Abstract
Objective:
Although intensive care medicine has evidenced a significant growth in recent decades, the number of patients requiring prolonged mechanical ventilation (PMV) still represents a considerable burden on health-care expenditure. The prediction of the need for PMV seems to provide a plausible cost-effective intervention. The objective of this study is to systematically review the predictors of the need for PMV of adult patients admitted to intensive care units (ICUs) due to medical and surgical needs.
Methods:
We conducted a systematic search on three online databases (PubMed, Embase, and MEDLINE) till February 20, 2019. The search process employed several combinations of specific keywords and Boolean operators.
Results:
A total of 15 articles were included in the study. Based on pooling the outcomes of odds ratios (ORs) and their respective 95% confidence intervals (CIs) as reported from logistic regression analyses, the pooled PMV incidence in 8220 patients (69.59% males) was 17.67 cases per 100 ICU admissions (95% CI 13.69–21.65). We could not conduct a meta-analysis of ORs and 95% CIs due to the significant heterogeneity observed between the included studies (P < 0.001, I2 = 97%). Pre-operative/preadmission kidney dysfunction and chronic obstructive pulmonary disease were the most significant independent predictors of the need for PMV. Following cardiac surgeries, repeated or emergency surgery, prolonged cardiopulmonary bypass time, and the need for blood transfusion were predictors of the need for PMV.
Conclusion:
Within the study limitations, several predictors were identified, which could be further investigated using a unified PMV definition. Successful prediction of the need for PMV would assist clinicians in identifying and adjusting a “weaning strategy” as well as improving patient care to reduce morbidity. Furthermore, establishing specialized weaning units could be warranted based on PMV incidence and prediction in the local settings.
Keywords: Critical illness, intensive care unit, tracheostomy, non-invasive mechanical ventilation, ventilator weaning
Introduction
Intensive care medicine has evidenced a steady growth in recent decades, supported by major technological advances accomplished in the scientific and medical fields. In addition to the supportive role provided by mechanical ventilation in patients undergoing surgical procedures, ventilatory support is needed when spontaneous ventilation is insufficient for life maintenance. This requirement for mechanical ventilation mandates admission to an intensive care unit (ICU), which usually lasts for a short period of time. However, a proportion of patients might be unable to recover rapidly, and hence, they need prolonged mechanical ventilation (PMV).[1]
The number of patients requiring PMV is growing globally and is predicted to increase parallel with the increased need for mechanical ventilation in ICUs, particularly in the elderly and those with comorbid conditions.[2,3] Indeed, such trends have attracted the attention of health-care planners since patients undergoing PMV usually require health-care resources and thus increased expenditure.[4] For example, patients on PMV showed higher rates of hospital readmission, ICU readmission, and total health-care costs as well as higher rates of morbidity in a recent prospective study in Canada.[5] Analysis of The National Inpatient Sample data from the United States revealed an increasing trend for the need for palliative care in patients receiving PMV.[6]
Therefore, the duration of mechanical ventilation seems to be an acceptable indicator of significant health complications as well as health-care costs. This way, there is a need to predict the length of mechanical ventilation, with various proposed equations and methodological approaches for such a purpose in the literature. Furthermore, predicting mechanical ventilation might be a robust rationale for quality improvement in a given ICU.[7] Collectively, the duration of mechanical ventilation is a valid measure for research. In this context, we sought to provide a systematic insight into the currently investigated predictors of the need for PMV among patients admitted to ICUs for medical and/or surgical purposes, focusing on the most significant factors, and to assist in clinical decision regarding critically ill patients.
Methods
A systematic review was carried out investigating the predictors of the need for PMV in patients admitted to ICUs based on the guidelines reported in the preferred reporting items for systematic reviews and meta-analyses statements.[8] The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42019125591).
Eligibility criteria
The included studies were those employing a retrospective design, or a prospective follow-up of adult patients admitted to ICUs due to trauma, surgeries, or other critical illnesses. The follow-up period should be at least 6 months. This is to overcome the potential confounding factors related to recovery from anesthesia and minor or transient complications, which may not actually be regarded as real risk factors for PMV, particularly in patients recovering from cardiac surgeries.[9] Eligible studies should be published in peer-reviewed journals. Studies conducted in long-term acute-care units or specialized weaning units (in which patients with the need for PMV were transferred from ICUs) were excluded from the study. In addition, randomized clinical trials, review articles, and systematic reviews, and meta-analyses were excluded from the study. In addition, studies recruiting pediatric patients and those written in a non-English language were ineligible for inclusion.
Types of outcome measures
The primary outcome variables were those comprising the occurrence of the need for PMV among adult patients. The primary outcomes were reported as odds ratios (ORs) with their respective confidence intervals (CIs) as computed by univariate or multivariate logistic regression analysis models.
Definitions
PMV was defined as prolonged invasive ventilation for more than 24 h, as recently indicated.[10] Patients with advanced ages were those aged > 60 years. Emergency surgery was referred to as the need to take a patient to the operating theatre before the next morning of the established operation schedule. Chronic obstructive pulmonary disease (COPD) was identified clinically through evidence of chronic bronchitis, small airway disease or emphysema, and spirometry was performed for confirmation. In this study, the duration of prolonged cardiopulmonary bypass (CPB) time was greater than 180 mins.
Search methods for identification of studies
A comprehensive search strategy was designated in the following scientific databases: PubMed, Embase, and MEDLINE. The last access to these databases was in February 2019. The search process employed several combinations of specific keywords and Boolean operators. An example of the used search strategy (as used in PubMed) is demonstrated in Appendix 1. Two independent researchers screened the eligible articles, and any disagreement was resolved by discussion.
Data collection process
For each included study, the following data were collected: (1) Study-related data, including the name of first author(s), year of publication, country, study period, design, and sample size, (2) patient’s data, including gender, the indication of admission to ICU, and number of patients who underwent PMV, (3) predictors of the need for PMV, including demographic data (advanced age, and sex), preweaning arterial blood gases, respiratory conditions (COPD, acute respiratory failure, and pneumonia), cardiovascular conditions (stroke, shock, elevated heart rate, and ejection fraction), kidney function (pre-operative serum creatinine, and blood urea nitrogen [BUN]), surgery-related predictors (redo surgery, emergency surgery, and CPB), and ICU scoring systems. The need for PMV predictors was extracted as reported by logistic regression analysis (ORs and 95% CI). They were extracted into a specifically designed spreadsheet in Microsoft Excel. The citations of the included articles were uploaded and compile using Endnote X7.0.1 (Thomson Reuters, USA).
Risk of bias across studies
Articles were assessed using the Newcastle–Ottawa quality assessment scale,[11] in which a scoring system ranging between 0 (bad) and 9 (excellent) is used to score each study based on its methodological quality. The assessed domains include selection, comparability, and outcomes. A score of ≥6 denotes a high-quality article.
Results
Outcomes of the search process
The main outcomes of the search process are depicted in Figure 1. Of a total of 542 records obtained during the initial search, 13 duplicates were omitted. An additional four records were identified from the bibliographies of the screened articles across different databases. Therefore, the titles/abstracts of 533 records were screened. The full-texts of 23 studies were assessed for eligibility. However, eight articles were excluded for the following reasons: Lack of access to the full-text version,[12] employing a prospective evaluation of multiple ICUs for 1 day only[13] or 1 month,[14] using different analytical models rather than logistic regression analyses,[15,16] defining PMV as a prolonged ventilation for more than 12 h,[17] and the investigation of patients admitted at a respiratory care center following 21 days in an ICU.[18,19] As such, a total of 15 articles were eventually included in the study.
Figure 1.
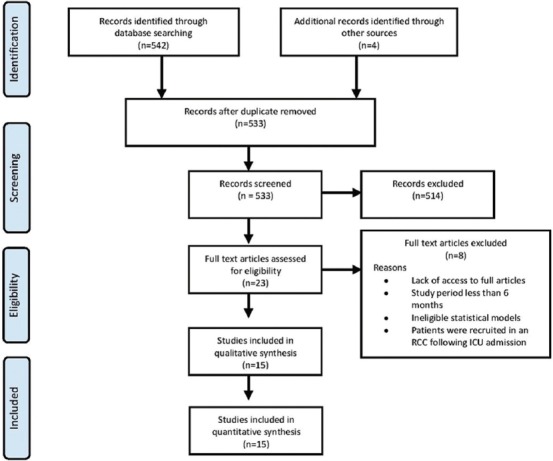
A flow diagram showing the employed search strategy in the current study
Characteristics of the included studies
Table 1 shows the main characteristics of the included studies, sorted in chronological order. Studies were published between 2001 and 2018 and they were conducted for periods ranging between 3 and 59 months. The included studies showed an approximately equal distribution across four continents; five studies were conducted in countries from Asia, [20-24] four studies in North America,[25-28] and three studies in either South America[7,29,30] or Europe.[31-33] PMV was established for at least 24 h in all articles, while it was defined as invasive mechanical ventilation for more than 7 days in seven studies,[7,24-27,30,32] and more than 14 days in three studies.[7,25,26] Patients were admitted to ICUs due to cardiac surgeries in six studies,[20,21,23,28,29,31] non-surgical admissions (medical ICU) in one study,[25] major torso trauma in one study,[27] and miscellaneous causes in the remaining studies. As for quality assessment, all of the included studies scored ≥6, indicating the inclusion of high-quality articles. Table 2 demonstrates PMV predictors as collected from the included studies.
Table 1.
Characteristics of the included studies
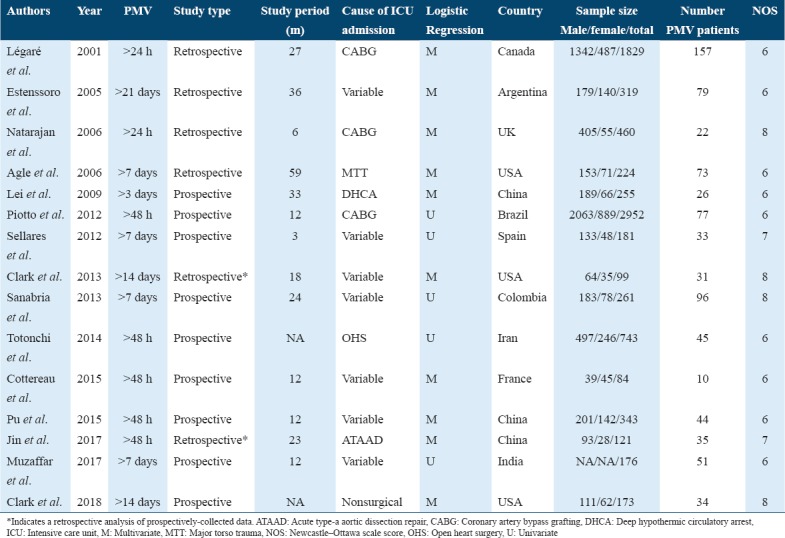
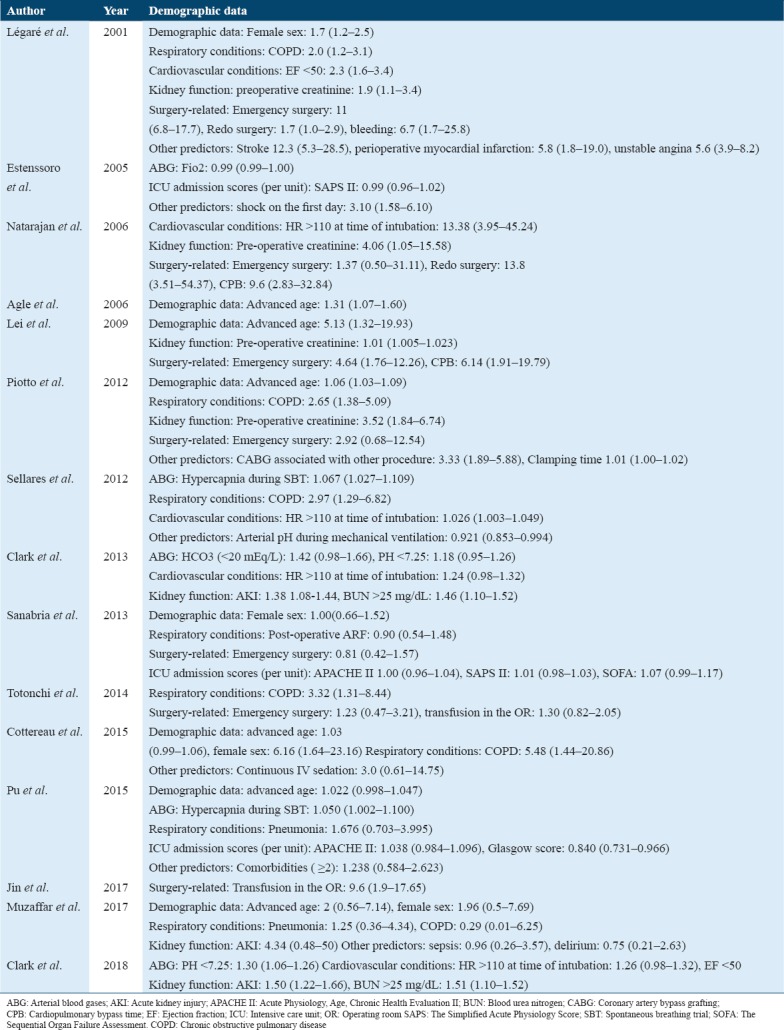
Table 2.
The investigated PMV predictors in the included studies. Results are presented as odds ratios (95% confidence intervals)
Demographic variables
The predicting capacity of demographic variables was investigated in seven studies. Two prospective studies showed that advanced age (more than 60 years) was a significant predictor for PMV for > 2 days[29] and > 3 days.[20] On the other hand, female patients were more likely to experience a need for PMV in a prospective investigation conducted in France[33] and a retrospective study in Canada.[28]
Preweaning arterial blood gases
The partial pressure of carbon dioxide (PaCO2) was the sole predictor of the need for PMV as a component of arterial blood gas analysis. However, it independently predicted the need for PMV only in two studies among patients admitted to ICUs for variable causes.[22,32] Interestingly, although low bicarbonate levels had no effect on the likelihood of experiencing PMV,[26] a pH of <7.25 was a significant predictor of the need for PMV for more than 14 days.[25,26]
Respiratory conditions
Both pneumonia and post-operative acute respiratory failure were not associated with an increased risk of the need for PMV. Notably, COPD was consistently reported as a significant PMV predictor in patients undergoing coronary artery bypass grafting (CABG)[28,29] and open-heart surgeries[21] as well as those admitted for other surgical and non-surgical causes.[32,33]
Cardiovascular factors
Bleeding mandating reoperation was demonstrated as increasing the likelihood of the need for PMV after CABG operations.[28] An increase in the heart rate (> 110 beats/min) also had a notable effect on PMV predilection for long periods, which ranged between > 7 days[32] and > 14 days.[25,26] In addition, low preoperative ejection fraction (< 50) has been identified as an independent predictor of the need for PMV for > 24 h in the patients after undergoing CABG operations.[28,31]
Shock on the day of ICU admission, defined as a reduction of systolic blood pressure of 40 mm Hg despite fluid resuscitation, was a significant predictor of the need for PMV in an early study conducted in Argentina.[7] The significance remained robust even after the authors performed a sensitivity analysis to ensure the validity of the used statistical model.
Kidney functions
There was strong evidence that patients with preoperative renal dysfunction, as indicated by a serum creatinine level of at least >150 µmol/L, showed a greater likelihood of PMV after CABG and deep hypothermic circulatory arrest (DHCA).[20,28,29,31] In addition, patients with an acute kidney injury, who showed a 50% elevation in serum creatinine during intubation as compared to baseline, were more likely to require PMV for >14 days.[25,26] Likewise, a cutoff value of >25 mg/dL of BUN was an efficient pre-operative predictor of the need for PMV.[25,26]
Surgery-related factors
Several surgery-attributable predictors have been investigated in literature. The need for a blood transfusion in the operating room (>2000 mL) was a strong predictor in patients following open-heart surgery[21] and acute type-A aortic dissection repair.[23] Despite its investigation in a total of six studies, performing emergency surgery was predictive of PMV in patients undergoing DHCA[20] and CABG.[28] Performing a redo surgery was also a significant predictor for PMV for > 24 h after CABG operations.[28,31] Moreover, prolonged CPB predicted the incidence of PMV in patients undergoing DHCA[20] and CABG.[31]
ICU scores
Results based on ORs of logistic regression analysis models showed no significant effects of the Acute Physiology and Chronic Health Evaluation (APACHE II) on the prediction of the need for PMV. However, Clark et al.[26] showed that an APACHE III score > 68 was highly predictive for the need for PMV for > 14 days, as indicated by its linear relationship to days of ventilation. Similarly, the need for PMV was more likely to occur when the Acute Physiology Score was > 48.[26]
Discussion
The prediction of the need for PMV in intensive care medicine is a challenging aspect. It frequently relies on clinical criteria, which are often subjective, unreliable, and must allow for taking late decisions. In addition, there are no currently validated instruments that can predict the need for PMV in patients undergoing intubation and early consideration for tracheostomy. In this study, we attempted to synthesize the current knowledge about the statistical potential for predicting the need for PMV using a logistic regression model. We identified several independent predictors related to patients’ demographics (advanced age and gender), health status (COPD, elevated heart rate, low ejection fraction, and kidney dysfunction), and surgery-related incidents (emergency and redo surgeries as well as the need to blood transfusion). However, we evidenced a significant heterogeneity between studies regarding their designs and, more importantly, PMV definitions.
Several definitions have been proposed for PMV in literature, where mechanical ventilation ranged between > 24 h and > 29 days.[34,35] For example, the National Association for Medical Direction of Respiratory Care defined PMV as the need for mechanical ventilation for > 21 days, where it is required for more than 6 h/day.[36] Furthermore, it was referred to as the need for > 7 days of weaning following the first spontaneous breathing trial (SBT) according to the European Respiratory Society task force.[37] A more recent definition has been proposed by the WIND study,[38] where prolonged ventilation was defined as those patients in whom weaning could not be achieved within 7 days. However, we adopted the most recent definition provided by the Society of Thoracic Surgeons[10] (mechanical ventilation for > 24 h) as this low cutoff value seems to be closely related to real clinical practice.
Although such a discrepancy in terminology might lead to epidemiological variations, we could not find significant differences in PMV incidence rates with different cutoffs periods for the duration of mechanical ventilation. This is supposedly attributable to the strict inclusion criteria, where only studies employing logistic regression analyses for PMV prediction were eligible. Differences in admission and discharge circumstances as well as the heterogeneity between populations might also explain these findings.
Intriguingly, in the present study, ICU admission severity scores did not show a high predictive power when using the employed statistical method. A plausible explanation is that these scores can lose their predictive capacity in patients with a prolonged length of hospital stay.[7] Likewise, Rojek-Jarmuła et al.[39] found that the APACHE II score could not predict successful freedom from mechanical ventilation or tracheostomy tube removal in patients admitted to a weaning center for PMV. However, it seems that predictive ability is relatively better in studies with short PMV cutoff values.[22] As such, future collective evidence in the form of systematic reviews is warranted concerning the predictive ability of ICU scores for PMV.
The clinical significance of predicting the need for PMV is multifaceted. Successful identification of patients prone to requiring PMV would help identify the proposed weaning strategy. The weaning strategy of patients who required PMV should differ from their mechanically-ventilated counterparts of shorter durations.[40] For successful weaning off PMV, Jubran et al.[41] have recently compared two strategies (pressure support vs. unassisted breathing through a tracheostomy collar), while Pellegrini et al.[42] compared pressure support versus SBT using a T-piece. However, there is a lack of robust evidence regarding particular weaning strategies. Therefore, the clinical significance of predicting the need for PMV extends to tailoring adequate weaning strategies that could be investigated in future clinical trials.
It is also imperative to identify PMV predictors in different cohorts, especially in the context of high morbidity risk that could be associated with PMV. Approximately one-quarter of PMV patients experience generalized and persistent muscular weakness, and recent estimates indicated that 1 million individuals could develop critical illness neuromyopathy every year.[43] Such an attitude would increase the consequences of weaning failure due to ineffective cough and secretion retention.[44] In patients undergoing cardiac surgery, PMV for > 24 h was associated with an increased risk of pulmonary complications, nosocomial pneumonia, and poor outcomes.[45] Moreover, PMV could be associated with high rates of morbidity.[35,46] This way, a successful prediction of the need for PMV would allow clinicians to provide better care to alleviate patient risk.
Finally, gaining insights into the potential independent predictors of the need for PMV in a given population would help to plan for the appropriate utilization of ICUs. This could be approached by providing specialized weaning units to render patient care more cost-effective.[47] This is because weaning units require lower staff-to-patient ratios when compared to ICUs. In addition, these units are beneficial for patients with a stable condition except for the requirement for PMV. The adequate identification of PMV predictors at a local level could eventually help decision-makers to implement policies and directions related to providing these specialized units.
Conclusion
Several independent predictors of the need for PMV among adults admitted to ICUs have been identified in this study. Pre-operative (or preadmission) renal dysfunction, as indicated by elevated serum creatinine and BUN levels, as well as acute kidney injury, elevated heart rate (> 110 beats/min), and COPD were significant PMV predicting factors. In patients undergoing cardiac surgery, patients were more likely to require PMV with repeated and emergency operations, the need for a blood transfusion in the operating room, and prolonged CPB. Future studies that aim to predict the need for PMV should adopt a standardized definition of PMV. Employing a prospective follow-up of patients is advised to reveal other confounding factors. It is also necessary to integrate the burden of PMV and its significance on patient’s morbidity and/or mortality, with cardiac surgery clinical practice guidelines to help clinicians in their decision-making regarding treatment.
References
- 1.Hasan A. The Indications for Mechanical Ventilation. In: Hasan A, editor. Understanding Mechanical Ventilation:A Practical Handbook. India: Springer Science and Business Media; 2010. pp. 9–18. [Google Scholar]
- 2.Laporte L, Hermetet C, Jouan Y, Gaborit C, Rouve E, Shea KM, et al. Ten-year trends in intensive care admissions for respiratory infections in the elderly. Ann Intensive Care. 2018;8:84. doi: 10.1186/s13613-018-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao KM, Chen YC, Cheng KC, Wang JJ, Ho CH. Trends in intensive care unit admissions of COPD patients from 2003 to 2013 in Taiwan. Int J Chron Obstruct Pulmon Dis. 2018;13:2007–12. doi: 10.2147/COPD.S163571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zilberberg MD, Luippold RS, Sulsky S, Shorr AF. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008;36:724–30. doi: 10.1097/CCM.0B013E31816536F7. [DOI] [PubMed] [Google Scholar]
- 5.Hill AD, Fowler RA, Burns KE, Rose L, Pinto RL, Scales DC, et al. Long-term outcomes and health care utilization after prolonged mechanical ventilation. Ann Am Thorac Soc. 2017;14:355–62. doi: 10.1513/AnnalsATS.201610-792OC. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee K, Goyal A, Kakkera K, Harrington S, Corwin HL. National trends (2009-2013) for palliative care utilization for patients receiving prolonged mechanical ventilation. Crit Care Med. 2018;46:1230–7. doi: 10.1097/CCM.0000000000003182. [DOI] [PubMed] [Google Scholar]
- 7.Estenssoro E, González F, Laffaire E, Canales H, Sáenz G, Reina R, et al. Shock on admission day is the best predictor of prolonged mechanical ventilation in the ICU. Chest. 2005;127:598–603. doi: 10.1378/chest.127.2.598. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- 9.Pulido JN. Prediction of prolonged mechanical ventilation after cardiac surgery:An imperfect crystal ball. J Thorac Cardiovasc Surg. 2017;153:116–7. doi: 10.1016/j.jtcvs.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 10.D'Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, et al. The society of thoracic surgeons adult cardiac surgery database:2019 update on outcomes and quality. Ann Thorac Surg. 2019;107:24–32. doi: 10.1016/j.athoracsur.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Cislaghi F, Condemi AM, Corona A. Predictors of prolonged mechanical ventilation in a cohort of 3,269 CABG patients. Minerva Anestesiol. 2007;73:615–21. [PubMed] [Google Scholar]
- 13.Li J, Zhan QY, Wang C. Survey of prolonged mechanical ventilation in intensive care units in Mainland China. Respir Care. 2016;61:1224–31. doi: 10.4187/respcare.04295. [DOI] [PubMed] [Google Scholar]
- 14.Peñuelas O, Frutos-Vivar F, Fernández C, Anzueto A, Epstein SK, Apezteguía C, et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am J Respir Crit Care Med. 2011;184:430–7. doi: 10.1164/rccm.201011-1887OC. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui MM, Paras I, Jalal A. Risk factors of prolonged mechanical ventilation following open heart surgery:What has changed over the last decade? Cardiovasc Diagn Ther. 2012;2:192–9. doi: 10.3978/j.issn.2223-3652.2012.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vieira JM, Jr, Castro I, Curvello-Neto A, Demarzo S, Caruso P, Pastore L., Jr Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35:184–91. doi: 10.1097/01.CCM.0000249828.81705.65. [DOI] [PubMed] [Google Scholar]
- 17.Cislaghi F, Condemi AM, Corona A. Predictors of prolonged mechanical ventilation in a cohort of 5123 cardiac surgical patients. Eur J Anaesthesiol. 2009;26:396–403. doi: 10.1097/EJA.0b013e3283232c69. [DOI] [PubMed] [Google Scholar]
- 18.Yang PH, Hung JY, Yang CJ, Tsai JR, Wang TH, Lee JC, et al. Successful weaning predictors in a respiratory care center in Taiwan. Kaohsiung J Med Sci. 2008;24:85–91. doi: 10.1016/S1607-551X(08)70102-5. [DOI] [PubMed] [Google Scholar]
- 19.Wu YK, Kao KC, Hsu KH, Hsieh MJ, Tsai YH. Predictors of successful weaning from prolonged mechanical ventilation in Taiwan. Respir Med. 2009;103:1189–95. doi: 10.1016/j.rmed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Lei Q, Chen L, Zhang Y, Fang N, Cheng W, Li L, et al. Predictors of prolonged mechanical ventilation after aortic arch surgery with deep hypothermic circulatory arrest plus antegrade selective cerebral perfusion. J Cardiothorac Vasc Anesth. 2009;23:495–500. doi: 10.1053/j.jvca.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Totonchi Z, Baazm F, Chitsazan M, Seifi S, Chitsazan M. Predictors of prolonged mechanical ventilation after open heart surgery. J Cardiovasc Thorac Res. 2014;6:211–6. doi: 10.15171/jcvtr.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu L, Zhu B, Jiang L, Du B, Zhu X, Li A, et al. Weaning critically ill patients from mechanical ventilation:A prospective cohort study. J Crit Care. 2015;30:862, 7–13. doi: 10.1016/j.jcrc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Jin M, Ma WG, Liu S, Zhu J, Sun L, Lu J, et al. Predictors of prolonged mechanical ventilation in adults after acute type-A aortic dissection repair. J Cardiothorac Vasc Anesth. 2017;31:1580–7. doi: 10.1053/j.jvca.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Muzaffar SN, Gurjar M, Baronia AK, Azim A, Mishra P, Poddar B, et al. Predictors and pattern of weaning and long-term outcome of patients with prolonged mechanical ventilation at an acute intensive care unit in North India. Rev Bras Ter Intensiva. 2017;29:23–33. doi: 10.5935/0103-507X.20170005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark PA, Inocencio RC, Lettieri CJ. I-TRACH: Validating A tool for predicting prolonged mechanical ventilation. J Intensive Care Med. 2018;33:567–73. doi: 10.1177/0885066616679974. [DOI] [PubMed] [Google Scholar]
- 26.Clark PA, Lettieri CJ. Clinical model for predicting prolonged mechanical ventilation. J Crit Care. 2013;28:880, 1–7. doi: 10.1016/j.jcrc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Agle SC, Kao LS, Moore FA, Gonzalez EA, Vercruysse GA, Todd SR, et al. Early predictors of prolonged mechanical ventilation in major torso trauma patients who require resuscitation. Am J Surg. 2006;192:822–7. doi: 10.1016/j.amjsurg.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 28.Légaré JF, Hirsch GM, Buth KJ, MacDougall C, Sullivan JA. Preoperative prediction of prolonged mechanical ventilation following coronary artery bypass grafting. Eur J Cardiothorac Surg. 2001;20:930–6. doi: 10.1016/s1010-7940(01)00940-x. [DOI] [PubMed] [Google Scholar]
- 29.Piotto RF, Ferreira FB, Colósimo FC, Silva GS, Sousa AG, Braile DM, et al. Independent predictors of prolonged mechanical ventilation after coronary artery bypass surgery. Rev Bras Cir Cardiovasc. 2012;27:520–8. doi: 10.5935/1678-9741.20120093. [DOI] [PubMed] [Google Scholar]
- 30.Sanabria A, Gómez X, Vega V, Domínguez LC, Osorio C. Prediction of prolonged mechanical ventilation in patients in the intensive care unit A cohort study. Colomb Med (Cali) 2013;44:184–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Natarajan K, Patil S, Lesley N, Ninan B. Predictors of prolonged mechanical ventilation after on-pump coronary artery bypass grafting. Ann Card Anaesth. 2006;9:31–6. [PubMed] [Google Scholar]
- 32.Sellares J, Ferrer M, Cano E, Loureiro H, Valencia M, Torres A, et al. Predictors of prolonged weaning and survival during ventilator weaning in a respiratory ICU. Intensive Care Med. 2011;37:775–84. doi: 10.1007/s00134-011-2179-3. [DOI] [PubMed] [Google Scholar]
- 33.Cottereau G, Dres M, Avenel A, Fichet J, Jacobs FM, Prat D, et al. Handgrip strength predicts difficult weaning but not extubation failure in mechanically ventilated subjects. Respir Care. 2015;60:1097–104. doi: 10.4187/respcare.03604. [DOI] [PubMed] [Google Scholar]
- 34.Gillespie DJ, Marsh HM, Divertie MB, Meadows JA., 3rd Clinical outcome of respiratory failure in patients requiring prolonged (greater than 24 hours) mechanical ventilation. Chest. 1986;90:364–9. doi: 10.1378/chest.90.3.364. [DOI] [PubMed] [Google Scholar]
- 35.Chelluri L, Im KA, Belle SH, Schulz R, Rotondi AJ, Donahoe MP, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32:61–9. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 36.MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S, et al. Management of patients requiring prolonged mechanical ventilation:Report of a NAMDRC consensus conference. Chest. 2005;128:3937–54. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 37.Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 38.Béduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, et al. Epidemiology of weaning outcome according to a new definition The WIND study. Am J Respir Crit Care Med. 2017;195:772–83. doi: 10.1164/rccm.201602-0320OC. [DOI] [PubMed] [Google Scholar]
- 39.Rojek-Jarmuła A, Hombach R, Krzych Ł J. APACHE II score cannot predict successful weaning from prolonged mechanical ventilation. Chron Respir Dis. 2017;14:270–5. doi: 10.1177/1479972316687100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang CT, Yu CJ. Conventional weaning parameters do not predict extubation outcome in intubated subjects requiring prolonged mechanical ventilation. Respir Care. 2013;58:1307–14. doi: 10.4187/respcare.01773. [DOI] [PubMed] [Google Scholar]
- 41.Jubran A, Grant BJ, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, et al. Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: A randomized trial. JAMA. 2013;309:671–7. doi: 10.1001/jama.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos Pellegrini JA, Boniatti MM, Boniatti VC, Zigiotto C, Viana MV, Nedel WL, et al. Pressure-support ventilation or T-piece spontaneous breathing trials for patients with chronic obstructive pulmonary disease a randomized controlled trial. PLoS One. 2018;13:0202404. doi: 10.1371/journal.pone.0202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, et al. An official american thoracic society clinical practice guideline:The diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190:1437–46. doi: 10.1164/rccm.201411-2011ST. [DOI] [PubMed] [Google Scholar]
- 44.Jiang C, Esquinas A, Mina B. Evaluation of cough peak expiratory flow as a predictor of successful mechanical ventilation discontinuation:A narrative review of the literature. J Intensive Care. 2017;5:33. doi: 10.1186/s40560-017-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Brien SM, Feng L, He X, Xian Y, Jacobs JP, Badhwar V, et al. The society of thoracic surgeons 2018 Adult cardiac surgery risk models:Part 2-statistical methods and results. Ann Thorac Surg. 2018;105:1419–28. doi: 10.1016/j.athoracsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Boniatti MM, Friedman G, Castilho RK, Vieira SR, Fialkow L. Characteristics of chronically critically ill patients:Comparing two definitions. Clinics (Sao Paulo) 2011;66:701–4. doi: 10.1590/S1807-59322011000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannan LM, Tan S, Hopkinson K, Marchingo E, Rautela L, Detering K, et al. Inpatient and long-term outcomes of individuals admitted for weaning from mechanical ventilation at a specialized ventilation weaning unit. Respirology. 2013;18:154–60. doi: 10.1111/j.1440-1843.2012.02266.x. [DOI] [PubMed] [Google Scholar]


