQR), the Bland–Altman method and the Clarke error grid, as well as according to guidelines for integrated CGM approvals (Class II–510(K)).16 The Clarke error grid is divided into zones to evaluate the risk caused by inaccuracy of measurement. Values in zone A reflect no effect on clinical action; values in zone B represent altered clinical action with small or no significant effect on clinical outcome; values in zone C represent altered clinical action with the probability of affecting clinical outcome; values in zone D represent altered clinical action that could have significant medical risk; and values in zone E represent altered clinical action that could have dangerous consequences. Overall assessment of data, excluding those concerning exercise, and data concerning exercise phases, excluding resting conditions, were stratified for glycaemic ranges, defined as hypoglycaemia level 1 (≤3.9 mmol/L), euglycaemia (4.0–9.9 mmol/L) and hyperglycaemia (≥10 mmol/L).17 Furthermore, data were stratified for day‐time (6:00 AM–12:00 AM) and night‐time (12:01 AM–5:59 AM) periods. Rate of change in glucose was calculated for the four 14‐hour inpatient phases, excluding exercise, and was analysed separately during exercise, during the post‐exercise day‐time period and during the night‐time period. This stratification was based on the expected rate of change in glucose for each period. Only data of participants who performed at least one 14‐hour in‐patient phase were used. A post‐hoc sample size calculation was performed, using data from the night‐time period, which was the most accurate, accompanied by the lowest numbers of points of comparison. Considering 273 points of comparison and α = 0.05, we achieved a power of ≥95% to detect an absolute median difference of 0.70 mmol/L in comparison of venous blood glucose and interstitial glucose concentration.
3. RESULTS
Among 23 screened individuals with type 1 diabetes, 19 were included in the analysis and were involved in at least one 14‐hour in‐patient phase. Four individuals were excluded according to pre‐defined inclusion criteria. Sixteen participants complied with all four trial visits, one participant made three trial visits and two participants made one trial visit. The two individuals who did not make all trial visits withdrew from the study for personal reasons and one individual was excluded because of unstable insulin therapy. The four women and 15 men who completed the study had a mean ± SD age of 35 ± 15 years, a body mass index of 26 ± 3 kg/m2, HbA1c of 56 ± 15 mmol/mol (7.3% ± 1.4%), a diabetes duration of 16 ± 11 years and a total daily insulin dose of 50 ± 23 IU. Before initiation of the study, all participants were using insulin aspart (Novo Nordisk, A/S) as bolus insulin, eight were using insulin glargine U100 (Sanofi), seven were using insulin detemir (Novo Nordisk, A/S), two were using insulin degludec U100 (Novo Nordisk, A/S) and one was using insulin glargine U300 (Sanofi) as basal insulin. Among 824 potential points of comparison for interstitial glucose and venous blood glucose, 821 were available. During 69 exercise sessions, 41 participants discontinued prematurely because of exercise‐induced hypoglycaemia (≤3.9 mmol/L). During exercise testing, 470 of 475 potential points of comparison were available. With the regular pre‐exercise bolus insulin dose, 28 episodes of hypoglycaemia occurred, whereas after a 50%‐reduced pre‐exercise bolus insulin dose only 13 episodes of hypoglycaemia occurred. Initial blood glucose concentration was 9.7 ± 3.1 mmol/L with the regular pre‐ and post‐exercise bolus insulin dose, 9.9 ± 2.4 mmol/L with the 50% reduced pre‐ and post‐exercise bolus insulin dose, 9.2 ± 1.9 mmol/L with the pre‐exercise regular and post‐exercise 50%‐reduced bolus insulin dose and 10.0 ± 2.3 mmol/L with the pre‐exercise 50%‐reduced and post‐exercise regular bolus insulin dose. The time until reaching exercise‐induced hypoglycaemia was 37 ± 11 minutes with the regular pre‐ and post‐exercise bolus insulin dose, 41 ± 7 minutes with the 50%‐reduced pre‐ and post‐exercise bolus insulin dose, 35 ± 7 minutes with the pre‐exercise regular and post‐exercise 50%‐reduced bolus insulin dose and 40 ± 9 minutes with the pre‐exercise 50%‐reduced and post‐exercise regular bolus insulin dose.
Overall MARD was 14.3% (IQR 6.9%–22.8%), during hypoglycaemia was 31.6% (16.2%–46.8%), during euglycaemia was 16.0% (8.5%–24.0%) and during hyperglycaemia was 9.4% (5.1%–15.7%). When data were stratified for time of day, based on the expected alternating rate of change in glucose, day‐time MARD was 18.0% (9.8%–27.5%), during exercise was 29.8% (17.5%–39.8%) and during the night was 8.6% (4.0%–14.5%). During exercise and periods of hypoglycaemia, MARD was 45.1% (35.2%–51.1%), during euglycaemia was 30.7% (18.7–39.2%) and during hyperglycaemia was 16.3% (10.0%–22.8%) (Table 1).
Table 1.
Median absolute relative difference (MARD) and interquartile range (IQR) between interstitial glucose and reference blood glucose
| Flash GM accuracy | ||
|---|---|---|
| MARD (IQR) | Overall |
14.3% (6.9%–22.8%) n = 821 |
| Hypoglycaemia (≤3.9 mmol/L) |
31.6% (16.2%–46.8%) n = 75 |
|
| Euglycaemia (3.9–9.9 mmol/L) |
16.0% (8.5%–24.0%) n = 508 |
|
| Hyperglycaemia (≥10 mmol/L) |
9.4% (5.1%–15.7%) n = 238 |
|
| Day‐time (6:00 AM–12:00 AM) |
18.0% (9.8%–27.5%) n = 548 |
|
| Night‐time (12:01 AM–05:59 AM) |
8.6% (4.0%–14.5%) n = 273 |
|
|
During exercise Overall |
29.8% (17.5%–39.8%) n = 470 |
|
|
During exercise Hypoglycaemia (≤3.9 mmol/L) |
45.1% (35.2%–51.1%) n = 70 |
|
|
During exercise Euglycaemia (3.9–9.9 mmol/L) |
30.7% (18.7%–39.2%) n = 306 |
|
|
During exercise Hyperglycaemia (≥10 mmol/L) |
16.3% (10.0%–22.8%) n = 94 |
Systematic assessment of the accuracy of the flash GM system in comparison with reference blood glucose concentration assessed using the Bland–Altman method resulted, overall, in an over‐estimation of flash GM values compared to reference blood glucose values (bias, 1.26 mmol/L, with 95% limits of agreement, from −1.67 mmol/L to 4.19 mmol/L). During exercise, the flash GM system overestimated reference blood glucose concentration by 2.27 mmol/L, with 95% limits of agreement from −0.64 mmol/L to 6.08 mmol/L (Figure 2).
Figure 2.
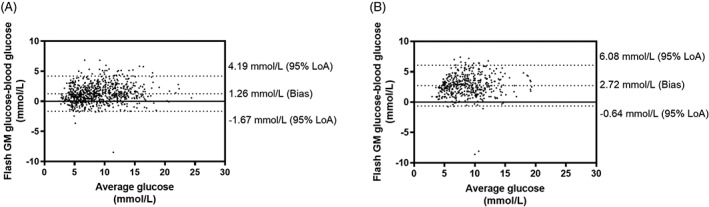
Comparison of interstitial glucose and reference blood glucose via the Bland–Altman method, displaying bias and 95% levels of agreement (95% LoA). A, Overall data; B, Exercise. Overall, the flash GM system overestimated the reference venous blood glucose concentration by 1.26 mmol/L, with 95% limits of agreement from −1.67 mmol/L to 4.19 mmol/L. During exercise, the flash GM system overestimated the reference capillary blood glucose concentration by 2.27 mmol/L, with 95% limits of agreement from −0.64 mmol/L to 6.08 mmol/L
Data from assessment of the performance of the flash GM system according to guidelines for integrated CGM approvals (Class II–510(K)) are shown in Table 2.
Table 2.
Assessment of performance of the flash GM system compared to venous blood glucose concentration according to guidelines for integrated CGM approvals (Class II–510(K))
| Measured accuracy: Lower bound of one‐sided 95% CI | Required accuracy: Lower bound of one‐sided 95% CI | |
|---|---|---|
| Overall | 68% within ±20% | >87% within ±20% |
| Euglycaemia (3.9–9.9 mmol/L) | 48% within ±15% | >70% within ±15% |
| Euglycaemia (3.9–9.9 mmol/L) | 97% within ±40% | >99% within ±40% |
| Hypoglycaemia (≤3.9 mmol/L) | 32% within ±0.8 mmol/L | >85% within ±0.8 mmol/L |
| Hypoglycaemia (≤3.9 mmol/L) | 39% within ±2.2 mmol/L | >98% within ±2.2 mmol/L |
| Hyperglycaemia (≥10 mmol/L) | 74% within ±15% | >80% within ±15% |
| Hyperglycaemia (≥10 mmol/L) | 100% within ±40% | >99% within ±40% |
The Clarke error grid showed that, overall, 56% of values were located in zone A, 35% in zone B, 9% in zone D, and no values were located in zones C and E. During exercise, 26% of values were located in zone A, 52% in zone B, 22% in zone D, and no values were located in zones C and E (Figure 3).
Figure 3.
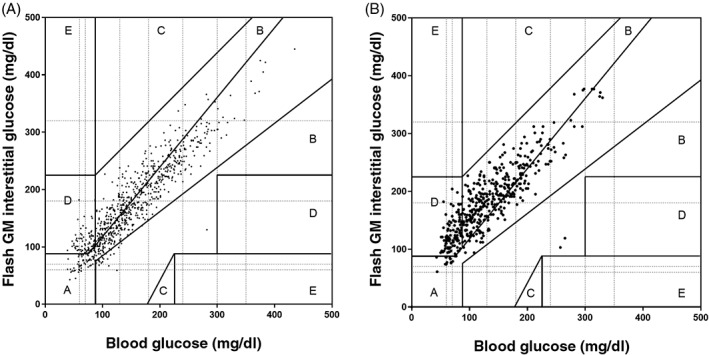
Clinical assessment of the flash GM system and its relationship to reference blood glucose levels using the Clarke error grid. A, Overall 56% of values were located in zone A, 35% in zone B, 9% in zone D and no values were in zones C and E. B, During exercise, 26% of values were located in zone A, 52% in zone B, 22% in zone D and no values were in zones C and E
The median and interquartile range of the rate of change in glucose was 0.15 mmol/L/min (0.098–0.2 mmol/L/min) during exercise, 0.03 mmol/L/min (0.014–0.061 mmol/L/min) during the post‐exercise day‐time period and 0.007 mmol/L/min (0.001–0.017 mmol/L/min) during the night.
4. DISCUSSION
This is the first study to assess the performance of the flash GM system with different rates of change in glucose in a clinical research facility setting, comprising acute daily‐life challenges such as carbohydrate‐rich meals, bolus insulin administration and physical exercise. MARDs' for the night‐time period were similar to findings of a recent study in which a mean absolute relative difference of 13% ± 11% was found for the flash GM system.8 During acute exercise, the results of our study using the flash GM system deviated considerably from those of Aberer et al., who found a mean absolute relative difference of 9% ± 6%, while we found a MARD of 29.8% (17.5%–39.8%). We have recently shown that use of the flash GM system during physical exercise, with the same mean exercise intensity as that in the present study, revealed a MARD of 22.0% (13.9%–29.7); however, in the previous study, participants' blood glucose concentration was deliberately kept stable, with a rate of change in glucose of 0.1 mmol/L/min.18 Furthermore, in the previous study, only capillary blood glucose concentration was used as a reference and the performance of the flash GM system was not assessed during meal‐induced and bolus insulin‐induced glycaemic challenge. One might assume that physical exercise per se did not deteriorate the performance of the flash GM system, because the rate of change in glucose appeared to result in inaccuracy. In the present study, the MARD during exercise was inherently higher than that with other CGM devices such as Minimed 640G (Medtronic), Dexcom G4 Platinum (Dexcom) or Paradigm Veo Enlite (Medtronic) (mean absolute relative difference/MARD, ~18%).6, 8, 19, 20, 21 A systematic overestimation of 2.7 mmol/L might suggest that anticipation of intervention to avoid exercise‐induced hypoglycaemia is required as soon as an interstitial glucose level of 7.2 mmol/L is reached. This result supports the need for adjuvant blood glucose measurements during moderate‐intensity exercise, as this inaccuracy leads to wrong clinical decisions that might entail serious health consequences. Although impairments were found in sensor performance during exercise, the flash GM system was more accurate under conditions of lower rates of change in glucose.
When assessing the overall accuracy of the device, based on pre‐specified glycaemic ranges, hypoglycaemia remained the weak spot, with a MARD of 31.6%. This is contrary to the findings of Aberer et al., who identified a mean absolute relative difference of 14.6% with the flash GM system.8 When comparing findings of our study to those concerning other CGM devices (iPro2, Enlite 2 and Minimed 640G, Medtronic), it appears that hypoglycaemia might be a challenge to the flash GM system.7 Additionally, during acute exercise accompanied by hypoglycaemia, flash GM data indicated a MARD of 45.1%; hence, glucose values must be interpreted very cautiously. When evaluating performance of the flash GM system according to the guidelines for integrated CGM approvals (Class II–510(K), the required accuracy was achieved only during hyperglycaemia, where 100% of the interstitial glucose values were within ±40% of the accompanying venous blood glucose values. Especially during hypoglycaemia, flash GM interstitial glucose values were far off the expected relative range (Class II–510(K)). When evaluating the clinical accuracy of the flash GM system using the Clark error grid, only 56% of values overall and 26% of values during exercise were located in zone A. Additionally, the fact that 9% of values overall and 22% of values during exercise were located in zone D clearly indicates that the flash GM system has major weaknesses, and decisions based on the displayed values are questionable.
Our study is limited by the rather small number of participants; however, this limitation was compensated for by four visits in a cross‐over fashion. Additionally, during exercise testing, we analysed the accuracy of the flash GM system in relation to capillary blood glucose concentration, which may have resulted in less accurate blood glucose values.
In conclusion, the flash GM system displayed an impaired performance during acute exercise and during periods of hypoglycaemia where it is inconvenient and difficult to perform capillary blood glucose measurements in individuals with type 1 diabetes. Our results also demonstrated that the performance of the flash GM system is linked to the rate of change in blood glucose. Adjuvant blood glucose measurements are encouraged during physical exercise and/or in the context of low blood glucose concentrations to avoid episodes of severe hypoglycaemia as the result of systematic overestimation of interstitial glucose levels as compared to blood glucose levels.
CONFLICT OF INTEREST
O. M. has received lecture fees from Medtronic, travel grants from Novo Nordisk A/S, Novo Nordisk AT, Novo Nordisk UK, Medtronic AT, research grants from Sêr Cymru II COFUND fellowship/European Union, Novo Nordisk A/S and Novo Nordisk AT, as well as material funding from Abbott Diabetes Care. M. L. E. has received a KESS2/European Social Fund scholarship and travel grants from Novo Nordisk A/S. H. S. has received honoraria, travel support or unrestricted research grants from Amgen, Astra Zeneca, Boehringer‐Ingelheim, Eli Lilly, MSD, Novo Nordisk and Sanofi‐Aventis. S. C. B. has received research grants, including those for principal investigator, collaborator or consultant and pending grants, as well as other grants, from Health Care and Research Wales (Welsh Government) and Novo Nordisk; has received research support from Healthcare and Research Wales (Welsh Government), honoraria from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck, and has an ownership interest in Glycosmedia, an on‐line news service concerning diabetes. R. M. B. has received honoraria as well as travel and educational grant support from Boehringer‐Ingelheim, Eli Lilly and Company, Novo Nordisk and Sanofi‐Aventis. The remaining authors have no relevant conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
O. M. is the guarantor and has written the study protocol, performed measurements, performed statistical analyses and has written the manuscript. M. L. E. performed measurements and statistical analyses and reviewed/edited the manuscript. O. Mc., R. D., J. P., D. M. W. and J. H. performed measurements and reviewed/edited the manuscript. H. S., S. C. B. and R. M. B contributed to writing the manuscript and reviewed/edited the manuscript.
ACKNOWLEDGMENTS
We want to thank the participants for their significant adherence to the study protocol.
Moser O, Eckstein ML, McCarthy O, et al. Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: A secondary outcome analysis of a randomized crossover trial. Diabetes Obes Metab. 2019;21:2505–2512. 10.1111/dom.13835
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13835.
Funding information This study was funded by Novo Nordisk A/S.
REFERENCES
- 1. Blum JR, Rayfield EJ. An endocrine clinic's perspective and experience with the Abbott Freestyle Libre Cgm. Endocr Pract. 2018;24:309‐311. [DOI] [PubMed] [Google Scholar]
- 2. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, Kröger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet. 2016;388:2254‐2263. [DOI] [PubMed] [Google Scholar]
- 3. Dover AR, Stimson RH, Zammitt NN, Gibb FW. Flash glucose monitoring improves outcomes in a type 1 diabetes clinic. J Diabetes Sci Technol. 2017;11:442‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J‐P, Rayman G. Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter, open‐label randomized controlled trial. Diabetes Ther. 2017;8:55‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real‐time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41:2265‐2274. [DOI] [PubMed] [Google Scholar]
- 6. Moser O, Yardley J, Bracken R. Interstitial glucose and physical exercise in type 1 diabetes: integrative physiology, technology, and the gap in‐between. Nutrients. 2018;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moser O, Pandis M, Aberer F, et al. A head‐to‐head comparison of personal and professional continuous glucose monitoring systems in people with type 1 diabetes: hypoglycaemia remains the weak spot. Diabetes Obes Metab. 2019;21:1043‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes, Obes Metab. 2017;19:1051‐1055. [DOI] [PubMed] [Google Scholar]
- 9. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real‐time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pleus S, Schoemaker M, Morgenstern K, et al. Rate‐of‐change dependence of the performance of two CGM systems during induced glucose swings. J Diabetes Sci Technol. 2015;9:801‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hofmann P, Tschakert G. Special needs to prescribe exercise intensity for scientific studies. Cardiol Res Pract. 2011;2011:209302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moser O, Eckstein ML, Mueller A, et al. Reduction in insulin degludec dosing for multiple exercise sessions improves time spent in euglycaemia in people with type 1 diabetes: a randomised cross‐over trial. Diabetes Obes Metab. 2019;21:349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pleus S, Kamecke U, Link M, Haug C, Freckmann G. Flash glucose monitoring: differences between intermittently scanned and continuously stored data. J Diabetes Sci Technol. 2018;12:397‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moser O, Tschakert G, Mueller A, et al. Short‐acting insulin reduction strategies for continuous cycle ergometer exercises in patients with type 1 diabetes mellitus. Asian J Sports Med. 2017;8. [Google Scholar]
- 15. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care. 2017;40:155‐157. 10.2337/dc16-2215 [DOI] [PubMed] [Google Scholar]
- 16. Garg SK, Akturk HK. A new era in continuous glucose monitoring: Food and Drug Administration creates a new category of factory‐calibrated nonadjunctive, interoperable class II medical devices. Diabetes Technol Ther. 2018;20:391‐394. [DOI] [PubMed] [Google Scholar]
- 17. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moser O, Eckstein ML, Mueller A, et al. The impact of physical exercise on sensor performance of the Abbott Freestyle® Libre intermittently‐viewed continuous glucose monitoring (iCGM) system in people with type 1 diabetes – a randomised cross‐over trial. Diabet Med. 2019;36:606‐611. 10.1111/dme.13909 [DOI] [PubMed] [Google Scholar]
- 19. Bally L, Zueger T, Pasi N, Carlos C, Paganini D, Stettler C. Accuracy of continuous glucose monitoring during differing exercise conditions. Diabetes Res Clin Pract. 2016;112:1‐5. [DOI] [PubMed] [Google Scholar]
- 20. Moser O, Mader JK, Tschakert G, et al. Accuracy of continuous glucose monitoring (CGM) during continuous and high‐intensity interval exercise in patients with type 1 diabetes mellitus. Nutrients. 2016;8:1‐15. 10.3390/nu8080489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taleb N, Emami A, Suppere C, et al. Comparison of two continuous glucose monitoring systems, Dexcom G4 platinum and Medtronic paradigm Veo Enlite system, at rest and during exercise. Diabetes Technol Ther. 2016;18:561‐567. [DOI] [PubMed] [Google Scholar]


